Abstract
Hyperproduced prostaglandin E2 by cyclooxygenase-2 and microsomal prostaglandin E synthase-1 evokes several pathophysiological responses such as inflammation and carcinogenesis. Our recent study demonstrated that Dioscorea japonica extract suppressed the expression of cyclooxygenase-2 and microsomal prostaglandin E synthase-1 and induced apoptosis in lung carcinoma A549 cells. In the present study, we investigated the effects of Dioscorea japonica on squamous cell carcinoma of mouse skin. Dioscorea japonica feeding and Dioscorea japonica extract topical application suppressed the expression of cyclooxygenase-2, microsomal prostaglandin E synthase-1, interleukin-1β and interleukin-6 and inhibited tumor formation, hyperplasia and inflammatory cell infiltration. Immunohistochemical analyses showed the immunoreactivities of cyclooxygenase-2 and microsomal prostaglandin E synthase-1 in tumor keratinocytes and stronger immunoreactivities of cyclooxygenase-2 and hematopoietic prostaglandin D synthase in epidermal dendritic cells (Langerhans cells). Treatment with Dioscorea japonica decreased the immunoreactivity of cyclooxygenase-2 and microsomal prostaglandin E synthase-1. These results indicate that Dioscorea japonica may have inhibitory effects on inflammation and carcinogenesis via suppression of the prostaglandin E2 synthetic pathway.
Keywords: wild yam, inflammation, carcinogenesis, skin cancer, prostaglandin E2
Introduction
Prostaglandin (PG) E2, a lipid mediator, is derived from arachidonic acid, and is involved in several pathophysiological responses such as inflammation, inhibition of gastric acid secretion, pain transmission, neurodegeneration and carcinogenesis.(1) In the PGE2 synthetic pathway, the isozymes cyclooxygenase (COX)-2 and microsomal PGE synthase-1 (mPGES-1) are induced by the pathophysiological conditions, and the hyperproduced PGE2 evokes the disease.(2,3) Clinically, COX inhibitors, namely non-steroidal anti-inflammatory drugs (NSAIDs), are commonly used for pyretolysis and pain relief,(2) however, most NSAIDs target constitutive COX-1 and inducible COX-2 and cause side effects such as gastrointestinal toxicity and cardiovascular risk.(4) On the other hand, a few mPGES-1 inhibitors have been found,(3) and none have been used clinically. Thus, it would be beneficial to identify natural substances from foods that have safe and functional effects on the regulation of PGE2 production.
Dioscorea japonica, a wild yam, is a relative of the Dioscoreaceae family native to Japan. Dioscoreaceae yam tubers are usually edible and are rich in many nutrients.(5,6) Dioscorea japonica is good for nutritional fortification,(7–10) and it has a gastric mucosal protective effect conferred by glycoproteins and digestive enhancement by glycosidase. Recently, we found that Dioscorea japonica extract (DJE) suppressed the expression of COX-2 and mPGES-1 in human non-small-cell lung carcinoma A549 cells and colon carcinoma Caco-2 cells, thereby inducing apoptosis in such cells.(11) DJE suppresses COX-2 mRNA with translocation of transcriptional factor nuclear factor-κB (NF-κB) to cytosol and a reduction of COX-2 promoter activity.(11)
In this study, to confirm the effects of Dioscorea japonica on inflammation and carcinogenesis via the suppression of COX-2 and mPGES-1 in vivo we demonstrate a two-stage cutaneous chemical carcinogenesis model of mouse skin.(12)
Materials and Methods
Animal rearing conditions and the method of preparing a model of squamous cell carcinoma
All protocols were approved by the Exclusive Committee on Animal Research at Okayama Prefectural University and the research was conducted in conformity with the Public Health Service (PHS) policy. Seven-week-old male Balb/c mice had free access to drinking water and food. Mice were fed a powder diet (CRF-1; Oriental Yeast Co., Ltd., Tokyo, Japan) with or without (w/w) 1% or 10% Dioscorea japonica powder. Dioscorea japonica was obtained from Autoraimu Yoshio Ltd. (Niimi, Japan). The outer skin of Dioscorea japonica was pared away, and it was dried and pulverized at 40°C. Feed consumption was measured twice a week, and weight was measured every second week. According to the procedure of Modi et al.,(12) a squamous cell carcinoma model was induced by topically applying the following to each mouse: firstly, 200 µl of 2 mM 7,12-dimethylbenz[a]anthracene (DMBA, as an initiator, Sigma-Aldrich, St. Louis, MO); then, after one week, 200 µl of 80 µM 12-O-tetradecanoylphorbol-13 acetate (TPA, as a promoter, Sigma-Aldrich) twice per week for 22 weeks (Fig. 1A). Numbers and volumes of cutaneous papillomas were measured. Mice in three experimental groups were applied with 100 µl of 0.05, 0.5 or 5 mg of Dioscorea japonica extract eluted from the powder with 50% ethanol 30 min before the application of TPA, and fed a basal diet (CRF-1) without Dioscorea japonica powder.
Fig. 1.
Treatment scheme and comparison of food intake and body weight among the experimental groups. Seven-week-old male Balb/c mice were treated with the respective regimens according to the treatment scheme (A). Animals were divided into four experimental groups: group “normal control” was given control diet and treatment with vehicle; group “carcinogenic control” was given normal diet and treatment with DMBA/TPA; group “Dioscorea japonica feeding” (D. japonica feeding) was given 1% or 10% Dioscorea japonica powder (w/w) containing diet and treatment with DMBA/TPA; group “Dioscorea japonica extract topical application” (DJE-application) was given a normal diet and treatment with DMBA/TPA and 0.05–5 mg of Dioscorea japonica extract/100 µl of 50% ethanol. Food intake (B) and body weight (C) were recorded. The values represent mean ± SD.
Quantitative reverse transcriptase (RT)-PCR
The gene expression was analyzed by quantitative RT-PCR (iQ5 real-time PCR system, Bio-Rad, Hercules, CA) using cDNA prepared from isolated total RNA. The quantitative PCR was performed using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), and the following PCR primer pairs: Ptgs1 (COX-1), 5'-CTTTGC ACAACACTTCACCCACC-3' (forward) and 5'-AGCAACCCA AACACCTCCTGG-3' (reverse); Ptgs2 (COX-2), 5'-GCATTC TTTGCCCAGCACTT-3' (forward) and 5'-AGACCAGGCACC AGACCAAAGA-3' (reverse); Ptges (mPGES-1), 5'-CTGCTG GTCATCAAGATGTACG-3' (forward) and 5'-CCCAGGTAG GCCACGGTGTGT-3' (reverse); Ptges2 [membrane-associated PGES-2 (mPGES-2)], 5'-AAGACATGTCCCTTCTGC-3' (forward) and 5'-CCAAGATGGGCACTTTCC-3' (reverse); Ptges3 [cytosolic PGES (cPGES)], 5'-AGTCATGGCCTAGGTTAAC-3' (forward) and 5'-TGTGAATCATCATCTGCTCC-3' (reverse); Ptgds2 [hematopoietic PGD synthase (H-PGDS)], 5'-CACGCTGGAT GACTTCATGT-3' (forward) and 5'-AATTCATTGAACATCCG CTCTT-3' (reverse); Il1b [interleukin (IL)-1β], 5'-GTCACAAGA AACCATGGCACAT-3' (forward) and 5'-GCCCATCAGAGG CAAGGA-3' (reverse); Il6 (IL-6), 5'-CTGCAAGAGACTTCC ATCCAGTT-3' (forward) and 5'-AGGGAAGGCCGTGGTTGT-3' (reverse); and gapdh [glyceraldehyde 3-phosphate dehydrogenase (GAPDH)], 5'-TGAACGGGAAGCTCACTGG-3' (forward) and 5'-TCCACCACCCTGTTGCTGTA-3' (reverse). The relative expression levels were shown against normal controls and represent mean ± SD.
Pathohistological and immunohistochemical analyses
Prepared paraffin-embedded sections of mouse skin were used for hematoxylin and eosin (HE) staining and immunohistochemical staining. For single immunolabeling, sections were treated with 3% hydrogen peroxide to block endogenous peroxidase activity, and were blocked with 12.5% Block Ace (DS Pharma Biomedical Co., Ltd., Tokyo, Japan) to block nonspecific bindings. For immunolabeling, we used the following first antibodies: goat anti-COX-2 antibody (1:50, catalogue no. sc-1745, lot no. E102, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit anti-mPGES-1 antibody (1:500, catalogue no. 160140, lot no. 0436398-1, Cayman Chemical Co., Ann Arbor, MI), rabbit anti-H-PGDS antiserum (1:1,000, catalogue no. 10004348, lot no. 0451181-1, Cayman Chemical Co.), rat anti-Ly-6G/Ly-6C (Gr-1) antibody (1:500, a marker of neutrophils; catalogue no. 108413, lot no. B141536, BioLegend, San Diego, CA), and mouse anti-Langerin/CD207 antibody (1:1000, a marker of Langerhans cells; catalogue no. DDX0361P-50, lot no. DDX0361-009, DENDRITICS, Lyon, France), and the following second antibodies: biotinylated anti-goat, biotinylated anti-rabbit, Cy3-labeled donkey anti-goat, FITC-labeled donkey anti-rabbit, FITC-labeled donkey anti-goat, FITC-labeled donkey anti-rat, FITC-labeled donkey anti-mouse IgGs (1:400, biotinylated IgGs from Vector Laboratories, Burlingame, CA, and fluorescein-labeled IgGs from Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). The biotin-labeling was enhanced by ABC Elite kit (Vector Laboratories) and was visualized in 50 mM Tris (pH 7.6) containing 0.1% 3,3'-diaminobenzidine tetrahydrochloride (DAB) and 0.01% hydrogen peroxide at 37°C. The fluorescein-labeled sections were examined by confocal laser scanning light microscopy (CLSM, Fluoview FV1000, Olympus Co., Tokyo, Japan).
Electrospray ionization mass spectrometry (ESI-MS)
All procedures were performed as described previously.(13) Tissues were homogenized with a Polytron homogenizer in methanol and were incubated overnight. As internal standards for determination, 100 pmol of d5-labeled eicosapentaenoic acid (EPA) and d8-labeled 15-hydroxyeicosatetraenoic acid (15-HETE), were added to the samples. The lipids in the sample were extracted using Oasis HLB cartridges (Waters, Milford, MA), and were dried up under nitrogen gas. The analysis was performed using a 4000Q-TRAP quadrupole-linear ion trap hybrid mass spectrometer (AB Sciex, Framingham, MA) with LC (NexeraX2 system; Shimadzu Co., Kyoto, Japan). The sample was applied to a C18 column (Kinetex C18, 2.1 × 150 mm, 1.7 µm, Phenomenex, Inc., Torrance, CA) coupled for ESI-MS/MS. For analyses of fatty acids, the samples are applied to a column and separated by a step gradient with mobile phase A (acetonitrile:MeOH:water = 1:1:1 (v/v/v) containing 5 µM phosphoric acid and 1 mM ammonium formate) and mobile phase B (2-propanol containing 5 µM phosphoric acid and 1 mM ammonium formate) at a flow rate of 0.2 ml/min at 50°C. Whereas, for analyses of oxidized fatty acids, the samples are applied to a column and separated by a step gradient with mobile phase C (water containing 0.1% acetic acid) and mobile phase D (acetonitrile:MeOH = 4:1; v/v) at a flow rate of 0.2 ml/min at 45°C. Signature ion fragments for each targeted lipid were monitored and quantified by a multiple reaction monitoring (MRM) method. Identification was conducted using MRM transition(14) and retention times. Quantification was performed based on peak area of the MRM transition and the calibration curve obtained with authentic standard for each compound.
Effect of diosgenin on expression of COX-2 and mPGES-1 in RAW264 cells
Mouse macrophage-like RAW264 cells was obtained from RIKEN BioResource Center (Tsukuba, Japan). The cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin. RAW264 cells were incubated with or without 100 nM diosgenin. After the incubation for 3 h, the cells were stimulated by 2.5 µg/ml lipopolysaccharide (LPS) for 6 h. mRNA expression of Ptgs2 and Ptges was measured by quantitative RT-PCR.
Statistics
Data were statistically evaluated by ANOVA with Bonferroni’s or Dunnett’s post-hoc test at significance level of p<0.01 or p<0.05.
Results
Comparison of tumor formation among the experimental groups
Figure 2 shows tumor formation in the experimental groups including Dioscorea japonica feeding group and DJE topical application group. Throughout the experiment, food intake and body weight were not significant differences among the experimental groups (Fig. 1B and C). After 22 weeks of tumor induction, there was no noticeable difference in tumor numbers between the experimental groups with the exception of the normal control (Fig. 2B and Supplemental Fig. 1A*). However, the tumor volumes were significantly decreased in the Dioscorea japonica feeding and DJE topical application groups (Fig. 2A, C and Supplemental Fig. 1B*). These data indicate that Dioscorea japonica treatment is more beneficial for tumor volumes than tumor numbers. Thus, Dioscorea japonica may inhibit tumor growth.
Fig. 2.
Comparison of tumor formation among experimental groups. Representative images of mice from each experimental group after 22 weeks of tumor induction are shown in (A). Tumors are identified to enable comparison between normal skin and swelling tissue, and tumor number (B) and volume (C) are indicated. The values represent mean ± SD of 6 mice per group; *p<0.01 compared with the carcinogenic control.
Suppression of COX-2, mPGES-1, inflammatory cytokines, and decrease of PG products by Dioscorea japonica treatment
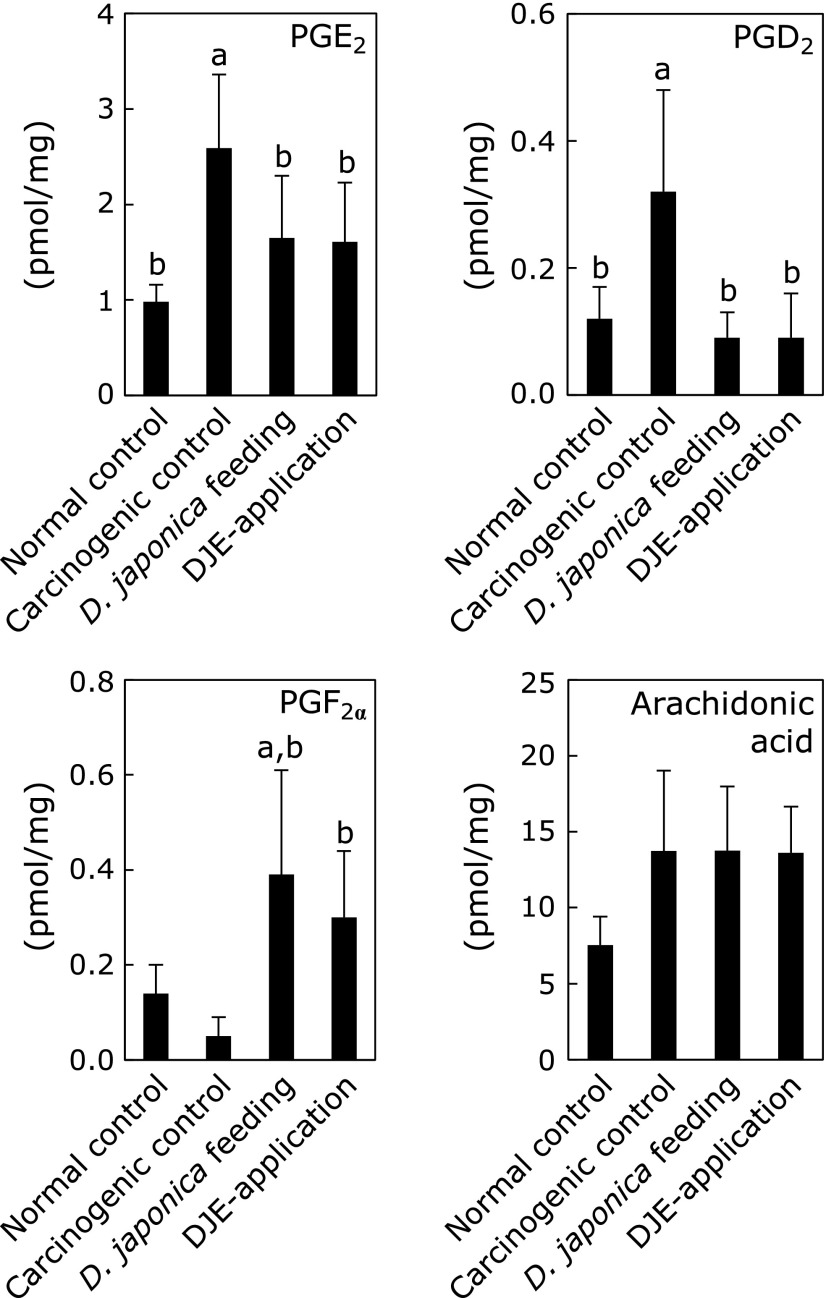
To examine the effect of Dioscorea japonica administration on mRNA expression in mouse skin (Fig. 3), Ptgs1 (COX-1), Ptgs2 (COX-2), Ptges (mPGES-1), Ptges2 (mPGES-2), Ptges3 (another cytosolic PGE2 synthase, cPGES), Ptgds2 (PGD2 synthesizing enzyme, H-PGDS), and inflammatory cytokines Il1b (IL-1β) and Il6 (IL-6) were analyzed by quantitative RT-PCR. In the carcinogenic control mice, Ptgs2, Ptges and Il1b increased approximately 25-fold compared with the normal control mice. On the other hand, Dioscorea japonica feeding led to the suppression of Ptgs2, Ptges and Il1b mRNAs to 47%, 46% and 15% compared with the carcinogenic control, respectively. In addition, DJE topical application decreased mRNA levels of these genes to 35%, 43% and 26% compared with the carcinogenic control, respectively. Moreover, mRNA expression of Il6 in Dioscorea japonica treatment was also decreased to almost the same level to that in normal control. Additionally, Dioscorea japonica treatment did not affect to the expression of Ptgds2 and Ptges3, although the expression of them was induced to approximately 5-fold and 2.5-fold, respectively in the carcinogenic control mice (Fig. 3). On the other hand, the expression of Ptgs1 and Ptges2 was not affected among all experimental groups (Fig. 3). Furthermore, lipid metabolome analysis in mouse skin was performed by liquid chromatography tandem mass spectrometry (ESI-MS, Fig. 4). We confirmed that the main lipid mediator in mouse skin was PGE2, and the production of PGE2 and PGD2 in Dioscorea japonica feeding mice decreased to 63.7% and 28.1%, respectively, compared with the carcinogenic control. DJE topical application also decreased PGE2 and PGD2 production to 62.2% and 28.1%, respectively. Whereas, PGF2α increased in Dioscorea japonica treatment groups.
Fig. 3.
Changes in gene expression. In normal or carcinogenic controls, 10% Dioscorea japnonica feeding, and 5 mg DJE topical application, mRNA expression of Ptgs1 (COX-1), Ptgs2 (COX-2), Ptges (mPGES-1), Ptges2 (mPGES-2), Ptges3 (cPGES), Ptgds2 (H-PGDS), Il1b (IL-1β), and Il6 (IL-6) was analyzed by quantitative RT-PCR. Expression levels of each mRNA were normalized to that of gapdh (GAPDH) mRNA. The relative expression levels are shown against normal control levels and represent mean ± SD of 6 mice per group; p<0.01 compared with anormal controls and bcarcinogenic controls.
Fig. 4.
Changes in amounts of PGs. The production of PGE2, PGD2, PGF2α and arachidonic acid was analyzed by ESI-MS. Amounts indicated represent mean ± SD of 10 samples per group; p<0.01 compared with anormal controls and bcarcinogenic controls.
Effect of Dioscorea japonica treatment on pathohistological and immunohistochemical analyses
Carcinogenic control mouse epidermis induced exhibited significant epidermal hyperplasia with hyperproliferation of the keratinocytes (upper part of HE staining in Fig. 5). Dioscorea japonica treatment markedly suppressed the epidermal hyperplasia. Moreover, infiltration of a lot of inflammatory cells in the epidermis in carcinogenic control was substantially inhibited by Dioscorea japonica feeding and DJE topical application (lower part of HE staining in Fig. 5). Immunohistochemical analyses indicated that COX-2 and mPGES-1 localized in tumor keratinocytes, and, additionally, COX-2 was strongly expressed in epidermal dendritic cells (Langerhans cells, LCs), but mPGES-1 was not observed in LCs (Fig. 5). Similarly, H-PGDS was also highly expressed in LCs, but not in keratinocytes. Interestingly, COX-2 staining was decreased in both tumor keratinocytes and LCs by Dioscorea japonica treatment. Additionally, we determined the localization of COX-2 and mPGES-1 in the mouse epidermis with inflammation and tumorigenesis by double immunofluorescent staining. Consistent with immunohistochemical analyses, COX-2 co-localized with CD207 (LC marker) and was present both in cancer cells and LCs. The immunoreaction of COX-2, especially in LCs, was stronger than that in cancer cells (Fig. 5 and 6A). In contrast, double staining for mPGES-1 and CD207, or for mPGES-1 and COX-2 showed that mPGES-1 co-localized with COX-2 in cancer cells, but not in LCs (Fig. 6A).
Fig. 5.
Histochemical analyses in mouse skin. Hematoxylin and eosin (HE) staining were pathologically analyzed. Brown-colored immunostaining of COX-2, mPGES-1 and H-PGES indicates each localization. Double-headed arrows indicate hyperplasia of accumulated cancer cells and arrowheads indicate Langerhans cells.
Fig. 6.
Double fluorostaining of COX-2, mPGES-1 or CD207 (A), and measurement of infiltrating cells in mouse skin (B–D). COX-2 and mPGES-1 expressing cells in carcinogenic control mouse epidermis were analyzed by immunofluorescent staining (A). COX-2 and CD207 (marker of Langerhans cells) were co-fluorostained, but mPGES-1 and CD207 were not. mPGES-1 and COX-2 were co-fluorostained in carcinogenic keratinocytes. For double immunofluorescent staining, merged signals are shown in yellow. The number of infiltrating neutrophils was counted in visualized by immunofluorescent staining of Ly-6G/Ly-6C (Gr-1) (green in upper B) and eosinophils were counted after HE staining (bottom B). The comparison of their number was represented as mean ± SD of 10 sections per group (C and D); *p<0.01 compared with the carcinogenic control. Arrowheads indicate Langerhans cells. ND, not detected.
Furthermore, we conducted immunostaining and HE staining, and counted the number of neutrophils and eosinophils to determine the effect of Dioscorea japonica on inflammatory cell infiltration. As shown in Fig. 6B, the greatest numbers of neutrophils and eosinophils were observed in carcinogenic controls. Counting the cells showed that Dioscorea japonica treatment decreased the infiltrating perilesional neutrophils and eosinophils to less than 50% and 20–35%, respectively (Fig. 6C and D).
Discussion
COX-2 is functionally coupled with mPGES-1 for PGE2 synthesis in pathophysiological conditions,(15) and these enzymes are overexpressed in various tumors and inflammation.(2,15–17) In skin cancers caused by some stimuli such as ultraviolet light and chemicals, COX-2 is induced and PGE2 is increased.(18,19) While COX-2 overexpression certainly induces skin carcinogenesis,(20) studies using COX-2 inhibitor(21) and COX-2 deficient mouse(22) demonstrate the prevention of tumor development in squamous cell carcinoma of the mouse skin. Our previous study demonstrated for the first time that DJE suppressed the expression of COX-2 and mPGES-1, causing PGE2 decrease and cancer cell apoptosis in lung cancer A549 cells.(11) In the present study, we confirmed the novel functionality of Dioscorea japonica that inhibited cancer evolution via suppression of COX-2 and mPGES-1, in vivo using a model of squamous cell carcinoma of the skin.
In the present model of squamous cell carcinoma, DMBA as an initiator is incorporated into LCs, and is metabolized to DMBA-3,4-diol-1,2-epoxide (DMBADE).(12) DMBADE is known to strongly induce keratinocytes during carcinogenesis.(12,23) We also showed that not only COX-2 but also mPGES-1 was overexpressed in squamous cell carcinoma of mouse skin, and they were co-localized in proliferated epidermal cancer cells. In addition, COX-2 was highly expressed, but mPGES-1 was not present in LCs. According to our results, COX-2 may be involved in carcinogenesis, tumor growth, and activation of LCs. On the other hand, main roles of mPGES-1 may be proliferation and development of cancer cells. PGE2 specific receptors EP1-4 are expressed in normal and tumor epidermis.(24,25) In tumors induced by UVB light exposure and COX-2 overexpression, EPs localize in epidermal hyperplasia.(24,25) The signaling pathways of PGE2/EP1 and PGE2/EP3 are involved in immune responses of the skin via T helper type (Th) 1 cells and dendritic cells, respectively.(26,27) Although the presence of EP4 in LCs is immunohistochemically unknown, PGE2/EP4 signaling promotes migration and maturation of Langerhance cells, and initiates skin immune responses.(28) Taken together with our results, it is suggested that the produced PGE2 by COX-2 and mPGES-1 induce tumor development and immune responses via the individual receptors in the autocrine or paracrine manner in squamous cell carcinoma of the skin.
In addition, H-PGDS was also predominantly expressed in LCs, and which may indicate a coupling of COX-2 and H-PGDS and the produced PGD2 regulates LCs activity. LCs play a key role in establishment of cutaneous immunity, and participate in squamous cell carcinoma.(12,29) Human dendritic cells including LCs in the skin express H-PGDS, and produce PGD2 in response to various stimuli.(30) Thus it is suggested that the produced PGD2 in autocrine manner in LCs is involved in cutaneous immune system and inflammatory reactions in the skin. Previous reports indicate the opposing immunomodulatory roles of PGD2 receptors, DP and CRTH2 (chemoattractant receptor homologous molecule expressed on Th2 cells) .(31,32) In inflammatory processes of the skin, PGD2 may play a role in the early stages via DP, and in eosinophil migration during the late stages via CRTH2. In each process of developing squamous cell carcinoma, further studies will be needed to determine the roles of H-PGDS and PGD2 in inflammation and carcinogenesis.
Our recent report demonstrates that Dioscorea japonica suppresses the expression of COX-2 and mPGES-1, and has an anti-carcinogenic effect on several model cell lines (reference 11 and unpublished data). In the present study, not only application, but also ingestion of Dioscorea japonica affects the expression of COX-2 and mPGES-1, and leads to decreased PGE2 in vivo. Previous studies concerning phytochemical effects on the PGE2 synthetic pathway report the suppression of COX-2 by resveratrol,(33) humulon,(34) chrysin,(35) and 6-shogaol(36) and of mPGES-1 by sulforaphane(37) and curcumin.(38) Moreover, we demonstrated that Dioscorea japonica was effective in suppressing both COX-2 and mPGES-1 mRNAs. Dioscorea japonica is rich in a lot of nutrients, and one of them is a plant steroidal saponin such as diosgenin. Diosgenin has been reported to have some preventive effects on mouse colon carcinogenesis,(39) mouse squamous cell carcinoma(40) and mouse Alzheimer’s disease.(41) Our previous study also showed that diosgenin suppressed COX-2 in A549 cells,(11) and additionally our recent experiment showed that it suppressed both of COX-2 and mPGES-1 in LPS-stimulated mouse macrophage-like RAW264 cells (Fig. 7). Therefore, diosgenin is likely one of the effective substances in the present study, and liposoluble and low molecular diosgenin may have effects via the oral and application routes.
Fig. 7.
Effects of diosgenin on the expression of Ptgs2 and Ptges in LPS-stimulated RAW264 cells. LPS-stimulated RAW264 were cultured with or without 100 nM diosgenin. mRNA expression of Ptgs2 (COX-2) and Ptges (mPGES-1) was measured by quantitative RT-PCR. The expression levels are shown as a relative value against control cells without LPS and diosgenin, and are represented mean ± SD of 3 separate experiments; p<0.05 compared with aLPS(–)/diosgenin(–) and bLPS(+)/diosgenin(–).
In concluding, our ex vivo(11) and in vivo (present study) results suggest that Dioscorea japonica may have inhibitory effects on inflammation and carcinogenesis caused by the hyperexpression of COX-2 and mPGES-1. In the present study, Dioscorea japonica also exerts a preventive effect on squamous cell carcinoma as a refractory skin disease, which has few known specific agents and treatments. Our results on the effects of Dioscorea japonica may pave the way for further therapeutic methods.
Acknowledgments
We are grateful to Professor Tetsuya Ogino and Ms. Yoko Watanabe (Okayama Prefectural University, Soja, Japan) for guidance regarding histological techniques. We also thank Mr. Soji Yoshio (Autoraimu Yoshio Ltd., Niimi, Japan) for providing Dioscorea japonica powder, and Enago (www.enago.jp) for English language review. This work was supported by JSPS KAKENHI Grant Number 15K00792, Japan Health Foundation (Kyoto, Japan) and Wesco Scientific Promotion Foundation (Okayama, Japan) to T.S.-Y., and by AMED-CREST to M.M.
Abbreviations
- COX
cyclooxygenase
- cPGES
cytosolic prostaglandin E synthase
- CRTH2
chemoattractant receptor homologous molecule expressed on Th2
- DJE
Dioscorea japonica extract
- DMBA
7,12-dimethylbenz[a]anthracene
- DMBADE
7,12-dimethylbenz[a]anthracene-3,4-diol-1,2-epoxide
- EPA
eicosapentaenoic acid
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HE
hematoxylin and eosin
- HETE
hydroxyeicosatetraenoic acid
- H-PGDS
hematopoietic prostaglandin D synthase
- IL
interleukin
- LCs
Langerhans cells
- LPS
lipopolysaccharide
- mPGES-1
microsomal prostaglandin E synthase-1
- mPGES-2
membrane-associated prostaglandin E synthase-2
- MRM
multiple reaction monitoring
- NF-κB
nuclear factor-κB
- NSAIDs
non-steroidal anti-inflammatory drugs
- PG
prostaglandin
- TPA
12-O-tetradecanoylphorbol-13 acetate
Conflict of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- 2.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 3.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007;59:207–224. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 4.Ng SC, Chan FKL. NSAID-induced gastrointestinal and cardiovascular injury. Curr Opin Gastroenterol. 2010;26:611–617. doi: 10.1097/MOG.0b013e32833e91eb. [DOI] [PubMed] [Google Scholar]
- 5.Wanasundera JPD, Ravindran G. Nutritional assessment of yam (Dioscorea alata) tubers. Plant Foods Hum Nutr. 1994;46:33–39. doi: 10.1007/BF01088459. [DOI] [PubMed] [Google Scholar]
- 6.Wanasundera JPD, Ravindran G. Effects of cooking on the nutrient and antinutrient contents of yam tubers (Dioscorea alata and Dioscorea esculenta). Food Chem. 1992;45:247–250. [Google Scholar]
- 7.Kwon CS, Sohn HY, Kim SH, et al. Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents. Biosci Biotechnol Biochem. 2003;67:1451–1456. doi: 10.1271/bbb.67.1451. [DOI] [PubMed] [Google Scholar]
- 8.Son IS, Kim JH, Sohn HY, Son KH, Kim JS, Kwon CS. Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.), on high-cholesterol fed rats. Biosci Biotechnol Biochem. 2007;71:3063–3071. doi: 10.1271/bbb.70472. [DOI] [PubMed] [Google Scholar]
- 9.Mbiantcha M, Kamanyi A, Teponno RB, Tapondjou AL, Watcho P, Nguelefack TB.Analgesic and anti-inflammatory properties of extracts from the bulbils of Dioscorea bulbifera L. var sativa (Dioscoreaceae) in mice and rats. Evid Based Complement Altern Med. 2011. DOI: 10.1155/2011/912935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon E, Lee SO, Kang TH, et al. Dioscorea extract (DA-9801) modulates markers of peripheral neuropathy in type 2 diabetic db/db mice. Biomol Ther (Seoul) 2014;22:445–452. doi: 10.4062/biomolther.2014.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki-Yamamoto T, Tanaka S, Tsukayama I, et al. Dioscorea japonica extract down-regulates prostaglandin E2 synthetic pathway and induces apoptosis in lung cancer cells. J Clin Biochem Nutr. 2014;55:162–167. doi: 10.3164/jcbn.14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modi BG, Neustadter J, Binda E, et al. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science. 2012;335:104–108. doi: 10.1126/science.1211600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto K, Miki Y, Sato H, Murase R, Taketomi Y, Murakami M. Secreted phospholipase A2 specificity on natural membrane phospholipids. Methods Enzymol. 2017;583:101–117. doi: 10.1016/bs.mie.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K, Miki Y, Sato M, et al. The role of group IIF-secreted phospholipase A2 in epidermal homeostasis and hyperplasia. J Exp Med. 2015;212:1901–1919. doi: 10.1084/jem.20141904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudo I, Murakami M. Prostaglandin E synthase, a terminal enzyme for prostaglandin E2 biosynthesis. J Biochem Mol Biol. 2005;38:633–638. doi: 10.5483/bmbrep.2005.38.6.633. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi M, Gokhale V, Meuillet EJ, Rosenberg DW. mPGES-1 as a target for cancer suppression: a comprehensive invited review “Phospholipase A2 and lipid mediators”. Biochimie. 2010;92:660–664. doi: 10.1016/j.biochi.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki Y, Kamei D, Ishikawa Y, et al. Microsomal prostaglandin E synthase-1 is involved in multiple steps of colon carcinogenesis. Oncogene. 2011;31:2943–2952. doi: 10.1038/onc.2011.472. [DOI] [PubMed] [Google Scholar]
- 18.Ali F, Khan BA, Sultana S. Wedelolactone mitigates UVB induced oxidative stress, inflammation and early tumor promotion events in murine skin: plausible role of NFkB pathway. Eur J Pharmacol. 2016;786:253–264. doi: 10.1016/j.ejphar.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Müller-Decker K. Cyclooxygenase-dependent signaling is causally linked to non-melanoma skin carcinogenesis: pharmacological, genetic, and clinical evidence. Cancer Metastasis Rev. 2011;30:343–361. doi: 10.1007/s10555-011-9306-z. [DOI] [PubMed] [Google Scholar]
- 20.Müller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Fürstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci U S A. 2002;99:12483–12488. doi: 10.1073/pnas.192323799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escuin-Ordinas H, Atefi M, Fu Y, et al. COX-2 inhibition prevents the appearance of cutaneous squamous cell carcinomas accelerated by BRAF inhibitors. Mol Oncol. 2014;8:250–260. doi: 10.1016/j.molonc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiano HF, Loftin CD, Akunda J, et al. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62:3395–3401. [PubMed] [Google Scholar]
- 23.Miyata M, Kudo G, Lee YH, et al. Targeted disruption of the microsomal epoxide hydrolase gene. Microsomal epoxide hydrolase is required for the carcinogenic activity of 7,12-dimethylbenz[a]anthracene. J Biol Chem. 1999;274:23963–23968. doi: 10.1074/jbc.274.34.23963. [DOI] [PubMed] [Google Scholar]
- 24.Neumann M, Dülsner E, Fürstenberger G, Müller-Decker K. The expression pattern of prostaglandin E synthase and EP receptor isoforms in normal mouse skin and preinvasive skin neoplasms. Exp Dermatol. 2007;16:445–453. doi: 10.1111/j.1600-0625.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 25.Tober KL, Thomas-Ahner JM, Kusewitt DF, Oberyszyn TM. Effects of UVB on E prostanoid receptor expression in murine skin. J Invest Dermatol. 2007;127:214–221. doi: 10.1038/sj.jid.5700502. [DOI] [PubMed] [Google Scholar]
- 26.Nagamachi M, Sakata D, Kabashima K, et al. Facilitation of Th1-mediated immune response by prostaglandin E receptor EP1. J Exp Med. 2007;204:2865–2874. doi: 10.1084/jem.20070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiraishi N, Nomura T, Tanizaki H, et al. Prostaglandin E2-EP3 axis in fine-tuning excessive skin inflammation by restricting dendritic cell functions. PLoS One. 2013;8:e69599. doi: 10.1371/journal.pone.0069599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabashima K, Sakata D, Nagamachi M, Miyachi Y, Inaba K, Narumiya S. Prostaglandin E2-EP4 signaling initiates skin immune responses by promoting migration and maturation of Langerhans cells. Nat Med. 2003;9:744–749. doi: 10.1038/nm872. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 30.Shimura C, Satoh T, Igawa K, et al. Dendritic cells express hematopoietic prostaglandin D synthase and function as a source of prostaglandin D2 in the skin. Am J Pathol. 2010;176:227–237. doi: 10.2353/ajpath.2010.090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirai H, Tanaka K, Yoshie O, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–261. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarashina H, Tsubosaka Y, Omori K, et al. Opposing immunomodulatory roles of prostaglandin D2 during the progression of skin inflammation. J Immunol. 2014;192:459–465. doi: 10.4049/jimmunol.1302080. [DOI] [PubMed] [Google Scholar]
- 33.Subbaramaiah K, Chung WJ, Michaluart P, et al. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998;273:21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto K, Wang J, Yamamoto S, Tobe H. Suppression of cyclooxygenase-2 gene transcription by humulon of beer hop extract studied with reference to glucocorticoid. FEBS Lett. 2000;465:103–106. doi: 10.1016/s0014-5793(99)01727-5. [DOI] [PubMed] [Google Scholar]
- 35.Ha SK, Moon E, Kim SY. Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-κB and JNK activations in microglia cells. Neurosci Lett. 2010;485:143–147. doi: 10.1016/j.neulet.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 36.Wu H, Hsieh MC, Lo CY, et al. 6-Shogaol is more effective than 6-gingerol and curcumin in inhibiting 12-O-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice. Mol Nutr Food Res. 2010;54:1296–1306. doi: 10.1002/mnfr.200900409. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, Joplin DG, Cross JV, Templeton DJ. Sulforaphane inhibits prostaglandin E2 synthesis by suppressing microsomal prostaglandin E synthase 1. PLoS One. 2012;7:e49744. doi: 10.1371/journal.pone.0049744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koeberle A, Northoff H, Werz O. Curcumin blocks prostaglandin E2 biosynthesis through direct inhibition of the microsomal prostaglandin E2 synthase-1. Mol Cancer Ther. 2009;8:2348–2355. doi: 10.1158/1535-7163.MCT-09-0290. [DOI] [PubMed] [Google Scholar]
- 39.Miyoshi N, Nagasawa T, Mabuchi R, et al. Chemoprevention of azoxymethane/dextran sodium sulfate-induced mouse colon carcinogenesis by freeze-dried yam sanyaku and its constituent diosgenin. Cancer Prev Res (Phila) 2011;4:924–934. doi: 10.1158/1940-6207.CAPR-10-0279. [DOI] [PubMed] [Google Scholar]
- 40.Das S, Dey KK, Dey G, et al. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. PLoS One. 2012;7:e46641. doi: 10.1371/journal.pone.0046641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tohda C, Urano T, Umezaki M, Nemere I, Kuboyama T. Diosgenin is an exogenous activator of 1,25D3-MARRS/Pdia3/ERp57 and improves Alzheimer’s disease pathologies in 5XFAD mice. Sci Rep. 2012;2:535. doi: 10.1038/srep00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.