Abstract
Several environmental factors during the prenatal period transgenerationally affect the health of newborns in later life. Because low-dose antibiotics have been used for promoting the growth of crops and livestock in agriculture, humans may have ingested residual antibiotics for several decades. However, the effect of prenatal administration of low-dose antibiotics on newborns’ health in later life is unclear. In the present study, we found that prenatal treatment of murine mothers with low-dose antibiotics increased the abundance of bacteria of the phylum Firmicutes and the genera Clostridium IV and XIVa in feces from pups. In addition, the body fat percentage of mice in the antibiotic-treated group was higher than those in the control group at 12 weeks of age even though all pups were fed a standard diet. The body fat percentage of all mice was correlated with the abundance of fecal bacteria of Clostridium IV and XIVa. These results predict that low-dose antibiotic administration during the prenatal period affects the gut microbiota of newborns and possibly their health in later life.
Keywords: transgenerational, maternal, adiposity, DGGE, CE-MS
Introduction
Human health is influenced by both genetic and environmental factors.(1) Maternal environmental factors, especially during the prenatal period, affect children’s future health.(2) For example, maternal malnutrition increases the risks of obesity, diabetes and psychiatric disorders in children,(3) and maternal smoking increases the risk of low birth weight and developmental disorders.(4) In addition, alcohol intake during pregnancy can affect the cognitive behavior of the child through childhood and adolescence,(5) and cocaine use during pregnancy induces hypoxia and growth disturbances in children.(6)
Antibiotics have been used in the agricultural setting to promote the growth of animals and plants as well in the medical setting to prevent and treat infectious diseases.(7) Nonetheless, antibiotic use during the perinatal period and disturbance of gut microbiota in early life due to cesarean delivery possibly increases the risk of body weight gain in early childhood.(8) Furthermore, administration of low-dose antibiotics to weaning mice was found to increase body fat mass and change gut microbiota and gene expression.(9,10) These findings suggest that the intake of antibiotics in the postnatal or weaning periods affects host gut microbiota and metabolism. However, it has been unclear how antibiotic exposure in the mother during the fetal period affects the future health of the child. In this study, we found that low-dose antibiotic treatment of female mice from 1 week before pregnancy to birth disturbed the gut microbiota of the pups and that increased amounts of Clostridium IV and XIVa in feces correlated with increased body fat percentage in pups.
Materials and Methods
Animals
We purchased six female C57BL/6J mice on day 7 of gestation from a local breeding colony (Charles River, Yokohama, Japan). After birth, twelve female pups were divided into the control group (n = 6) and antibiotics (Ab) group (n = 6) at 7 weeks of age. Mice in the Ab group were administered a mixture of three different kinds of antibiotics at subtherapeutic levels: penicillin V (Tokyokasei, Tokyo, Japan), chlortetracycline (Sigma, St. Louis, MO) and vancomycin (WAKO, Osaka, Japan) per day at 1 µg/g body weight(10) from 7 weeks of age. The mice in the control group were administered a vehicle. After 7 days of administration of antibiotics or vehicle, mice were mated with male mice for 4 days. Nine female mice were pregnant (control group, n = 5; Ab group, n = 4). Administration of antibiotics or vehicle continued until the time of birth. Fecal samples were collected before and after administration of antibiotics and stored at −80°C until analysis. Pups were co-housed with the mothers until weaning. After weaning, pups were fed a standard diet (MF; Oriental Yeast Co., Tokyo, Japan) and tap water ad libitum. Mice were housed in cages maintained at constant temperature (23 ± 2°C) and humidity (65–75%) with a 12-h light (8:00–20:00), 12-h dark (20:00–8:00) cycle. During the experimental period, their body weights were measured weekly. At 8 weeks old, their fecal samples were collected and stored at −80°C. At 12 weeks old, their body fat percentage was analyzed by CT. At 13 weeks old, all pups were dissected, and liver, serum, cecum and fecal samples were collected and rapidly stored at −80°C. The University of Tokushima Animal Use Committee approved the study (T14010), and mice were maintained according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Fecal bacteria analysis by quantitative real-time PCR
DNA was extracted from fecal samples using the QIAamp DNA Stool Mini Kit (QIAGEN, Tokyo, Japan). The extracted DNA concentration was adjusted to 10 ng/µl, and the relative abundance of total bacteria and specific bacteria [phylum or genus, including Firmicutes, Bacteroidetes, Lactobacillus, Clostridium IV, Clostridium XIVa and Bacteroides (Table 1)] was measured using quantitative real-time PCR using SYBR Premix Ex Taq (TaKaRa, Otsu, Japan).
Table 1.
Oligonucleotide primer
| Primer name | Sequence (5'-3') | Amplicon size (bp) | Reference |
|---|---|---|---|
| Eub338F | ACTCCTACGGGAGGCAGCAG | 180 | (19) |
| Eub518R | ATTACCGCGGCTGCTGG | ||
| F341GC | CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGcctacgggaggcagcag | (20) | |
| Bact934F | GGARCATGTGGTTTAATTCGATGAT | 126 | (21) |
| Bact934GC-F | CGCCCGCCGCGCCCCGCGCCCGTCCCGCCGCCCCCGCCCGggarcatgtggtttaattcgatgat | ||
| Bact1060R | AGCTGACGACAACCATGCAG | ||
| Firm934F | GGAGYATGTGGTTTAATTCGAAGCA | 126 | (21) |
| Firm934GC-F | CGCCCGCCGCGCCCCGCGCCCGTCCCGCCGCCCCCGCCCGggagyatgtggtttaattcgaagca | ||
| Firm1060R | AGCTGACGACAACCATGCAC | ||
| Lactobacillus-F | AGCAGTAGGGAATCTTCCA | 341 | (22) |
| Lactobacillus-R | CACCGCTACACATGGAG | ||
| Clostridia IV-F | GCACAAGCAGTGGAGT | 239 | (23) |
| Clostridia IV-R | CTTCCTCCGTTTTGTCAA | ||
| Clostridia XIVa-F | AAATGACGGTACCTGACTAA | 438–441 | (24) |
| Clostridia XIVa-R | CTTTGAGTTTCATTCTTGCGAA | ||
| Bacteroides-F | GAGAGGAAGGTCCCCCAC | 106 | (25) |
| Bacteroides-R | CGCTACTTGGCTGGTTCAG | ||
Fecal bacterial analysis by denaturing gradient gel electrophoresis
Denaturing gradient gel electrophoresis (DGGE) analysis was carried out using the DCodeTM Universal Mutation Detection System according to the manufacturer’s instructions (Bio-Rad Labs, Hercules, CA). The specific region of the 16S rRNA genes of all bacteria (GC-Eubacteria, positions 341 to 518 in Escherichia coli) and specific phyla (GC-Firmicutes, positions 934 to 1060; GC-Bacteroidetes, positions 934 to 1060) in fecal DNA was amplified by KOD FX Neo (TOYOBO, Osaka, Japan) (Table 1). The denaturing gradient was formed with 6% (for all bacteria) or 8% (for specific phyla) acrylamide (acrylamide-bis 37.5:1) with the denaturing gradient ranging from 20% to 80% (for all bacteria) or 50% to 60% (for specific phyla) for analysis of amplified 16S rRNA fragments. PCR products were electrophoresed at 200 V for 4 h. After electrophoresis, gels were stained with Gelstar (Lonza Japan, Tokyo, Japan) for 30 min and analyzed by Chemi-Doc image analysis equipment (Bio-Rad Labs). Image Lab software, ver. 5.0 (Bio-Rad Labs) was used for identification of bands and normalization of band patterns from DGGE gels.
Measure of body fat percentage by CT
The body fat percentage of male mice was measured using X-ray computed tomography (CT) Latheta LCT-200 for experimental animals (Hitachi Aloka, Tokyo, Japan). Mice were anesthetized with isoflurane (Abbott Japan Co., LTD, Tokyo, Japan), fixed to Latheta and abdominal part L4–L5 was measured. Measurement conditions were: rotational speed, standard; slice thickness, 192 µm × 107 sheet; voxel size, 96 × 192 µm; x-ray tube voltage, low; shooting conditions, 48 mm view, 180°, 436, asynchronous. Filmed images were analyzed by computer, and body fat percentage was calculated.
Metabolome analysis of cecal contents
Cecal contents from 13 weeks old mice which stored at −80°C were weighed and completely homogenized in 500 µl methanol containing 50 µM methionine sulfone and camphor-10-sulfonic acid as internal standards. The homogenates were mixed with 500 µl chloroform and 200 µl Milli-Q water. Samples were centrifuged (2,300 g, 5 min, 4°C), and then the supernatant was centrifugally filtered using 5-kDa cut-off filters (Millipore, Bedford, MA) until all was filtered (9,100 g, 4°C). The filtrate was centrifugally concentrated in a vacuum evaporator, dissolved in Milli-Q water and analyzed by capillary electrophoresis electrospray ionization time-of-flight mass spectrometry (CE-TOFMS).
CE-TOFMS analysis was performed by an Agilent CE system combined with a TOFMS (Agilent Technologies, Palo Alto, CA) as reported by Human Metabolome Technologies Inc. (HMT, Tsuruoka, Japan).(11,12) Each metabolite was identified and quantified based on the peak information including m/z, migration time and peak area.
Statistical analysis
The mean and standard deviation were calculated for all results. The t test or Mann-Whitney U test (p<0.05) was used to compare two groups. Principal component analysis (PCA) was carried out with Excel Tokei 2010 (SSPZ) and Mass Profiler Professional software (Agilent Technology).
Results
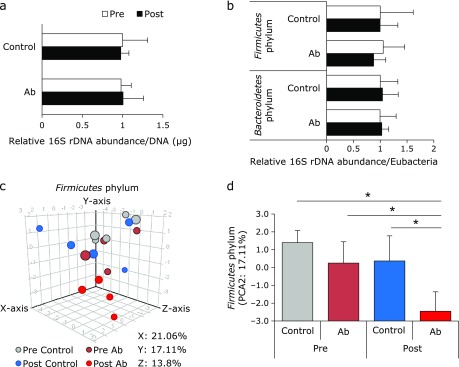
Cho et al.(10) reported that low dose antibiotics (1 µg/g BW/day of penicillin V, vancomycin, and chlortetracycline) administration in early life alters the adiposity in mice. To investigate the effects of the administration of low-dose antibiotics to pregnant mothers on the future health of their pups, we exposed female mice to penicillin V, chlortetracycline and vancomycin from 1 week before pregnancy to birth. During the exposure period, changes in the mothers’ body weight (25.0 ± 0.9 g in the control group and 24.8 ± 1.1 g in the Ab group) and water consumption (3.6 ± 0.4 g/g BW/day in the control group and 3.6 ± 0.5 g/g BW/day in the Ab group) did not differ between the control and Ab groups. Low-dose antibiotic administration did not affect the number of pups (mean 7.2 ± 1.9 in the control group and 5.6 ± 0.8 in the Ab group), the ratio of males to females (control group, 48%:52%; Ab group, 59%:41%). We could not define any differences between the relative amounts of total bacteria, Firmicutes or Bacteroidetes in the mothers’ feces before and after antibiotic administration (Fig. 1a, b). In order to analyze the mother’s gut microbiota in more detail, we conducted DGGE analysis targeting Eubacteria (as total bacteria), Firmicutes and Bacteroidetes. Band patterns from amplified DNA (16s RNA V2 to V3 region) (Fig. 1 and Supplemental Fig. 1*) and PCA (Fig. 1 and Supplemental Fig. 1*) indicated that the compositions of fecal microbiota were not significantly different between the control and Ab groups. We analyzed the Firmicutes phylum by three-dimensional PCA, which clearly showed differences among the post-Ab group and other groups (Fig. 1c, d). Although the composition of gut microbiota was heterogeneous (Supplemental Fig. 1*), the individual distance of the Firmicutes in the Ab group was significantly lower than the pre-treatment Ab group and the pre- and post-control group (Fig. 1h and Supplemental Fig. 1*). These results suggest that low-dose antibiotics affect the bacterial composition of the mothers’ feces, especially bacteria in the phylum Firmicutes.
Fig. 1.
Fecal microbiota of the mother mice. (a) The relative amounts of mothers’ total fecal bacteria before antibiotic administration (pre), and after antibiotic administration (post) were analyzed by RT-PCR for Eubacteria (as total bacteria) and the phyla Bacteroidetes and Firmicutes. Total bacteria were corrected for fecal DNA concentration. Bacteroidetes and Firmicutes were corrected for the amounts of total bacteria. (c) Three-dimensional of PCA plot of DGGE band pattern in Firmicutes phylum. (d) Scale of Y axis in PCA of Firmicutes phylum. Control group (n = 5), Antibiotics group (Ab, n = 4). Data are shown as average and SD. *p<0.05.
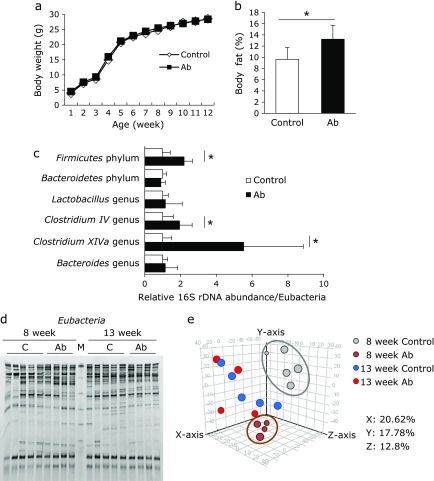
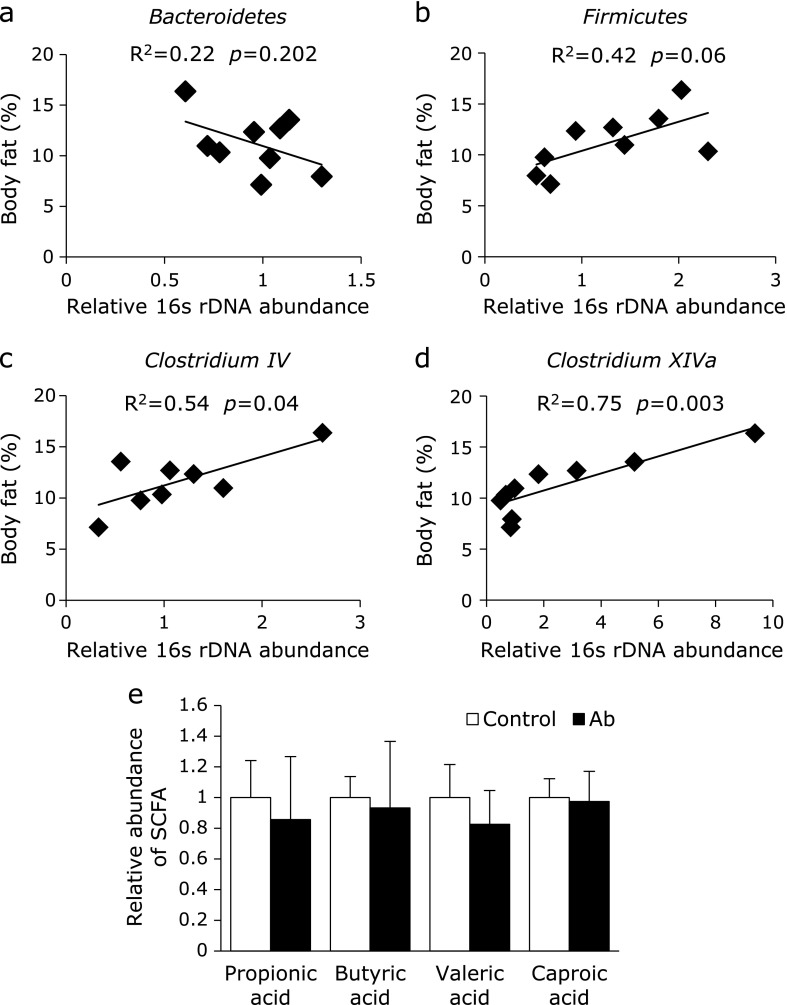
Next, we analyzed the body composition and gut microbiota of the pups. Body weight was not significantly different over time between pups whose mothers had received antibiotics or the control (Fig. 2a). However, pups in the Ab group had a significantly higher abdominal body fat percentage at 12 weeks compared with the control group (Fig. 2b). The number of bacteria from the phylum Firmicutes and genera Clostridium IV and XIVa were significantly higher in the Ab group than those in the control group (Fig. 2c). According to PCR-DGGE analysis for total bacteria, PCA and hierarchical clustering of the gut microbiota of the pups at 8 weeks was different between the two groups (Fig. 2d, e and Supplemental Fig. 2*). These differences in the gut microbiota of the pups were reduced at 13 weeks. A similarity of the gut microbiota between mother and pups (at 8 weeks) were not different between the two group (Supplemental Fig. 3*). However, there is a limitation of this results that individual variances of the composition of gut microbiota were remarkable in the much pairs. We then analyzed the association between body fat percentage and gut microbiota. There was no correlation between body fat percentage and the abundance of Bacteroidetes and Firmicutes, but the abundance of Clostridium IV and XIVa were significantly positively correlated with body fat percentage (Fig. 3a–d).
Fig. 2.
Fecal microbiota and body composition in mouse pups. (a) Changes in body weight of pups from weeks 1 to 12. (b) Percentage of abdominal body fat at 12 weeks. (c) The relative amounts of pups’ fecal bacteria at 8 weeks was analyzed by RT-PCR. The number of all bacteria was corrected by the amount of Eubacteria. (d) Band image of DGGE analysis of DNA from feces at 8 and 13 weeks. (e) PCA plots of each DGGE band pattern in total bacteria. M, DNA marker; Control group (n = 5); Antibiotics group (Ab, n = 4). Data are shown as average and SD. *p<0.05.
Fig. 3.
Correlation between fecal microbiota and percentage of body fat and the concentration of luminal short chain fatty acids in pups. (a–d) Relationship between the abundance of fecal bacterial groups and percentage of body fat in pups. Data were analyzed using Spearman’s rank correlation test. Control group (n = 5); Antibiotics group (Ab, n = 4). (e) Changes in the relative concentration of luminal short chain fatty acids (SCFA) at 13 weeks old. Control group (n = 4); Antibiotics group (Ab, n = 4). Data are shown as average and SD.
Finally, we analyzed luminal metabolites in the pups by CE-MS. Based on their m/z values and migration times, 160 metabolites were measured in the luminal contents. There was no metabolite that was significantly different between the two groups, including short-chain fatty acids (SCFA) (Fig. 3e).
Discussion
In the present study, we found that administration of low-dose antibiotics from 1 week before pregnancy to birth affected the gut microbiota and abdominal fat percentage of the pups but not body weight. The changes in gut microbiota, especially in Firmicutes and Clostridium, could be observed at 8 weeks of age. In addition, the genera Clostridium IV and XIVa were correlated with increased abdominal fat percentage.
Cho et al.(10) reported that antibiotic treatment of pups at subtherapeutic levels (penicillin, vancomycin, chlortetracycline, at 1 µg/g body weight per day) for 7 weeks beginning at the time of weaning increased the relative concentrations of fecal Firmicutes compared to Bacteroidetes, which accompanied the observed increases in adiposity with body weight gain. The effects of low-dose antibiotics on the adiposity of pups may depend on the timing of antibiotic administration. Treatment with low-dose penicillin from 1 week prior to birth to 20 weeks resulted in increased body fat and body weight,(9) while the present study showed that administration of low-dose antibiotics only to mothers prior to birth promoted the deposition of energy as abdominal fat without obesity. Because these metabolic changes were emphasized by a high fat diet after weaning,(9) we should carefully select the low-dose antibiotics or antibiotic dietary compounds that are exposed to the mothers.
We found that there are large individual differences in intestinal bacterial flora. Gut microbial communities vary even within adult monozygotic and dizygotic twin pairs.(13) Moreover, Andoh et al.(14) reported that obesity associated gut microbiota (higher amount of the order Clostridiales) in the Japanese population was different from that in western people. Some environmental factors including diet, lifestyle, stress and aging contribute to the variance in gut microbiota.(13,15,16) The expansion of bacterial diversity slowly occurs in early life, as gut microbial diversity is lower in children than in adults.(16) The differences in gut microbiota between the two groups were reduced in 13 weeks compared to in 8 weeks in our study. We only treated mothers; therefore, diet, housing and other environmental factors of the pups were not different between the groups. These results suggest that the differences in gut microbial composition between the two groups at 8 weeks may have been masked due to the increase of gut microbial diversity over time.
We also observed that individual differences in the gut microbiota of the mothers, especially in Firmicutes, were enhanced by subtherapeutic antibiotic treatment. Even at a low dose, we should consider continuous antibiotic treatments as an environmental factor and realize that they may affect host gut microbiota. Recent progress in this field showed that not only antibiotics, but also some food derivatives and food additives, affect host gut microbiota.(17,18) We need to carefully study the effect of dietary components during the prenatal period on the maturation of infants’ gut microbiota and health in future life. Therefore, we should administer antibiotics thoughtfully.
In conclusion, we found that maternal subtherapeutic antibiotic exposure during the fetal period affected the gut microbiota and abdominal fat percentage of the pups without increasing body weight.
Author Contributions
T. U., M. N., T. S., K. M. and A. T. designed research; T. U. and A. Y. conducted research and analyzed data; A. Y., T. U. and A. T. wrote paper and had responsibility for final content. All authors read and approved the final manuscript.
Acknowledgments
This work was financially supported by Pilot Research program in Tokushima University, Yakult Bio-Science Foundation and JSPS KAKENHI Grant Numbers JP15H0564710 and JP16K15191. This study was supported by Support Center for The Special Mission Center for Metabolome Analysis, School of Medical Nutrition, Faculty of Medicine of Tokushima University and Support Center for Advanced Medical Sciences, Institute of Biomedical Sciences, Tokushima University Graduate School in material. We gratefully acknowledge the excellent assistance of Yumi Harada, Rumiko Masuda, Akiko Uebanso and Metabolome Tokumei-Unit in Tokushima University. We also wish to thank Division for Animal Research Resources and Genetic Engineering Support Center for Advanced Medical Sciences, Institute of Biomedical Sciences, Tokushima University Graduate School for care of mice.
Conflict of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Faluk C, Dolinoy DC. Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics. 2011;6:791–797. doi: 10.4161/epi.6.7.16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parlee SD, MacDougald OA. Maternal nutrition and risk of obesity in offspring: the Trojan horse of developmental plasticity. Biochim Biophys Acta. 2014;1842:495–506. doi: 10.1016/j.bbadis.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuoka H. Epigenetic changes caused by intrauterine malnutrition as potential disease mediator and early prevention in developmental stages. Nihon Eiseigaku Zasshi. 2014;69:82–85. doi: 10.1265/jjh.69.82. [DOI] [PubMed] [Google Scholar]
- 4.Knopik VS, Maccani MA, Francazio S, McGeary JE. The epigenetics of maternal cigarette smoking during pregnancy and effects on child. Dev Psychopathol. 2012;24:1377–1390. doi: 10.1017/S0954579412000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis CE, Thomas KG, Dodge NC, et al. Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2015;39:724–732. doi: 10.1111/acer.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grewen K, Burchinal M, Vachet C, et al. Prenatal cocaine effects on brain structure in early infancy. Neuroimage. 2014;101:114–123. doi: 10.1016/j.neuroimage.2014.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiling S, Lee P, Breslow L. Curtailing antibiotic use in agriculture: it is time for action: this use contributes to bacterial resistance in humans. West J Med. 2002;176:9–11. doi: 10.1136/ewjm.176.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox LM, Yamanishi S, Sohn J, et al. Altering the interstinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohashi Y, Hiratama A, Ishikawa T, et al. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol Biosyst. 2008;4:135–147. doi: 10.1039/b714176a. [DOI] [PubMed] [Google Scholar]
- 12.Kami K, Fujimori T, Sato H, et al. Metabolomic profiling of lung and prostate tumor tissues by capillary electrophoresis time-of-flight mass spectrometry. Metabolomics. 2013;9:444–453. doi: 10.1007/s11306-012-0452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Hamady M, Yatsuneko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andoh A, Nishida A, Takahashi K, et al. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J Clin Biochem Nutr. 2016;59:65–70. doi: 10.3164/jcbn.15-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 18.Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fierer N, Jackson JA, Vilgalys R, Jackson RB. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbial. 2005;71:4117–4120. doi: 10.1128/AEM.71.7.4117-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo , X , Xia X, Tang R, Zhou J, Zhao H, Wang K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Environ Microbiol. 2008;47:367–373. doi: 10.1111/j.1472-765X.2008.02408.x. [DOI] [PubMed] [Google Scholar]
- 22.Walter J, Hertel C, Tannock GW, Lis CM, Munro K, Hammers WP. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl Environ Microbiol. 2001;67:2578–2585. doi: 10.1128/AEM.67.6.2578-2585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuki T, Watanabe K, Fujimoto J, Takada T, Yanaka R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol. 2004;70:7220–7228. doi: 10.1128/AEM.70.12.7220-7228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuki T, Watanabe K, Fujimoto J, et al. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl Environ Microbiol. 2002;68:5445–5451. doi: 10.1128/AEM.68.11.5445-5451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Layton A, McKay L, Williams D, Garrett V, Gentry R, Sayler G. Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl Environ Microbiol. 2006;72:4214–4224. doi: 10.1128/AEM.01036-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.