Abstract
In this study, an alpha-amylase enzyme from a locally isolated Aspergillus flavus NSH9 was purified and characterized. The extracellular α-amylase was purified by ammonium sulfate precipitation and anion-exchange chromatography at a final yield of 2.55-fold and recovery of 11.73%. The molecular mass of the purified α-amylase was estimated to be 54 kDa using SDS-PAGE and the enzyme exhibited optimal catalytic activity at pH 5.0 and temperature of 50 °C. The enzyme was also thermally stable at 50 °C, with 87% residual activity after 60 min. As a metalloenzymes containing calcium, the purified α-amylase showed significantly increased enzyme activity in the presence of Ca2+ ions. Further gene isolation and characterization shows that the α-amylase gene of A. flavus NSH9 contained eight introns and an open reading frame that encodes for 499 amino acids with the first 21 amino acids presumed to be a signal peptide. Analysis of the deduced peptide sequence showed the presence of three conserved catalytic residues of α-amylase, two Ca2+-binding sites, seven conserved peptide sequences, and several other properties that indicates the protein belongs to glycosyl hydrolase family 13 capable of acting on α-1,4-bonds only. Based on sequence similarity, the deduced peptide sequence of A. flavus NSH9 α-amylase was also found to carry two potential surface/secondary-binding site (SBS) residues (Trp 237 and Tyr 409) that might be playing crucial roles in both the enzyme activity and also the binding of starch granules.
Keywords: α-Amylase, Aspergillus flavus NSH9, Characteristic, cDNA, Nucleotide sequence
Introduction
Alpha amylase (α-1, 4 glucan-glucanohydrolase, EC 3.2.1.1) belongs to a family of endo-acting amylases that hydrolyses α-1,4 glycosidic bonds randomly throughout the starch molecule producing oligosaccharides and monosaccharides including maltose, glucose, and alpha limit dextrin at α-anomeric configuration (Bhanja et al. 2007). Most α-amylases are metalloenzymes, which require calcium ions (Ca2+) as co-factor for their activity, structural integrity, and stability. Ever since the establishment of a sequence-based classification of all glycoside hydrolases in 1991, the α-amylases family has been known as family 13 of glycoside hydrolases (GH) (Henrissat 1991). GH13 is the largest member of the GH-H clan which also contains GH-70 and GH-77 (MacGregor 2005). In 2006, Stam et al. further divided members of GH13 into 35 subfamilies based on their sequence similarity and phylogenetic reconstruction criteria. To date, there are up to 42 subfamilies in GH13 and the number is still being updated (http://www.cazy.org/Glycoside-Hydrolases.html) (Valk et al. 2016). Fungal α-amylases are mainly classified into subfamilies of GH13_1 and GH13_5 with members in subfamily GH13_1 being extracellular and fungal specific, while those in subfamily GH13_5 are intracellular and have high sequence similarities to the bacterial α-amylases (Stam et al. 2006; van der Kaaij et al. 2007). A more recent study by Da Lage et al. (2013) reported on an additional family of GH13_32 for Basidiomycetes α-amylase which originated from Actinobacteria.
Having approximately 25% of the world enzyme market, amylase such as α-amylase is one of the most popular and important forms of industrial amylases due to its ability to hydrolyze starch (Reddy et al. 2003). In the conventional industrial starch processing for the production of glucose and fructose syrup, for example, α-amylase is needed to catalyze the first step of this process. The starch is first cooked at high temperature with incorporation of α-amylase to disrupt the granular structure to bring the amylose and amylopectin into the solution. The liquefied starch can only then be simultaneously saccharified by glucoamylase to release glucose (van der Maarel et al. 2002; Sundarram and Murthy 2014). With the advances in biotechnology, α-amylase has found its application into a wide range of industries including food, baking, brewing, distilling, fermentation, textile, paper, pharmaceutical, and even for bioconversion of solid waste (Gupta et al. 2003; Couto and Sanromán 2006).
Alpha amylases are widely distributed in animals, plants, and microorganisms including fungus and bacteria. Despite being widely distributed, however, enzymes derived from fungal and bacterial sources have dominated applications in the industrial sectors (Gupta et al. 2003). The major reason for the increasing interest in using microbes for the production of amylases is the fact that microbes are much easier to manipulate using genetic engineering or any other means to produce enzymes of desired characteristics (Souza 2010). Microorganisms also grow much faster as compared to both plants and animals thus help to speed up enzyme production (Sundarram and Murthy 2014).
Commercially available α-amylase derived from bacteria is commonly obtained from Bacillus species such as B. amyloliquefaciens and B. licheniformis. α-amylase of fungal origin on the other hand are much confined to terrestrial isolates such as Aspergillus niger, A. oryzae and a few Penicillium species (Souza 2010; Saranraj and Stella 2013; Sundarram and Murthy 2014). Alpha amylases of fungal origin, however are much preferred over any other microbial sources due to their more accepted GRAS (Generally Recognized as Safe) status (Gupta et al. 2003). That is also the reason for the increase in number of fungi being continuously screened for α-amylase production with properties that better suit various industrial applications (Negi and Banerjee 2009; Sanghvi et al. 2011; Fadahunsi and Garuba 2012). The properties of each α-amylase such as thermostability, pH profile, pH stability, and Ca-independency are critical in the development of fermentation process (Souza 2010).
With an expected annual increment of 3.3% in the global enzyme market and the ever-increasing demand for amylase enzyme, users are continuously trying to increase the productivity of amylases by a variety of approaches such as optimizing current production process, using much cheaper substrates and also selection for a high enzyme producing strain (Saxena et al. 2010). Aspergillus flavus has previously been reported to be a good and active producer of amylase enzyme (El-Abyad et al. 1992; Fadahunsi and Garuba 2012). Alpha amylases produced by several different strains of A. flavus have also been purified and characterized (Khoo et al. 1994; Abou-Zeid 1996; El-Safey and Ammar 2004). An example is A. flavus isolated from mangrove that was reported to be a potent strain for industrial production of α-amylase (Bhattacharya et al. 2011; Bhardwaj et al. 2012).
We have previously isolated an amylase producing A. flavus strain NSH9 from sago humus and in this study, we described the production, purification, and characterization of α-amylase derived from this A. flavus NSH9 isolate. The genetic sequence and molecular information of this α-amylase were further elucidated at the second part of this study. Here, we also reported, for the first time, the presence of potential substrate-binding site (SBS) residues in the deduced α-amylase peptide sequence of A. flavus NSH9.
Materials and methods
Production of α-amylase enzyme
Aspergillus flavus NSH9 (isolated from sago humus) was obtained from the Molecular Biology Microbial Collection, Universiti Malaysia Sarawak, and used as inoculum for amylase production in liquid culture. For enzyme induction, the actively growing fungal mycelium was transferred from potato dextrose agar (PDA) plate to a minimal salt culture medium (MSM) containing (g l−1): 20 g of raw sago starch, 3 g of KH2PO4, 1 g of (NH4)2SO4, 0.5 g of MgSO4.7H2O, and 4 g of yeast extract (pH 5.0). One piece of a 7-day-old fungal culture (approximately 5 mm2 in diameter) grown in PDA was used in the fermentation of 50 ml MSM medium containing 2% (w/v) raw sago starch (sterilized separately by dry heat). The incubation was carried out at room temperature (25–27 °C) for a period of 5 days on a rotary shaker, at the speed of 150 rpm. The samples were then filtered through a Whatman filter paper no. 1, before it was centrifuged at 10,000×g for 20 min at 4 °C to remove the fungal mycelia and any suspended particles. The crude enzyme supernatant was collected and stored at − 20 °C for further analysis.
Alpha amylase assay and protein assay
Alpha amylase enzyme activity was estimated based on starch–iodine method of Xiao et al. (2006) with slight modification. Assay reactions were initiated by adding 200 µl of starch (Merck) solution (2.0 g l−1) prepared in 0.1 M of phosphate buffer (pH 7.0) to 200 µl of appropriately diluted enzyme in a 1.5 ml microfuge tube. After 30 min of incubation at 50 °C, enzymes were inactivated by adding 100 µl of 1 M HCl, followed by the addition of 500 µl of iodine reagent (5 mM I2 and 5 mM KI). Following color development, starch–iodine complex formed were measured at a wavelength of 580 nm. A standard curve of starch–iodine complex was also prepared using different amount of starch ranging from 50–400 µg. Alpha amylase activity unit (U) was expressed in the starch–iodine assay as the disappearance of an average of 1 mg of iodine binding starch material per min in the assay reaction. The enzyme activity was calculated using the following Eq. 1:
| 1 |
where A580 control = the absorbance obtained from the starch without the addition of enzyme; A580 sample = the absorbance for the starch digested with enzyme; A580/mg starch = the absorbance for 1 mg of starch as derived from the standard curve; 30 min = the assay incubation time; and 0.2 ml = the volume of the enzyme used in the assay.
The protein content was determined by the method of Bradford (1976) with bovine serum albumin (BSA) as the protein standard. The specific activity of α-amylase was taken as units/mg protein.
Purification of α-amylase
Ammonium sulfate precipitation
The enzyme α-amylase was first partially purified from the culture filtrate by fractional ammonium sulfate precipitation. Solid ammonium sulfate was sequentially added to the culture filtrate to 30, 50, and 80% saturation and was left overnight at 4 °C before centrifugation at 15,000×g at 4 °C for 20 min. All proteins precipitated with 30 and 50% ammonium sulfate were discarded. Protein pellets obtained after 80% ammonium sulfate precipitation were re-suspended in 50 mM potassium phosphate buffer (pH 7.0) and dialyzed against the same buffer to remove salt.
Ion-exchange chromatography
The partially purified α-amylase after 80% ammonium sulfate precipitation was further purified by means of ion-exchange chromatography. The enzyme was loaded onto a 20 ml open column containing Amberlite IRA-400 beads (from Sigma, anion exchanger) pre-equilibrated with 50 mM potassium phosphate buffer (pH 7.0). Proteins bound to the anion-exchange beads were then washed using potassium phosphate buffer (50 mM, pH 7.0) before eluted with a linear concentration gradient of NaCl (0–1 M) in potassium phosphate buffer (50 mM, pH 7.0) at a flow rate of 2.5 ml min−1. Fractions of 5 ml each were collected (total 85 ml elution) and assayed for α-amylase activity. Fractions containing amylase activity were pooled and concentrated with 80% ammonium sulfate and re-suspended in 50 mM potassium phosphate buffer (pH 7.0) before dialyzed overnight against the same buffer to remove salt.
Polyacrylamide gel electrophoresis and zymogram
The homogeneity and molecular weight of the purified α-amylase enzyme was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli (1970). Electrophoresis was carried out using a 12% (w/v) acrylamide resolving gel and 6% (w/v) acrylamide stacking gel, in a Mini-Protean Tetra Cell electrophoresis system (Bio-Rad, Richmond, CA, USA). Protein bands formed were visualized after staining with Coomassie Brilliant Blue R-250.
Zymogram for amylase activity was also carried out using the same conditions of SDS-PAGE except that the protein samples were not heated prior to sample loading and no β-mercaptoethanol was used in the sample buffer. After electrophoresis, SDS in the gel was removed by washing for 30 min using 0.2% (v/v) Triton X-100 prepared in 0.1 M sodium acetate buffer (pH 5.0). The gel was then incubated with 0.1 M sodium acetate buffer (pH 5.0) containing 1% soluble starch for 10–30 min at 50 °C before activity staining with iodine reagent (0.15% (w/v) of I2 and 0.5% (w/v) of KI) (Chen et al. 2005; Karim et al. 2016). Amylase activities were visualized as the formation of a clear band on a dark blue background.
Desired protein band in SDS-PAGE after de-staining from Coomassie was cut out and subjected to peptide sequencing via LC/MS/MS. The analysis was performed by Proteomics International Pty Ltd, Broadway, Nedlands, Western Australia.
Characterization of the purified α-amylase
Effect of pH and temperature
The optimum pH for activity was determined by measuring the purified α-amylase activity at 50 °C for 30 min using various buffers. The following 0.1 M buffer systems were used: sodium citrate (pH 3.0); sodium acetate (pH 4.0–5.0); potassium phosphate (pH 6.0–7.0); and Tris-HCI (pH 8.0–9.0). The optimum temperature for activity was assayed by measuring activity at optimum pH 5 (0.1 M sodium acetate buffer) over different temperatures ranging from 20 to 90 °C.
pH stability and thermostability
For pH stability of the purified α-amylase, the enzyme was dispersed (1:1) in 0.1 M buffer solution of different pH and incubated at 25 °C for 24 h. An aliquot was used to determine the remaining activity at optimum pH and temperature. Thermal stability of purified enzyme was determined by incubating the enzyme in 0.1 M sodium acetate buffer pH 5.0, at 50–70 °C for 120 min. Time course aliquots were withdrawn, and cooled in ice bath and residual activity determined under optimum pH and temperature (Karim et al. 2017).
Enzyme kinetics
The initial reaction rate of the purified α-amylase was determined at different concentrations of starch ranging from 0.05 to 0.8% (w/v). After 30 min of incubation at 50 °C, enzyme activity per unit time was determined in each starch concentration (Negi and Banerjee 2009). Both the Km and Vmax values were calculated from Lineweaver–Burk plot.
Effect of metal ions
The effect of metal ions (Na+, K+, Cu2+, Fe2+, Ca2+, Mg2+, and Zn2+) on the activity of α-amylase was measured by incubating the enzyme in the presence of metal ion (with the final concentrations of 1 and 5 mM) for 30 min at 37 °C. The relative activity was measured under standard assay condition with a control having no metal ions in the assay and taken as 1.00 (Karim et al. 2016).
Molecular characterization of α-amylase gene
Total RNA, DNA extraction, and first-strand cDNA synthesis of α-amylase
The fungal mycelia were collected after 4 days of growth in the culture media at room temperature (28 °C) with shaking at 150 rpm. Total RNA was extracted using TRIzol Reagent according to the protocol by Schumann et al. (2013) and genomic DNA was extracted according to the method of Cubero et al. (1999). Total RNA was treated with DNase prior to first-strand cDNA synthesis using a first-strand cDNA synthesis kit (Thermo Scientific). The product generated was used for subsequent PCR analysis.
Isolation, subcloning, and sequencing of α-amylase
The α-amylase gene of A. flavus NSH9 was isolated using primers designed based on result of LC/MS/MS peptides hits in combination with consensus of closely matching nucleotide sequences from the National Center for Biotechnology Information (NCBI). The consensus sequence of closely matching α-amylase gene from NCBI starting from the start codon until the stop codon was used as templates in designing the forward and reverse primers, respectively. PCR was carried out in a volume of 50 µl with 5 µl of 10× Pol Buffer A, 5 mM MgCl2, 0.2 mM dNTP, 0.1–0.5 µM gene-specific primers Amy-F (5´-AAG ATG ATG GTC GCG TGG TGG-3´) and Amy-R (5´-CGC TCA CGA GCT ACT ACA GAT C-3´), < 0.5 µg gDNA as template, and 1.25 U Taq DNA polymerase (EURx, Gdansk Poland). The PCR reaction was carried out with the following conditions: initial denaturation at 94 °C for 2 min, 35 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C for 45 s, elongation at 72 °C for 1.45 min, and final elongation at 72 °C for 5 min. The full α-amylase cDNA sequence was also PCR amplified under the same PCR reaction and condition using cDNA as template. The purified PCR product was cloned into pGEMT-Easy vector (Promega) and propagated in Escherichia coli XL1-Blue following the manufacturer’s protocols before sequenced using T7 (5′ TAATACGACTCACTATAGGG 3′) and SP6 (5´ ATTTAGGTGACACTATAG 3´) universal primers.
Bioinformatic analysis of α-amylase gene sequence
The positions of introns and exons in α-amylase gene sequence were determined by aligning α-amylase gDNA with its cDNA using Clustal Omega. The predicted amino acid sequence was deduced using Translate tool (http://web.expasy.org/translate/). The molecular weight and isoelectric point of the deduced protein was predicted using Compute pI/Mw tool (http://www.expasy.org/tools/pi_tool.html). The presence of signal peptide was predicted using SignalP4.1 (http://www.cbs.dtu.dk/services/SignalP/) and possible N-glycosylation sites (Asn-X-Ser/Thr) were predicted using NetGlyc 1.0 (http://www.cbs.dtu.dk/ services/NetNGlyc/). The family and conserved domains of the protein were also predicted using Interpro (https://www.ebi.ac.uk/interpro/) and Conserved Domain Database (CDD) from NCBI (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). The sequence homology between α-amylase amino acid sequence of A. flavus NSH9 and α-amylase of other fungi including Aspergillus oryzae RIB40 (Accession: P0C1B4, P0C1B3, 2TAA) was analyzed using BLAST, UniProt (http://www.uniprot.org/), and Clustal Omega (https://www.ebi.ac.uk/ Tools/msa/clustalo/). A neighbor-joining tree was also constructed using MEGA version 6 (Tamura et al. 2013) and the evolutionary distances between sequences computed using the Jones–Taylor–Thornton (JTT) matrix-based model (Jones et al. 1992). Bootstrap analysis was performed with 1000 replications to assess the confidence limits of the branching (Felsenstein 1985).
Results
Production and purification of α-amylase
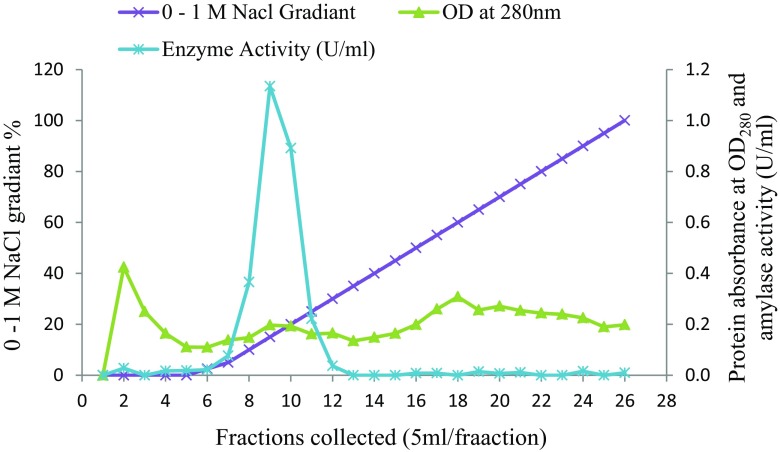
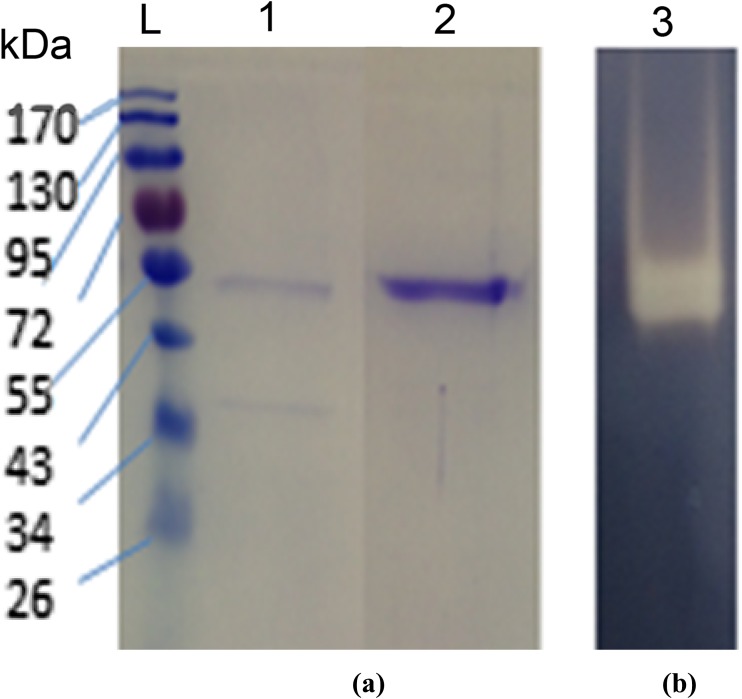
Aspergillus flavus NSH9 was found to produce 2.87 U ml−1 of amylase activity after 5 days of incubation in MSM containing raw sago starch. Proteins in the culture supernatant were fractionally precipitated using ammonium sulfate before α-amylase was further purified by anion-exchange chromatography. Two protein peaks were observed from the anion-exchange chromatogram after the column was eluted with increasing concentration of NaCl (Fig. 1). Alpha amylase activity, however, was only found in fractions collected from the first protein peak. Active fractions containing α-amylase activity after being pooled and analyzed by SDS-PAGE were found to contain only one protein band of the size around 54 kDa (Fig. 2a). Further zymogram of the purified sample which showed only one clear band after staining by iodine reagent confirmed that α-amylase of A. flavus NSH9 has been successfully purified (Fig. 2b). The enzyme was 3.4-fold purified at a yield of 11.73% and with a specific activity of 48.10 U/mg (Table 1).
Fig. 1.
Anion-exchange chromatography of A. flavus NSH9 α-amylase in Amberlite IRA-400 beads packed column
Fig. 2.
a SDS-PAGE and b zymogram of α-amylase. Lane 1: SDS-PAGE of ammonium sulfate (80%) precipitated protein; Lane 2: SDS-PAGE of purified α-amylase after ion-exchange chromatography (54 kDa); Lane 3: Zymogram of purified α-amylase. L: EZ-Run Prestained Rec Protein Ladder
Table 1.
Purification of α-amylase from A. flavus NSH9
| Purification step | Total activity (U) | Total protein (mg) | Specific activity (U/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Crude culture | 86.10 | 4.560 | 18.88 | 1.0 | 100.0 |
| 80% (NH4)2SO4 precipitation | 26.42 | 0.759 | 34.81 | 1.84 | 30.69 |
| Anion-exchange chromatography | 10.1 | 0.210 | 48.10 | 2.55 | 11.73 |
Further identification of the purified enzyme by LC/MS/MS using both Ludwig NR and Swiss-Prot database confirmed that the amylase enzyme purified is α-amylase. Peptide fragments generated were all matched with the same enzyme which is α-amylase from A. flavus (Tax-Id = 332952) and also α-amylase from A. sojae (Tax-Id = 41058), both with up to 28% of protein sequence coverage. Protein identification using Swiss-Prot database also resulted in similar match with α-amylase of A. oryzae RIB 40 (Tax-Id = 510516) showing 24% protein sequence coverage. The molecular weight of the purified α-amylase determined by LC/MS/MS (54.8 kDa) was also very similar with the 54 kDa that was observed earlier from SDS-PAGE (Fig. 2).
Characteristics of α-amylase enzyme
Effect of pH and temperature
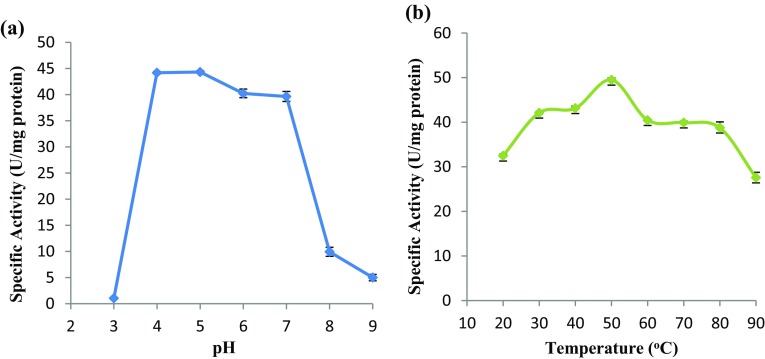
The purified α-amylase was found to be sensitive towards pH. When assayed at different pH ranging from pH 3–9, the enzyme exhibited optimal activity between pH 4–7 (Fig. 3a), but activity was significantly reduced when the enzyme was assayed at both acidic (pH 3) and alkaline condition (pH 8–9) (p ≤ 0.05 by one-way ANOVA). Highest α-amylase activity of 44.34 U mg−1 protein was observed at pH 5.0. It was significantly higher than enzyme activity found at all pH except activity at pH 4 (p ≤ 0.05, by Tukey HSD test).
Fig. 3.
Effect of a pH and b temperature on α-amylase activity. Error bars show standard deviation among three independent observations
When assayed at different temperatures (20–90 °C), the purified α-amylase showed the highest activity at a temperature of 50 °C (significant at p ≤ 0.05, Tukey HSD test) (Fig. 3b). Alpha amylase activity increased with increasing temperature and peaked at 50 °C (49.51 U mg−1 proteins) before gradually decreases as temperature further increases.
pH stability and thermostability
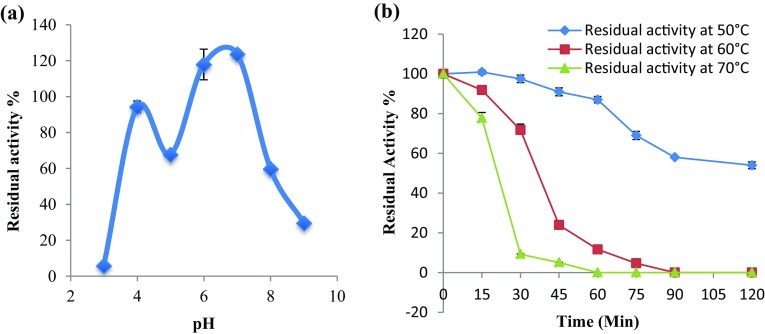
The purified α-amylase, when tested for its pH stability by incubating for 24 h in buffer with different pH, was found to be most stable at pH 6–7 (Fig. 4a). The enzyme was able to retain around 100% of its initial activity even after 24 h of incubation at pH 6 and pH 7. Incubation at both acidic (pH 3) and alkaline (pH 9) condition, on the other hand, significantly reduced the enzyme activity to less than 40% (p ≤ 0.05).
Fig. 4.
Residual activity of purified α-amylase from A. flavus NSH9: a after 24 h of incubation at different pH and b after incubation at different temperatures. Each value represents the mean of three independent observations. Error bars show standard deviation among three independent observations
The purified α-amylase from A. flavus NSH9 was thermally stable at a temperature of 50 °C as it retained about 87% of its initial activity after 60 min of incubation at this temperature (Fig. 4b). At higher temperature of 60 and 70 °C, the purified α-amylase was also stable at least for 30 and 15 min, respectively, retaining more than 70% of its initial activity. Longer incubation at these temperatures, however, rapidly reduces the enzyme activity.
Enzyme kinetics and effect of metal ions
The Michaelis–Menten constant, Km, and maximum velocity, Vmax, of the purified α-amylase calculated using Lineweaver–Burk plot were found to be 4.22 mg ml−1 and 65.52 U mg−1 protein, respectively. When the purified α-amylase was further tested on metal ions, it was found that metal ions such as Na+, Ca2+, and Fe2+ ions at a concentration of 1 mM had no significant effect on enzyme activity, but at higher concentration of 5 mM, Na+ and Fe2+ had negative effect whilst Ca2+ had positive effect on enzyme activity (Table 2). Enzyme activity, however, was significantly inhibited by K+, Cu2+, Zn2+ and Mg2+ ions at both 1 and 5 mM concentrations. Of all the different metal ions tested, only the presence of increased Ca2+ ions concentration significantly stimulates the activity of the purified α-amylase.
Table 2.
Effect of metal ions on α-amylase activity
| Metals salt | Relative α-amylase activity at different metal salt concentrations | |
|---|---|---|
| 1 mM | 5 mM | |
| Control (H2O) | 1.00 | 1.00 |
| Na+ | 0.88 | 0.72* |
| K+ | 0.89* | 0.79* |
| Ca2+ | 1.05 | 1.14* |
| Mg2+ | 0.77* | 0.76* |
| Fe2+ | 0.92 | 0.73* |
| Zn2+ | 0.74* | 0.27* |
| Cu2+ | 0.46* | 0.24* |
*Significant difference on metal either positive or negative (p = < 0.05)
Isolation and identification of α-amylase gene
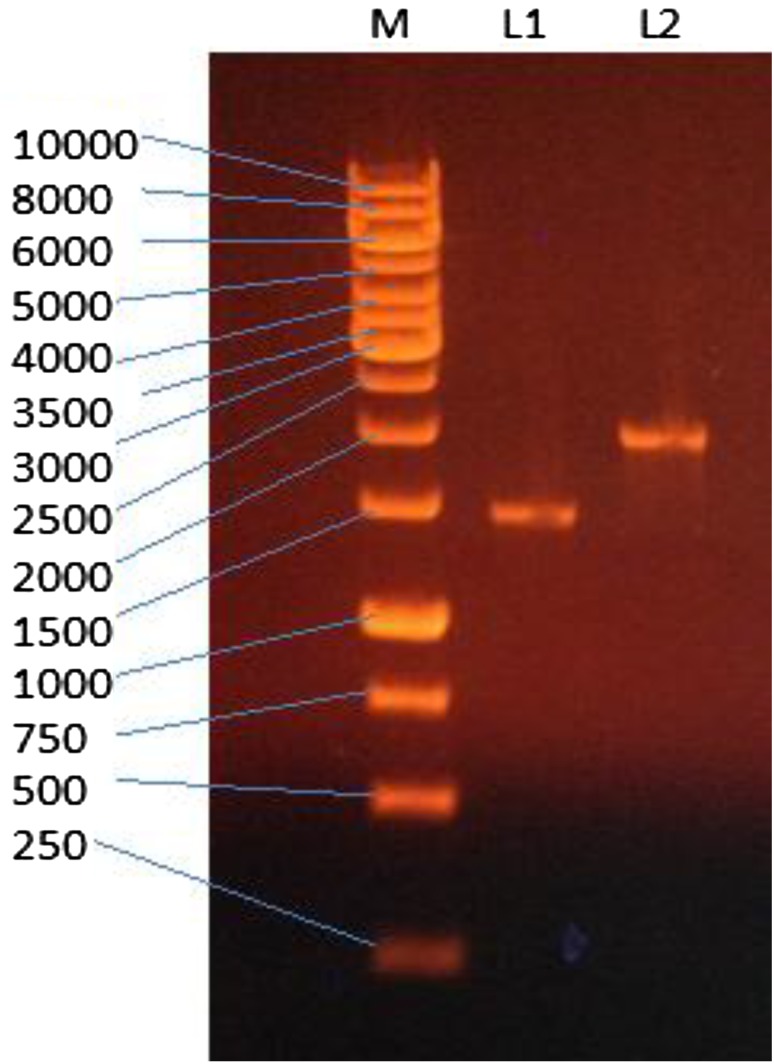
Isolation of A. flavus NSH9 α-amylase gene by PCR from gDNA resulted in the amplification of a 2.05 kb gene sequence (Fig. 5). PCR using cDNA as the template, on the other hand, produced a band of smaller size, 1.5 kb (Fig. 5). The difference in sizes between these two PCR products suggested the presence of introns. A comparison of the gDNA (2049 bp) and cDNA (1500 bp) sequences indicated the presence of eight introns varying in sizes of 55, 90, 69, 68, 58, 65, 65, and 79 bp; respectively. The BLAST search further confirmed that these gene sequences obtained are the full α-amylase gene sequence with up to 99% of sequence identity with α-amylase gene of Aspergillus species including Aspergillus flavus NRRL3357 (XM_002374083.1), Aspergillus oryzae RIB40 (XM_001823889.3), Aspergillus niger CBS 513.88 (XM_001390741.2), Aspergillus awamori (AB083159.1), and Aspergillus shirousami (D10461.1). The full α-amylase gene sequences of A. flavus NSH9 were submitted to GenBank as α-amylase cDNA (KU378618) and α-amylase gDNA (KU378619), respectively.
Fig. 5.
Agarose gel electrophoresis of PCR amplified A. flavus NSH9 full α-amylase cDNA (L1 = 1.5 kb) and full α-amylase gDNA (L2 = 2.05 kb)
Analysis of A. flavus NSH9 α-amylase cDNA shows that the open reading frame (ORF) of the gene is consisted of 1500 nucleotides, which encode for a polypeptide of 499 amino acid residues preceded by a signal peptide of 21 amino acids. The mature α-amylase protein should be a protein of 478 amino acids with a calculated molecular weight of 52.5 kDa and an isoelectric point of 4.62. Peptide sequence deduced from α-amylase cDNA was found to contain all the peptide fragments generated from LC/MS/MS. This proves that the α-amylase gene isolated is the gene that encodes for the α-amylase enzyme that was successfully purified from A. flavus NSH9 (Fig. 2). The deduced α-amylase protein sequence was found to contain two putative asparagine-linked N-glycosylation sites (Asn-X-Ser/Thr) at the 218th and 422nd amino acid residue. The peptide sequence (22nd–399th amino acids) was homologous to glycoside hydrolase superfamily (IPR017853) in which contain glycosyl hydrolase, family 13, catalytic domain (34th–390th amino acids) (IPR006047–Interpro), and α-amylase catalytic domain, AmyAc_euk_AmyA (26th–395th amino acids) (cd11319–CDD NCBI). Near the C-terminal (407th–496th amino acids), the deduced peptide sequence also contains α-amylase domain of unknown function DUF1966 (IPR015340, pfam09260).
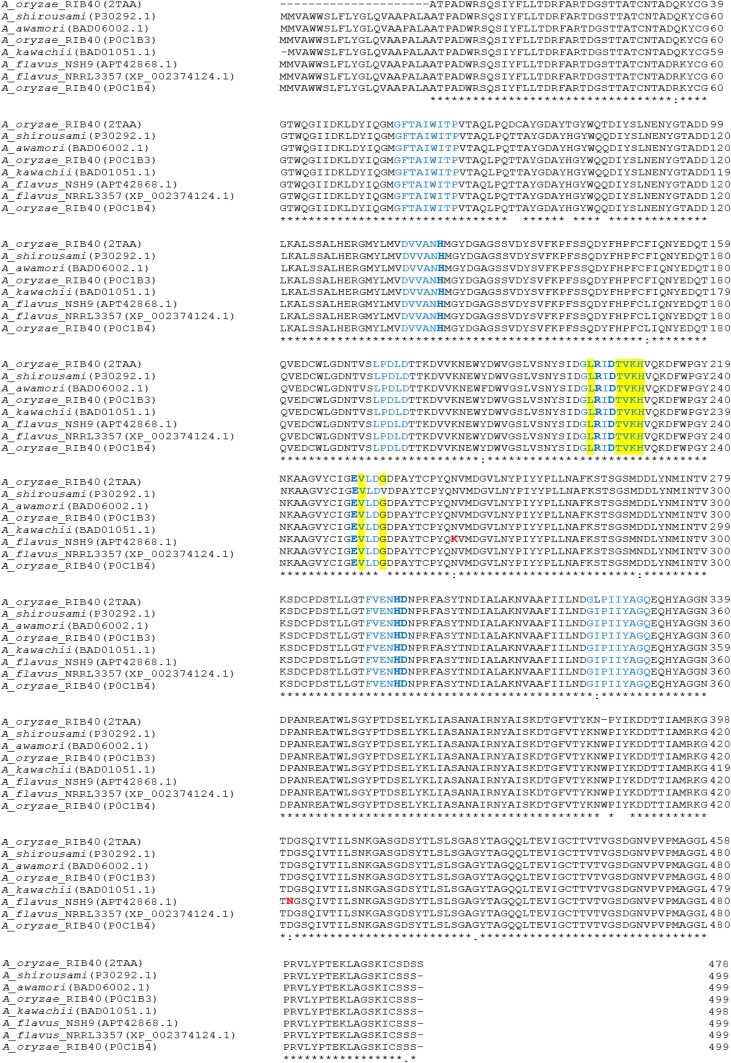
Aligning the deduced peptide sequence with other α-amylase revealed that α-amylase from A. flavus NSH9 has three catalytic residues: Asp 227 which function as the nucleophile, Glu 251 as the proton donor, and Asp 318 which is the transition state stabilizer for substrate binding; the invariantly conserved arginine, Arg 225 of α-amylase; two amino acids that function as Ca2+-binding sites (Asn 142 and Asp 196); and several other active site at 101, 103, 104, 143, 225, 227, 228, 230, 231, 251, 253, 255, 277, 317, 318, 361, and 365 (Fig. 6). Up to seven of the conserved peptide sequence of α-amylase: DVVANH, GLRIDTVKH, EVLD, FVENHD, LPDLD, GFTAIWITP, and GIPIIYAGQ can also be easily located in the deduced α-amylase peptide sequence of A. flavus NSH9 (indicated in blue, Fig. 6). Two amino acid residues unique only to α-amylase of A. flavus NSH9 were also observed (Lys 265 which is near the catalytic proton donor Glu 251 and Asn 422 located inside α-amylase domain of unknown function DUF1966) (Fig. 6). Further peptide sequence alignment with other structurally proven α-amylase and raw starch degrading α-amylase of fungal origin also shows that the α-amylase of A. flavus NSH9 lacks a carbohydrate-binding modules (CBM) but potentially carry two surface/secondary-binding sites (SBS) (Trp 237 and Tyr 409) and four maltose-binding sites (bold and highlighted in grey) (Fig. 7).
Fig. 6.
Alignment of A. flavus NSH9 α-amylase deduced amino acid sequence with other Aspergillus α-amylase. The seven conserved peptide sequences are indicated in blue. The conserved three catalytic residues, two His and one Arg, are indicated in bolded blue. Amino acid residues indicating enzyme acting only on α-1,4-bonds are highlighted. The two amino acids in red are unique residues presence only in α-amylase of A. flavus NSH9
Fig. 7.
Sequence alignment of A. flavus NSH9 α-amylase with two other proven raw starch degrading fungal α-amylase to date (Cryptococcus sp. S-2 Accession no: BAA12010.1; Saccharomycopsis fibuligera Accession no: ADD80242.1) as compared to α-amylase of proven crystal structure (Human pancreatic α-amylase PDB-5TD4; Human saliva α-amylase PDB-1SMD; Barley α-amylase PDB-1RPK; A. niger NSH9 α-amylase PDB-2GUY). Amino acids in red indicate GH13 family catalytic domain; in blue indicate domain of unknown function/α-amylase C-terminal domain; and in green indicate CBM20. α-amylase surface/secondary-binding sites (SBS) residues from human saliva and pancreatic were shown in bold and highlighted in green and blue; SBS from barley were shown in bold and highlighted yellow, while maltose-binding sites from A. niger were shown in bold and highlighted in grey
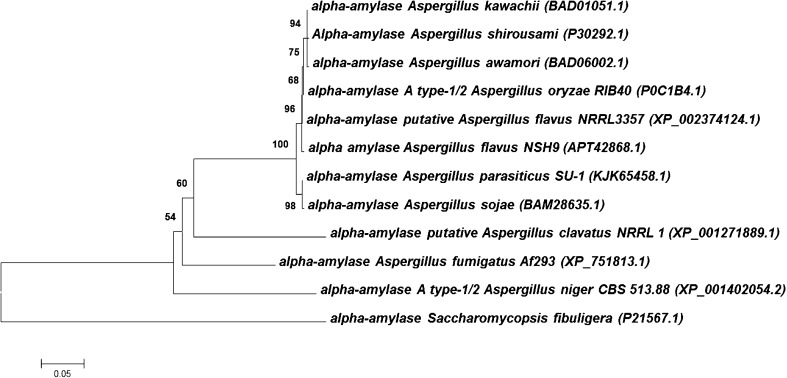
When analyzed for sequence homology using NCBI protein BLAST, the deduced peptide sequence of A. flavus NSH9 α-amylase was found to exhibit a high degree of sequence identity with other fungal α-amylase. The deduced peptide sequence shared up to 99% of sequence identity with α-amylase of A. flavus NRRL3357 (XP_002374124.1), A. oryzae RIB40 (XP_003189619.1), A. awamori (BAD06002.1), A. kawachii (BAD01051.1), A. shirousami (P30292.1), A. parasiticus SU1 (KJK65458.1), and also A. sojae (BAM28635.1). This high degree of sequence similarity observed thus explained the close grouping of this fungal α-amylase when these sequences were represented in a neighbor-joining tree (Fig. 8).
Fig. 8.
Phylogenetic tree showing the relationship of A. flavus NSH9 α-amylase with α-amylase from other Aspergillus species. Bootstrap values are shown on branches and accession number in bracket. Alpha amylase protein sequence from Saccharomycopsis fibuligera (P21567.1) was used as outgroup
Discussion
Alpha amylase produced by A. flavus NSH9 was successfully purified by sequential purification using ammonium sulfate precipitation followed by anion-exchange chromatography. The purification yield of α-amylase in this study was determined at 11.73%, which was higher compared to several previous attempts by Abou-Zeid (1996) and Shaw et al. (1995) with reported yield of 0.57 and 2.6%; respectively. The yield obtained in this study, however, was very much lower than the 70% yield reported by Khoo et al. (1994) during their purification of A. flavus LINK α-amylase.
The purified α-amylase of A. flavus NSH9 shared many similar physicochemical properties with other known fungal α-amylase. Molecular weights of microbial α-amylase have been commonly reported to be between 50 and 60 kDa (Gupta et al. 2003). Similar to this study, A. flavus α-amylase purified by Bhattacharya et al. (2011) (55 kDa) and Khoo et al. (1994) (52.5 ± 2.5 kDa) were also found to be within this range of molecular weight. In addition, α-amylase purified from Chrysosporium asperatum was also reported to have a molecular weight of 55 kDa (Sanghvi et al. 2011). In this study, the highest activity of purified α-amylase was observed at pH 5, which is similar with many reported findings. Kariya et al. (2003) and Madihah et al. (2000) reported that the optimum pH for α-amylase activity were pH 4.5 and pH 5.3, respectively. Optimal pH for A. flavus α-amylase has also been reported to be pH 6 (Khoo et al. 1994) and pH 7 (Abou-Zeid 1996). Optimal temperature for α-amylase activity found in this study was similar with the 50 °C reported for a thermostable α-amylase (Chakraborty et al. 2000). Optimal temperature for α-amylase of A. flavus has been found to be ranging from 30 °C (Abou-Zeid 1996), 35 °C (El-Safey and Ammar 2004) to 55 °C (Bhattacharya et al. 2011).
Alpha amylase with high pH and thermostability are normally the desirable criteria that people look into when screening for new source of novel enzyme. The α-amylase purified in this study was stable at 50 °C for at least 2 h, which is much longer than the 1 h as reported for A. flavus α-amylase at the same temperature (Khoo et al. 1994). The enzyme was also much more stable than the α-amylase of Lactobacillus manihotivorans (Aguilar et al. 2000). The increased thermostability observed could be due to the stabilization of enzyme conformation by Ca2+ ions that are normally found in metalloenzymes such as α-amylase. Similar to the findings of Nguyen et al. (2002), supplementation of Ca2+ significantly increases the α-amylase enzyme activity. The presence of Ca2+ helps the protein to adapt to a compact structure by salting out hydrophobic residues (Goyal et al. 2005). Further supporting this was the discovery of at least two Ca2+-binding sites (Asn 142 and Asp 196) in the peptide sequence deduced from the isolated α-amylase cDNA sequence. The fact that α-amylase is not inhibited by Ca2+ ions makes it suitable for use in the starch processing industry (Bhattacharya et al. 2011).
Further analysis of the isolated α-amylase gene sequence also identified the presence of introns with the length of less than 100 bp, typical for most fungal introns (Gurr et al. 1987). The splice sites of these introns correspond to the fungal consensus sequences, GT–AG boundaries, and internal consensus for lariat formation. The 21 amino acid residues that made up the signal peptide concur to Amyl1 of the Aspergillus awamori KT-11 (Matsubara et al. 2004). The deduced peptide sequence was also found to carry two glycosylation site which are more than the single glycosylation site found in the peptide of A. flavus NRRL3357 α-amylase (GenBank accession number: XP_002374124.1). As the number of glycosylation sites affects the stability of a protein (Imperiali and O’Connor 1999), this increase in the number of glycosylation site observed might be one of the reason for the improved thermostability of A. flavus NSH9 α-amylase in comparison to α-amylase of other A. flavus strain (Khoo et al. 1994).
The deduced peptide sequence of A. flavus NSH9 α-amylase showed high sequence similarity with other known fungal α-amylase. It contain the three conserved catalytic site amino acid residues (Asp 227, Glu 251 and Asp 318), the invariantly conserved β4 arginine, Arg 225 (equivalent to the Arg 204 in Taka-amylase A) (Janecek 2002), the additional two histidine residues His 143 and His 317 postulated as being crucial for Taka-amylase A (Matsuura et al. 1984), the four conserved peptide sequence which is one of the main criteria for deciding whether or not a new sequence belongs to the α-amylase family (I: 138 DVVANH, II: 223 GLRIDTVKH, III: 252 EVLD, IV: 313 FVENHD) (Nakajima et al. 1986), and also three other conserved peptide sequence of α-amylase (V: 194 LPDLD, VI: 77 GFTAIWITP and VII: 344 GIPIIYAGQ) (Janecek 2002). These seven conserved peptide sequence regions covering the strands β2, β3, β4, β5, β7, and β8 of the catalytic (β/α)8-barrel domain and domain B were identified and used for defining α-amylase in the α-amylase family (Janecek 2002). These conserved amino acid residues and peptide sequences are all important for the catalytic activity of α-amylase enzyme particularly the three catalytic amino acid residues (Asp 227, Glu 251, and Asp 318). Mutating these conserved catalytic sites via site-directed mutagenesis has been reported to result in 15,000-fold decrease in the specific activity of the enzyme (Takase et al. 1992) and almost complete enzyme inactivation (Vihinen et al. 1990).
Although protein modelling and study of its specificity and product profile were not carried out in this study, the high sequence similarity observed between A. flavus NSH9 α-amylase and other structurally proven α-amylases of A. oryzae RIB40 suggested that the enzyme to be also containing 3 domains: domain A, B, and C. Domain A is a (β/α)8-barrel containing the catalytic residues; domain B is a long loop between the β3 strand and α3 helix of domain A, while domain C is the C-terminal extension characterized by a Greek key with eight-stranded antiparallel beta-sandwich structure. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu, and Asp) performs catalysis (Marchler-Bauer et al. 2017; Zhang et al. 2017). Furthermore, according to MacGregor et al. (2001), the presence of amino acid Thr 228, Val 229, Lys 230, and His 231, while not Ile, Trp, and Glu at position 224, 252, and 255, respectively, also indicates that the α-amylase of A. flavus NSH9 is an enzyme acting on α-1,4-bonds only (Fig. 6).
Some members of family GH13 also bear a variable numbers of supplemental N- or C-terminal extensions such as starch-binding modules (families CBM26, CBM41, CBM34, CBM20 in CAZy) and other modules of still unknown function (Janecek 1997). Despite the lack of CBM20 in α-amylase of A. flavus NSH9 which is needed for starch binding, however, the enzyme carries two potential SBS residues (Trp 237 and Tyr 409) that have been proven to be crucial in the binding of starch granules for barley (Tyr 380), human saliva, and pancreatic α-amylase (Trp 203) (Ragunath et al. 2008; Nielsen et al. 2012; Zhang et al. 2016). The Tyr 380 of barley α-amylase (AMY1) constituting the pair of sugar-tong (SBS2), for instance, has been shown to be an important binding site in starch amylopectin depolymerisation and alteration of this amino acids resulted in reduced binding to starch granules by the mutant enzyme (Nielsen et al. 2009, 2012; Cockburn et al. 2015). Further structural analysis of enzyme–ligand complexes primarily by X-ray crystallography of A. flavus NSH9 α-amylase will be needed if we are to confirm on the presence of SBS as these binding sites have been previously reported to be non-conserved (Cockburn and Svensson 2013). An α-amylase of Saccharomycopsis fibuligera KZ capable of raw starch degradation was also reported to be lacking both the CBM and similarity with all other known SBS to which the author suggested to be having unique SBS (Hostinová et al. 2010).
With the aim of producing a recombinant enzyme with higher thermostability, more works are currently being carried out to heterologously express α-amylase of A. flavus NSH9 in Pichia pastoris for further comparative studies. Previous heterologous expression attempt on A. flavus NSH9 glucoamylase gene using the same yeast expression host has resulted in the production of a biologically active thermostable recombinant glucoamylase (Karim et al. 2016).
Conclusion
In this study, both the protein and gene sequence A. flavus NSH9 α-amylase have been successfully purified and characterized. The purified α-amylase showed some potent properties for industrial exploitation such as elevated activity by Ca2+ ions and better thermostability at 50 °C as compared to other reported A. flavus α-amylase.
Acknowledgements
This research project was financially supported by the Ministry of Higher Education Malaysia (MoHE) under the Research Acculturation Grant Scheme (RAGS) RAGS/b(9)/931/2012(32).
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Abou-Zeid AM. Production, purification and characterization of an extracellular alpha-amylase enzyme isolated from Aspergillus flavus. Microbios. 1996;89(358):55–66. [PubMed] [Google Scholar]
- Aguilar G, Morlon-Guyot J, Trejo-Aguilar B, Guyot JP. Purification and characterization of an extracellular α-amylase produced by Lactobacillus manihotivorans LMG 18010 T, an amylolytic lactic acid bacterium. Enzyme Microb Technol. 2000;27(6):406–413. doi: 10.1016/S0141-0229(00)00230-1. [DOI] [PubMed] [Google Scholar]
- Bhanja T, Rout S, Banerjee R, Bhattacharya BC. Comparative profiles of α-amylase production in conventional tray reactor and GROWTEK bioreactor. Bioprocess Biosyst Eng. 2007;30:369–376. doi: 10.1007/s00449-007-0133-0. [DOI] [PubMed] [Google Scholar]
- Bhardwaj S, Vedamurthy AB, Bhattacharya S, Das A. Effect of inorganic salts and surfactants on the production of [alpha]-amylase by a mangrove isolate of Aspergillus flavus using solid-state fermentation. J Chem Biol Phys Sci (JCBPS) 2012;2(3):1390. [Google Scholar]
- Bhattacharya S, Bhardwaj S, Das A, Anand S. Utilization of sugarcane bagasse for solid-state fermentation and characterization of α-amylase from Aspergillus flavus isolated from Muthupettai Mangrove, Tamil Nadu, India. Aust J Basic Appl Sci. 2011;5(12):1012–1022. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the determination of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Bhattacharyya BK, Sen SK. Purification and characterization of a thermostable α-amylase from Bacillus stearothermophilus. Folia Microbiologica (Praha) 2000;45(3):207–210. doi: 10.1007/BF02908945. [DOI] [PubMed] [Google Scholar]
- Chen J, Li DC, Zhang YQ, Zhou QX. Purification and characterization of a thermostable glucoamylase from Chaetomium thermophilum. J Gen Appl Microbiol. 2005;51:175–181. doi: 10.2323/jgam.51.175. [DOI] [PubMed] [Google Scholar]
- Cockburn D, Svensson B. Surface binding sites in carbohydrate active enzymes: an emerging picture of structural and functional diversity. Carbohydr Chem. 2013;39:204–221. doi: 10.1039/9781849737173-00204. [DOI] [Google Scholar]
- Cockburn D, Nielsen MM, Christiansen C, Andersen JM, Rannes JB, Blennow A, Svensson B. Surface binding sites in amylase have distinct roles in recognition of starch structure motifs and degradation. Int J Biol Macromol. 2015;75:338–345. doi: 10.1016/j.ijbiomac.2015.01.054. [DOI] [PubMed] [Google Scholar]
- Couto SR, Sanromán MA. Application of solid-state fermentation to food industry—a review. J Food Eng. 2006;76:291–302. doi: 10.1016/j.jfoodeng.2005.05.022. [DOI] [Google Scholar]
- Cubero OF, Crespo A, Fatehi J, Bridge PD. DNA extraction and PCR amplification method suitable for fresh, herbarium-stored, lichenized and other fungi. Plant Syst Evol. 1999;216:243–249. doi: 10.1007/BF01084401. [DOI] [Google Scholar]
- Da Lage JL, Binder M, Hua-Van A, Janeck S, Casanel D. Gene make-up: rapid and massive intron gains after horizontal transfer of a bacterial α-amylase gene to Basidiomycetes. BMC Evol Biol. 2013;13:40. doi: 10.1186/1471-2148-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Abyad MS, Fawzeya A, El-Sayed A, Hafez M. Effects of culture conditions on amylase production by some soil fungi. Zentralblatt fur Mikrobiologie. 1992;147:23–34. doi: 10.1016/S0232-4393(11)80359-8. [DOI] [Google Scholar]
- El-Safey EM, Ammar MS. Purification and characterization of α-amylase isolated from Aspergillus falvus var. columnaris. Assiut Univ Bull Environ Res. 2004;7(1):93–100. [Google Scholar]
- Fadahunsi IF, Garuba OE. Amylase production by Aspergillus flavus associated with the bio-deterioration of starch-based fermented foods. NY Sci J. 2012;5(1):13–18. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Goyal N, Gupta JK, Soni SK. A novel raw starch digesting thermostable α-amylase from Bacillus sp. I-3 and its use in the direct hydrolysis of raw potato starch. Enzyme Microb Technol. 2005;37(7):723–734. doi: 10.1016/j.enzmictec.2005.04.017. [DOI] [Google Scholar]
- Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B. Microbial α-amylases: a biotechnological perspective. Process Biochem. 2003;38:1599–1616. doi: 10.1016/S0032-9592(03)00053-0. [DOI] [Google Scholar]
- Gurr SJ, Unkles SE, Kinghorn JR. The structure and organization of nuclear genes of filamentous fungi. In: Kinghorn JR, editor. Gene structure in eukaryotic microbes. Oxford: IRL; 1987. pp. 93–139. [Google Scholar]
- Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1991;280(2):309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinová E, Janeček Š, Gašperík J. Gene sequence, bioinformatics and enzymatic characterization of α-amylase from Saccharomycopsis fibuligera KZ. Protein J. 2010;29(5):355–364. doi: 10.1007/s10930-010-9260-6. [DOI] [PubMed] [Google Scholar]
- Imperiali B, O’Connor SE. Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr Opin Chem Biol. 1999;3(6):643–649. doi: 10.1016/S1367-5931(99)00021-6. [DOI] [PubMed] [Google Scholar]
- Janecek S. α-Amylase family: molecular biology and evolution. Prog Biophys Mol Biol. 1997;67(1):67–97. doi: 10.1016/S0079-6107(97)00015-1. [DOI] [PubMed] [Google Scholar]
- Janecek S. How many conserved sequence regions are there in the α-amylase family? Biologia Bratislava. 2002;57(Suppl. 11):29–41. [Google Scholar]
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci CABIOS. 1992;8(3):275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Karim KMR, Husaini A, Hossain M, Sing NN, Mohd Sinang F, Hussain MHM, Roslan HA. Heterologous, expression, and characterization of thermostable glucoamylase derived from Aspergillus flavus NSH9 in Pichia pastoris. BioMed Res Int. 2016;2016:5962028. doi: 10.1155/2016/5962028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim KMR, Husaini A, Tasnim T. Production and characterization of crude glucoamylase from newly isolated Aspergillus flavus NSH9 in liquid culture. Am J Biochem Mol Biol. 2017;7:118–126. doi: 10.3923/ajbmb.2017.118.126. [DOI] [Google Scholar]
- Kariya M, Shigemi Y, Yano M, Konno H, Takii YJ. Purification and properties of alpha-amylase from Aspergillus oryzae MIBA316. J Biol Macromol. 2003;2:57–60. [Google Scholar]
- Khoo SL, Amirul AA, Kamaruzaman M, Nazalan N, Azizan MN. Purification and characterization of alpha amylase from Aspergillus. Folia Microbiol. 1994;39:392–398. doi: 10.1007/BF02814445. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacGregor EA. An overview of clan GH-H and distantly related families. Biologia. 2005;60(Suppl 16):5–12. [Google Scholar]
- MacGregor EA, Janeček Š, Svensson B. Relationship of sequence and structure to specificity in the α-amylase family of enzymes. Biochimica et Biophysica Acta (BBA) Protein Struct Mol Enzymol. 2001;1546(1):1–20. doi: 10.1016/S0167-4838(00)00302-2. [DOI] [PubMed] [Google Scholar]
- Madihah MS, Ariff AB, Khalil MS, Suraini AA, Karim MIA. Partial purification and some properties of alpha amylase and glucoamylase obtained as by-product from direct fermentation of sago starch to solvent by Clostridium acetobutylicum. Pak J Biol Sci. 2000;5:744–749. [Google Scholar]
- Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017;45(D1):D200–D203. doi: 10.1093/nar/gkw1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Ammar YB, Anindyawati T, Yamamoto S, Ito K, Iizuka M, Minamiura N. Molecular cloning and determination of the nucleotide sequence of raw starch digesting α-Amylase from Aspergillus awamori KT-11. J Biochem Mol Biol. 2004;37(4):429–438. doi: 10.5483/bmbrep.2004.37.4.429. [DOI] [PubMed] [Google Scholar]
- Matsuura A, Ishii Y, Yuasa H, Narita H, Kon S, Takami T, Kikuchi K. Rat T lymphocyte antigens comparable with mouse Lyt-1 and Lyt-2, 3 antigenic systems: characterization by monoclonal antibodies. J Immunol. 1984;132(1):316–322. [PubMed] [Google Scholar]
- Nakajima R, Imanaka T, Aiba S. Comparison of amino acid sequences of eleven different α-amylases. Appl Microbiol Biotechnol. 1986;23:355–360. doi: 10.1007/BF00257032. [DOI] [Google Scholar]
- Negi S, Banerjee R. Characterization of amylase and protease produced by Aspergillus awamori in a single bioreactor. Food Res Int. 2009;42(4):443–448. doi: 10.1016/j.foodres.2009.01.004. [DOI] [Google Scholar]
- Nguyen QD, Rezessy-Szabo JM, Claeyssens M, Stals I, Hoschke A. Purification and characterization of amylolytic enzymes from thermophilic fungus Thermomyces lanuginosus strain ATCC 34626. Enzyme Microb Technol. 2002;31:345–352. doi: 10.1016/S0141-0229(02)00128-X. [DOI] [Google Scholar]
- Nielsen MM, Bozonnet S, Seo ES, Mótyán JA, Andersen JM, Dilokpimol A, Abou Hachem M, Gyémánt G, Næsted H, Kandra L, Sigurskjold BW. Two secondary carbohydrate binding sites on the surface of barley α-amylase 1 have distinct functions and display synergy in hydrolysis of starch granules. Biochemistry. 2009;48(32):7686–7697. doi: 10.1021/bi900795a. [DOI] [PubMed] [Google Scholar]
- Nielsen JW, Kramhøft B, Bozonnet S, Hachem MA, Stipp SLS, Svensson B, Willemoës M. Degradation of the starch components amylopectin and amylose by barley α-amylase 1: role of surface binding site 2. Arch Biochem Biophys. 2012;528(1):1–6. doi: 10.1016/j.abb.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Ragunath C, Manuel SG, Venkataraman V, Sait HB, Kasinathan C, Ramasubbu N. Probing the role of aromatic residues at the secondary saccharide-binding sites of human salivary α-amylase in substrate hydrolysis and bacterial binding. J Mol Biol. 2008;384(5):1232–1248. doi: 10.1016/j.jmb.2008.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy NS, Nimmagadda A, Rao KS. An overview of the microbial α-amylase family. Afr J Biotechnol. 2003;2(12):645–648. doi: 10.5897/AJB2003.000-1119. [DOI] [Google Scholar]
- Sanghvi GV, Koyani RD, Rajput KS. Isolation, optimization and partial purification of amylase from Chrysosporium asperatum by submerged fermentation. J Microbiol Biotechnol. 2011;21(5):470–476. doi: 10.4014/jmb.0910.10014. [DOI] [PubMed] [Google Scholar]
- Saranraj P, Stella D. Fungal amylase—a review. Int J Microbiol Res. 2013;4(2):203–211. [Google Scholar]
- Saxena L, Iyer BK, Ananthanarayan L. Purification of a bifunctional amylase/protease inhibitor from ragi (Eleusine coracana) by chromatography and its use as an affinity ligand. J Chromatogr B. 2010;878(19):1549–1554. doi: 10.1016/j.jchromb.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Schumann U, Smith NA, Wang MB. A fast and efficient method for preparation of high-quality RNA from fungal mycelia. BMC Res Notes. 2013;6:71. doi: 10.1186/1756-0500-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JF, Lin FP, Chen SC, Chen HC. Purification and properties of an extracellular α-amylase from Thermus sp. Bot Bull Acad Sinica. 1995;36:195–200. [Google Scholar]
- Souza PMD. Application of microbial α-amylase in industry—a review. Braz J Microbiol. 2010;41(4):850–861. doi: 10.1590/S1517-83822010000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam MR, Danchin EGJ, Rancurel C, Coutinho PM, Henrissat B. Dividing the large glycoside hydrolase family 13 into subfamilies: towards improved functional annotations of alpha-amylase-related proteins. Protein Eng Des Sel. 2006;19:555–562. doi: 10.1093/protein/gzl044. [DOI] [PubMed] [Google Scholar]
- Sundarram A, Murthy TPK. α-Amylase production and applications: a review. J Appl Environ Microbiol. 2014;2(4):166–175. [Google Scholar]
- Takase K, Matsumoto T, Mizuno H, Yamane K. Site-directed mutagenesis of active site residues in Bacillus subtilis α-amylase. Biochimica et Biophysica Acta (BBA) Protein Struct Mol Enzymol. 1992;1120(3):281–288. doi: 10.1016/0167-4838(92)90249-D. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk V, Van Der Kaaij RM, Dijkhuizen L. Characterization of the starch-acting MaAmyB enzyme from Microbacterium aurum B8. A representing the novel subfamily GH13_42 with an unusual, multi-domain organization. Sci Rep. 2016;6:36100–36111. doi: 10.1038/srep36100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kaaij RM, Janecek S, van der Maarel MJEC, Dijkhuizen L. Phylogenetic and biochemical characterization of a novel cluster of intracellular fungal alpha-amylase enzymes. Microbiology. 2007;153:4003–4015. doi: 10.1099/mic.0.2007/008607-0. [DOI] [PubMed] [Google Scholar]
- van der Maarel MJEC, van der Veen B, Uitdehaag JCM, Leemhuis H, Dijkhuizen L. Properties and applications of starch-converting enzymes of the alpha-amylase family. J Biotechnol. 2002;94(2):137–155. doi: 10.1016/S0168-1656(01)00407-2. [DOI] [PubMed] [Google Scholar]
- Vihinen M, Ollikka P, Niskanen J, Meyer P, Suominen I, Karp M, Holm L, Knowles J, Mäntsälä P. Site-directed mutagenesis of a thermostable α-amylase from Bacillus stearothermophilus: putative role of three conserved residues. J Biochem. 1990;107(2):267–272. doi: 10.1093/oxfordjournals.jbchem.a123037. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Storms R, Tsang A. A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal Biochem. 2006;351:146–148. doi: 10.1016/j.ab.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Zhang X, Caner S, Kwan E, Li C, Brayer GD, Withers SG. Evaluation of the significance of starch surface binding sites on human pancreatic α-amylase. Biochemistry. 2016;55(43):6000–6009. doi: 10.1021/acs.biochem.6b00992. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Han Y, Xiao H. Microbial α-amylase: a biomolecular overview. Process Biochem. 2017;53:88–101. doi: 10.1016/j.procbio.2016.11.012. [DOI] [Google Scholar]