Abstract
Bacillus thuringiensis (Bt) is a Gram-positive, spore-forming, soil bacterium, which is very popular bio-control agent in agricultural and forestry. In general, B. thuringiensis secretes an array of insecticidal proteins including toxins produced during vegetative growth phase (such as secreted insecticidal protein, Sip; vegetative insecticidal proteins, Vip), parasporal crystalline δ-endotoxins produced during vegetative stationary phase (such as cytolytic toxin, Cyt; and crystal toxin, Cry), and β-exotoxins. Till date, a wide spectrum of Cry proteins has been reported and most of them belong to three-domain-Cry toxins, Bin-like toxin, and Etx_Mtx2-like toxins. To the best of our knowledge, neither Bt insecticidal toxins are exclusive to Bt nor all the strains of Bt are capable of producing insecticidal Bt toxins. The lacuna in their latest classification has also been discussed. In this review, the updated information regarding the insecticidal Bt toxins and their different mode of actions were summarized. Before applying the Bt toxins on agricultural field, the non-specific effects of toxins should be investigated. We also have summarized the problem of insect resistance and the strategies to combat with this problem. We strongly believe that this information will help a lot to the budding researchers in the field of modern pest control biotechnology.
Keywords: Bacillus thuringiensis, Insecticides, Cry toxins, Cyt toxins, Mechanism of action, Bt resistance
Introduction
Bacillus thuringiensis or Bt is an important bacterium that possesses entomopathogenic activity and thus has high demand in agricultural sectors. Due to its colonization ability in the gut of insect, Bt is often used in agriculture (Deist et al. 2014), forestry (Zhang et al. 2016), and public health programmes as a bio-control agent (Ibrahim et al. 2010; De Schrijver et al. 2015). Bt israelensis (Bti) was the first subspecies observed to be toxic against the dipteran larvae of noxious species such as mosquitoes, black flies, and chironomid midges. The demand for Bt-based insecticides in agriculture sectors declined in the mid 1970s, due to availability of more effective chemical pesticides. However, in the 1980s, progress in biotechnology has again accelerated the demand for Bt products. In an investigation, Schnepf and Whiteley (1981) cloned a crystal toxin gene from Bt kurstaki (Btk) and expressed it into Escherichia coli. Since then, much studies have been performed to improve the target spectra and to discover more infectious strains of Bt. According to Tabashnik et al. (2013), the commercial application of Bt genes in plant (expressing Bt toxins) has been started from mid 1990s. In 1999, different types of Bt products such as Bt corn, Bt cotton, and Bt potatoes were produced worldwide.
During spore-forming stage, B. thuringiensis secretes different types of δ-endotoxins [belong to the cytolytic (Cyt) or crystal (Cry) families], which vary greatly in structure, mode of action, and specificity (de Maagd et al. 2003; Deist et al. 2014; Land and Miljand 2014), whereas the other insecticidal toxins such as Vip are secreted during the vegetative phase of growth (Palma et al. 2014). While, another group of novel Bt proteins, termed as parasporins (Brasseur et al. 2015), have unique cytocidal activity. The parasporins are parasporal-like protein that are non-hemolytic in nature, but have the ability to preferentially kill cancer cells (Xu et al. 2014). We have not discussed parasporin in this review, because it is beyond the scope of insecticidal activity.
In this review, we have categorically analyzed the chemical arsenal of Bt, which are insecticidal in nature. We have also discussed about Cyt, vegetative insecticidal proteins (Vip), and secreted insecticidal protein (Sip) toxins along with different type of Cry toxins. The sequential-binding model or classical model of Cry toxins has already studies intensively by researchers. In this review along with the sequential-binding model, we also have summarized the signaling pathway model or the alternate action pathway of Cry toxins which trigger apoptosis in gut cells of the pest. We also have summarized the problems of insect resistance and the strategies to combat with these problems.
Insecticidal Bt strains and Bt toxins
The use of Bt strains has increased tremendously, and currently, it is about 90% of the world microbial pest control agent (MCPA) market. Due to beneficial effects, about 84 strains of Bt were recorded in the world data centre for microorganism (WDCM) (http://www.wfcc.info/); however, all the strains of Bt are not insecticidal in nature (Ibrahim et al. 2010). For example, Bt strain NTB-88 possess non-insecticidal parasporal inclusions and flagellar antigen H8a8b (Ibrahim et al. 2010).
There are several strains of Bt with different host specificity. The toxins produced by Bt israeliensis (Bti) are very specific and effective against dipteran pest, including mosquito and black fly, and thus has great demand in public health programmes (kills 95–100% of mosquito larvae within 24 h) (Land and Miljand 2014). Most of the Bt kurstaki (Btk) strains are active against lepidopteran pests, while strains of Bt tenebrionis (Btt) are active against coleopteran pests (Azizoglu et al. 2015). The commercial Bt strains, their target pest, and their use in different sectors (agriculture, forestry, and public health programmes) are presented in Table 1.
Table 1.
Selection of Bt strains and strains originated from Bt used as commercial pesticides in agriculture, forestry, and public health programmes
| Pest control sectors | Insecticidal Bt strains/genes | Target pest |
|---|---|---|
| Agriculture | Natural strains | |
| Bt kurstaki (Btk) | Lepidoptera | |
| Bt san diego (Btsd) | Coleoptera | |
| Bt tenebrionis (Btt) | Coleoptera | |
| Improved strains | ||
| Btk HD1 | Lepidoptera | |
| Btk SA-11 | Lepidoptera | |
| Btk SA-12 | Lepidoptera | |
| Conjugated strains | ||
| EG2424 (Btk × Btt) | Lepidoptera and Coleoptera | |
| EG2348 (Btk × Bta) | Lepidoptera | |
| Recombinant strains | ||
| E. coli expressing Cry1Ac (x2), Cry3A Cry3Bb | Lepidoptera | |
| E. coli expressing Cry1Ac (x3), Cry2A Cry1C | Lepidoptera | |
| E. coli expressing Cry1Aa, Cry1Ac (x2), Cry2A Cry1F-1Ac | Lepidoptera | |
| Forestry | Natural strains | |
| Bt aizawai (Bta) | Lepidoptera | |
| Btk | Lepidoptera | |
| Improved strains | ||
| Btk HD1 | Lepidoptera | |
| Btk SA-11 | Lepidoptera | |
| Public health | Natural strains | |
| Bt israelensis (Bti) | Diptera | |
| Bt medellin (Btm) | Diptera | |
Bt toxins produced by other bacterial candidates
The expression of Bt toxins like Cry and Cyt is not limited to Bt strains, and several other microorganisms like Clostridium bifermentans, Paenibacillus popiliae, P. lentimorbus, and B. sphaericus have been reported as good sources of Bt toxin. The sphaericolysin/anthrolysin family of toxins is highly conserved across isolates from Lysinibacillus sphaericus, Bt, Bacillus cereus, and Paenibacillus alvei (Berry 2012; Castagnola and Patricia Stock 2014). Mtx2 protein and aerolysin were also identified from Clostridium sp. and Aeromonas hydrophila, respectively (Szczesny et al. 2011; Palma et al. 2014). On the other hand, different strains of L. sphaericus also produce the BinA/B toxin, Mtx1, Mtx2, Mtx3, Mtx4, sphaericolysin, Cry48, and Cry49 (Berry 2012). Photorhabdus strains were reported to produce PirA/B and Mcf toxins (Sato et al. 2014).
Genetically modified organism with Bt toxins
To combat with the insect pest, the diversity of Bt toxin must be enriched, and this can be attained by conjugation or recombinant DNA technology [for example, EG2348 (Btk × Bta) and EG2424 (Btk × Btt)] (Carlton and Gawron-Burke 1993).
Bacteria producing Bt toxins are non-systemic insecticides and are, therefore, ineffective against insects that do not come into direct contact with the crystals (which includes sap sucking and piercing insects, root dwelling pests, stem, and fruit borer, etc.). This problem has been effectively addressed by creating genetically modified plants (GMP) that express the crystal proteins. The problem of narrow insect pest specificity has been effectively solved by simultaneously expressing of multiple Bt toxins through gene ‘stacking’ or ‘pyramiding’ (de Maagd et al. 1999). The US Environmental Protection Agency (EPA) first approved the registration of Bt crops (potato, corn, and cotton) in 1995. First marked GMP with Bt toxin was a NewLeaf potato variety expressing Cry3A developed by Monsanto. Presently, the United States is the global leader in producing GMP with Bt toxins followed by Argentina and Brazil. China and India are in top among countries taking most rapid adoption of GMP expressing Bt toxins.
Ethical, socio-economic, and regulatory issues related to Bt toxins
Bt is a natural insecticide and is, therefore, its field application as bacterial suspension (or any products that use live Bt spores and crystal toxins) is not restricted. However, the production company has to ensure that the strain used is not producing any non-specific β-exotoxins. However, the question of stringent rule, regulation, and requirement of approval is enforced in case of GMO with Bt toxins (Altieri and Rosset 1999). There have been several issues raised at different platforms on the use of GMP with Bt toxins (example included Galgene’s FlavrSavr and Ciba Giegy’s Bt corn). The issue of antibiotic resistance markers in selection of GMP has been tackled with auxotropic or food grade markers (Braun et al. 2001). GMPs need to be evaluated for toxicity rigorously in animal models before their release for human consumption. In most of the countries, GMP with Bt toxins are require to be clearly labeled biotech (Maghari and Ardekani 2011). It is always recommended that before applying the Bt toxins on agricultural field in any form, the non-specific effects of toxins should be investigated. Otherwise, it will disturb the natural insect diversity and hamper the ecosystem.
Insecticidal Bt toxins nomenclature
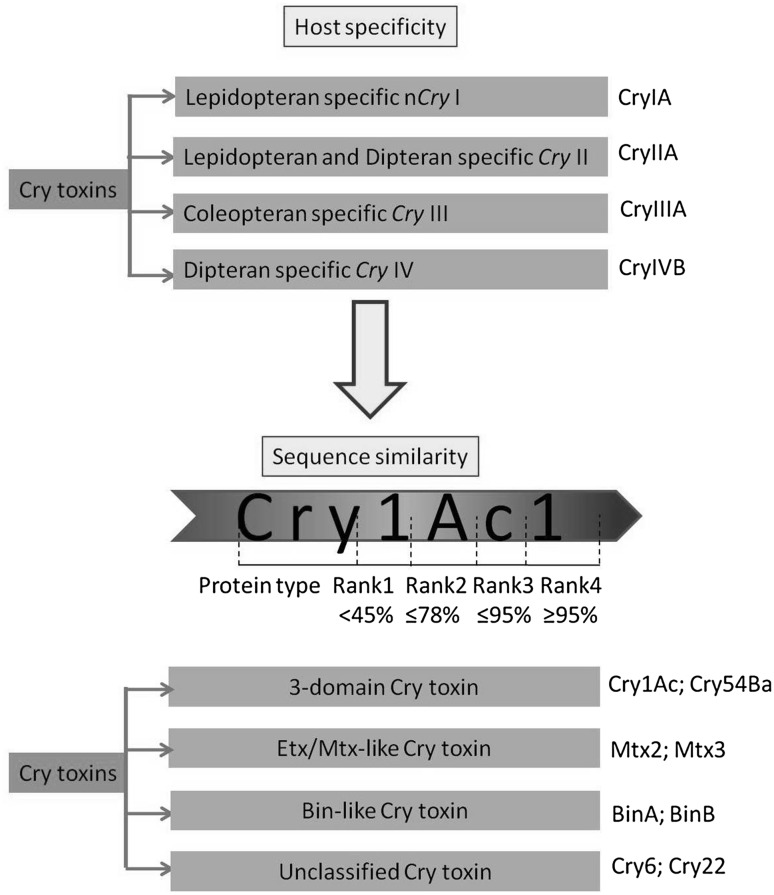
Höfte and Whiteley (1989) classified Cry proteins into four major classes according to the specificity of the toxin against different insect classes (Fig. 1). However, several workers have reported the overlapping toxicity of Bt toxins to different insect classes. To overcome such problem, Crickmore et al. (1998) have introduced another classification scheme. According to the scheme, the protein class named CryIC (genes involved: cry1Ca1, cry1Ca2, cry1Ca3, cry1Ca4, etc.) was recorded to have toxic effects in dipterans and lepidopterans pest (Crickmore et al. 1998). Finally, in the year 1993, the Bt δ-endotoxin nomenclature committee (http://www.btnomenclature.info/) has divided the Cry, Cyt, and Vip toxins based on amino acid identity (Fig. 1). The nomenclature committee has assigned a distinctive name for each toxin and placed closely related toxins in group or rank. Each rank was again divided into four sub ranks. Furthermore, the nomenclature style also has been changed (Roman numerals to Arabic numerals) in the primary rank (e.g., CryIIIA became Cry3A).
Fig. 1.
Schematic overview of the evolution of nomenclature system used by the Bt Toxin Nomenclature Committee for δ-endotoxins (Cry and Cyt) and secretable (Vip and Sip) toxins. In this example, numbers indicate different Cry proteins changing rank 1 depending of percentage amino acid similarity. The same rule applies for ranks 2, 3, and 4 assigning a different identification digit/letter
The classification scheme was developed on the basis of identity in primary sequence. However, it is conceivable that despite the diversity in primary sequence, many of the non three-domain Cry toxins could share significant structural/functional homology (Xu et al. 2014). On the other hand, Etx_Mtx2 like cry toxins or Bin-like cry toxins are considerable diverge than three-domain Cry (3d-Cry) proteins in their tertiary structure. Therefore, inclusion of these two groups into this present nomenclature scheme is quite misleading. Parasporins shows significant structural similarity with 3d-Cry, but is not considered into the present nomenclature scheme. Moreover, the numbers of unclassified cry toxins into this present scheme of nomenclature are ever increasing which further emphasizes the need of re-evaluation of the Bt toxins nomenclature.
Cry toxins
Cry proteins or parasporal inclusion proteins produced by Bt and other bacteria are investigated deeply in the last few years. In general, the molecular weight of Cry proteins varies between 70 and 140 kDa (de Maagd et al. 2003; Pardo-López et al. 2013; Palma 2015). It is well established that most of the Cry toxin belongs to the three-domain family protein, though two minor groups of Cry toxins such as Mtx-like and Bin-like were also introduced (Fig. 1). However, parasporins (Cry family) are the most important and studies proteins, as it possess anticancer activity (Berlitz et al. 2013).
Three-domain Cry toxin
Among insecticidal Bt toxins, the family of three-domain Cry (3d-Cry) proteins belong to the main section having > 53 subgroups (Bravo et al. 2013). In general, the 3d-Cry toxin contains two subunits: 65 and 130 kDa. The 130 kDa subunit is inactive and designated as protoxin. The removal of C-terminal extension by the proteolytic activity of larval midgut proteases converts it as an active one (de Maagd et al. 1999; Bravo et al. 2013). The N-terminal regions of all 3d-Cry toxins contain approximately 20–60 amino acid residues (Pardo-López et al. 2013). It also contains eight conserved amino acid blocks; and among these, first five blocks are highly conserved and are concentrated mainly in the centre of the domains and at the junctions between the domains (Bravo et al. 2013). Pardo-López et al. (2013) have stated that the domains I and II have evolved together, whereas the domain III showed a different structural geometry due to its swapping nature (Pardo-López et al. 2013). In general, 3d-Cry proteins (or protoxin) are activated into toxin by trypsin and are highly specific in nature (e.g., Cry2Aa for dipteran–lepidopteran; Cry1Aa for lepidopteran; Cry4Aa and Cry4Ba for dipteran; Cry3Aa, Cry3Bb and Cry8Ea for coleopteran) (Guo et al. 2012; Pardo-López et al. 2013).
Epsilon toxin_ metaxin 2 (Etx_Mtx2)-like Cry toxins
Along with three-domain Cry toxins, Cry nomenclature committee also has classified some other families of unrelated toxins (Fig. 1). Etx_Mtx2 like Cry toxins such as Cry15, Cry33, Cry60, etc. share a significant structural homology, and are very similar to toxin like aerolysin. Aerolysin is well-studied toxin (having pore-forming capability) produced by Aeromonas hydrophila (Szczesny et al. 2011). Palma et al. (2014) have evaluated the mode of action of Etx_Mtx2 like Cry toxins that actively form a pore in the host target cell. In the aerolysin family of proteins, the conserved beta sheet or aerolysin fold is believed to adopt a barrel conformation within the membrane (Szczesny et al. 2011).
Binary (Bin)-like Cry toxins
Majority of Bin-like Cry toxins such as Cry35 and Cry36 (Toxin_10 family) have high antagonistic activity against different agricultural insect pests (Fig. 1). The structural topology of Cry35Ab1 (Kelker et al. 2014) and BinB (Srisucharitpanit et al. 2014) is very similar to those of Etx_Mtx2 like Cry toxins, as they possess aerolysin-like fold. Cry35 contains a QxW motif, which is structurally very similar to carbohydrate-binding domains of ricin and Mtx1. In recent years, a few other Bin-like Cry toxins also have been characterized such as Cry37, Cry34, and Cry23Aa, also known as ET33 (de Maagd et al. 2003; Contreras et al. 2013; Kelker et al. 2014). In an investigation, Ekobu et al. (2010) have reported the combined effects of Cry37 (ET34) and Cry23Aa against various coleopteran insects.
Unclassified Cry toxins
In recent years, several Bt strains have been reported which produce insecticidal toxins; however, the Bt δ-endotoxin nomenclature committee was unable to place these toxins into any category, due to different structure and amino acid sequences. For example, Palma et al. (2014) have reported a 42-kDa toxin from Bt strain, which exhibited high similarity to BinA and BinB. Similarly, Palma et al. (2014) also have reported a sphaericolysin (possess unique N-terminal sequence and cholesterol-dependent cytolysins motif) producing Bt strain. Sphaericolysin is considered to be a potent insecticidal toxin, which is very effective to control the Blattella germanica and Spodoptera litura (Castagnola and Patricia Stock 2014; Palma et al. 2014). Few other unclassified Bt toxins have also been studied against different insect pest such as Helicoverpa armigera (Tabashnik et al. 2015) and Aedes aegypti (Deist et al. 2014). Some other toxins (such as Cry6, Cry22, Cry55, Mcf, etc.) which belong to unclassified category also have been reported (Fig. 1).
Cyt toxins
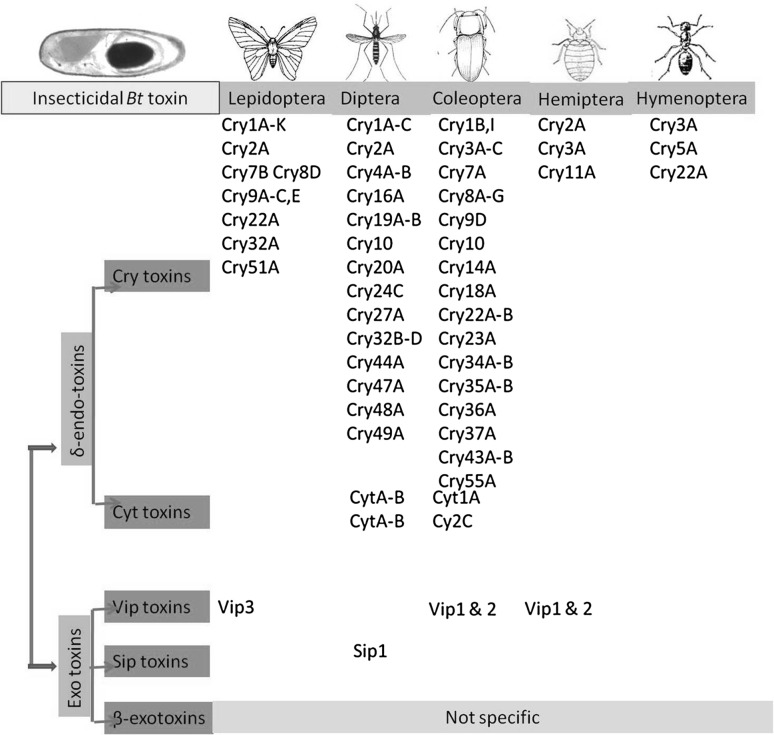
Like Cry toxin, Cyt toxins are another group of Bt crystal toxins that possess an in vitro and in vivo activity against dipteran pests (Fig. 2). Cyt toxins are able to bind with non-saturated membrane lipids (Chougule et al. 2013) and thus do not require any specific receptor. In general, Cyt toxins are ~25 kDa protein containing a single α–β domain wrapped by two layers of α-helix hairpins (Cantón et al. 2014). The conversion of inactive protoxin Cyt1Aa (27 kDa) to active toxin (24 kDa) is achieved by the proteolytic cleavage at the amino (N) and carboxy-terminal (C) ends (Chougule et al. 2013), which is vital for membrane attachment and piercing. The topology of Cyt2Aa reveals a three-layer α–β domain with a unique fold, which permits the α-helix layers to move away and push the β-sheet into the host membrane. Furthermore, the two components of Cyt2Aa: loop β6–αE and part of β7 also possess high affinity to Cry11Aa (Cadavid-Restrepo et al. 2012). The cytolysin fold has critical importance, as the binding of toxin to epithelial membrane depends on the conformational geometry of the fold (Coates et al. 2013). The Cyt1Aa toxins do not have any sequence similarity to Cry polypeptides (Bravo et al. 2013).
Fig. 2.
Schematic representation of insecticidal Bt toxins classification with example of toxins and their target pest in each class
Vip toxins
Like other Bt toxin, Vip are also important insecticidal protein in integrated pest management system (Baranek et al. 2015). Based on amino acid sequence, the Vip proteins may be divided into four different families: Vip1, Vip2, Vip3, and Vip4 (Crickmore et al. 2014) (Fig. 2). Like Cyt toxins, Vip proteins are also inactive in its native form. Thus, Vip protein also undergoes a conformational changes at conserved signal peptide sequences before or after the secretion in the membrane (Baranek et al. 2015). Vip1 and Vip2 are insecticidal binary toxins against a wide range of coleopteran and Hemipteran pests (Sattar and Maiti 2011). Furthermore, in a review, Palma et al. (2014) have stated that Vip2 exhibits structural similarity with active domain of CdtA toxin produced by Clostridium difficile. Whereas, Vip3 (single-chain) toxins are effective against a wide range of agricultural lepidopteran pest (Palma et al. 2014; Palma 2015).
Sip toxins
The secreted insecticidal protein (Sip) is the member of Bt insecticidal family, which are very effective against coleopteran larvae (Fig. 2). Like Cyt and Vip toxins, a conformational change in amino acid sequence converts the inactive Sip toxin into an active one (Palma et al. 2014). Palma et al. (2014) have reported the production of Sip protein named Sip1Aa1 from the Bt strain EG2158. The protein Sip1Aa1 is approximately 41 kDa long that contains 367 amino acid residues. The Sip1Aa1 toxin contains a 30 amino acid long secretion signal and is recorded to be lethal for Leptinotarsa decemlineata, Diabrotica undecimpunctata howardi, and D. virgifera virgifera (Palma et al. 2014).
β-Exotoxins
β-Exotoxins are thermostable, non-proteinaceous secondary metabolites secreted by a few strains of Bacillus thuringiensis. Unlike δ-endotoxins, the target of β-exotoxins is non-specific; and sometimes, it also affects mammals, along with insect pest (Liu et al. 2014), and thus, the use of β-exotoxins producing bt strains is restricted in several countries of Europe and USA. β-Exotoxins are also known as thuringiensin, which are analogues of adenine (Obeidat et al. 2012) or uracil (Levinson et al. 1990). It is well established that β-exotoxins play a negative role in RNA biosynthesis (Obeidat et al. 2012).
Mode of action of Bt toxin
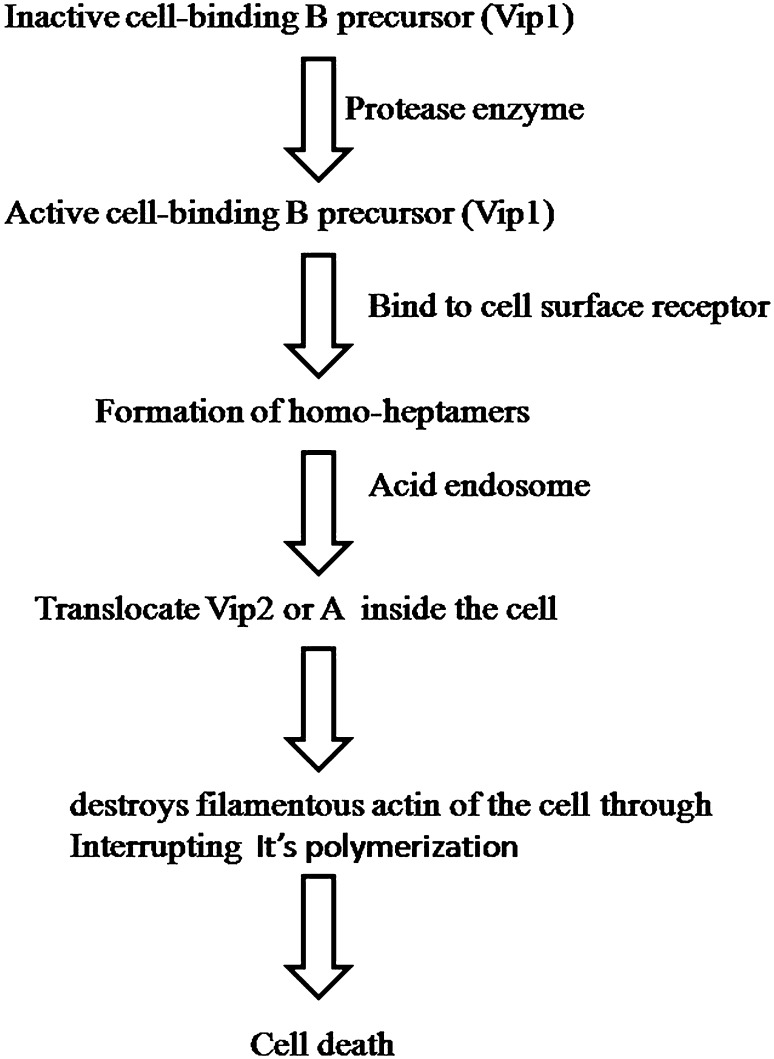
In the last few years, a wide spectrum of Bt toxins have been introduced in agricultural and forestry. All these toxins have great ability to kill different insect pests; however, their mechanism of action is different. The mechanism of cell death caused by VIP toxins is presented in Fig. 3 (de Maagd et al. 2003; Bravo et al. 2013; Palma et al. 2014). On the other hand, the exact working mechanism of Sip toxins is not clear to date; however, Sip1Aa1, a toxin of Sip class, is reported to be a pore-forming agent (Palma et al. 2014). In this review, we have discussed the mechanism of action of Cry toxins and Cyt toxins in details, as they are considered to be the most studied Bt toxins till date.
Fig. 3.
Schematic overview of the mode of action of Vip toxin triggering apoptotic pathways
Working mechanism of insecticidal Cry toxins
The working mechanism of Cry toxin is well established. At present, two models have been proposed based on the working mechanism of Cry toxins. The most studied one is the sequential-binding model or classical mode of action of Cry toxins, whereas new arrival is the signaling pathway model or alternate mode of action of Cry toxins. In both the models, upon ingestion, Cry toxins are dissolved in the insect gut juices to yield the protoxin. The conversation of protoxin to active toxin in the insect gut is only possible due to the suitable pH condition. Then, the active toxin binds with his specific receptor present in the membrane of the gut cells. The use of Cry toxin is popular to the agricultural field due to its target specificity (insect pest) and does not hamper the other insect (non-target). The strategy of such specificity is assumed to be caused by the (1) gut pH, (2) domain II and III, and (3) the presence of the toxin-specific receptors in the cell membrane. However, the binding affinity of Cry toxin to its receptor varies greatly (due to varying number of binding sites) from one insect to others (Bravo et al. 2013; Lee 2013).
The sequential-binding model (classical mode of action)
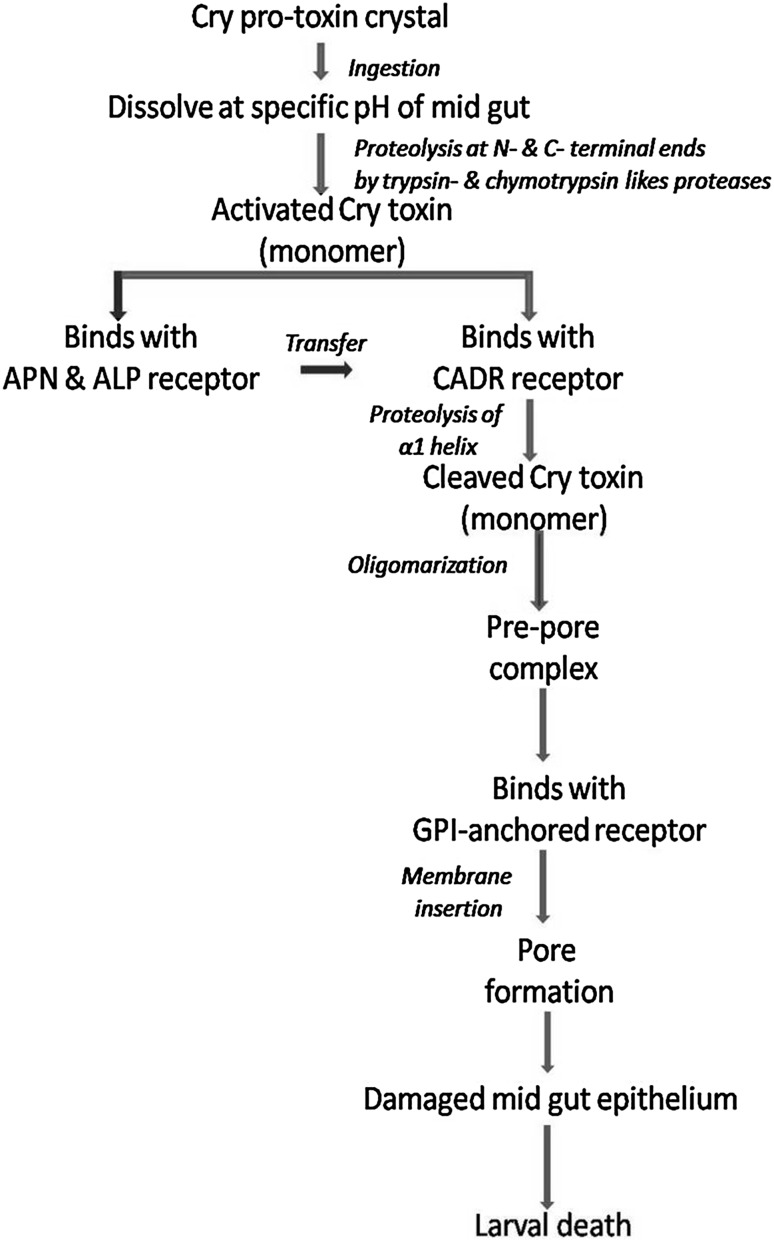
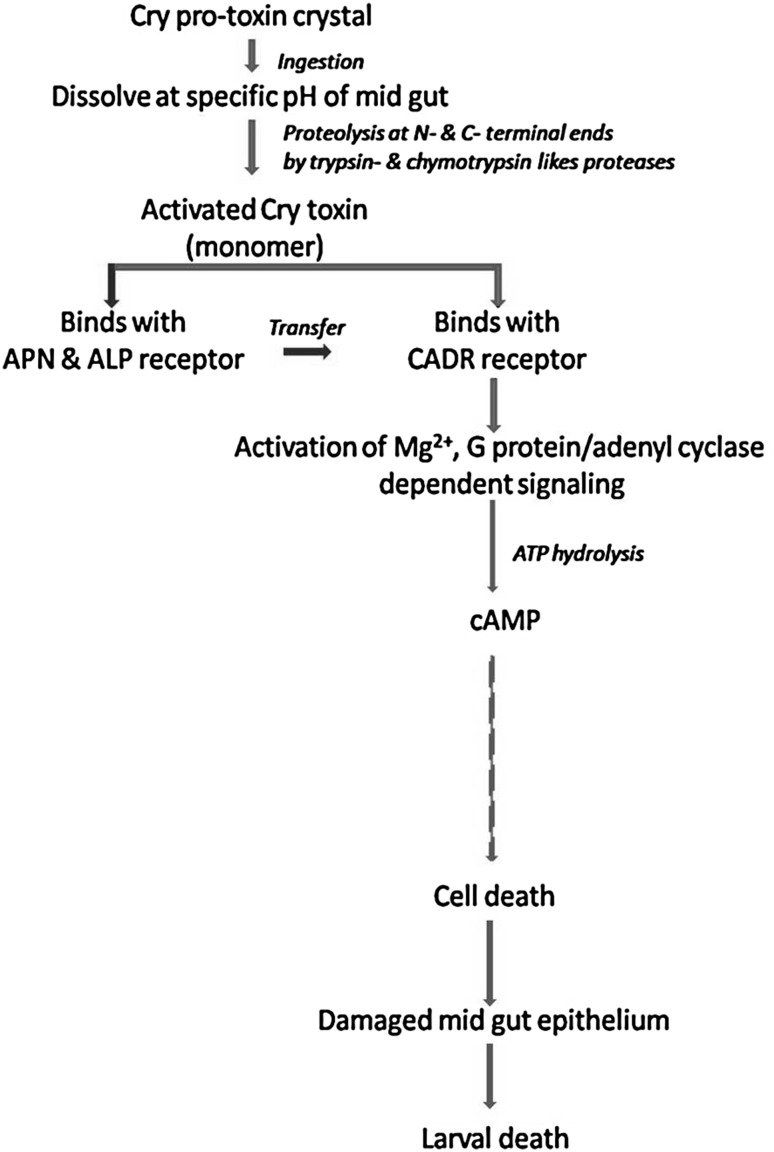
It is a multiple step process of receptor-mediated complex pathway that leads to the formation of pore in the cell membrane (Pacheco et al. 2009; Palma et al. 2014). Upon ingestion, the crystalline inclusions of the endotoxin are dissolved in the insect gut juices to yield the protoxin (Fig. 4). The conversation of protoxin to active toxin (removal of N- and C-terminal amino acids) in the insect gut is only possible due to the suitable pH condition. The removal of N-terminal amino acid by the proteolytic action by gut enzymes varies from one Cry toxin to another (for example, 49, 58, and 25–30 amino acid residues are removed in case of Cry2A, Cry3A, and Cry1 toxins, respectively) (Silvia Denise et al. 2013) (Fig. 4). However, The C-terminal end of the protoxin plays the major role in receptor-binding phenomena. During last few years, researchers have investigated and identified different types of receptor [ABC transporters (ABCC2, ABCC3), GPI-anchored alkaline phosphatase (ALP), cadherin-like protein (CADR), and glycosylphosphatidyl-inositol (GPI)-anchored aminopeptidase-N (APN)] in Cry1A toxins, which are required for toxin activation (Yudina et al. 2007; Tabashnik et al. 2015). Upon binding on membrane receptor, the hydrophobic amino acid residues accelerate the pore formation process. Lepidopteran-specific Cry1Aa specifically bind with lepidopteran ABCC transporter ABCC2 and ABCC3, whereas coleopteran ABCC transporter specifically responded to a coleopteran-specific Cry8Ca toxin (Endo et al. 2017). Ben-Dov (2014) has reported that Cry4Aa or Cry4Ba are the most important parts of pre-pore structure. Finally, it enters within the membrane of the gut epithelium cells and forms a stable membrane channel (Tabashnik et al. 2015), through which different types of cation can enter freely. The entrance of outer ions dis-balances the cell physiology and osmotic balance and ultimately led to the lysis of the cell. Furthermore, the pore allows leaking out the leak of the gut content to the haemocoel, including bacteria that causes septicemia (Ben-Dov 2014) (Fig. 4).
Fig. 4.
Sequential-binding model (classical mode of action) of Cry toxin showing toxin activation, binding with specific receptors, formation of pores, and subsequent death of pest insect larvae
The signaling pathway model (alternate mode of action)
Unlike sequential-binding model, the pore formation is not an important event in signaling pathway model (Fig. 4). Palma et al. (2014) have reported that upon attachment of Cry1A toxin to cadherin receptor accelerates the events of intracellular apoptotic pathways that lead to the destruction of the cell (Palma et al. 2014). However, the interaction of toxin and receptor in insect gut depends on extracellular signals (Palma et al. 2014) (Fig. 5).
Fig. 5.
Signaling pathway model (alternate mode of action) of Cry toxin showing attachment of Cry toxin to cadherin receptor accelerates the events of intracellular apoptotic pathways
Mechanism of insecticidal Cyt toxins
Like Cry toxin, the mechanism of action of Cyt toxins is also very complicated; and at present, two general mechanistic pathways have been investigated.
Pore formation model
The pore-forming ability of the Cyt toxins highly depends on the structural topology of the protein. This model was suggested for the toxins like Cyt1Aa and Cyt2Ba that contain a cytolysin fold and exhibit hemolytic activity (Ben-Dov 2014). Cyt toxins may form pore in the membrane either alone or synergistically with Cry toxin. In the insect midgut, digestive juice solubilizes the Cyt protein and activates it. Upon activation, the Cyt proteins bind to the membrane receptor and exposes the specific binding sites for the Cry toxins (Deist et al. 2014). The interaction of Cry11Aa and Cyt1Aa is important for membrane insertion, which depend on the charged amino acids residues (domain II loop α8 of Cry11Aa and β-4 of Cyt1Aa). Ultimately, it forms a pore and destabilizes the cell physiology and osmotic balance.
Detergent action model
This model has proposed the detergent action mechanism of Cyt toxins. Upon binding on the cell surface, it disrupts the lipid packing pattern and forms a stable channel through which the cell content comes out by all-or-nothing mechanism (Doggett et al. 2013). Like Cry toxins, the modes of action of Cyt toxin may not be mutually exclusive. Bravo et al. (2007) have reported that the pore formation process of Cyt toxins (e.g., Cyt1A) and Cry toxins is quite different. Unlike Cry toxin, Cyt toxins are directly absorbed on the surface and form the pore using detergent-like mechanism (Bravo et al. 2007). In this direction, Ben-Dov (2014) has reported that the efficacy of pore formation of Cyt toxins depends on the toxin concentration and time of exposure.
Bt toxin effect on non-target insects
Until now, several reports have been published regarding the effects of Bt toxins on agricultural insect pest (target pest) and their control; however, the information regarding the non-target insects or unspecific effects of Bt toxins (specially Cyt toxin) is scanty (Bøhn et al. 2008; Resende et al. 2016). Previously, people are not so concern about the adverse effects of Bt toxins on non-target insect. In the year 1999, Losey et al. have reported a huge mortality of Monarch butterfly (Danaus plexippus) larvae fed with pollen from Cry1Ab expressing maize plants, and from then, it becomes a regulatory test approved by USA Government. The effects of Bt plant (such as transgenic Cry1Ab maize and Cry3A-expressing potato foliage) on non-target insects (Spodoptera littoralis and Chrysoperla carnea) have been recorded (Hussein et al. 2006; Romeis et al. 2014; Resende et al. 2016). The toxicity of Bt toxins was found sublethal for Neobelleria bullata depending on the dose of applied toxin (Cerstiaens et al. 2001). Brandt et al. (2004) have detected the presence of Cry2Ab in the Lygus hesperus tissues; however, the binding of Cry2Ab alone is not sufficient for toxicity. Furthermore, Rodrigo-Simon et al. (2006) stated that the gut of C. carnea larvae does not contain any specific receptors for Cry1Ab and Cry1Ac, and thus, the actions of these toxins are unspecific. In a review, Deist et al. (2014) also have mentioned the toxic effects of Cry3A toxins on non-target insect pests [as, for example, pea aphid (Acyrthosiphon pisum)]. Interestingly field studies on the NewLeaf potato (Cry3Aa) showed that the toxin specifically affects the Colorado potato beetle has no deleterious effect on other insects in the potato field, including the beetle’s natural predators (Reed et al. 2001). After detailed experimental design, Lawo et al. (2009) have concluded that Bt cotton has no negative impact on beneficial insects in the cotton ecosystem.
Bt resistance
Till date, there are only few instances of resistance development in the agricultural field. In laboratory conditions, due to regular use, the agricultural pests are turned to be resistant to Bt toxins. Emergence of resistant pest is a big threat for agricultural sectors. In general, three major events are considered that confer resistant property to the insect pests; (1) alteration of toxin–receptor interactions, (2) alterations in toxin, and (3) alterations in toxin solubilization (Ferry et al. 2004).
The attachment of Bt toxins on the cell surface is a receptor-binding phenomenon and any defects in receptor topology do not allow the toxin to bind on the surface. Until now, several types of receptor defects such as defects on cadherin-like receptor, defects on GPI-anchored receptor, and defects on channel protein have been investigated (Pardo-López et al. 2013). In a study Elzak (2016) has reported a mutation in the cadherin-like gene in Heliothis virescens, which confers resistant to Cry1Ac-resistant line YHD2 (cross resistant to Cry1A and Cry1Fa toxins). In a similar study, Chougule and Bonning (2012) have indentified mutated CADR alleles in Pectinophora gossypiella that confer resistant property against a wide range of Cry toxins. Furthermore, Coates et al. (2013) have stated that Cry1C-resistant colony of Spodoptera exigua did not express APN1 in the gut, which clearly indicates the correlation between toxin-resistant and receptor protein production. Pardo-López et al. (2013) also have established the relation between ALP receptor expression and Cry1Ac toxin-resistant property of H. virescens YHD2 larvae. In this direction, Park et al. (2014) have identified several types of mutation in ABCC2 transporter protein, which confer resistant property against Bt toxin. Recently Chakroun et al. (2016) also have reported Vip3Aa-resistant pest Helicoverpa armigera in Australia, which cause a huge damage in agricultural production.
Strategies for combating against Bt resistance problems
Regular and constitutive application of Bt 3d-Cry proteins contributed in insect resistance to these toxins. Agricultural methods like refuge strategy may only partially address the problem by delaying the appearance of resistance in insect pests (P. gossypiella) as discussed by Tabashnik et al. (2010). Therefore, precise strategies are being formulated to combat with this problem in real time. Here, we have discussed and summarized different technical strategies that are being applied in agricultural field.
Discovery of novel Bt toxins
Bt toxin plays an important role in agriculture and forestry, and thus, searching and characterization of novel Bt toxin are a never-ending process. Several molecular, biological, and biotechnological techniques such as DNA microarray, HPLC, and southern hybridization have been introduced to purify and characterize new Bt toxin (Kelker et al. 2014). Furthermore, next-generation sequencing (NGS) technologies are also available to discover unrecognized insecticidal-toxin genes (Palma et al. 2014). The combination of novel Bt toxins produced by other bacteria and already known 3d-Cry toxins produced by Bt might be useful in controlling insect pest (Bravo et al. 2011).
Combination among Cry proteins
The information regarding the 3d-Cry toxins genes, expressed proteins, and their receptors is already available. Therefore, one of the available technologies is ‘stacking’ or ‘pyramiding’ different Bt toxins together to tackle multiple pest attack in the agriculture field. Till date, the best example cited may be SmartStax® (Monsanto, MI and Dow AgroSciences, IN). SmartStax® is a brand of genetically modified seed made through several combinations of Cry proteins which are very effective against resistant pest. These combinations of toxins not only kill the insect pest but also slowdown the resistance evolution process. Long-term use of Bt toxin creates a selective pressure for emerging Bt toxin-resistant pest; however, synergistic effect of two or more toxins might be an alternative way to combat with such issues. In this direction, researchers have reported the synergistic effects of Cry4Aa and Cry4Ba, Cry4Aa, and Cry11Aa, or the three Cry’s, against larvae of Culex, Aedes, and Anopheles, whereas Cry4Ba and Cry11Aa exhibited synergistic effect against Ae. aegypti (Ibrahim et al. 2010; Elleuch et al. 2015).
Synergies between Cry and Cyt proteins
Several investigations have reported the low toxicity of Cyt1Aa and of Cyt2Aa produced by Bt kyushuensis; however, their combined insecticidal effect is very high (Ben-Dov 2014; Deist et al. 2014). Similar synergistic effect of Bti Cry toxins–Cyt1Aa and Cyt toxins–Cry toxins against several insect pests, including Culex quinquefasciatus, also have been studies (Sena et al. 2009; Hayakawa et al. 2016). However, better result against C. quinquefasciatus was observed in case of recombinant acrystalliferous Bti strain that produce a mixture of Bt toxins (Ben-Dov 2014).
Synergies between different Bt toxins
The combination of VIPs and immunosuppressive proteins (animals or plants origin) may tackle the resistance problem (Price and Gatehouse 2008). Based on combinational effect of Bt toxin, researchers have developed alternative killing mechanism such as chitin degrading enzyme (digest chitin membrane) and Trypsin Modulating Oostatic Factor (responsible for larval starvation) (Ajamhassani et al. 2011; Han et al. 2015).
Bt toxin protein engineering
Protein engineering has opened the new window of controlling mechanism against resistant pest through several strategies such as loop substitution, domain swapping, and shuffling strategies (Deist et al. 2014). Cry1AMod toxins could effectively reduce the resistance levels in the six major crop pest species (Franklin et al. 2009; Elzak 2016). Cry1Ab.105 introduced by Monsanto (Monsanto, MI and Dow AgroSciences, IN) for their product SmartStax® is a brilliant example of Bt toxin protein engineering. It is very effective way to control insect pest, as well as to check the insect pest resistance problem.
Conclusion and future perspective
In agriculture, forestry, and public health sectors, insecticidal Bt toxins are valuable tools for pest control management. Currently, different chemical pesticides are available in the market; however, the use of such chemicals is not good for health, and, thus, have been restricted in several countries. It is now well established that the mutual interactions among the Bt toxins (Cry4Aa, Cry4Ba, and Cry11Aa and Cyt1Aa) confer the resistant property of Bti. This knowledge turns to be fundamental for the development of strategies to combat resistance problems faced in the agricultural sectors. Before applying the Bt toxins on agricultural field, the non-specific effects of toxins should be investigated. The specific larvicidal properties of Bt toxins are attributed to the complex interactions between and among Cry, Cyt, Vip, and Sip proteins and their specific receptors. In virtue of the discovery of novel insecticidal Bt proteins and emerging technologies like protein engineering, transgenic plants are expected to serve mankind for a longer time period than previously expected. In conclusion, the discovery of new insecticidal Bt toxins, development of chimeric toxins, and their expression in different combinations will definitely open a new window for the researcher in future. It is evident that neither Bt insecticidal toxins are exclusive to Bt nor all the strains of Bt are capable of producing insecticidal Bt toxins. Therefore, the scope of research on insecticidal Bt toxins is not limited to Bt only.
Acknowledgements
Authors are very much thankful to Visva Bharati University, India, University of Calcutta, India, and Gauhaati University, India for proving necessary supports. This work is not funded by any agencies. Authors express their sincere respects to the honorable reviewers for their constructive criticism to make the manuscript even better.
Compliance with ethical standards
Conflict of interest
We declared that none of the authors have any conflict of interest.
References
- Ajamhassani M, Ghadamyary M, Borovsky D. Effect of trypsin modulating oostatic factor (TMOF) on trypsin and chymotrypsin in Glyphodes pyloalis Walker (Lep.: Pyralidae) and Hyphantria cunea Drury (Lep.: Arctiidae) Pestycydy/Pesticides. 2011;1–4:35–39. [Google Scholar]
- Altieri MA, Rosset P. Strengthening the case for why biotechnology will not help the developing world: a response to McGloughlin. AgBioForum. 1999;2:226–236. [Google Scholar]
- Azizoglu U, Yılmaz S, Ayvaz A, Karabörklü S. Effects of Bacillus thuringiensis subsp. kurstaki HD1 spore-crystal mixture on the adults of egg parasitoid Trichogramma evanescens (Hymenoptera: Trichogrammatidae) Biotechnol Biotechnol Equip. 2015;29:653–658. doi: 10.1080/13102818.2015.1038303. [DOI] [Google Scholar]
- Baranek J, Kaznowski A, Konecka E, Naimov S. Activity of vegetative insecticidal proteins Vip3Aa58 and Vip3Aa59 of Bacillus thuringiensis against lepidopteran pests. J Invertebr Pathol. 2015;130:72–81. doi: 10.1016/j.jip.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Ben-Dov E. Bacillus thuringiensis subsp. israelensis and its dipteran-specific toxins. Toxins. 2014;6:1222–1243. doi: 10.3390/toxins6041222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlitz DL, de Athayde Sau D, Machado V, de Cássia Santin R, Guimarães AM, Matsumura ATS, Ribeiro BM, Fiuza LM. Bacillus thuringiensis: molecular characterization, ultrastructural and nematoxicity to Meloidogyne sp. J Biopest. 2013;6:120–128. [Google Scholar]
- Berry C. Lysinibacillus sphaericus, as an insect pathogen. J Invertebr Pathol. 2012;109:1–10. doi: 10.1016/j.jip.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Bøhn T, Primicerio R, Hessen DO, Traavik T. Reduced fitness of Daphnia magna fed a Bt-transgenic maize variety. Arch Environ Contam Toxicol. 2008;55:584–592. doi: 10.1007/s00244-008-9150-5. [DOI] [PubMed] [Google Scholar]
- Brandt SL, Coudron TA, Habibi J, Brown GR, Ilagan OM, Wagner RM, Wright MK, Backus EA, Huesing JE. Interaction of two Bacillus thuringiensis delta-endotoxins with the digestive system of lygus hesperus. Curr Microbiol. 2004;48(1):1–9. doi: 10.1007/s00284-003-4056-y. [DOI] [PubMed] [Google Scholar]
- Brasseur K, Auger P, Asselin E, Parent S, Côté J-C, Sirois M. Parasporin-2 from a new Bacillus thuringiensis 4R2 strain induces caspases activation and apoptosis in human cancer cells. PLoS One. 2015;10(8):e0135106. doi: 10.1371/journal.pone.0135106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R, Bennett DJ, Secretariat EFB, Delft O. Antibiotic resistance markers in genetically modified (GM) crops. European Federation of Biotechnology: Task group on public perceptions of biotechnology; 2001. [Google Scholar]
- Bravo A, Soberón M. How to cope with resistance to Bt toxins? Trends Biotechnol. 2008;26:573–579. doi: 10.1016/j.tibtech.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Bravo A, Gill SS, Soberón M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 2007;49:423–435. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo A, Likitvivatanavong S, Gill SS, Soberón M. Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem Mol Biol. 2011;41:423–431. doi: 10.1016/j.ibmb.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo A, Gómez I, Porta H, García-Gómez BI, Rodriguez-Almazan C, Pardo L, Soberón M. Evolution of Bacillus thuringiensis Cry toxins insecticidal activity. Microbial Biotechnol. 2013;6(1):17–26. doi: 10.1111/j.1751-7915.2012.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid-Restrepo G, Sahaza J, Orduz S. Treatment of an Aedes aegypti colony with the Cry11Aa toxin for 54 generations results in the development of resistance. The Memórias do Inst Oswaldo Cruz. 2012;107(1):74–79. doi: 10.1590/S0074-02762012000100010. [DOI] [PubMed] [Google Scholar]
- Cantón PE, López-Díaz JA, Gill SS, Bravo A, Soberón M. Membrane binding and oligomer membrane insertion are necessary but insufficient for Bacillus thuringiensis Cyt1Aa toxicity. Peptides. 2014;53:286–291. doi: 10.1016/j.peptides.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton BC, Gawron-Burke C. Genetic improvement of Bacillus thuringiensis for bioinsecticide development. In: Kim L, editor. Advanced Engineered Biopesticides. NY: Marcel Dekker Inc; 1993. pp. 43–61. [Google Scholar]
- Castagnola A, Patricia Stock S. Common virulence factors and tissue targets of entomopathogenic bacteria for biological control of lepidopteran pests. Insects. 2014;5(1):139–166. doi: 10.3390/insects5010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerstiaens A, Verleyen P, van Rie J, van Kerkhove E, Schwartz J-L, Laprade R, de Loof A, Schoofs L. Effect of Bacillus thuringiensis Cry1 toxins in insect hemolymph and their neurotoxicity in brain cells of Lymantria dispar. Appl Environ Microbiol. 2001;67:3923–3927. doi: 10.1128/AEM.67.9.3923-3927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakroun M, Banyuls N, Walsh T, Downes S, James B, Ferré J. Characterization of the resistance to Vip3Aa in Helicoverpa armigera from Australia and the role of midgut processing and receptor binding. Sci Rep. 2016;6:24311. doi: 10.1038/srep24311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chougule NP, Li H, Liu S, Linz LB, Narva KE, Meade T, Bonning BC. Retargeting of the Bacillus thuringiensis toxin Cyt2Aa against hemipteran insect pests. Proc Natl Acad Sci USA. 2013;110:218465–218470. doi: 10.1073/pnas.1222144110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chougule NP, Bonning BC. Toxins for transgenic resistance to hemipteran pests. Toxins (Basel) 2012;4(6):405–429. doi: 10.3390/toxins4060405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates BS, Sumerford DV, Siegfried BD, Hellmich RL, Abel CA. Unlinked genetic loci control the reduced transcription of aminopeptidase N 1 and 3 in the European corn borer and determine tolerance to Bacillus thuringiensis Cry1Ab toxin. Insect Biochem Mol Biol. 2013;43(12):1152–1160. doi: 10.1016/j.ibmb.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Contreras E, Rausell C, Real MD. Proteome response of Tribolium castaneum larvae to Bacillus thuringiensis toxin producing strains. PLoS One. 2013;8(1):e55330. doi: 10.1371/journal.pone.0055330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crickmore N, Zeigler DR, Feitelson J, Schnepf E, Van-rie J, Lereclus D, Baum J, Dean DH. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:807–813. doi: 10.1128/mmbr.62.3.807-813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crickmore N, Zeigler DR, Schnepf E, van Rie J, Lereclus D, Baum J, Bravo A, Dean DH (2014) Bacillus thuringiensis toxin nomenclature. Available online: http://www.lifesci.sussex.ac.uk/Home/Neil_Crickmore/Bt/
- de Maagd RA, Bosch D, Stiekema W. Bacillus thuringiensis toxin-mediated insect resistance in plants. Trends Plant Sci. 1999;4:9–13. doi: 10.1016/S1360-1385(98)01356-9. [DOI] [PubMed] [Google Scholar]
- de Maagd RA, Bravo A, Berry C, Crickmore N, Schnepf HE. Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Ann Rev Genet. 2003;37:409–433. doi: 10.1146/annurev.genet.37.110801.143042. [DOI] [PubMed] [Google Scholar]
- de Schrijver A, De Clercq P, de Maagd RA, van Frankenhuyzen K. Relevance of Bt toxin interaction studies for environmental risk assessment of genetically modified crops. Plant Biotechnol J. 2015;13:1221–1223. doi: 10.1111/pbi.12406. [DOI] [PubMed] [Google Scholar]
- Deist BR, Rausch MA, Fernandez-Luna MT, Adang MJ, Bonning BC. Bt toxin modification for enhanced efficacy. Toxins. 2014;6(10):3005–3027. doi: 10.3390/toxins6103005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett NA, Stubben CJ, Chertkov O, Bruce DC, Detter JC, Johnson SL, Han CS. Complete genome sequence of Bacillus thuringiensis serovar israelensis strain HD-789. Genome Announce. 2013;1:e01023-13. doi: 10.1128/genomeA.01023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekobu M, Solera M, Kyamanywa S, Mwanga RO, Odongo B, Ghislain M, Moar WJ. Toxicity of seven Bacillus thuringiensis Cry proteins against Cylas puncticollis and Cylas brunneus (Coleoptera: Brentidae) using a novel artificial diet. J Econ Entomol. 2010;103:1493–1502. doi: 10.1603/EC09432. [DOI] [PubMed] [Google Scholar]
- Elleuch J, Jaoua S, Darriet F, Chandre F, Tounsi S, Zghal RZ. Cry4Ba and Cyt1Aa proteins from Bacillus thuringiensis israelensis: Interactions and toxicity mechanism against Aedes aegypti. Toxicon. 2015;104:83–90. doi: 10.1016/j.toxicon.2015.07.337. [DOI] [PubMed] [Google Scholar]
- Elzak MEA. Resistance to Bt crops; influence, mechanisms and management strategies. Biotechnol Mol Biol Rev. 2016;11:1–5. doi: 10.5897/BMBR2016.0256. [DOI] [Google Scholar]
- Endo H, Tanaka S, Imamura K, Adegawa S, Kikuta S, Sato R. Cry toxin specificities of insect ABCC transporters closely related to lepidopteran ABCC2 transporters. Peptides. 2017;98:86–92. doi: 10.1016/j.peptides.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Ferry N, Edwards MG, Mulligan EA, Emami K, Petrova AS, Frantescu M, Davison GM, Gatehouse AMR. Engineering resistance to insect pests. In: Christou P, Klee H, editors. Handbook of Plant Biotechnology. Chichester: Wiley; 2004. pp. 373–394. [Google Scholar]
- Franklin MT, Nieman CL, Janmaat AF, Soberón M, Bravo A, Tabashnik BE, Myers JH. Modified Bacillus thuringiensis toxins and a hybrid B. thuringiensis strain counter greenhouse-selected resistance in Trichoplusia ni. Appl Environ Microbiol. 2009;75:5739–5741. doi: 10.1128/AEM.00664-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CH, Zhao ST, Ma Y, Hu JJ, Han XJ, Chen J, Lu MZ. Bacillus thuringiensis Cry3Aa fused to a cellulase-binding peptide shows increased toxicity against the longhorned beetle. Appl Environ Microbiol. 2012;93:1249–1256. doi: 10.1007/s00253-011-3523-9. [DOI] [PubMed] [Google Scholar]
- Han G, Li X, Zhang T, Zhu X, Li J. Cloning and tissue-specific expression of a chitin deacetylase gene from Helicoverpa armigera (Lepidoptera: Noctuidae) and its response to Bacillus thuringiensis. J Insect Sci. 2015;15(1):95. doi: 10.1093/jisesa/iev076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Yoneda N, Okada K, Higaki A, Howlader MTH, Ide T. Bacillus thuringiensis Cry11Ba works synergistically with Cry4Aa but not with Cry11Aa for toxicity against mosquito Culex pipiens (Diptera: Culicidae) larvae. Appl Entomol Zool. 2016 [Google Scholar]
- Höfte H, Whiteley HR. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein HM, Habuštová O, Turanli F, Sehnal F. Potato expressing beetle–specific Bacillus thuringienis Cry3Aa toxin reduces performance of a moth. J Chem Ecol. 2006;32:1–13. doi: 10.1007/s10886-006-9347-x. [DOI] [PubMed] [Google Scholar]
- Ibrahim MA, Griko N, Junker M, Bulla LA. Bacillus thuringiensis: a genomics and proteomics perspective. Bioeng Bugs. 2010;1(1):31–50. doi: 10.4161/bbug.1.1.10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelker MS, Berry C, Evans SL, Pai R, McCaskill DG, Wang NX, Russell JC, Baker MD, Yang C, Pflugrath JW, Wade M, Wess TJ, Narva KE. Structural and biophysical characterization of Bacillus thuringiensis insecticidal proteins Cry34Ab1 and Cry35Ab1. PLoS One. 2014;9(11):e112555. doi: 10.1371/journal.pone.0112555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M, Miljand M (2014) Biological control of mosquitoes using Bacillus thuringiensis israelensis: a pilot study of effects on target organisms, non-target organisms and humans. Mistra EviEM Pilot Study PS4 (www.eviem.se)
- Lawo NC, Wäckers FL, Romeis J. Indian Bt cotton varieties do not affect the performance of cotton aphids. PLoS One. 2009;4:e4804. doi: 10.1371/journal.pone.0004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB (2013) Toxin binding receptors and the mode of action of Bacillus thuringiensis subsp. israelensis Cry toxins. UC Riverside: Environmental Toxicology. Retrieved from: http://escholarship.org/uc/item/6cr35910
- Levinson BL, Kasyan KJ, Chiu SS, Currier TC, González JM., Jr Exotoxin, β-exotoxin production, plasmids encoding & β Identification of and a new exotoxin in Bacillus thuringiensis by using high performance liquid chromatography. J Bacteriol. 1990;172:3172–3179. doi: 10.1128/jb.172.6.3172-3179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ruan L, Peng D, Li L, Sun M, Yu Z. Thuringiensin: a thermostable secondary metabolite from Bacillus thuringiensis with insecticidal activity against a wide range of insects. Toxins. 2014;6:2229–2238. doi: 10.3390/toxins6082229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losey JE, Raynor LS, Carter ME. Transgenic pollen harms monarch larvae. Nature. 1999;399:214. doi: 10.1038/20338. [DOI] [PubMed] [Google Scholar]
- Maghari BM, Ardekani AM. Genetically modified foods and social concerns. Avicenna J Med Biotechnol. 2011;3:109. [PMC free article] [PubMed] [Google Scholar]
- Obeidat M, Khyami-Horani H, Al-Moman F. δ-Exotoxins and & βToxicity of Bacillus thuringiensis endotoxins to Drosophila melanogaster, Ephestia kuhniella and human erythrocytes. Afr J Biotechnol. 2012;11(46):10504–10512. [Google Scholar]
- Pacheco S, Gomez I, Arenas I, Saab-Rincon G, Rodriguez-Almazan C, Gill SS, Bravo A, Soberon M. Domain II loop 3 of Bacillus thuringiensis Cry1Ab toxin is involved in a “ping-pong” binding mechanism with Manduca sexta aminopetidase-N and cadherin receptors. J Biol Chem. 2009;284:32750–32757. doi: 10.1074/jbc.M109.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma L. Protocol for the fast isolation and identification of insecticidal Bacillus thuringiensis strains from soil. Bt Res. 2015;6:1–3. [Google Scholar]
- Palma L, Muñoz D, Berry C, Murillo J, Caballero P. Draft genome sequences of two Bacillus thuringiensis strains and characterization of a putative 41.9-kDa insecticidal toxin. Toxins. 2014;6:1490–1504. doi: 10.3390/toxins6051490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-López L, Soberón M, Bravo A. Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiol Rev. 2013;37:3–22. doi: 10.1111/j.1574-6976.2012.00341.x. [DOI] [PubMed] [Google Scholar]
- Park Y, González-Martínez RM, Navarro-Cerrillo G, Chakroun M, Kim Y, Ziarsolo P, Blanca J, Cañizares J, Ferré J, Herrero S. ABCC transporters mediate insect resistance to multiple Bt toxins revealed by bulk segregant analysis. BMC Biol. 2014;14:46. doi: 10.1186/1741-7007-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed GL, Jensen AS, Riebe J, Head G, Duan JJ. Transgenic Bt potato and conventional insecticides for Colorado potato beetle management: comparative efficacy and non-target impacts. Entomol Exp Appl. 2001;100:89–100. doi: 10.1046/j.1570-7458.2001.00851.x. [DOI] [Google Scholar]
- Resende DC, Mendes SM, Rosangela C, Marucci RC, de Carvalho Silva A, Campanha MM, Waquil JM. Does Bt maize cultivation affect the non-target insect community in the agro ecosystem? Rev Bras Entomol. 2016;60:82–93. doi: 10.1016/j.rbe.2015.12.001. [DOI] [Google Scholar]
- Rodrigo-Simon A, de Maagd RA, Avilla C, Bakker PL, Molthoff J, González-Zamora JE, Ferre J. Lack of detrimental effects of Bacillus thuringiensis Cry toxins on the insect predator Chrysoperla carnea: a toxicological, histopathological, and biochemical analysis. Appl Environ Microbiol. 2006;72:1595–1603. doi: 10.1128/AEM.72.2.1595-1603.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis J, Meissle M, Naranjo SE, Li Y, Bigler F. The end of a myth—Bt (Cry1Ab) maize does not harm green lacewings. Front Plant Sci. 2014;5:391. doi: 10.3389/fpls.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Yoshiga T, Hasegawa K. Activated and inactivated immune responses in Caenorhabditis elegans against Photorhabdus luminescens TT01. Springer Plus. 2014;3:274. doi: 10.1186/2193-1801-3-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar S, Maiti MK. Molecular characterization of a novel vegetative insecticidal protein from Bacillus thuringiensis effective against sap-sucking insect pest. J Microbiol Biotechnol. 2011;21:937–946. doi: 10.4014/jmb.1105.05030. [DOI] [PubMed] [Google Scholar]
- Schnepf HE, Whiteley HR. Cloning and expression of the Bacillus thuringiensis crystal protein gene in Escherichia coli. Proc Natl Acad Sci USA. 1981;78:2893–2897. doi: 10.1073/pnas.78.5.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena JAD, Hernandez-Rodrigues CS, Ferre J. Interaction of Bacillus thuringiensis Cry1 and Vip3A proteins with Spodoptera frugiperda midgut binding sites. Appl Environ Microbiol. 2009;75(7):2236–2237. doi: 10.1128/AEM.02342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia Denise PB, Eduardo PM, Benjamín VC. Recombinant protein detection and its content in total protein, lipids and toxic antinutritional substances in Mexican maize. Health. 2013;5:9–13. doi: 10.4236/health.2013.510A1002. [DOI] [Google Scholar]
- Soberón M, Pardo L, Muñóz-Garay C, Sánchez J, Gómez I, Porta H, Bravo A. Pore formation by Cry toxins. Adv Exp Med Biol. 2010;677:127–142. doi: 10.1007/978-1-4419-6327-7_11. [DOI] [PubMed] [Google Scholar]
- Srisucharitpanit K, Yao M, Promdonkoy B, Chimnaronk S, Tanaka I, Boonserm P. Crystal structure of BinB: a receptor binding component of the binary toxin from Lysinibacillus sphaericus. Proteins. 2014;82:2703–2712. doi: 10.1002/prot.24636. [DOI] [PubMed] [Google Scholar]
- Szczesny P, Iacovache I, Muszewska A, Ginalski K, van der Goot FG, Grynberg M. Extending the aerolysin family: from bacteria to vertebrates. PLoS One. 2011;6(6):e20349. doi: 10.1371/journal.pone.0020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik BE, Sisterson MS, Ellsworth PC, Dennehy TJ, Antilla L, Liesner L, Whitlow M, Staten RT, Fabrick JA, Unnithan GC, Yelich AJ, Ellers-Kirk C, Harpold VS, Li X, Carrière Y. Suppressing resistance to Bt cotton with sterile insect releases. Nat Biotechnol. 2010;28(12):1304–1307. doi: 10.1038/nbt.1704. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Brévault T, Carrière Y. Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol. 2013;31:510–521. doi: 10.1038/nbt.2597. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Zhang M, Fabrick JA, Wu Y, Gao M, Huang F, Wei J, Zhang J, Yelich A, Unnithan GC, Bravo A, Soberón M, Carrière Y, Li X. Dual mode of action of Bt proteins: protoxin efficacy against resistant insects. Sci Rep. 2015;5:15107. doi: 10.1038/srep15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wang B-C, Yu Z, Sun M. Structural Insights into Bacillus thuringiensis Cry. Cyt and Parasporin Toxins. Toxins. 2014;6(9):2732–2770. doi: 10.3390/toxins6092732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudina TG, Brioukhanov AL, Zalunin IA, Revina LP, Shestakov AI, Voyushina NE, Chestukhina GG, Netrusov AI. Antimicrobial activity of different proteins and their fragments from Bacillus thuringiensis parasporal crystals against clostridia and archaea. Anaerobe. 2007;13:6–13. doi: 10.1016/j.anaerobe.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Lan J, Wang J, Liu J, Yang M. Temporal and spatial changes in Bt toxin expression in Bt-transgenic poplar and insect resistance in field tests. J For Res. 2016;27:1249–1256. doi: 10.1007/s11676-016-0254-x. [DOI] [Google Scholar]