Abstract
Objective
This study was designed to directly compare the outcomes of tofacitinib therapy for methotrexate-refractory rheumatoid arthritis (RA) between biologic-naïve patients and patients who had experienced an inadequate response to biological agents.
Methods
We prospectively enrolled and followed 113 patients who had a high or moderate clinical disease activity index (CDAI) (36 biologic-naïve patients and 77 biologic-experienced patients). Patients received 5 mg of tofacitinib twice daily. Effectiveness and adverse events were examined at month 6 of treatment.
Results
At month 6, 65 patients (57.5%) reached CDAI50, which is defined as achieving ≥50% improvement. The number of previous biological agents was twice as high in CDAI50 non-responders as in responders (2.2 versus 1.1, p<0.001), but there was no significant difference in the type of previous agents or the reason for discontinuation. According to a multivariate logistic regression analysis, the previous use of biological agents [odds ratio (OR) 4.48, p=0.002] and the concurrent use of prednisolone (OR 2.40, p=0.047) were associated with a failure to achieve a CDAI 50 response. Biologic-naïve patients were more likely to achieve CDAI50 than biologic-experienced patients (80.6% versus 46.8%, p=0.001). Mean CDAI values were higher in biologic-experienced patients (11.4 versus 4.8, p=0.001), and remission rates were higher in biologic-naïve patients (41.7% versus 11.7%, p=0.001). Biologic-naïve patients more rapidly achieved remission. Rates of discontinuation resulting from adverse events were similar in both groups.
Conclusion
Although tofacitinib can provide an effective treatment option for intractable RA patients, its impact on outcomes is lower in patients with previous biologic failure.
Keywords: tofacitinib, rheumatoid arthritis, JAK inhibitor, effectiveness, antirheumatic drugs
Introduction
Owing to advances in pharmaceutical therapy over the past decade, the prognosis of patients with rheumatoid arthritis (RA) has improved dramatically (1, 2). Since the approval of infliximab in March 2003 as the first biological agent in Japan, five tumor necrosis factor (TNF) inhibitors (infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol), an interleukin (IL)-6 receptor-blocking antibody (tocilizumab), and a T-cell co-stimulation inhibitor (abatacept) have mainly been used in the treatment of methotrexate (MTX)-refractory RA. From daily clinical practice, however, we have learned that many RA patients have inadequate responses, including a lack or loss of efficacy and intolerance, to one or more biological agents. Randomized, double-blind trials have also shown that approximately 30-50% of individuals treated with biological therapy fail to achieve a 20% clinical improvement, according to the American College of Rheumatology (ACR) criteria (3-7). A recent systemic review using data from drug registries and health care databases indicated that overall discontinuation rates of TNF inhibitors at 0.5, 1, 2, 3, and 4 years were 21%, 27%, 37%, 44%, and 52%, respectively (8).
RA patients with an inadequate response to TNF inhibitors are recommended to switch to other TNF inhibitors, abatacept, or tocilizumab, but many patients do not respond well, lose initial clinical response, or experience adverse events during a subsequent biological treatment (9-19). Direct comparison studies between biologic-naïve patients and patients switched onto TNF inhibitors have shown that response and remission outcomes were consistently inferior for the switched patients compared with the biologically naïve patients (20, 21). Therefore, treating RA patients with response failure to TNF inhibitors remains a challenging task for rheumatologists.
Tofacitinib is a novel, small-molecule, oral inhibitor of Janus kinases (JAKs). Recent phase III trials have produced favorable results in the treatment of patients with active RA (22-27). However, there are few direct comparison studies examining its impact on the therapeutic outcomes between biologic-naïve patients and biologic-experienced patients in a real-world clinical setting. In addition, there are limited data regarding tofacitinib efficacy and its safety for inadequate responders to non-TNF inhibitors such as abatacept and tocilizumab.
To address these issues, we performed a six-month prospective follow-up study that investigated the outcomes of tofacitinib therapy for RA and compared data between biologic-naïve patients and patients who experienced inadequate response to previous biological agents, including TNF inhibitors, abatacept, and tocilizumab.
Materials and Methods
Patients and study design
Tofacitinib was approved for the treatment of RA in Japan on March 25, 2013, and was launched on July 30, 2013. For this study, 113 patients with RA who had a high or moderate clinical disease activity index (CDAI>10) (36 biologic-naïve patients and 77 biologic-experienced patients) were prospectively enrolled and followed from August 1, 2013, through April 30, 2016. All participants fulfilled the 1987 ACR criteria or the 2010 ACR/European League Against Rheumatism (EULAR) criteria for the diagnosis of RA (28, 29), and they had to have experienced a failure to achieve a low CDAI or remission by MTX therapy for ≥3 months. At enrollment, we classified all participants into biologic-experienced and biologic-naive groups according to the following definition: biologic-experienced patients were defined as patients who had experienced an inadequate response, including lack of efficacy, loss of efficacy, or an adverse event, during treatment with one or more biological agents. Exclusion criteria were as follows: patients with current infectious disease, those with hematopoietic disorders (hemoglobin levels <8.0 mg/dL, neutrophil counts <1,000/μL, or lymphocyte counts <500/μL), those with an abnormal hepatic function [direct bilirubin, aspartate transaminase, or alanine transaminase >2× the upper limit of normal (ULN)], those with an estimated glomerular filtration rate <40 mL/min/1.73 m2, and those with malignancy.
For each patient, the demographic characteristics and RA-related characteristics such as RA duration, stages, positivity of anti-cyclic citrullinated peptide antibodies (anti-CCP Abs), disease activity, and the use of MTX and prednisolone were examined and recorded at enrollment in this study. The number and type of previous biological agents that had produced an inadequate response were also recorded. The median (range) of MTX dose previously used was 8 mg weekly (4 to 14 mg/week). Previous biological agents included TNF inhibitors (56.8%), tocilizumab (32.2%), and abatacept (10.9%).
Patients were scheduled to receive tofacitinib at a 5-mg twice-daily regimen, as monotherapy or in combination with MTX. For patients who were receiving MTX at enrollment, a stable dose of MTX (4 to 14 mg/week) was allowed to be continuously used during tofacitinib therapy. Patients who were receiving prednisolone at the start of tofacitinib therapy were allowed to continuously receive the same dose (2 to 7.5 mg/day) concomitantly throughout this therapy. The end of follow-up was set as the point when tofacitinib therapy was discontinued or 6 months after the initiation of this therapy, whichever came first. Discontinuation was defined as tofacitinib withdrawal because of an adverse event, lack or loss of efficacy, or loss to follow-up before the end of the 6-month follow-up period. The therapeutic outcomes at month 6 were compared between the biologic-naïve group and the biologic-experienced group.
Effectiveness analyses
The CDAI was used to quantify the RA disease activity (30). In this study, many patients had received tocilizumab, which is known to be a strong inhibitor of acute phase reactant production. Because the CDAI does not include acute-phase reactant values, a more accurate disease activity status can be obtained for such patients. Cut-off values for disease activity states were defined as follows: high disease activity, CDAI>22; moderate disease activity, CDAI>10 and ≤22; low disease activity, CDAI>2.8 and ≤10; and remission, CDAI≤2.8 (31). During the follow-up period, CDAI assessments were performed at months 3 and 6. Clinical responses during tofacitinib therapy were evaluated according to the new CDAI improvement criteria, in which minor, moderate, and major responses were defined as achieving ≥50% improvement of CDAI (CDAI50), ≥70% (CDAI70), and ≥85% (CDAI85), respectively (32). ACR20, ACR50, and ACR70 responses were reported to correspond to CDAI50, CDAI70, and CDAI85, respectively (32). CDAI improvement based on the minimally clinically improved difference (MCID), which was defined as a CDAI reduction >12 for patients starting with a high CDAI and >6 for those starting with a moderate CDAI, was also used to evaluate clinical responses to tofacitinib therapy (33). In this study, CDAI50 responders were defined as those who had achieved and maintained a CDAI50 response during the 6 months of tofacitinib therapy. Non-responders included those who had completed the 6-month therapy but had not achieved a CDAI50 response and those who had discontinued this therapy because of a lack or loss of efficacy, adverse events, or loss to follow-up.
Ethics approval
This study was conducted in accordance with the principles of the Declaration of Helsinki (2008). The protocol of this study also meets the requirements of the Ethical Guidelines for Medical and Health Research Involving Human Subjects, Japan (2014) and was approved by the Human Research Ethics Committees of NHO Kumamoto Saishunsou National Hospital. Written informed consent was obtained from all patients.
Statistical analyses
Data comparisons of continuous variables between the two patient groups were performed using the independent-measures t-test. The chi-squared test and Fisher's exact probability test were used for statistical analyses of categorical variables. To compare the CDAI values at month 6, missing data on patients who withdrew from the study were replaced with the last observed values.
A multivariate logistic regression analysis was performed to evaluate the association between the failure to achieve a CDAI50 response as a dependent variable and a set of independent variables considered to be significant factors in univariate analyses. We also included, as independent variables, factors that were not significant in univariate analyses but might be confounding or clinically relevant variables. A backward stepwise selection procedure was used to select significant independent variables. The strength of the association between CDAI50 failure and these independent variables was estimated using odds ratios (ORs) and 95% confidence intervals (95% CIs). In addition, the receiver operating characteristic (ROC) curve and the corresponding area under the curve (AUC) value were calculated to provide an index of validity for the multivariate logistic regression model.
We confirmed that the sample size of this study provided >90% power to detect an efficacy difference between biologic-naïve and experienced patients at month 6.
For all tests, a probability value (p value) of <0.05 was considered to indicate statistical significance. All calculations were performed using the PASW Statistics software program version 22 (SPSS Japan, Tokyo, Japan).
Results
Comparisons of baseline characteristics of CDAI50 responders and non-responders
Among the participants, 65 (57.5%) achieved a CDAI50 response within 6 months of tofacitinib therapy and had no relapses during the follow-up period (CDAI50 responders) (Table 1). Forty-eight patients (42.5%) failed to exhibit a CDAI50 outcome at month 6 (non-responders): 16 (14.2%) completed the 6-month tofacitinib therapy without achieving CDAI50; the remaining dropped out before the end of follow-up because of a lack or loss of efficacy [17 patients (15.0%)], adverse events [4 patients (3.5%)], and loss to follow-up [11 patients (9.7%)]. When compared with CDAI50 responders, non-responders more often had advanced stages of disease (stages III/IV, 68.8% versus 38.5%, p=0.002) and were more likely to have received previous biological therapy (85.4% versus 55.4%, p=0.001). In addition, non-responders used twice the number of biological agents (2.2 versus 1.1, p<0.001). RA duration was longer in non-responders than in responders, but this difference was marginal (13.1 years versus 9.6 years, p=0.048). MTX use was more often observed in CDAI50 responders (81.5% versus 60.4%, p=0.019), and rates of prednisolone use were higher in non-responders (41.7% versus 21.5%, p=0.024). There was no significant difference in other RA-related indexes, including positive rates of anti-citrullinated peptide antibodies, mean CDAI values at baseline, or rates of high CDAI patients between the two patient groups. Rates of previous treatment episodes with TNF inhibitors, tocilizumab, or abatacept were not significantly different between CDAI50 responders and non-responders (p=0.53). The reasons for discontinuation of previous biological therapy did not influence the achievement of CDAI50 (TNF inhibitors, p=0.24; tocilizumab, p=0.42; and abatacept, p=0.08).
Table 1.
Comparison of Baseline Characteristics between CDAI50 Responders and Non-responders after 6 Months of Tofacitinib Therapy.
| Total (n=113) |
CDAI50 responders* (n=65) |
Non-responders* (n=48) |
p† | |
|---|---|---|---|---|
| Age, years, mean (95% CI) | 63.7 (61.4-66.0) | 63.7 (60.6-66.9) | 63.7 (60.2-67.3) | 1.00 |
| Male/female | 26/87 | 17/48 | 9/39 | 0.38 |
| RA duration, years, mean (95% CI) | 11.1 (9.3-12.9) | 9.6 (7.2-12.0) | 13.1 (10.6 -15.7) | 0.048 |
| Anti-CCP (+), patient number (%) | 99 (87.6) | 58 (89.2) | 41 (85.4) | 0.58 |
| Stage III/IV, patient number (%) | 58 (51.3) | 25 (38.5) | 33 (68.8) | 0.002 |
| CDAI, mean (95% CI) | 24.5 (22.5-26.6) | 25.3 (22.3-28.3) | 23.4 (20.7-26.2) | 0.37 |
| High CDAI (>22), patient number (%) | 49 (43.4) | 28 (43.1) | >21 (43.8) | 1.000 |
| Concomitant MTX use, patient number (%) | 82 (72.6) | 53 (81.5) | 29 (60.4) | 0.019 |
| Concomitant PLS use, patient number (%) | 34 (30.1) | 14 (21.5) | 20 (41.7) | 0.024 |
| Previous biologic use, patient number (%) | 77 (68.1) | 36 (55.4) | 41 (85.4) | 0.001 |
| Number of agents per patient, mean (95% CI) | 1.6 (1.3-1.8) | 1.1 (0.8-1.4) | 2.2 (1.8-2.6) | <0.001 |
| ≥3 biological agents, patient number (%) | 26 (23.0) | 10 (15.4) | 16 (33.3) | 0.026 |
| Previous treatment episodes | ||||
| Total number of episodes | 183 | 76 | 107 | - |
| TNFi, episode number (%) | 104 (56.8) | 40 (52.6) | 64 (59.8) | 0.53‡ |
| Primary lack, episode number (%) | 21 (20.2) | 5 (12.5) | 16 (25) | 0.24§ |
| Secondary loss, episode number (%) | 66 (63.5) | 29 (72.5) | 36 (57.8) | - |
| Adverse events, episode number (%) | 17 (16.3) | 6 (15) | 11 (17.2) | - |
| TCZ, episode number (%) | 59 (32.2) | 28 (36.8) | 31 (29.0) | - |
| Primary lack, episode number (%) | 10 (16.9) | 5 (17.9) | 5 (16.1) | 0.42§ |
| Secondary loss, episode number (%) | 40 (67.8) | 17 (60.7) | 23 (74.2) | - |
| Adverse events, episode number (%) | 9 (15.3) | 6 (21.4) | 3 (9.7) | - |
| ABT, episode number (%) | 20 (10.9) | 8 (10.5) | 12 (11.2) | - |
| Primary lack, episode number (%) | 3 (15) | 2 (25) | 1 (8.3) | 0.08§ |
| Secondary loss, episode number (%) | 15 (75) | 4 (50) | 11 (91.7) | - |
| Adverse events, episode number (%) | 2 (10) | 2 (25) | 0 | - |
*CDAI50-responders were defined as patients who had achieved and maintained a CDAI50 response during 6 months of tofacitinib therapy. The other patients were classified as non-responders.
†Compared between CDAI50 responders and non-responders
‡Rates of previous treatment episodes with TNFi, TCZ, or ABT were compared between the two groups.
§Rates of primary lack, secondary loss, or adverse events were compared between the two groups.
RA: rheumatoid arthritis, anti-CCP: anti-citrullinated peptide antibodies, CDAI: clinical disease activity index, MTX: methotrexate, PSL: prednisolone, TNFi: tumor necrosis factor inhibitor, TCZ: tocilizumab, ABT: abatacept, CI: confidence interval
Multivariate logistic regression analyses
A multivariate logistic regression analysis was performed to evaluate the possible risk factors associated with the failure to achieve a CDAI50 response. RA duration, RA stages III/IV, CDAI>22, the concurrent use of MTX and prednisolone, and the previous use of biological agents were selected as independent variables. The analysis revealed that the previous use of biological agents was a strong risk factor for the failure to achieve CDAI50 (OR 4.48, 95% CI 1.73-11.63, p=0.002). The concurrent use of prednisolone was also a factor associated with CDAI50 failure, but its association was marginal (OR 2.40, 95% CI 1.01-5.71, p=0.047). The final logistic regression model yielded an AUC-ROC of 0.70 (95% CI 0.61-0.80, p<0.001), which showed that this model was moderately accurate.
Comparison of baseline RA characteristics and therapeutic outcomes between biologic-naïve and experienced patients
Regarding RA characteristics at baseline, advanced-stage RA was more frequently seen in patients who had shown inadequate response (biologic-experienced patients) than in biologic-naïve patients (66.2% versus 19.4%, p<0.001; Table 2). RA duration was also longer in the biologic-experienced group than in the biologic-naïve group (12.8 years versus 9.6 years, p=0.004). There was no significant difference in the mean CDAI values or rates of high CDAI between the two groups. MTX was more often used for biologic-naïve than biologic-experienced patients (100% versus 59.7%, p<0.001).
Table 2.
Baseline RA Characteristics and Month-6 Outcomes of Patients who Received Tofacitinib Therapy, Stratified by Previous Use of Biological Agents.
| Total (n=113) |
Biologic-naïve patients (n=36) |
Biologic-experienced patients (n=77) |
p* | |
|---|---|---|---|---|
| Baseline characteristics of RA | ||||
| Age, years, mean (95% CI) | 63.7 (61.4-66.0) | 61.0 (56.3-65.8) | 65.0 (62.4, 67.6) | 0.11 |
| Male/female | 26/87 | 6/30 | 20/57 | 0.34 |
| RA duration, years, mean (95% CI) | 11.7 (9.8-12.9) | 9.6 (5.3-13.8) | 12.8 (10.9-14.8) | 0.004 |
| Anti-CCP (+), patient number (%) | 99 (87.6) | 30 (83.3) | 69 (89.6) | 0.37 |
| Stage III/IV, patient number (%) | 58 (51.3) | 7 (19.4) | 51 (66.2) | <0.001 |
| CDAI, mean (95% CI) | 24.5 (22.5-26.6) | 26.5 (22.1-30.9) | 23.6 (21.4-25.9) | 0.20 |
| High CDAI (>22), patient number (%) | 49 (43.4) | 16 (44.4) | 33 (42.9) | 1.00 |
| Concomitant MTX use, patient number (%) | 82 (72.6) | 36 (100) | 46 (59.7) | <0.001 |
| Concomitant PSL use, patient number (%) | 34 (30.1) | 8 (22.2) | 26 (33.8) | 0.27 |
| Therapeutic outcomes at month 6 | ||||
| CDAI, mean (95% CI)† | 9.3 (7.5-11.1) | 4.8 (2.4-7.2) | 11.4 (9.1-17.7) | 0.001 |
| Dropout, patient number (%) | 32 (28.3) | 5 (13.9) | 27 (35.1) | 0.025 |
| Adverse events | 4 (3.5) | 2 (5.6) | 2 (2.6) | 0.59 |
| Lack or loss of efficacy | 17 (15.0) | 1 (2.8) | 16 (20.8) | 0.011 |
| Loss to follow-up | 11 (9.7) | 2 (5.6) | 9 (11.7) | 0.50 |
| Remission (CDAI≤2.8), patient number (%) | 24 (21.2) | 15 (41.7) | 9 (11.7) | 0.001 |
| Low CDAI (<2.8 and ≤10), patient number (%) | 40 (35.4) | 13 (36.2) | 27 (35.1) | 1.00 |
| Moderate CDAI (>10 and ≤22), patient number (%) | 14 (12.4) | 3 (8.3) | 11 (14.3) | 0.55 |
| High CDAI (>22), patient number (%) | 3 (2.7) | 0 | 3 (3.9) | 0.55 |
| Improvement rates of CDAI at month 6 | ||||
| CDAI improvement‡, patient number (%) | 69 (61.1) | 30 (83.3) | 39 (50.6) | 0.001 |
| CDAI50§ (minor response), patient number (%) | 65 (57.5) | 29 (80.6) | 36 (46.8) | 0.001 |
| CDAI70§ (moderate response), patient number (%) | 49 (43.4) | 25 (69.4) | 24 (31.2) | <0.001 |
| CDAI85§ (major response), patient number (%) | 36 (31.9) | 19 (52.8) | 17 (22.1) | 0.002 |
*Compared between biologic-naïve patients and biologic-experienced patients.
†For dropout patients, missing data were replaced by the last observed values.
‡Defined as MCID-based CDAI improvement, i.e., CDAI reduction >12 for patients starting with a high CDAI and >6 for those starting with a moderate CDAI.
§Defined as achieving and maintaining ≥50% improvement of CDAI (CDAI50), ≥70% (CDAI70), and ≥85% (CDAI85) during the 6-month tofacitinib therapy.
RA: rheumatoid arthritis, IR: inadequate response, anti-CCP: anti-citrullinated peptide antibodies, CDAI: clinical disease activity index, MCID: minimally clinically important difference, MTX: methotrexate, PSL: prednisolone, CI: confidence interval
At month 6, the mean CDAI values remained significantly higher in patients who experienced an inadequate response to previous biological therapy (11.4 versus 4.8, p=0.001) than in biologic-naïve patients. Remission rates were significantly increased in biologic-naïve patients compared with experienced patients (41.1% versus 11.7%, p=0.001), while rates of low, moderate, and high CDAI were similar between the biologic-naïve and experienced groups. Rates of dropout because of an adverse event or loss to follow-up were not significantly different between the groups, but the rates of discontinuation because of lack or loss of efficacy were significantly higher in the biologic-experienced group than in the biologic-naïve group (20.8% versus 2.8%, p=0.011). Biologic-naïve patients were more likely to achieve MCID-based CDAI improvement (83.3% versus 50.6%, p=0.001), CDAI50 (80.6% versus 46.8%, p=0.001), CDAI70 (69.4% versus 31.2%, p<0.001), and CDAI85 (52.8% versus 22.1%, p=0.002) responses than biologic-experienced patients.
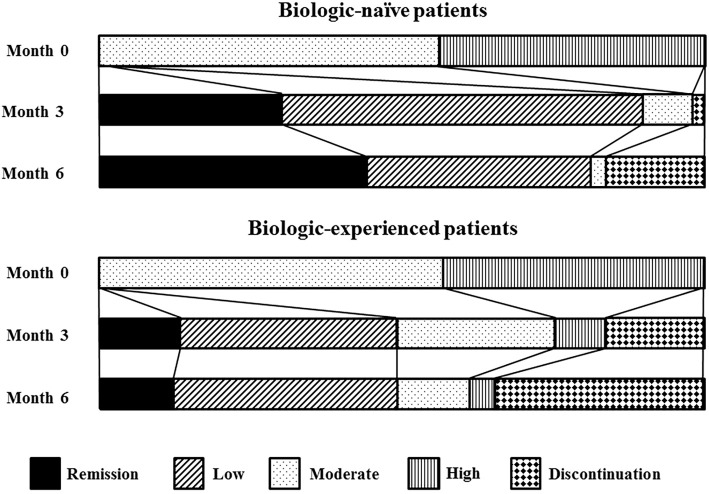
As shown in Figure, biologic-naïve patients achieved remission more rapidly than biologic-experienced patients. Discontinuation of tofacitinib therapy occurred much earlier in the biologic-experienced group than in the biologic-naïve group.
Figure.
Changes in CDAI rates during tofacitinib therapy for biologic-naïve patients and biologic-experienced patients. CDAI: clinical disease activity index
The four patients who discontinued tofacitinib by month 6 because of adverse events included two biologic-experienced patients who were hospitalized for severe lymphopenia, one biologic-naïve patient who developed organizing pneumonia, and one biologic-naïve patient who developed systemic eruption.
Discussion
In this prospective follow-up study, biologic-naïve RA patients were more likely to achieve a CDAI50 response at month 6 than inadequate responders to previous biological therapy. The remission rate was also higher and achieved more rapidly in biologic-naïve patients. The rate of tofacitinib discontinuation because of adverse events was similar between both groups. A higher number of previous biological agents was significantly associated with failure of tofacitinib therapy. The type of previous biological agents and the reason for previous discontinuation were not significantly different between CDAI50-repsponders and non-responders. In a multivariate logistic regression analysis, we confirmed that a previous use of biological agents was a strong risk factor for failure to achieve a CDAI 50 response. The current use of prednisolone was also identified as a factor associated with the tofacitinib treatment failure, although its association was marginal. This association may be explained by the trend of rheumatologists often prescribing prednisolone for patients who have experienced an inadequate response during treatment with biological agents.
RA patients with biologic failure may represent the main target for tofacitinib therapy. In the present study, the rates of CDAI50 response and remission at month 6 were 46.8% and 11.7%, respectively, in RA patients with inadequate response to previous biological agents. Similar response rates were reported in the phase III ORAL Step study, which reported that for active RA patients with an inadequate response to TNF inhibitors, 51.5% achieved an ACR20 response, which is considered to correspond to CDAI50, after 6 months of tofacitinib therapy (5 mg twice a day) in combination with MTX. The DAS28 remission rate was 8.2% at month 6 (25). Data on patient-reported outcomes (PROs) from this trial study also showed that tofacitinib therapy produced significant, clinically meaningful improvements in multiple PROs in patients with a previous inadequate response to TNF inhibitors (34). A recent pooled analysis showed, using data from phase II and phase III trials, that tofacitinib (5 mg twice-daily regimen) as monotherapy or with background MTX was effective in reducing signs and symptoms of RA in biologic-naïve patients and patients who had failed previous treatment with biological agents. The efficacy parameters at month 6 were not significantly different in both groups (ACR20, 51.9% versus 45.6%; DAS28 remission, 7.1% versus 7.2%, overlapping 95% CIs), although neither direct comparisons nor formal statistical analyses were performed (35). Given the present and those previous findings, tofacitinib may prove an effective treatment option for patients with an inadequate response to biological agents. However, approximately half of such patients failed to achieve CDAI50 or ACR20 improvements at month 6 of tofacitinib therapy.
For MTX-refractory biologic-naïve patients, the phase III ORAL-Standard and ORAL-Scan studies showed that 51.5% reached an ACR20 response at month 6 of tofacitinib therapy (5 mg twice a day) with background MTX, and the remission rate was 6.2% at the same point. Progression of structure damage was also prevented by this therapy (22, 26). These patients also reported improvements across a broad range of PROs (36). In the present study, 80.6% of MTX-refractory, biologic-naïve patients reached CDAI50, and 41.7% achieved and maintained remission throughout the 6-month tofacitinib therapy. The efficacy parameters were different between our data and others, which may be explained by differences in MTX dosing used for RA treatment between Japan and Western countries. A phase II study of tofacitinib conducted in Japan revealed that the ACR20 response rate at month 12 was 96.3% in MTX-refractory RA patients who had received a 5 mg twice-daily regimen in combination with background MTX (37). At that time, the maximum MTX dosage approved in Japan was 8 mg weekly. Although a dose escalation to 16 mg weekly has recently been approved, 6 to 8 mg weekly dosing remains a routine MTX regimen for RA patients in Japan. In contrast, in the ORAL Standard and ORAL Scan studies, participants were receiving 7.5 to 25 mg of MTX weekly (22, 26).
The positioning of tofacitinib in treatment approaches to MTX-refractory RA patients has been debated. In the 2013 update of the EULAR recommendations, it was stated that tofacitinib may be considered after biological treatment has failed. The EULAR Executive Committee was not convinced that tofacitinib has a similar safety profile, especially with regard to its long-term safety, to tocilizumab or other biological agents (1). The 2015 ACR guidelines stated that, for established RA patients with moderate or high disease activity despite monotherapy with disease-modifying antirheumatic drugs, tofacitinib therapy is one possible treatment option, although they also mentioned that the long-term safety of this drug is unclear at present (2). This year, however, Cohen et al. reported long-term safety data on tofacitinib therapy (exposures up to 8.5 years) obtained by following all patients who had participated in previous phase I, II, and III trial studies as well as long-term extension studies. In that study, the incidence rates of serious infectious events with tofacitinib were generally consistent with those of biological agents; adverse events with tofacitinib were also stable over time, and no new safety signals were observed compared with previous tofacitinib studies (38). In the present study, tofacitinib therapy was more effective in the treatment of biologic-naïve RA patients than of those who had failed previous biological therapy. Considering the oral availability of tofacitinib and its lower immunogenicity than biological agents, it may be useful to consider tofacitinib more often to treat MTX-refractory, biologic-naïve RA patients.
The main limitation of the present study is its small sample size, which prevented us from drawing a more definitive conclusion. However, this is the first study that directly compares the therapeutic outcomes of tofacitinib between biologic-naïve and biologic-experienced patients. In addition, the present study included a large number of previous treatment episodes with tocilizumab compared with other studies. Second, since real-world studies follow less restrictive methodological standards than phase III trials, the inherent methodological problems can hamper the interpretation of results. Increased variance due to many types of cofounders at baseline should be considered carefully. In this study, biologic-experienced patients had a longer RA duration, more advanced RA stages, and less frequent use of MTX than biologic-naïve patients. To address this problem, we performed a multivariate logistic regression analysis including these possible confounders and showed that the previous use of biological agents was a strong risk factor for failure to achieve CDAI50. In addition, the AUC-ROC analysis confirmed the validity of the final model in our multivariate logistic regression analysis.
In conclusion, tofacitinib is an effective treatment option for MTX-refractory RA patients in daily clinical practice. However, the present study showed that its impact on therapeutic outcomes is significantly lower in patients who have failed previous treatment with biological therapy than in biologic-naïve patients. Further studies are needed to determine the positioning of tofacitinib in the treatment of MTX-refractory RA patients.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73: 492-509, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 68: 1-26, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Patel AM, Moreland LW. Interleukin-6 inhibition for treatment of rheumatoid arthritis: a review of tocilizumab therapy. Drug Des Devel Ther 4: 263-278, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther 33: 679-707, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orme ME, Macgilchrist KS, Mitchell S, Spurden D, Bird A. Systematic review and network meta-analysis of combination and monotherapy treatments in disease-modifying antirheumatic drug-experienced patients with rheumatoid arthritis: analysis of American College of Rheumatology criteria scores 20, 50, and 70. Biologics 6: 429-464, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nam JL, Ramiro S, Gaujoux-Viala C, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 73: 516-528, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Aaltonen KJ, Virkki LM, Malmivaara A, et al. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS One 7: e30275, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology (Oxford) 55: 523-534, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 353: 1114-1123, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Reino JJ, Carmona L, Group B. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther 8: R29, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 67: 1516-1523, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 374: 210-221, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Schiff M, Pritchard C, Huffstutter JE, et al. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: the ARRIVE trial. Ann Rheum Dis 68: 1708-1714, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiff M, Keiserman M, Codding C, et al. Clinical response and tolerability to abatacept in patients with rheumatoid arthritis previously treated with infliximab or abatacept: open-label extension of the ATTEST Study. Ann Rheum Dis 70: 2003-2007, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rendas-Baum R, Wallenstein GV, Koncz T, et al. Evaluating the efficacy of sequential biologic therapies for rheumatoid arthritis patients with an inadequate response to tumor necrosis factor-alpha inhibitors. Arthritis Res Ther 13: R25, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Pan SM, Scherer A, Gabay C, Finckh A. Differential drug retention between anti-TNF agents and alternative biological agents after inadequate response to an anti-TNF agent in rheumatoid arthritis patients. Ann Rheum Dis 71: 997-999, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Smolen JS, Kay J, Matteson EL, et al. Insights into the efficacy of golimumab plus methotrexate in patients with active rheumatoid arthritis who discontinued prior anti-tumour necrosis factor therapy: post-hoc analyses from the GO-AFTER study. Ann Rheum Dis 73: 1811-1818, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis 68: 25-32, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genovese MC, Schiff M, Luggen M, et al. Efficacy and safety of the selective co-stimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti-tumour necrosis factor therapy. Ann Rheum Dis 67: 547-554, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg JD, Reed G, Decktor D, et al. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann Rheum Dis 71: 1134-1142, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann Rheum Dis 69: 817-821, 2010. [DOI] [PubMed] [Google Scholar]
- 22.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 367: 508-519, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 367: 495-507, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Kremer J, Li ZG, Hall S, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 159: 253-261, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 381: 451-460, 2013. [DOI] [PubMed] [Google Scholar]
- 26.van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 65: 559-570, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 370: 2377-2386, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62: 2569-2581, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31: 315-324, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 64: 640-647, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 23: S100-S108, 2005. [PubMed] [Google Scholar]
- 32.Aletaha D, Martinez-Avila J, Kvien TK, Smolen JS. Definition of treatment response in rheumatoid arthritis based on the simplified and the clinical disease activity index. Ann Rheum Dis 71: 1190-1196, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Curtis JR, Yang S, Chen L, et al. Determining the minimally important difference in the clinical disease activity index for improvement and worsening in early rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 67: 1345-1353, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strand V, Burmester GR, Zerbini CA, et al. Tofacitinib with methotrexate in third-line treatment of patients with active rheumatoid arthritis: patient-reported outcomes from a phase III trial. Arthritis Care Res (Hoboken) 67: 475-483, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Charles-Schoeman C, Burmester G, Nash P, et al. Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs. Ann Rheum Dis 75: 1293-1301, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strand V, van Vollenhoven RF, Lee EB, et al. Tofacitinib or adalimumab versus placebo: patient-reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatology (Oxford) 55: 1031-1041, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka Y, Takeuchi T, Inoue E, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan: one-year clinical outcomes (RECONFIRM-2). Mod Rheumatol 18: 146-152, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 76: 1253-1262, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]