Abstract
Accessory spleen (AS) is common anomaly, and 20% of AS cases occur in the pancreatic tail. An intrapancreatic AS can be difficult to distinguish from pancreatic neoplasms. In most cases, an AS is described as a hypervascular and solitary tumor, but an AS sometimes takes other forms. We herein report a rare case of an intrapancreatic AS with temporal changes in its appearance after splenectomy, which mimicked aspects of pancreatic cancer. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and 99mTc sulfur colloid scintigraphy were useful for the diagnosis.
Keywords: accessory spleen, EUS-FNA, splenectomy
Introduction
An accessory spleen (AS) is a common congenital anomaly which is sometimes detected incidentally. It is present in 10-15% of the general population; in about 16% of cases, the AS is located in the pancreas (1,2). An intrapancreatic AS has the appearance of a hypervascular and solitary lesion. The differential diagnoses include neuroendocrine neoplasms, metastatic tumors, and solid pseudopapillary tumors (3,4). It is difficult to diagnose an intrapancreatic AS because it sometimes shows uncharacteristic imaging findings. We herein report a rare case involving an intrapancreatic AS that was difficult to distinguish from pancreatic cancer.
Case Report
A 62-year-old man was diagnosed with a pancreatic tumor that was detected by contrast-enhanced computed tomography (CECT). He had undergone hepatic resection and splenectomy for hepatocellular carcinoma and hypersplenism one year previously. The tumor was described as a 2-cm hypovascular mass that had slowly grown after the previous surgeries (Fig. 1, 2). In the arterial phase, the tumor was visualized as a hypovascular lesion on the pancreatic parenchyma, and the imaging effect did not change until the equilibrium phase (Fig. 1). The tumor had been observed as an intrapancreatic AS since before splenectomy, because its imaging characteristics were typical of AS, including hypervascularity, small size, and a circular lesion. It had undergone gradual growth and the hemodynamics had changed from hypervascular to hypovascular (Fig. 2). On magnetic resonance imaging (MRI), the tumor showed low signal intensity on T1-weighted images, faintly high signal intensity on T2-weighted images, high signal intensity on diffusion-weighted MRI, and a low signal intensity on an apparent diffusion coefficient (ADC) map (Fig. 3). No uptake was observed on positron emission tomography imaging CT (PET/CT) (Fig. 4). The laboratory data on admission showed that the patient's carcinoembryonic antigen (CEA) level was elevated (Table). We could not deny pancreatic cancer in view of the course. Then, endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) was performed. The tumor was visualized as a low echoic lesion without cystic change by EUS. EUS-FNA revealed the presence of lymphocytes (Fig. 5); however, immunohistochemical staining revealed that the cells were negative for CD8. Thus, 99mTc sulfur colloid scintigraphy was performed to differentiate the tumor as a malignant tumor and the uptake of the tracer was observed within the mass (Fig. 6). These results indicated that the tumor was an intrapancreatic AS with compensatory growth after splenectomy. The patient was followed closely without treatment, and the tumor has shown no change after one year.
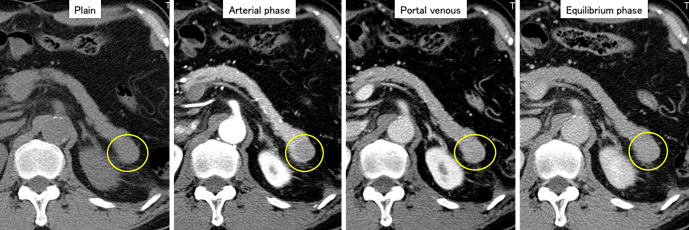
Figure 1.
CECT showed a hypovascular tumor in the pancreatic tail (yellow circle). The tumor was visualized as a hypovascular lesion on the pancreatic parenchyma, and the imaging effect did not change from the arterial phase to the equilibrium phase. CECT: contrast-enhanced computed tomography
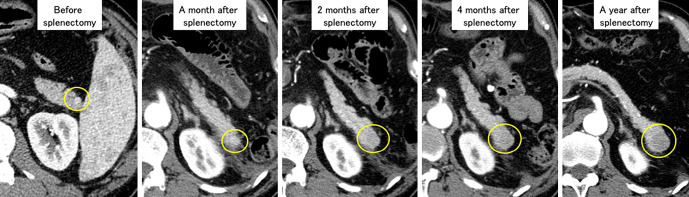
Figure 2.
A series of arterial-phase CT images. The tumor (yellow circle) grew slowly and the hemodynamics changed from hypervascular to hypovascular after splenectomy.
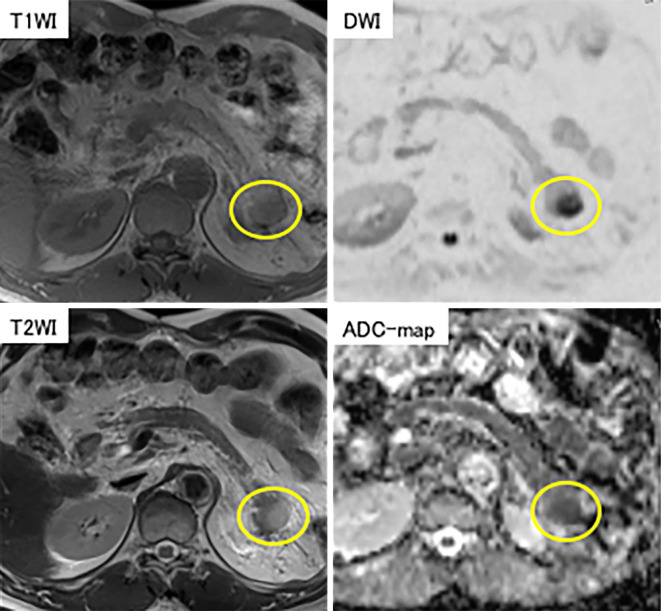
Figure 3.
MRI showed a nodule with low signal intensity on T1-weighted images and faintly high signal intensity on T2-weighted images (yellow circle).
Figure 4.
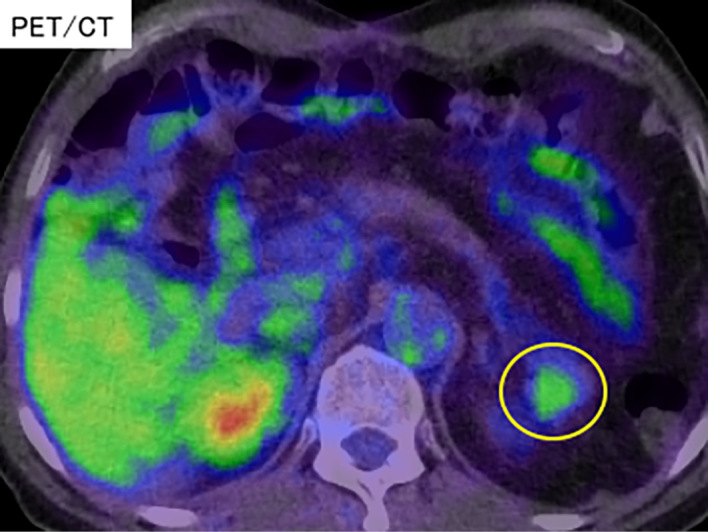
A PET/CT image; no abnormal uptake was observed in the tumor at 90 minutes after the administration of FDG (yellow circle). PET/CT: positron emission tomography imaging CT, FDG: fluorodeoxyglucose
Table.
Laboratory Data on Admission.
| Blood count | AST | 32 | IU/L | Infection | ||||
| WBC | 7.97 | ×103/μL | ALT | 25 | IU/L | HBs Ag | (-) | |
| RBC | 2.98 | ×106/μL | ALP | 438 | IU/L | HBs Ab | (-) | |
| Ht | 28.1 | % | γGTP | 41 | IU/L | HCV Ab | (-) | |
| Hb | 8.8 | g/dL | ChE | 186 | IU/L | |||
| Plt | 33.9 | ×104/μL | LDH | 225 | IU/L | Tumor marker | ||
| Cr | 0.77 | mg/dL | AFP | 27 | ng/mL | |||
| Chemistry | BUN | 8.3 | mg/dL | PIVKA-2 | 2.6 | mAU/mL | ||
| TP | 7.3 | g/dL | Na | 138 | mEq/L | CEA | 14.6 | ng/mL |
| Alb | 3.9 | g/dL | K | 4.3 | mEq/L | CA19-9 | 12 | U/mL |
| T-BIL | 0.65 | mg/dL | Cl | 106 | mEq/L | DUPAN-2 | 82 | U/mL |
| D-BIL | 0.12 | mg/dL | CRP | 0.5 | mg/dL | SPAN-1 | <10 | ng/mL |
WBC: White blood cell, RBC: Red blood cell, Ht: Hematocrit, Hb: Hemoglobin, Plt: Platelet, TP: Total protein, Alb: Albumin, T-Bil: Total bilirubin, D-Bil: Direct bilirubin, AST: Asparate transaminase, ALT: Alanine transaminase, ALP: Alkaline phosphatase, γGTP: gamma guanosine triphosphate, ChE: Cholinesterase, LDH: Lactate dehydrogenase, Cr: Creatinine, BUN: Blood urea nitrogen, Na: Natrium, K: Kalium, Cl: Crawl, CRP: C-reactive protein, HBs Ag: Hepatitis B surface antigen, HBs Ab: Hepatitis B surface antibody, HCV Ab: Hepatitis C virus antibody, AFP: Alpha phetoprotein, PIVKA-2: protein induced by Vitamin K absence or antagonists-II, CEA: Carcinoembryonic antigen, CA19-9: Carbohydrate antigen 19-9, DUPAN-2: duke pancreatic monoclonal antigen type 2
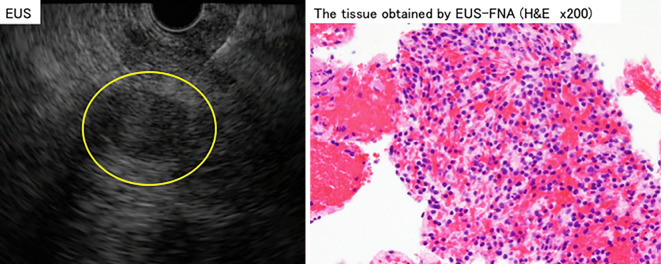
Figure 5.
Left: A low echoic lesion of 2 cm in length in the pancreatic tail was detected by EUS (yellow circle). The lesion did not include a cystic lesion. It was punctured with a 22-gauge needle and tumor tissue was collected. Right: Hematoxylin and Eosin staining showed lymphocytes, as is observed in splenic tissue. EUS: endoscopic ultrasound, EUS-FNA: endoscopic ultrasound-guided fine-needle aspiration
Figure 6.
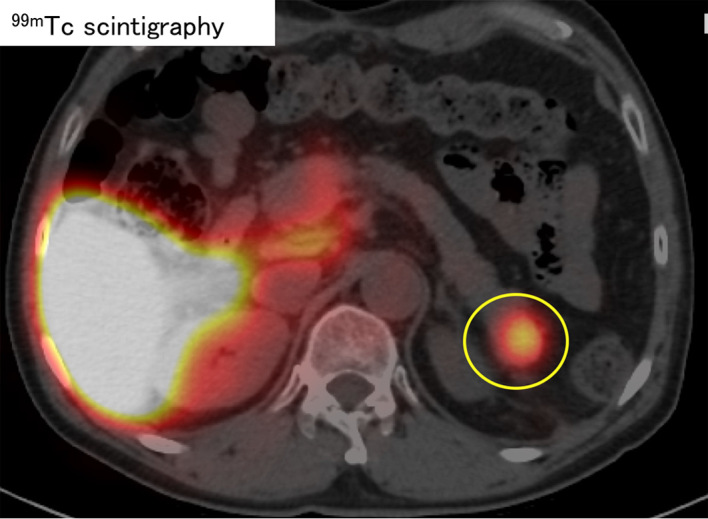
99mTc sulfur colloid scintigraphy showed a high uptake into the tumor of the pancreatic tail (yellow circle).
Discussion
An AS is a common congenital anomaly, and an intrapancreatic AS can be confusing to diagnose because it take various forms; it sometimes appears similar to a pancreatic neoplasm (5-7). It is widely known that epidermoid cysts may arise in association with an intrapancreatic AS; however, in the present case, a growing tumor was detected in the pancreatic tail, and the hemodynamic changes in the tumor vessels developed during follow-up. An AS generally tends to be a hypervascular lesion, but the tumor was a hypovascular lesion without a cystic lesion that could be detected by MRI, and which showed slow growth. Additionally, the elevated CEA level complicated the diagnosis. Previous studies have reported the utility of EUS-FNA in the diagnosis of an AS (7,8); we also performed EUS-FNA to make a definitive diagnosis. EUS-FNA revealed that it was a lymphocyte-rich tumor, but did not detect CD8-positive cells, which prove the presence of endothelial cells of the splenic sinus. 99mTc sulfur colloid scintigraphy has also been reported to be effective for differentiating tumors from an AS (9). In this case, the uptake of 99mTc by the tumor was detected, and we concluded that the tumor was actually an AS. Some studies have reported the compensated growth of an AS after splenectomy; we therefore considered that the imaging findings of this tumor were caused by hemodynamic changes that occurred due to splenectomy (10,11). However, the reason for the hypovascular change remains unclear. We suspected that epidermoid cysts arose from the AS; however, no cystic lesions were detected by MRI or EUS. The patient was followed for one year without significant changes. These findings suggest that an AS can turn grow and change from hypervascular to hypovascular after splenectomy, and a careful diagnosis must be made using various diagnostic methods.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Halpert B, Alden ZA. Accessory spleens in or at the tail of the pancreas. A survey of 2,700 additional necropsies. Arch Pathol 77: 652-654, 1964. [PubMed] [Google Scholar]
- 2.Takayama T, Shimada K, Inoue K, Wakao F, Yamamoto J, Kosuge T. Intrapancreatic accessory spleen. Lancet 344: 957-958, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Hayward I, Mindelzun RE, Jeffrey RB. Intrapancreatic accessory spleen mimicking pancreatic mass on CT. J Comput Assist Tomogr 16: 984-985, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Schreiner AM, Mansoor A, Faigel DO, Morgan TK. Intrapancreatic accessory spleen: mimic of pancreatic endocrine tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy. Diagn Cytopathol 36: 262-265, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Cho JH. Rare nonneoplastic cysts of pancreas. Clin Endosc 48: 31-38, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugiyama Y, Kawamoto T, Sasajima J, Koizumi K, Karasaki H, Mizukami Y. A rare case of epidermoid cyst in the pancreatic tail invaginated from the splenic hilum: the long-term changes in the imaging findings. Intern Med 55: 3591-3594, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez E, Netto G, Li QK. Intrapancreatic accessory spleen: a case report and review of literature. Diagn Cytopathol 41: 466-469, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Saunders TA, Miller TR, Khanafshar E. Intrapancreatic accessory spleen: utilization of fine needle aspiration for diagnosis of a potential mimic of a pancreatic neoplasm. J Gastrointest Oncol 7: S62-S65, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castellani M, Cappellini MD, Cappelletti M, et al. Tc-99m sulphur colloid scintigraphy in the assessment of residual splenic tissue after splenectomy. Clin Radiol 56: 596-598, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Zhu HX, Lou WH, Kuang TT, Wang DS. Post-splenectomy intrapancreatic accessory spleen mimicking endocrine tumor of the pancreas. Int J Surg Case Rep 5: 1151-1153, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.d'Amico A, Cofalik A, Przeorek C, et al. Role of nuclear medicine imaging in differential diagnosis of accessory spleens in patients after splenectomy. Pol J Radiol 77: 68-71, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]