Abstract
The exact pathogenesis of diarrhea-dominant irritable bowel syndrome (IBS) is not known, but the abnormal microbiota of the gastrointestinal tract is considered to be one of the important contributing factors as in other gastrointestinal diseases such as inflammatory bowel disease, antibiotic-associated diarrhea, and colorectal cancer as well as systemic diseases. Though diverse trials of probiotics had been continued in the treatment of diarrhea-IBS, only a few proved by randomized clinical trial. To prove the efficacy of Lactobacillus gasseri BNR17 isolated from breast milk in patients with diarrhea-IBS, prospective, randomized, placebo controlled clinical trial was done including health related-quality of life analysis, colon transit time, and the changes of fecal microbiota. BNR17 significantly improved the symptoms of diarrhea compared to control group. Health related-QOL analysis showed significant improvement of abdominal pain, distension, disturbed daily life, and mean defecation frequency with BNR17. On comparative CTT before and after BNR17, 6 out of 24 subjects showed significant correction of rapid colon transit pattern, while only 2 out of 24 in placebo (p<0.01). Upon fecal microbiota analysis, BNR17 significantly increased B. fecalis, E. rectale, C. aerofaciens, F. prausnitzil and B. steroris. Conclusively, Lactobacillus gasseri BNR17 can be a potential probiotics to ameliorate diarrhea-IBS.
Keywords: diarrhea-dominant IBS, Lactobacillus gasseri BNR17, IBS-QOL, colon transit time, microbiota
Introduction
Probiotics have been extensively studied over the past several years in the treatment of diverse diarrheal diseases.(1) However, in spite that most randomized controlled trials and subsequent meta-analyses suggest benefit for probiotics in the treatment or prevention of irritable bowel syndrome (IBS), antibiotic-associated diarrhea,(2) inflammatory bowel disease (IBD),(3) the real clinical efficacy is still inconclusive. Therefore, the efforts to develop effectiveprobioticlactic acid bacilli (LAB) strain are actively on-going since reported benefits were different depending on strain.(4,5)
IBS patients are known to have a substantially reduced health related-quality of life (HR-QOL),(6) by which the improvement of IBS-QOL reflects much better than the subjective changes of patients symptoms and visual analogue changes.(7,8) Rapid colon transit time (CTT) has been acknowledged as basis for diarrhea symptom in patients with IBS(9) possibly through increased intestinal permeability,(10) increased visceral hypersensitivity and lowered water resorption.(11) Also, an “imbalance of the microbiota”, a dysbiosis, has been associated with different GI diseases including Helicobacter pylori-associated gastritis, IBD, IBS, some infectious intestinal diseases such as Clostridium difficile colitis, and even colon carcinogenesis.(12,13) Until now, the trials regarding the efficacy assessment of probiotics were mostly based on the changes of symptoms by visual analogue scale (VAS) or other subjective symptom changes, after which the results of Cochrane meta-analysis did not show significant impact.
Lactobacillus gasseri (L. gasseri), in the genus of Lactobacillus acidophilus, is the most well-known probiotics and one of the most critical constituents of gut flora.(14) L. gasseri BNR17 was isolated from breast milk collected from healthy lactating females within two weeks of parturition in our author’s laboratory. During the study that L. gasseri BNR17 showed utmost efficacy on obesity, we have noticed breast milk derived BNR17 might improve bowel function,(15,16) in the current study, we evaluated the efficacy of probiotic L. gasseri BNR17 in subjects with diarrhea-dominant IBS incorporating specific IBS-QOL scale, the changes of CTT and blood chemistry, and the fecal microbiota along with the changes of diarrhea symptoms.
Methods
Study design
A single center, randomized, double-blind and placebo-controlled clinical trial was carried out to examine the efficacy and safety of L. gasseri BNR17 in patients with diarrhea dominant IBS. Treatment duration was 8 weeks with 2 follow-up visits of 4 week intervals. This trial was approved by the Institutional Review Board in CHA Bundang Medical Center, Seongnam, Korea (BD #2013-064) and registered in the International Clinical Trials Registry Platform of WHO with the following identification number of KCT0000969. All volunteers gave written informed consent prior to participation in this trial. Subjects between 20–55 years of age with diarrhea dominant IBS according to the Rome III criteria were eligible for inclusion. Subjects were excluded if they took any medication for IBS, previous intake of probiotics, antibiotics or anti-inflammatory drugs within 4 weeks before first visit. Subjects who had liver, kidney or psychiatric disorder were also excluded and pregnant or breast feeding women, alcoholics were also excluded. Compliance was calculated as the percentage of planned ingestion of the product and subjects at least 80% of compliance were analyzed. As randomization, subjects received number according to the registration order and assignment was performed using computer generated random list. The subjects and investigators remained blind until completion of this trial.
Study products
The test product contained L. gasseri BNR17 (250 mg per capsule, Bioneer Inc., Daejeon, Korea), maltodextrin (6 mg), microcrystalline cellulose (35%) and magnesium stearate (1%). Placebo capsules were identical in all aspects, but maltodextrin was the substitution of L. gasseri BNR17. All subjects were instructed to take 4 capsules per day (2 capsules after breakfast and dinner) and subjects in test group could take 1010 CFU/day of L. gasseri BNR17.
Assessments and study endpoints
The primary endpoint of this study was the improvement of the bowel function. Secondary endpoints included the improvement on blood glucose control and safety of this product. Bowel function was assessed by IBS-QOL questionnaire, Questionnaire for health status, Questionnaire for the degree of IBS symptom, Subjective global assessment of IBS symptom improvement, Bristol stool scale at week 0, 4 and 8, colon transit time at week 0 and 8 and pyrosequencing in feces at week 0 and 8. Colon transit time was calculated with 4-day method. Subjects took a capsule which contains radiopaque markers (Kolomark®) a day for 3 days and followed by abdominal X-ray on day 4. Pyrosequencing study of fecal microbiota was performed only in participants, whose body mass index (BMI) was over 25/m2 sent with written consent. Safety was assessed with vital sign on every visit and blood laboratory test such as CBC c diff., AST, ALT, BUN, creatinine, uric acid, total protein, albumin and urinalysis (blood, protein, glucose, pH). Serum glucose, insulin and HbA1c at week 0 and 8 were also analyzed to assess the effect of L. gasseri BNR17 on blood glucose control.
Statistics
Per-protocol analysis, which included the participants who consumed at least 80% of the study material provided, was performed. Variables were tested for normal distribution using the Shapiro-Wilk test. Normally distributed variables were analyzed using the Student’s t test for the between-group comparisons and the paired t test for the within-group comparisons. Non-normally distributed variables were analyzed using the Wilcoxon rank sum test for the between-group comparisons and the Wilcoxon signed rank test for the within-group comparisons. Within-group comparisons were performed between week 0 and week 8. Categorical variables were analyzed using Chi-square test and Fisher’s exact test. All statistical analyses were performed using the SAS program package ver. 9.3 (SAS Institute, Cary, NC). A two-tailed value of p<0.05 was considered to be significantly different.
Results
RCT of L. gasseri BNR17 in diarrhea-dominant IBS
60 subjects were included and were divided into two groups. 30 subjects were assigned to take L. gasseri BNR17 group and other 30 subjects to take placebo through randomization assignment. Onto per protocol analysis (PP), in L. gasseri BNR17 group, 2 subjects were discontinued intervention and 4 subjects did not achieve medication adherence rate at 80%, after which 24 subjects were included in the analysis. In placebo group, 2 subjects were discontinued intervention and 1 subject did not achieve medication adherence rate at 80%, after which 27 subjects were included in the analysis. As seen in Table 1, there were no significant differences in the sex ratio, age, body weight, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP) and smoking between placebo and BNR17 group. However, L. gasseri BNR17 group had significantly higher drinker than placebo group (p<0.05). As results of RCT, according to the changes of visual analogue scale (0–5 scale), especially the diarrhea symptoms were improved in 7 of 27 (25.9% in placebo group) and in 16 of 24 (66.7% in L. gasseri BNR17 group), significant difference between group (p<0.01).
Table 1.
Baseline characteristics†
| Placebo (n = 27) | BNR17 (n = 24) | p value‡ | |
|---|---|---|---|
| Gender (male/female) | 10/17 | 12/12 | 0.3508 |
| Age (years) | 38.0 (30.0, 46.0) | 35.0 (32.0, 40.5) | 0.2639 |
| Weight (kg) | 62.7 (54.0, 77.0) | 68.2 (56.4, 82.5) | 0.2736 |
| BMI (kg/m2) | 22.9 (20.1, 27.9) | 23.9 (21.6, 27.6) | 0.4335 |
| SBP (mmHg) | 118.0 (104.0, 134.0) | 119.5 (110.0, 135.5) | 0.4848 |
| DBP (mmHg) | 70.0 (64.0, 77.0) | 70.5 (67.0, 81.5) | 0.6706 |
| Alcohol consumption (current drinker/former drinker/non-drinker) | 15/3/9 | 22/1/1 | 0.0066 |
| Smoking (current smoker/former smoker/non-smoker) | 2/4/21 | 5/5/14 | 0.2714 |
†Median, interquartile range (25%, 75%) in parentheses (all such values). ‡Between-group comparisons using Student’s t test or Wilcoxon rank sum test for continuous variables and Chi-square test or Fisher’s exact test for categorical variables.
Comparative changes of IBS-QOL questionnaire in diarrhea-dominant IBS
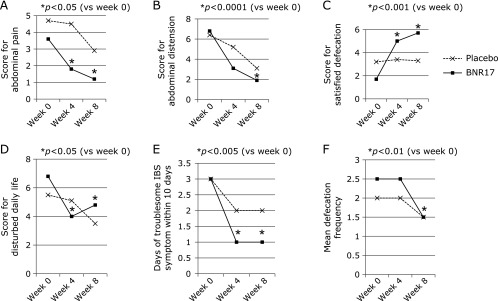
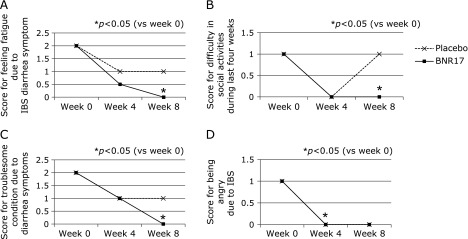
IBS-QOL questionnaire was specifically developed for the current study.(17) In 24 questionnaires out of 29 questionnaires, the both groups showed improvement of symptoms during 8 weeks, signifying that the diverse symptoms concurring in diarrhea-dominant IBS can be improved with placebo. However, as shown in Table 2 and Fig. 1, the mean score for abdominal pain, abdominal distension, satisfied defecation and disturbed daily life and changes of days of troublesome IBS symptoms within 10 days were statistically significantly reduced in L. gasseri BNR17 group compared to placebo group (p<0.05). In additional questionnaires shown in Table 3 and Fig. 2, L. gasseri BNR17 group showed significant improved scores compared to placebo group at 8 week in generalized symptoms like fatigue due to IBD diarrhea symptom (p<0.05), difficulty in social activities last four weeks (p<0.05), troublesome diarrhea symptoms (p<0.05), exhausted due to IBS symptoms (p<0.05), angry due to irritable symptoms of IBS (p<0.05), and restricted and limited in familial activities (p<0.05).
Table 2.
Questionnaire score changes regarding IBS symptoms†
| Placebo (n = 27) | BNR17 (n = 24) | |
|---|---|---|
| Q1. Abdominal pain | ||
| Week 0 → 4 → 8 | 4.7 → 4.5 → 2.9 | 3.6 → 1.8 → 1.2 |
| p value | 0.1142 | 0.0032 |
| Q2. Abdominal distension | ||
| Week 0 → 4 → 8 | 6.4 → 5.2 → 3.1 | 6.8 → 3.1 → 1.9 |
| p value | 0.1172 | <0.0001 |
| Q3. Satisfied defecation | ||
| Week 0 → 4 → 8 | 3.2 → 3.4 → 3.3 | 1.7 → 5.0 → 5.7 |
| p value | 0.8426 | 0.0008 |
| Q4. Disturbed daily life | ||
| Week 0 → 4 → 8 | 5.5 → 5.1 → 3.5 | 6.8 → 4.0 → 4.8 |
| p value | 0.1792 | 0.05 |
| Q5. Days of troublesome IBS symptoms within 10 days | ||
| Week 0 → 4 → 8 | 3.0 → 2.0 → 2.0 | 3.0 → 1.0 → 1.0 |
| p value | 0.3424 | 0.0051 |
| Q6. Mean defecation frequency | ||
| Week 0 → 4 → 8 | 2.0 → 2.0 → 1.5 | 2.5 → 2.5 → 1.5 |
| p value | 0.0172 | 0.0966 |
†Median, interquartile range (25%, 75%) in parentheses (all such values). Q1–Q4: Visual analogue scale, 0 (not at all)–10 (extremely), Q5: day, Q6: number of frequency. p value; Within-group comparisons using Wilcoxon signed rank test.
Fig. 1.
The changes of IBS-QOL questionnaire after placebo or L. gasseri BNR17, symptom changes. Among IBS-QOL scale, statistically significant changes in score after L. gasseri BNR17 were as follows; the changes of abdominal pain (p<0.05), abdominal distension (p<0.0001), satisfied defecation (p<0.001), disturbed daily life (p<0.05), the days of troublesome IBS symptoms (p<0.005), and mean defecation frequency (p<0.01). Detailed changes in score were presented in Table 2.
Table 3.
Diarrhea-IBS QOL assessment [from 0 (not at all) to 4 (extremely)]†
| Q8. Feels fatigue due to IBS diarrhea symptom | Q6. Difficulty in social activities last four weeks? | |||||||
| Week 0 | 2.0 (1.0, 3.0) | 2.0 (1.0 2.5) | 0.9604 | Week 0 | 1.0 (0.0, 1.0) | 1.0 (0.0, 1.5) | 0.9272 | |
| Week 4 | 1.0 (0.0, 1.0) | 0.5 (0.0, 1.0) | 0.6264 | Week 4 | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.9584 | |
| Week 8 | 1.0 (1.0, 1.0) | 0.0 (0.0, 1.0) | 0.0275 | Week 8 | 1.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.028 | |
| ΔWeek 8–0 | −1.0 (−1.0, −1.0) | −1.0 (−2.0, −1.0) | 0.2209 | Δ Week 8–0 | 0.0 (−1.0, 0.0) | −0.5 (−1.0, 0.0) | 0.2047 | |
| p value‡ | <0.0001 | <0.0001 | ||||||
| Q12. Troublesome and not stable due to diarrhea symptoms | Q7–9. Exhausted due to IBS symptoms | |||||||
| Week 0 | 2.0 (2.0, 4.0) | 2.0 (1.0, 3.0) | 0.1826 | Week 0 | 2.0 (2.0, 3.0) | 3.0 (3.0, 4.0) | 0.0485 | |
| Week 4 | 1.0 (0.0, 10.) | 1.0 (0.0, 1.5) | 0.8487 | Week 4 | 3.0 (2.0, 3.0) | 3.0 (2.0, 4.0) | 0.3831 | |
| Week 8 | 1.0 (0.0, 2.0) | 0.0 (0.0, 1.0) | 0.0487 | Week 8 | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 0.3913 | |
| ΔWeek 8–0 | −1.0 (−2.0, 0.0) | −1.5 (−3.0, 0.0) | 0.8773 | ΔWeek 8–0 | 0.0 (0.0, 1.0) | 0.0 (−1.0, 1.0) | 0.0159 | |
| p value‡ | <0.0001 | 0.0054 | p value‡ | 0.0041 | 0.293 | |||
| Q13. Angry due to irritable symptoms of IBS | Q10. Restricted and limited in familial activities? | |||||||
| Week 0 | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 0.5648 | Week 0 | 3.0 (2.0, 3.0) | 3.0 (2.5, 4.0) | 0.3878 | |
| Week 4 | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | 0.0968 | Week 4 | 3.0 (3.0, 4.0) | 3.5 (3.0, 4.0) | 0.1214 | |
| Week 8 | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | 0.0156 | Week 8 | 3.0 (3.0, 4.0) | 4.0 (3.0, 4.0) | 0.0393 | |
| ΔWeek 8–0 | −1.0 (−1.0, 0.0) | −0.5 (−1.5, 0.0) | 0.5997 | ΔWeek 8–0 | 1.0 (0.0, 1.0) | 0.5 (0.0, 1.0) | 0.8218 | |
| p value‡ | 0.0176 | 0.0005 | ||||||
†Median, interquartile range (25%, 75%) in parentheses (all such values). ‡Within-group comparisons using Wilcoxon signed rank test.
Fig. 2.
The changes of IBS-QOL questionnaire after placebo or L. gasseri BNR17, life style changes. Among IBS-QOL scale, significant changes among IBS-QOL were as follows; feels fatigue due to IBD diarrhea symptom (p<0.05), difficulty in social activities last four weeks (p<0.05), troublesome and not stable due to diarrhea symptoms (p<0.05), exhausted due to IBS symptoms (p<0.05), angry due to irritable symptoms of IBS (p<0.05), and restricted and limited in familial activities (p<0.05). Detailed changes in score were presented in Table 3.
Subjective global assessment of IBS symptom improvement
The number of subjects who responded that have improved or not improved symptom are shown in Table 4. The number of subjects with symptom improved was larger in L. gasseri BNR17group than placebo group, but it was not significant statistically.
Table 4.
Subjective global assessment of IBS symptom improvement
| Placebo (n = 27) | BNR17 (n = 24) | p value† | |
|---|---|---|---|
| (improved/not improved) | |||
| Week 0 | 0/27 | 0/24 | — |
| Week 4 | 14/13 | 15/9 | 0.4435 |
| Week 8 | 14/13 | 18/6 | 0.0879 |
†Between-group comparisons using Chi-square test.
The changes of metabolic parameters including glucose, HbA1c, and insulin
Since the preliminary study showed that L. gasseri BNR17 improved metabolic syndrome, in this study,(14–16) we had measured the changes of glucose, HbA1c, and serum level of insulin according group. As seen in Table 5, the mean fasting blood glucose was significantly decreased during 8 weeks in L. gasseri BNR17 group (101.5 mg/dl at week 0 and 97.5 mg/dl at week 8, p<0.05) but not in placebo group. However, HbA1c and insulin level did not show significant changes in any group. Since the most subjects were without diabetes, these changes only suggest the possible application for metabolic advantage in patients with diabetes.
Table 5.
The changes of glucose, HbA1c and insulin in fasting blood according to group†
| Placebo (n = 27) | BNR17 (n = 24) | p value§ | |
|---|---|---|---|
| Glucose (mg/dl) | |||
| Week 0 | 99.0 (93.0, 112.0) | 101.5 (98.0, 105.5) | 0.4499 |
| Week 8 | 99.0 (92.0, 106.0) | 97.5 (94.5, 102.0) | 0.6985 |
| ΔWeek 8–0 | −2.0 (−5.0, 5.0) | −3.0 (−8.0, 0.5) | 0.307 |
| p value‡ | 0.4402 | 0.0138 | |
| HbA1c (%) | |||
| Week 0 | 5.5 (5.3, 5.8) | 5.4 (5.3, 5.8) | 0.5184 |
| Week 8 | 5.5 (5.3, 5.7) | 5.5 (5.2, 5.7) | 0.5308 |
| Δ Week 8–0 | 0.0 (−0.1, 0.2) | 0.0 (−0.1, 0.1) | 0.9772 |
| p value‡ | 0.788 | 0.7894 |
†Median, interquartile range (25%, 75%) in parentheses (all such values). ‡Within-group comparisons using Wilcoxon signed rank test. §Between-group comparisons using Wilcoxon rank sum test.
The changes of colonic transit time (CTT)
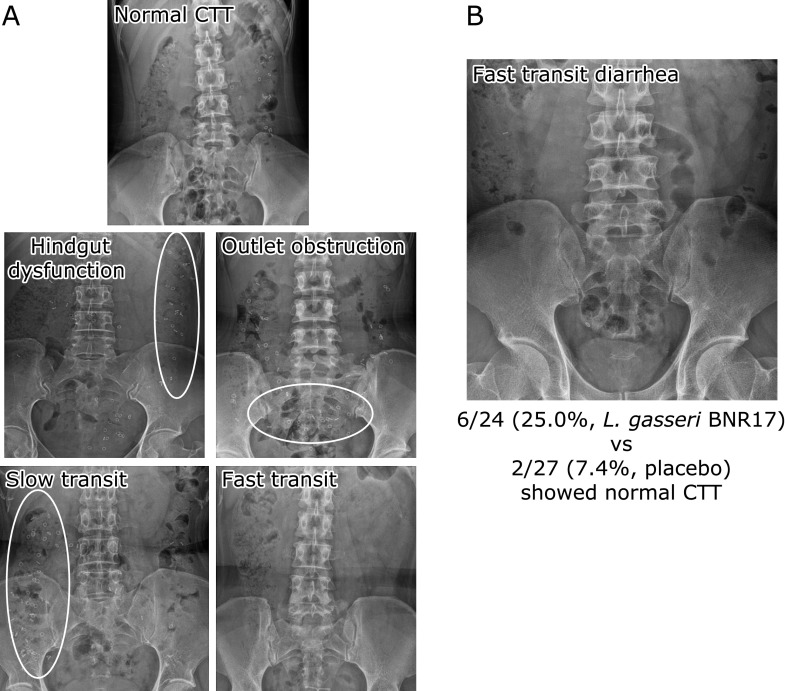
The CTT changes according to group are shown in Table 5. The mean CTT was 5.4 h at week 0 and 19.2 h at week 8 in subjects with diarrhea dominant IBS treated with BNR17 and 8.4 h week 0 and 13.2 h at week 8 in placebo treated group. CTT was significantly increased during 8 weeks in L. gasseri BNR17 group but not in placebo group (p<0.05). Various types of colonic transit type were found in this study and typical cases are shown in Fig. 3A. The numbers of subject who changed from fast transit time to normal transit time are 2 out of placebo group, but was noted in 6 out of L. gasseri BNR17 group (p<0.01, Fig. 3B).
Fig. 3.
CTT pattern and the changes of CTT with L. gasseri BNR17. (A) CTT patterns, hindgut dysfunction, oulet obstruction, slow transit, fast transit (B) CTT changes from fasting transit diarrhea to normal pattern after L. gasseri BNR17 in 6 subjects with diarrhea-dominant IBS.
Changes of fecalmicrobiota according to group
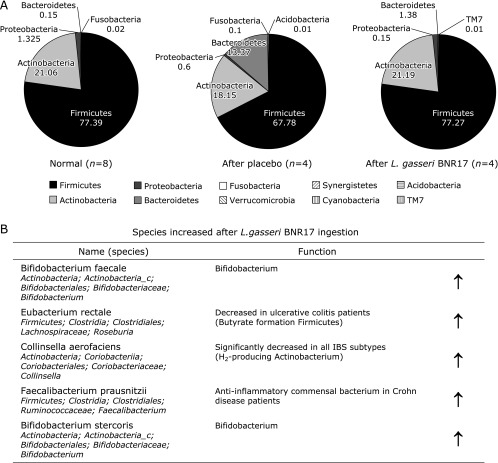
Among 51 subjects, 8 subjects who presented with BMI >25/m2 were included in these analysis of fecal microbiota as written in inclusion criteria. On phylum assessment, subjects receiving L. gasseri BNR17 showed significant increment in Actinobacteria (8.1% to 21.19%), decrement in Proteobacteria (2.2% to 0.15%). On placebo administered cases, no significant changes were noted except significant increment of Bacteroidetes. On further Genus analysis, significant changes were prominently noted in BNR17 administration that increment in Lactobacillus and Bifidobacterium, whereas decrement in Blautia and fecalibacterium (Fig. 4). Significantly increasing species were described in Fig. 4B, all of these microbiome changes relevant to L. gasseri BNR17 were, we inferred, compatible with afore-mentioned improvement of diarrhea dominant IBS.
Fig. 4.
Microbiota changes. (A) phylum levels. (B) Genus level. Significant increments in Bifidobacteriumfaecalis, Eubacteriumrectale, CollinsellaAerofaciens, Faecalibacteriumprausnitzii, and Bifidobacteriumstercoris were noted with L. gasseri BNR17 as part of improved symptom of diarrhea-dominant IBS.
Safety assessment
Vital sign, CBC with differentiation, biochemical changes including AST, ALT, BUN, creatinine, uric acid, total protein and albumin, and urinalysis were not changed significantly in the two groups (data not shown).
Discussion
From the current a randomized, double blinded, prospective, comparative clinical trial, we found that 8-week ingestions of breast milk derived L. gasseri BNR17 significantly improved the IBS-QOL in subjects with diarrhea-dominant IBS accompanied with healthymicrobiota changes, improved colon transit in some. Thoughmeta-analyses that have evaluated the efficacy of probiotics in physiological and pathological conditions such as antibiotic or chemotherapy-associated diarrhea, Clostridium difficile-associated pseudomembranous colitis, IBS, constipation, inflammatory bowel disease, respiratory tract infection, ventilator-associated pneumonia, non-alcoholic fatty liver disease, periodontitis, vaginosis, urinary tract infections, pancreatitis, various hospital infection, necrotisingenterocolitis in premature infants,(5,18) the results are contradictory except antibiotic- and Clostridium difficile-associated diarrhea. After the current status, we can add evidence that L. gasseri BNR17 can be effective for diarrhea-dominant IBS.
Though there had been many clinical trials to investigate the effect of probiotics in patients with IBS, most trials were open-labeled proof of the effectivenessof probiotics compared to placeboin IBS symptoms,(19–22) only some were blinded trial, but mostly focused on visual analogue scaled symptom changes and some more parameters.(23–28) Since a meta-analysis of RCT of IBS patient showed that the placebo response rate ranged from 16.0 to 71.4% in assessed global response,(29) the placebo effect is important in functional disorder and psychological factors also affect significant levels of IBS symptoms.(30) In our trial, more than 80% of IBS-QOL were improved with placebo trial, both L. gasseri BNR17 and placebo improved symptoms of subjects with IBS in some points of QOL. However, we could clearly show significant beneficiary effect of L. gasseri BNR17 onto diarrhea-dominant IBD including the following analysis that statistically significant in some IBS-QOL analysis, improved CTT, and enrichments of gut microbiota relevant to patient symptoms.
Regarding the implication of CTT, some studies showed accelerated CTT in diarrhea-dominant IBS patients,(31) whereas not in other studies.(32,33) In studies performed with Korean population, the mean CTT was 20 to 30 h in asymptomatic normal subjects,(34) but in this current study, average CTT of all subjects was 7.0 h, which was considerably accelerated compared to average transit time of normal Korean population, a part of pathogenetic mechanism explaining of diarrhea-dominant IBS. Similar to previous studies,(35) L. gasseri BNR17 ingestion prolonged CTT to the near average level of Korean population. Because of disturbances of colonic motility play an important role in diarrhea symptom of IBS, the effect of L. gasseri BNR17 on the colon motility is very contributoryto IBS and imposes a potential treatment option to subjects with diarrhea-dominant IBS.
The deranged intestinal microbiota is thought to play important roles in the pathogenesis of IBS since many recent studies demonstrated gut microbial dysbiosis in IBS patients. The putative mechanism show intestinal microbiota orchestrate intestinal homeostasis and function include the alteration of the microbiota-gut-brain axis, quantitative and qualitative changes in the microbiota, activation of mucosal immunity and inflammation, altered mucosal permeability, and the epithelial barrier and sensory-motor disturbances.(36) In this presented study, Firmicutes took the most microbiome at the phylum level in the both groups of subjects with IBS before intake L. gasseri BNR17. Although we did not compare the microbiome to healthy subjects in whole subjects, the Firmicutes levels are high consistent to previous studies which showed the increased ratio of Firmicutes to Bacteroidetes and reduced quantity of Actinobacteria insubjects with IBS.(37–39) The decrease of Firmicutes and increase of Actinobacteria and Bacteroidetes after L. gasseri BNR17 ingestion is the outcome suggestive of the correction of the dysbiosis observedin IBS patient. Therefore, we could conclude that L. gasseri BNR17 may improve the bowel function in subjects with diarrhea dominant IBS through the correction of the symptomatic related dysbiosis. However, more clear explanation can be possible with more extensive inclusion of subject number as well as elucidations of further mechanistic exploration.
Previous study with L. gasseri BNR17 in db⁄db mice which have similar characteristics to humans with type 2 diabetes demonstrated the anti-diabetic effect of L. gasseri BNR17.(40) We proved the anti-diabetic effect of L. gasseri BNR17 in this study, but seem insignificant because included subjects were mostly not diabetic. Although the amount of reduction was only 4 mg/dl during 8 weeks, the difference was significant statistically. The number of subjects whose fasting glucose was above 110 mg/dl was 5 in placebo and L. gasseri BNR17 group equally. The mean changes of fasting glucose level in these subgroups were 10.4 mg/dl in L. gasseri BNR17 group and 2.8 mg/dl in placebo group. This result implies the possibility that L. gasseri BNR17 can be preferred in subjects with DM-related intestinal motility disorder or metabolic syndrome-related IBS. Also, longer study duration and larger amount of subjects with diabetes are needed in this aspect.
L. gasseri BNR17 reported not a serious adverse reaction but slightly decrease of hematocrit.(16) In this study, only a slightly decrease of hematocrit was noted without other adverse reactions, but the reduction rated was minimal and the change of hemoglobin was insignificant. Although it is hard to conclude that L. gasseri BNR17 inducesanemia, study with larger number of subject with longer duration is needed to clarify it. Another limitation of current study was that we could not explain why the degree of alcohol consumption was different between the two groups, that is, in the L. gasseri BNR17 group, subjects took more alcohol, even though assignment was very randomized in clinical trial. The subjects who drink might reduce or stop the alcohol during the study period, which can affect the efficacy of L. gasseri BNR17. However, the withdrawal of alcohol increased the CTT,(41) by which no statistical significance of CTT was seen between placebo and L. gasseri BNR17. In spite of these limitations, correction of CTT by L. gasseri BNR17 was observed in some subjects. Though still the poor understanding of IBS-QOL questionnaire was limitation, since the subtle description regarding questions made participants hard to response, but overall assessment was performed in excellence.
Though more extensive clinical trials are needed with longer duration and larger number of subjects to evaluate the long term effect of BNR17 on bowel function, we could conclude that L. gasseri BNR17 derived from human breast milk significantly improved diarrhea-dominant IBS, based on significant microbiota change, bowel function improvement, and fasting glucose control without any serious complication, deserving clinical application in patients with diarrhea-dominant IBS as rescue and ethical medication.
Table 6.
The changes of colonic transit time (CTT) according to group† (unit: h)
| Placebo (n = 27) | BNR17 (n = 24) | p value§ | |
|---|---|---|---|
| Week 0 | 8.4 (2.4, 31.2) | 5.4 (0.0, 28.7) | 0.3578 |
| Week 8 | 13.2 (0.0, 26.4) | 19.2 (2.4, 40.8) | 0.146 |
| ΔWeek 8–0 | −2.4 (−14.4, 10.8) | 2.4 (−1.2, 20.4) | 0.0155 |
| p value‡ | 0.2762 | 0.0464 |
†Median, interquartile range (25%, 75%) in parentheses (all such values). ‡Within-group comparisons using Wilcoxon signed rank test. §Between-group comparisons using Wilcoxon rank sum test.
Acknowledgments
This study was supported by the Ministry of Agriculture, Food, and Rural Affairs (High Value-Added Food Technology Development Program 111006-3, The Ministry of Science, ICT and Future Planning (NRF 2012M3A9C4048761).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Guandalini S. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol. 2011;45 Suppl:S149–S153. doi: 10.1097/MCG.0b013e3182257e98. [DOI] [PubMed] [Google Scholar]
- 2.Issa I, Moucari R. Probiotics for antibiotic-associated diarrhea: do we have a verdict? World J Gastroenterol. 2014;20:17788–17795. doi: 10.3748/wjg.v20.i47.17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guandalini S, Cernat E, Moscoso D. Prebiotics and probiotics in irritable bowel syndrome and inflammatory bowel disease in children. Benef Microbes. 2015;6:209–217. doi: 10.3920/BM2014.0067. [DOI] [PubMed] [Google Scholar]
- 4.Whelan K, Quigley EM. Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29:184–189. doi: 10.1097/MOG.0b013e32835d7bba. [DOI] [PubMed] [Google Scholar]
- 5.Yoon H, Park YS, Lee DH, Seo JG, Shin CM, Kim N. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Biochem Nutr. 2015;57:129–134. doi: 10.3164/jcbn.15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou X, Chen S, Zhang Y, et al. Quality of life in patients with Irritable Bowel Syndrome (IBS), assessed using the IBS-Quality of Life (IBS-QOL) measure after 4 and 8 weeks of treatment with mebeverine hydrochloride or pinaverium bromide: results of an international prospective observational cohort study in Poland, Egypt, Mexico and China. Clin Drug Investig. 2014;34:783–793. doi: 10.1007/s40261-014-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monnikes H. Quality of life in patients with irritable bowel syndrome. J Clin Gastroenterol. 2011;45 Suppl:S98–S101. doi: 10.1097/MCG.0b013e31821fbf44. [DOI] [PubMed] [Google Scholar]
- 8.Park JM, Choi MG, Kim YS, et al. Quality of life of patients with irritable bowel syndrome in Korea. Qual Life Res. 2009;18:435–446. doi: 10.1007/s11136-009-9461-7. [DOI] [PubMed] [Google Scholar]
- 9.Ma AD, Kessler CM, Al-Mondhiry HA, Gut RZ, Cooper DL. Use of recombinant activated factor VII for acute bleeding episodes in acquired hemophilia: final analysis from the Hemostasis and Thrombosis Research Society Registry acquired hemophilia study. Blood Coagul Fibrinolysis. 2016;27:753–760. doi: 10.1097/MBC.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishimoto H, Oshima T, Sei H, et al. Claudin-2 expression is upregulated in the ileum of diarrhea predominant irritable bowel syndrome patients. J Clin Biochem Nutr. 2017;60:146–150. doi: 10.3164/jcbn.16-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belkind-Gerson J, Tran K, Di Lorenzo C. Novel techniques to study colonic motor function in children. Curr Gastroenterol Rep. 2013;15:335. doi: 10.1007/s11894-013-0335-3. [DOI] [PubMed] [Google Scholar]
- 12.Biedermann L, Rogler G. The intestinal microbiota: its role in health and disease. Eur J Pediatr. 2015;174:151–167. doi: 10.1007/s00431-014-2476-2. [DOI] [PubMed] [Google Scholar]
- 13.Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 14.Kang JH, Yun SI, Park HO. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J Microbiol. 2010;48:712–714. doi: 10.1007/s12275-010-0363-8. [DOI] [PubMed] [Google Scholar]
- 15.Kang JH, Yun SI, Park MH, Park JH, Jeong SY, Park HO. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS One. 2013;8:e54617. doi: 10.1371/journal.pone.0054617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung SP, Lee KM, Kang JH, et al. Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: a randomized, double-blind clinical trial. Korean J Fam Med. 2013;34:80–89. doi: 10.4082/kjfm.2013.34.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EH, Kwon O, Hahm KB, et al. Irritable bowel syndrome-specific health-related quality of life instrument: development and psychometric evaluation. Health Qual Life Outcomes. 2016;14:22. doi: 10.1186/s12955-016-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rondanelli M, Faliva MA, Perna S, Giacosa A, Peroni G, Castellazzi AM.Using probiotics in clinical practice: Where are we now? A review of existing meta-analyses. Gut Microbes. 2017. DOI: 10.1080/19490976.2017.1345414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyonnet D, Chassany O, Ducrotte P, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475–486. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzo-Zúñiga V, Llop E, Suárez C, et al. I.31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J Gastroenterol. 2014;20:8709–8716. doi: 10.3748/wjg.v20.i26.8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 22.Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 23.O'Sullivan MA, O'Morain CA. Bacterial supplementation in the irritable bowel syndrome. A randomised double-blind placebo-controlled crossover study. Dig Liver Dis. 2000;32:294–301. doi: 10.1016/s1590-8658(00)80021-3. [DOI] [PubMed] [Google Scholar]
- 24.Sen S, Mullan MM, Parker TJ, Woolner JT, Tarry SA, Hunter JO. Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig Dis Sci. 2002;47:2615–2620. doi: 10.1023/a:1020597001460. [DOI] [PubMed] [Google Scholar]
- 25.Newcomer AD, Park HS, O'Brien PC, McGill DB. Response of patients with irritable bowel syndrome and lactase deficiency using unfermented acidophilus milk. Am J Clin Nutr. 1983;38:257–263. doi: 10.1093/ajcn/38.2.257. [DOI] [PubMed] [Google Scholar]
- 26.Niv E, Naftali T, Hallak R, Vaisman N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome--a double blind, placebo-controlled, randomized study. Clin Nutr. 2005;24:925–931. doi: 10.1016/j.clnu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Choi CH, Jo SY, Park HJ, Chang SK, Byeon JS, Myung SJ. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679–683. doi: 10.1097/MCG.0b013e318204593e. [DOI] [PubMed] [Google Scholar]
- 28.Kabir MA, Ishaque SM, Ali MS, Mahmuduzzaman M, Hasan M. Role of Saccharomyces boulardii in diarrhea predominant irritable bowel syndrome. Mymensingh Med J. 2011;20:397–401. [PubMed] [Google Scholar]
- 29.Patel SM, Stason WB, Legedza A, et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17:332–340. doi: 10.1111/j.1365-2982.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 30.Barbara G, De Giorgio R, Stanghellini V, Cremon C, Salvioli B, Corinaldesi R. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20 Suppl 2:1–9. doi: 10.1111/j.1365-2036.2004.02036.x. [DOI] [PubMed] [Google Scholar]
- 31.Corbett CL, Thomas S, Read NW, Hobson N, Bergman I, Holdsworth CD. Electrochemical detector for breath hydrogen determination: measurement of small bowel transit time in normal subjects and patients with the irritable bowel syndrome. Gut. 1981;22:836–840. doi: 10.1136/gut.22.10.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu WZ, Song GH, Gwee KA. Ho KY. The effects of melatonin on colonic transit time in normal controls and IBS patients. Dig Dis Sci. 2009;54:1087–1093. doi: 10.1007/s10620-008-0463-z. [DOI] [PubMed] [Google Scholar]
- 33.Horikawa Y, Mieno H, Inoue M, Kajiyama G. Gastrointestinal motility in patients with irritable bowel syndrome studied by using radiopaque markers. Scand J Gastroenterol. 1999;34:1190–1195. doi: 10.1080/003655299750024698. [DOI] [PubMed] [Google Scholar]
- 34.Lee OY. Asian motility studies in irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:120–130. doi: 10.5056/jnm.2010.16.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee KN, Lee OY. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J Gastroenterol. 2014;20:8886–8897. doi: 10.3748/wjg.v20.i27.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeffery IB, O'Toole PW, Öhman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 38.Rajilić-Stojanović M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 39.Krogius-Kurikka L, Lyra A, Malinen E, et al. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009;107:1681–1686. doi: 10.1111/j.1365-2672.2009.04350.x. [DOI] [PubMed] [Google Scholar]
- 41.Bouchoucha M, Nalpas B, Berger M, Cugnenc PH, Barbier JP. Recovery from disturbed colonic transit time after alcohol withdrawal. Dis Colon Rectum. 1991;34:111–114. doi: 10.1007/BF02049982. [DOI] [PubMed] [Google Scholar]