Abstract
Mutations in the guanosine diphosphate mannose (GDP-mannose) pyrophosphorylase B (GMPPB) gene encoding a key enzyme of the glycosylation pathway have been described in families with congenital (CMD) and limb girdle (LGMD) muscular dystrophy with reduced alpha-dystroglycan (α-DG) at muscle biopsy.
Patients typically display a combined phenotype of muscular dystrophy, brain malformations, and generalized epilepsy. However, a wide spectrum of clinical severity has been described ranging from classical CMD presentation to children with mild, yet progressive LGMD with or without intellectual disability. Cardiac involvement, including a long QT interval and left ventricular dilatation, has also been described in four cases.
Two missense mutations in GMPPB gene, one novel and one already reported, have been identified in a 21-year-old man presenting with elevated CK (38,650 UI/L; normal values <150 UI/L) without overt muscle weakness. Major complaints included limb myalgia, exercise intolerance, and several episodes of myoglobinuria consistent with a form of metabolic myopathy. Muscle biopsy showed only minimal alterations, whereas a marked reduction of glycosylated α-DG was evident.
This case further expands the phenotypic spectrum of GMPPB mutations and highlights the importance of exhaustive molecular characterization of patients with reduced glycosylation of α-DG at muscle biopsy.
Introduction
Congenital muscular dystrophies with hypoglycosylation of alpha-dystroglycan (α-DG) are a heterogeneous group of disorders of varying severity often associated with brain and eye defects in addition to muscular dystrophy.
Seventeen genes have been implicated in defective α-DG glycosylation, including proteins with putative or uncertain glycosyltransferase function such as POMT1, POMT2, POMGNT1, LARGE, GTD2, B4GAT1, B3GALNT2; one protein implicated in a glycan phosphorylation SGK-196; proteins involved in mannose, dolichol, and ribitol metabolism such as GMPPB, DPM1, DPM2, DPM3, DOLK, and other largely uncharacterized genes such as FKTN, FKRP, ISPD, and TMEM5 (Bouchet-Seraphin et al. 2015; Gerin et al. 2016). Allelic mutations in some of these genes have been also linked to milder limb-girdle muscular dystrophy phenotype such as LGMD2I (FKRP), LGMD2K (POMT1), LGMD2M (FKTN), LGMD2N (POMT2), LGMD2O (POMGnT1), and LGMD2U (ISPD) (Gerin et al. 2016). Among them mutations in FKRP are quite common, leading to at least 10% of all LGMD (Nigro and Savarese 2014).
The most recently added gene to the list is the guanosine diphosphate mannose pyro phosphorylase B (GMPPB) gene. The guanosine diphosphate mannose pyro phosphorylase B encodes an enzyme involved in α-DG glycosylation; in particular, it catalyzes the formation of GDP-mannose, which is required for O-mannosylation of proteins. As expected, a defect of GMPPB results in hypoglycosylation of α-DG in human cells and animal models (Carss et al. 2013; Raphael et al. 2014; Cabrera-Serrano et al. 2015; Bharucha-Goebel et al. 2015; Belaya et al. 2015; Jensen et al. 2015; Oestergaard et al. 2016; Montagnese et al. 2016; Gerin et al. 2016).
GMPPB causative variants have been described so far in 49 cases with a spectrum of clinical phenotypes ranging from forms with structural brain and eye anomalies (OMIM615350) to forms with a limb-girdle phenotype (LGMD2T – OMIM 615352). In particular a predominant mild LGMD phenotype associated with GMPPB mutation is described in 29 patients.
Recently also congenital myasthenic syndromes have been reported with patients with GMPPB mutation (CMS-GMPPB) and EMG myasthenic patterns have been observed in LGMD-GMPPB patients (Belaya et al. 2015; Montagnese et al. 2016).
Generalized epilepsy has been described in 12 patients (Carss et al. 2013; Raphael et al. 2014; Cabrera-Serrano et al. 2015; Bharucha-Goebel et al. 2015; Belaya et al. 2015; Jensen et al. 2015) and evidence of cardiorespiratory involvement including a long QT interval, sino-atrial block, wandering atrial pacemaker, bicuspid aortic valve, and left ventricular dilatation in seven patients (Carss et al. 2013; Cabrera-Serrano et al. 2015; Jensen et al. 2015). Furthermore, mild to severe mental retardation is reported in 24 patients (Carss et al. 2013; Raphael et al. 2014; Cabrera-Serrano et al. 2015; Bharucha-Goebel et al. 2015; Belaya et al. 2015; Jensen et al. 2015; Oestergaard et al. 2016) and ophthalmologic features as cataracts or strabismus have been described in 9 cases (Carss et al. 2013; Bharucha-Goebel et al. 2015; Jensen et al. 2015). Cerebellar hypoplasia at brain MRI is reported in 6 patients with severe mental retardation and CMD phenotype (Carss et al. 2013 and Jensen et al. 2015). Muscle MRI data from 11 patients have been published so far (Bharucha-Goebel et al. 2015; Belaya et al. 2015; Oestergaard et al. 2016; Montagnese et al. 2016). In one of the most recent papers (Oestergaard et al. 2016) the authors defined a specific muscle MRI pattern associated with LGMD-GMPPB patients which preferentially involves paraspinal and hamstring muscles.
Here we describe a further case harboring two missense mutations in GMPPB and presenting mild features consistent with a metabolic muscle disorder. We also provide a summary of the main characteristics observed in previously described LGMD-GMPPB patients (Table 1).
Table 1.
Summary of the main clinical, lab and genetic features of previously described GMPPB patient with LGMD phenotype
| Sex | Origin | Onset | Muscular symptoms | Neurological features | Cardiorespiratory features | Ophthalmologic features | CK level(UI/L) | Neurophysiology | Brain MRI findings | Muscle MRI findings | Genetical findings | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | F | Indian | Birth | Not shown | Mild intellectual disability Epilepsy |
None | None | 4,500 | Not performed | No structural abnormality | Not performed | c.64C>T c.1000G>A |
Carss et al. (2013) |
| P2 | M | English | 4 years | Mild exercise intolerance | None | None | None | 3,000 | Not performed | Not performed | Not performed | c.79G>C c.988G>A |
Carss et al. (2013) |
| P3 | M | Egyptian | 2.5 years | Mild lower limb weakness | Mild intellectual disability Epilepsy |
Wandering atrial pacemaker Cardiomyopathy Respiratory insufficiency |
Cataract, nystagmus | 5,200 | Not performed | No structural abnormality | Not performed | c.553C>T c.553C>T |
Carss et al. (2013) |
| P4 | M | Australian | 15 years | Difficulties in running Exercises intolerance Calves hypertrophy |
Behavioral problems | None | None | 5,000 | Myopathic EMG | Not performed | Not performed | c.79G>C c.95C>T |
Cabrera-Serrano et al. (2015) |
| P5 | M | Australian | 26 years | Lower limb weakness Calves hypertrophy |
None | Right bundle branch block | None | 3,600–17,000 | Not performed | Not performed | Not performed | c.79G>C c.95C>T |
Cabrera-Serrano et al. (2015) |
| P6 | F | Italian | 28 years | Upper and lower limb weakness Calves hypertrophy |
None | Respiratory insufficiency at 70 years | None | Not shown | Myopathic EMG | Not performed | Not performed | c.79G>C c.797G>A |
Cabrera-Serrano et al. (2015) |
| P7 | M | Italian | 35 years | Upper and lower limb weakness Calves hypertrophy |
None | Sino-atrial block | None | 1,000 | Not performed | Not performed | Not performed | c.79G>C c.797G>A |
Cabrera-Serrano et al. (2015) |
| P8 | M | Caucasian | 22 years | Upper and lower limb weakness | Learning difficulties Autism |
None | None | 1,400 | Not performed | Not performed | Not performed | c.79G>C c.1036C>A |
Cabrera-Serrano et al. (2015) |
| P9 | M | Caucasian | 22 years | Upper and lower limb weakness | Learning difficulties | None | None | 1,400 | Not performed | Not performed | Not performed | c.79G>C c.1036C>A |
Cabrera-Serrano et al. (2015) |
| P10 | M | Unknown | 13 years | Recurrent rhabdomyolysis | Rolandic epilepsy | None | None | 700 baseline 35,000 in crises |
Not performed | Not performed | Not performed | c.860G>A c.95C>T |
Cabrera-Serrano et al. (2015) |
| P11 | M | Unknown | 12 years | Mild exercise intolerance Upper and lower limb weakness (MRC4/5) Calves hypertrophy |
Intellectual disability (IQ 49) ADHD Epilepsy (6 years) |
None | Cataract (severe) | 18,000 | Myopathic EMG | Bilateral cortical thinning | Normal muscle bulk, mild T1 signal increase | c.79G>C c.790C>T |
Bharucha-Goebel et al. (2015) |
| P12 | F | Unknown | 12 years | Muscle pain Upper and lower limb weakness (MRC4/5) Calves hypertrophy |
Intellectual disability (IQ 58) | None | Cataract (mild) | 15,000 | Myopathic EMG | Normal | Normal muscle bulk, mild T1 signal increase | c.79G>C c.790C>T |
Bharucha-Goebel et al. (2015) |
| P13 | F | Unknown | 13 years | Mild exercise intolerance Upper and lower limb weakness (MRC4/5) Calves hypertrophy |
Mild intellectual disability (IQ 81) | Not investigated | Cataract (mild) | 3,000 | Not performed | Not performed | Normal muscle bulk, mild T1 signal increase | c.79G>C c.790C>T |
Bharucha-Goebel et al. (2015) |
| P14 | F | Caucasian | 15 years | Episodes of generalized sudden weakness | None | Not investigated | None | 3,000 | Myopathic EMG decrement on repetitive nerve stimulation | Not performed | Not performed | c.559C>T c.578T>C |
Belaya et al. (2015) |
| P15 | M | Caucasian | 2.5 years | Upper and lower limb weakness | Mild intellectual disability | Not investigated | None | 3,000 | Myopathic EMG Increase in jitter + block on SFEMG | Not performed | Not performed | c.656T>C c.860G>A |
Belaya et al. (2015) |
| P16 | M | Caucasian | 2 years | Upper and lower limb weakness | Moderate intellectual disability | Not investigated | None | 2,832 | Increase in jitter | Not performed | Not performed | c.656T>C c.860G>A |
Belaya et al. (2015) |
| P17 | F | Asian | 2.5 years | Upper and lower limb weakness | Moderate intellectual disability Epilepsy |
Not investigated | None | 2,500 | Normal | Not performed | Not performed | c.64C>T c.1000G>A |
Belaya et al. (2015) |
| P18 | M | Unknown | 17 years | Muscle weakness | None | Ischemic heart disease | None | 1,331 | Not performed | Not performed | Not performed | c.79G>C c.1069G>A |
Jensen et al. (2015) |
| P19 | M | Unknown | 17 years | Mild exercise intolerance with myalgias and myoglobinuria | None | None | None | 7,250 baseline 52,000 in crises |
Not performed | Not performed | Not performed | c.79G>C c.1069G>A |
Jensen et al. (2015) |
| P20 | M | Unknown | 15 years | Muscle weakness | None | None | None | 3,693 | Not performed | Not performed | Not performed | c.79G>C c.760G>A |
Jensen et al. (2015) |
| P21 | M | Unknown | 23 years | Incidental hyperCKemia Myoglobinuria | None | Atrio-ventricular block (Ist degree) | None | 5,900 | Not performed | Not performed | Not performed | c.79G>C c.402+1G>A |
Jensen et al. (2015) |
| P22 | F | Unknown | 5 years | Muscle weakness | Moderate intellectual disability | Moderate restrictive lung disease | None | 7,112 | Not performed | Not performed | Not performed | c.721C>T c.1034T>C |
Jensen et al. (2015) |
| P23 | M | Caucasian | 15 years | Difficulties in running | None | FVC 59% | None | 2,390 | Myopathic EMG decrement on repetitive nerve stimulation | Normal | Severe T1 signal increase in paraspinal (MS 4) and hamstring (MS 4) muscles | c.79G>C c.859C>T |
Oestergaard et al. (2016) |
| P24 | F | Caucasian | 30 years | Difficulties in climbing stairs | None | FVC 66% | None | 1,520 | Not performed | Normal | Severe T1 signal increase in paraspinal (MS 4) and hamstring (MS 4) muscles | c.79G>C c.859C>T |
Oestergaard et al. (2016) |
| P25 | F | Caucasian | 12 years | Difficulties in running | None | FVC 76% | None | 1,604 | Myopathic EMG decrement on repetitive nerve stimulation | Normal | Mild T1 signal increase in paraspinal (MS 3) and hamstring (MS 2) muscles | c.464G>A c.1039_1043 dup |
Oestergaard et al. (2016) |
| P26 | F | Caucasian | 18 years | Exercise intolerance | MMSE 27/30 | None | None | 1,200 | Myopathic EMG decrement on repetitive nerve stimulation | Normal | Mild T1 signal increase in paraspinal (MS 2) and hamstring (MS 2) muscles | c.79G>C c.760G>A |
Oestergaard et al. (2016) |
| P27 | M | Caucasian | 5 years | Walked at 5 years | Not reported | FVC 61% | None | 1,327 | Not performed | Normal | Not performed | c.79G>C c.859C>T |
Oestergaard et al. (2016) |
| P28 | M | Caucasian | 25 years | Difficulties in walking | MMSE 28/30 | FVC 43% Ejection fraction 48% |
None | 619 | Myopathic EMG decrement on repetitive nerve stimulation | Normal | Not performed | c.902C>G c.1069G>A |
Oestergaard et al. (2016) |
| P29 | M | Unknown | 8 years | Calves hypertrophy Myoglobinuria Action tremor |
None | None | None | 3,000 baseline 25,000 in crises |
Myopathic EMG normal repetitive nerve stimulation | Normal | Mild edematous changes of soleus muscles | c.79G>C c.859C>T |
Montagnese et al. (2016) |
| P30 | M | Caucasian | 15 years | Mild exercise intolerance with myalgias and myoglobinuria | Moderate intellectual disability (IQ 45) | WPW syndrome | None | 2,000 baseline 38,000 in crises |
Myopathic EMG normal repetitive nerve stimulation | Slight pericerebellar subarachnoid space enlargement | Mild T1 signal increase in hamstring muscles (MS2) | c.79G>C c.943C>A |
This report |
MRC Medical Research Council evaluation of strength, MMSE Mini-Mental State Examination, WPW Wolf-Parkinson-White, FVC forced vital capacity, SFEMG single fibre EMG, MS Mercuri scale
Case Report
The propositus, a 23-year-old man, is the second child of healthy unrelated parents. He was born at term after uneventful pregnancy and normal delivery. Psychomotor development was reported normal. At 6 years, he showed generalized epileptic seizures and he was put on valproate therapy which was continued until 12 years of age despite no further epileptic manifestations. Last EEG, performed at the age of 14 years, was normal.
At the age of 15 years he came to observation for a first episode of acute rhabdomyolysis after moderate exercise. CK level at the time was 38,650 UI/L and myoglobinuria was reported. Subsequently, serum CK level dropped to 2,100 UI/L in follow-up measurements and remained elevated thereafter, despite substantial abstinence from physical activity.
On neurological examination there was no motor defect. We only observed mild calf hypertrophy and flat feet. Wechsler Adult Intelligence Scale – Revised (WAIS-R) showed a generalized moderate mental retardation (verbal IQ 48; performance IQ 45).
Respiratory muscles function was spared with normal spirometry. ECG documented short PR interval and prolonged QRS, with occasional delta waves, indicating Wolf–Parkinson–White syndrome. Echocardiography was normal for chambers dimensions, morphology, and ejection fraction. At 23 years the patient underwent ablation of the abnormal electrical pathway by radiofrequency catheter.
At 16 years, a muscle biopsy revealed moderate fiber size variability with several hypotrophic fibers (Fig. 1a) and increased staining for acid phosphatase (Fig. 1b). Most hypotrophic fibers were type I. The immunofluorescence study of sarcolemmal proteins revealed a significant reduction of α-DG binding (Fig. 1c–e). Western blot analysis with anti-IIH6 antibody showed a 15% reduction of α-DG levels compared to the control (Fig. 1f). Serum CK level was 1,062 UI/L at the time of the muscle biopsy.
Fig. 1.
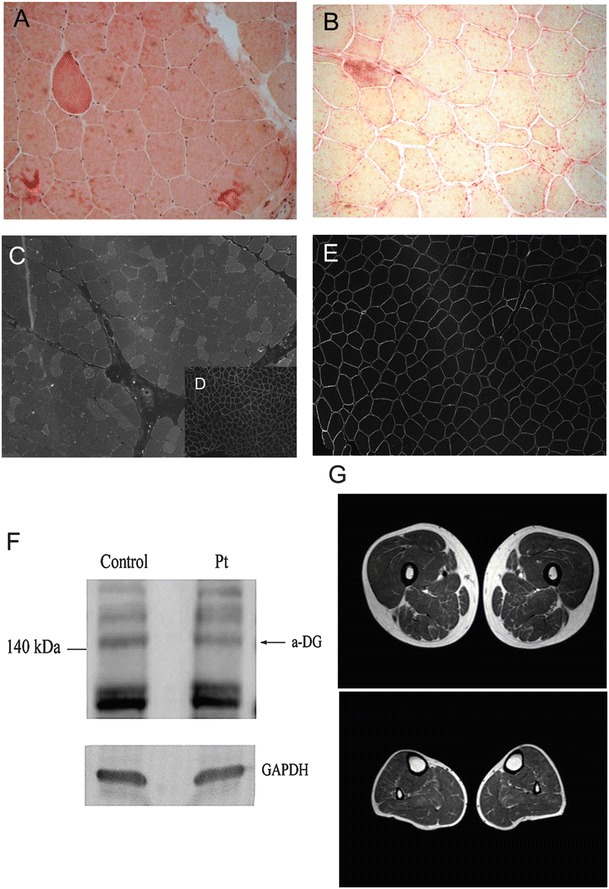
(A, B) Histology of skeletal muscle from the patient. Hematoxylin eosin staining showed moderate fiber size variability with several hypotrophic fibers and some hypercontracted fibers (A). The staining for acid phosphatase was increased (B). (C–E) Immunofluorescence with IIH6C4 antibody showing profound reduction of immunodetectable glycosylated α–DG (C) in comparison to control muscle stained with the same antibody (D). In (E) immunofluorescence for caveolin-3, showing normal protein expression. (F) Western blotting showed an expected band of 140 kDa with a 15% reduction in glycosylated α-DG compared to control. Anti-α-Dystroglycan, clone IIH6, monoclonal antibody (Millipore, Temecula, CA) was used at 1:100 dilution, according to manufacturer’s instruction. GADPH expression is used as control for protein loading. Band intensities were evaluated by densitometry using the ImageQuant 350 system (Amersham Biosciences). (G) Muscle MRI performed at 21 years disclosed a moderate fatty infiltration in muscles of the posterior thigh (adductor magnus hamstrings, and partially sartorius) and minimal fatty infiltration of soleus muscle at leg level
Muscle MRI at 21 years revealed a moderate increase in T1 hyperintensity in posterior tights muscles, hamstrings, and very mild involvement of soleus (Fig. 1g). A subsequent muscle MRI at 23 years showed a stable picture of muscle involvement (data not shown).
Brain MRI performed at the age of 23 years did not reveal definite abnormalities except for slight pericerebellar subarachnoid space enlargement.
Latest neurological examination at the age of 23 years showed minimal muscle strength reduction (MRC 4) of pelvic girdle muscles. Deep tendon reflexes were reduced in all limbs. Pseudo-hypertrophy of calves and quadriceps, and a mild scoliotic curve were evident. In view of the recent work by Belaya et al. (2015) reporting myasthenic features in GMPPB patients, we performed repetitive nerve stimulation of the left median nerve which did not reveal any pathological findings.
Genetic analysis of the most common form of alpha-dystroglycanopathy with milder phenotype did not show pathogenic mutations in FKRP, POMT-1, POMT-2, LARGE, FKTN, and ISPD. This case was included in a group of undiagnosed muscular dystrophy patients to be analyzed by Dystroplex, an extended NGS testing panel able to investigate, in a single tube, the coding regions of 93 genes linked to CMDs, LGMDs, and related diseases. We identified a c.79G>C (p.D27H) on the maternal allele and a c.943C>A (p. G315S) on the paternal allele. The latter variant is novel and affects a highly conserved residue and it is considered damaging on Polyphen prediction software.
Mutation c.79G>C (p.D27H) has been previously reported in 19 cases presenting with LGMD phenotype, whereas it has never been described in association with CMD.
Discussion
Exercise myalgia and rhabdomyolysis are commonly suggestive of an underlying metabolic condition, such as a glycogen storage disorder, fatty acid oxidation disorder, or mitochondrial cytopathy. However, there are anecdotal reports of pseudometabolic dominant clinical manifestations of defined genetic muscular diseases.
Pseudometabolic symptoms have been frequently reported in patients with dystrophin gene mutations with neither fixed muscle weakness nor calf hypertrophy. Patients harbored in-frame deletion, rarely missense mutations. In some cases immunohistochemical analysis demonstrated normal dystrophin staining for antibodies against the COOH-terminus and rod domain, and dystrophin western blot analysis confirmed normal dystrophin quantity (Minetti et al. 1993; Figarella-Branger et al. 1997; Veerapandiyan et al. 2010; Liewluck et al. 2015). Exertional myalgia and rhabdomyolysis may also be a presenting feature in female carriers of X-linked dystrophinopathies (Itagaki et al. 1993).
In 2007 Nguyen et al., retrospectively examining clinical data from 40 patients with dysferlin deficiency associated with LGMD2B phenotype, found that four patients had distal leg painful swelling without any weakness or atrophy (Nguyen et al. 2007).
Likewise two different series of patients with genetically confirmed LGMD2I due to mutations in the FKRP gene displayed a high incidence (26–37%) of pseudometabolic presentation (Mathews et al. 2011; Lindberg et al. 2012). Most of these patients were homozygous for the common p.L276I mutation and exercise-induced muscle cramps and myoglobinuria were the only presenting symptom occurring before the onset of muscle weakness.
There are a few reports on pseudometabolic presentation in patients affected by LGMD1C, LGMD2A, LGMD2C, LGMD2D, LGMD2E, and LGMD2L (Aboumousa et al. 2008; Penisson-Besnier et al. 1998; Pena et al. 2010; Ceravolo et al. 2014; Mongini et al. 2002; Cagliani et al. 2001; Lahoria et al. 2014; Quinlivan and Jungbluth 2012).
So far among patients carrying disease-related variants in GMMPB only four other similar cases have been reported, three of them carrying the same c.79G>C p.D27H variant presented by our patient (Jensen et al. 2015; Montagnese et al. 2016). One child showed also a severe muscle impairment beside the metabolic presentation, whereas the other patients showed exclusively post-exercise rhabdomyolysis with myoglobinuria.
Some hypotheses have been postulated to account for the pseudometabolic presentation in patients with muscular dystrophies, even if the mechanisms involved are not completely clear. It is widely accepted that deficiency of one or more components of the dystrophin associated protein complex (DPC) leads to an increased membrane susceptibility to contraction-induced damage. This process has been largely studied in mdx mice, the animal model of DMD, and seems to be due to a structural weakness of the sarcolemma and to impaired homeostasis resulting from increased membrane permeability to Ca2+ and Na+ (Gillis 1999; Allen 2004). It has been suggested that the cytoskeletal disarrangement, due to dystrophin defect, can induce abnormalities of enzyme complexes organization and function, which in turn alter the control of energy metabolism in the muscle cells (Even et al. 1994). Finally it has been demonstrated that the level of sarcolemmal damage is directly correlated with the magnitude of mechanical stress (Petrof et al. 1993).
Thus it is possible that the milder end of the muscular dystrophies is able to a heavier workload, and as a consequence is more prone to rhabdomyolysis. The present patient, carrying two mutations in GMPPB gene, presented with exertional myalgias and myoglobinuria. In addition muscle biopsy showed increased acid phosphatase staining which can be considered the hallmark of several metabolic myopathies such as glycogenosis type 2. The muscle MRI disclosed a mild involvement of the hamstring muscles that has recently been defined as a common finding in LGMD2T (Oestergaard et al. 2016). Interestingly, our patient, carrying the c. c.79G>C p.D27H substitution which shows an allelic frequency of 32.7% among LGMD-GMPPB patients, confirms the mild muscular phenotype associated with this GMPPB variant (Carss et al. 2013; Oestergaard et al. 2016; Montagnese et al. 2016). It has also been suggested that the p.D27H retains more functional activity than other mutants found in more severe phenotypes as demonstrated by C2C12 myoblasts transfection experiments (Carss et al. 2013). Aside from the predominant pseudometabolic presentation, the boy suffered from epileptic seizures in childhood which remitted in adolescence. These did not differ from common idiopathic benign epilepsy of childhood. There is limited information on the type and severity of epilepsia in GMPPB. Drug resistant epileptic seizure has been mostly associated with more severe phenotypes but to our knowledge no cases have been described with benign epilepsy. The boy also suffered from WPW syndrome and underwent ablation of the abnormal electrical pathway. WPW has a prevalence of 1/450, and it has never been described in association with mutations in GMMPB. Taking all this into account a possible concomitant presentation of both epilepsy and WPW cannot be excluded. Albeit a decremental result at repetitive nerve stimulation (RNS) and good response to pyridostigmine have been reported in some GMPPB patients (Belaya et al. 2015), we have not pursued this therapy because of lack of suggestive RNS findings and in fear of low compliance from the patient who suffers from moderate mental retardation.
In conclusion, our case extends the number of patients with pseudometabolic presentation of limb girdle muscle dystrophies. An alpha-dystroglycanopathy should be considered in the differential diagnosis of the most common metabolic myopathies in order to provide a correct genetic counseling and long-term surveillance.
Acknowledgments
The authors wish to thank the patient and his family for the collaboration, and Paolo Broda and Annagloria Incontrera for technical assistance.
Take Home Message
In this report we highlight the importance of considering an α-DG glycosylation defect when a pseudometabolic phenotype occurs.
Compliance With Ethics Guidelines
Conflict of Interest
Chiara Panicucci, Chiara Fiorillo, Francesca Moro, Guja Astrea, Giacomo Brisca, Federica Trucco, Marina Pedemonte, Paola Lanteri, Lucia Sciarretta, Carlo Minetti, Filippo M. Santorelli, and Claudio Bruno declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Author Contributions
Study concept and design: Panicucci, Fiorillo, Bruno.
Acquisition of data: Panicucci, Fiorillo, Moro, Astrea, Trucco, Lanteri, Sciarretta.
Analysis and interpretation of data: Panicucci, Fiorillo, Brisca, Pedemonte, Lanteri, Sciarretta, Bruno.
Drafting of the manuscript: Panicucci, Fiorillo, Pedemonte, Bruno.
Critical revision of the manuscript for important intellectual content: Fiorillo, Minetti, Santorelli, Bruno.
Obtained funding: Santorelli, Bruno.
Administrative, technical, and material support: Santorelli, Bruno.
Study supervision: Fiorillo, Bruno.
Footnotes
“Chiara Panicucci” and “Chiara Fiorillo” equally contributed to this work.
Contributor Information
Claudio Bruno, Email: claudio2246@gmail.com.
Collaborators: Matthias Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Aboumousa A, Hoogendijk J, Charlton R, Barresi R, Herrmann R, Voit T, Hudson J, Roberts M, Hilton-Jones D, Eagle M, Bushby K, Straub V. Caveolinopathy-new mutations and additional symptoms. Neuromuscul Disord. 2008;18(7):572–578. doi: 10.1016/j.nmd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Allen DG. Brief review skeletal muscle function: role of ionic changes in fatigue, damage and disease. Clin Exp Pharmacol Physiol. 2004;31:485–493. doi: 10.1111/j.1440-1681.2004.04032.x. [DOI] [PubMed] [Google Scholar]
- Belaya K, Rodríguez Cruz P, Liu W, Maxwell S, McGowan S, Farrugia M, Petty R, Walls T, Sedghi M, Basiri K, Yue W, Sarkozy A, Bertoli M, Pitt M, Kennett R, Schaefer A, Bushby K, Parton M, Lochmuller H, Palace J, Muntoni F, Beeson D. Mutations in GMPPB cause congenital myasthenic syndrome and bridge myasthenic disorders with dystroglycanopathies. Brain. 2015;138:2493–2504. doi: 10.1093/brain/awv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha-Goebel DX, Neil E, Donkervoort S, Dastgir J, Wiggs E, Winder TL, Moore SA, Iannaccone ST, Bönnemann CG. Intrafamilial variability in GMPPB-associated dystroglycanopathy: broadening of the phenotype. Neurology. 2015;84(14):1495–1497. doi: 10.1212/WNL.0000000000001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchet-Seraphin C, Vuillaumier-Barrot S, Seta N. Dystroglycanopathies: about numerous genes involved in glycosylation of one single glycoprotein. J Neuromuscul Dis. 2015;2:27–38. [PubMed] [Google Scholar]
- Cabrera-Serrano M, Ghaoui R, Ravenscroft G, Johnsen RD, Davis MR, Corbett A, Reddel S, Sue CM, Liang C, Waddell LB, Kaur S, Lek M, North KN, MacArthur DG, Lamont PJ, Clarke NF, Laing NG. Expanding the phenotype of GMPPB mutations. Brain. 2015;138(Pt 4):836–844. doi: 10.1093/brain/awv013. [DOI] [PubMed] [Google Scholar]
- Cagliani R, Comi GP, Tancredi L, Sironi M, Fortunato F, Giorda R, Bardoni A, Moggio M, Prelle A, Bresolin N, Scarlato G. Primary beta-sarcoglycanopathy manifesting as recurrent exercise-induced myoglobinuria. Neuromuscul Disord. 2001;11:389–394. doi: 10.1016/S0960-8966(00)00207-8. [DOI] [PubMed] [Google Scholar]
- Carss KJ, Stevens E, Foley AR, Cirak S, Riemersma M, Torelli S, Hoischen A, Willer T, van Scherpenzeel M, Moore SA, Messina S, Bertini E, Bönnemann CG, Abdenur JE, Grosmann CM, Kesari A, Punetha J, Quinlivan R, Waddell LB, Young HK, Wraige E, Yau S, Brodd L, Feng L, Sewry C, MacArthur DG, North KN, Hoffman E, Stemple DL, Hurles ME, van Bokhoven H, Campbell KP, Lefeber DJ, Lin YY, Muntoni F, UK10K Consortium Mutations in GDP-mannose pyrophosphorylase B cause congenital and limb-girdle muscular dystrophies associated with hypoglycosylation of α-dystroglycan. Am J Hum Genet. 2013;93(1):29–41. doi: 10.1016/j.ajhg.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceravolo F, Messina S, Rodolico C, Strisciuglio P, Concolino D. Myoglobinuria as first clinical sign of a primary alpha-sarcoglycanopathy. Eur J Pediatr. 2014;173:239–242. doi: 10.1007/s00431-013-2151-z. [DOI] [PubMed] [Google Scholar]
- Even PC, Decrouy A, Chinet A. Defective regulation of energy metabolism in mdx-mouse skeletal muscles. Biochem J. 1994;304:649–654. doi: 10.1042/bj3040649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figarella-Branger D, Baeta Machado AM, Putzu GA, Malzac P, Voelckel MA, Pellissier JF. Exertional rhabdomyolysis and exercise intolerance revealing dystrophinopathies. Acta Neuropathol. 1997;94:48–53. doi: 10.1007/s004010050671. [DOI] [PubMed] [Google Scholar]
- Gerin I, Ury B, Breloy I, et al. ISPD produces CDP-ribitol used by FKTN and FKRP to transfer ribitol phosphate onto alpha-dystroglycan. Nat Commun. 2016;7:11534. doi: 10.1038/ncomms11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis JM. Understanding dystrophinopathies: an inventory of the structural and functional consequences of the absence of dystrophin in muscles of the mdx mouse. J Muscle Res Cell Motil. 1999;20:605–625. doi: 10.1023/A:1005545325254. [DOI] [PubMed] [Google Scholar]
- Itagaki Y, Saida K, Nishitani H, Matsuo M, Nishio H. Manifesting carriers of duchenne muscular dystrophy over two generations. Rinsho Shinkeigaku. 1993;33:377–381. [PubMed] [Google Scholar]
- Jensen B, Willer T, Saade D, Cox M, Mozaffar T, Scavina M, Stefans V, Winder T, Campbell K, Moore S, Mathews K. GMPPB-associated dystroglycanopathy: emerging common variants with phenotype correlation. Hum Mutat. 2015;36:1159–1163. doi: 10.1002/humu.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoria R, Winder TL, Lui J, Al-Owain MA, Milone M. Novel ANO5 homozygous microdeletion causing myalgia and unprovoked rhabdomyolysis in an Arabic man. Muscle Nerve. 2014;50(4):610–613. doi: 10.1002/mus.24302. [DOI] [PubMed] [Google Scholar]
- Liewluck T, Tian X, Wong LJ, Pestronk A. Dystrophinopathy mimicking metabolic myopathies. Neuromuscul Disord. 2015;25:653–657. doi: 10.1016/j.nmd.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Lindberg C, Sixt C, Oldfors A. Episodes of exercise-induced dark urine and myalgia in LGMD 2I. Acta Neurol Scand. 2012;125:285–287. doi: 10.1111/j.1600-0404.2011.01608.x. [DOI] [PubMed] [Google Scholar]
- Mathews KD, Stephan CM, Laubenthal K, Winder TL, Michele DE, Moore SA, Campbell KP. Myoglobinuria and muscle pain are common in patients with limb-girdle muscular dystrophy 2I. Neurology. 2011;76(2):194–195. doi: 10.1212/WNL.0b013e3182061ad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti C, Tanji K, Chang HW, et al. Dystrophinopathy in two young boys with exercise-induced cramps and myoglobinuria. Eur J Pediatr. 1993;152:848–851. doi: 10.1007/BF02073385. [DOI] [PubMed] [Google Scholar]
- Mongini T, Doriguzzi C, Bosone I, Chiadò-Piat L, Hoffman EP, Palmucci L. Alpha-sarcoglycan deficiency featuring exercise intolerance and myoglobinuria. Neuropediatrics. 2002;33:109–111. doi: 10.1055/s-2002-32374. [DOI] [PubMed] [Google Scholar]
- Montagnese F, Klupp E, Karampinos DC, Biskup S, Gläser D, Kirschke JS, Schoser B. Two patients with GMPPB mutation: the overlapping phenotypes of limb-girdle myasthenic syndrome and LGMD2T dystroglycanopathy. Muscle Nerve. 2016 doi: 10.1002/mus.25485. [DOI] [PubMed] [Google Scholar]
- Nguyen K, Bassez G, Krahn M, Bernard R, Laforet P, Labelle V, et al. Phenotypic study in 40 patients with dysferlin gene mutations: high frequency of atypical phenotypes. Arch Neurol. 2007;64:1176–1182. doi: 10.1001/archneur.64.8.1176. [DOI] [PubMed] [Google Scholar]
- Nigro V, Savarese M. Genetic basis of limb-girdle muscular dystrophies: the 2014 update. Acta Myol. 2014;33(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- Oestergaard ST, Stojkovic T, Dahlqvist JR, Bouchet-Seraphin C, Nectoux J, Leturcq F, Cossèe M, Solè G, Thomsen C, Krag TO, Vissing J. Muscle involvement in limb-girdle muscular dystrophy with GMPPB deficiency (LGMD2T) Neurol Genet. 2016;2(6):e112. doi: 10.1212/NXG.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena L, Kim K, Charrow J. Episodic myoglobinuria in a primary gamma-sarcoglycanopathy. Neuromuscul Disord. 2010;20:337–339. doi: 10.1016/j.nmd.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Penisson-Besnier I, Richard I, Dubas F, Beckmann JS, Fardeau M. Pseudometabolic expression and phenotypic variability of calpain deficiency in two siblings. Muscle Nerve. 1998;21:1078–1080. doi: 10.1002/(SICI)1097-4598(199808)21:8<1078::AID-MUS15>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90(8):3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlivan R, Jungbluth H. Myopathic causes of exercise intolerance with rhabdomyolysis. Dev Med Child Neurol. 2012;54(10):886–891. doi: 10.1111/j.1469-8749.2012.04320.x. [DOI] [PubMed] [Google Scholar]
- Raphael AR, Couthouis J, Sakamuri S, Siskind C, Vogel H, Day JW, Gitler AD. Congenital muscular dystrophy and generalized epilepsy caused by GMPPB mutations. Brain Res. 2014;1575:66–71. doi: 10.1016/j.brainres.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerapandiyan A, Shashi V, Jiang YH, Gallentine WB, Schoch K, Smith EC. Pseudometabolic presentation of dystrophinopathy due to a missense mutation. Muscle Nerve. 2010;42:975–979. doi: 10.1002/mus.21823. [DOI] [PMC free article] [PubMed] [Google Scholar]


