Abstract
Primary hepatic angiosarcoma is a rare tumor originating from endothelial cells in the liver and accounts for approximately 1% of all hepatic malignant tumors. It is difficult to diagnose due to the lack of specific symptoms or tumor markers. No effective treatment exists, but complete surgical resection may achieve a good outcome. Since most primary hepatic angiosarcomas are already at an advanced stage at diagnosis, few reports describe tumors smaller than 2 cm. We report a case of surgery for a 1.7-cm sized primary hepatic angiosarcoma. Further studies are required to improve the preoperative diagnosis of primary hepatic angiosarcoma.
Keywords: primary hepatic angiosarcoma, hemangiosarcoma, hepatosarcoma, surgical resection, diagnosis
Introduction
Primary hepatic angiosarcoma is a rare tumor that originates from the endothelial cells in the liver. These tumors account for approximately 1% of all primary hepatic carcinomas and are characterized by a highly malignant nature and rapid progression (1,2). They are related to exposure to carcinogens such as polyvinyl chloride and arsenic (3). However, most primary hepatic angiosarcomas are unexpectedly found in patients without these risk factors and with no specific symptoms or tumor marker aberrations (4). As a further complication, most patients with hepatic angiosarcoma present at an advanced stage and are unresectable at the time of the diagnosis. In 2016, a review of the literature on primary hepatic angiosarcoma showed that unresectable cases have a poorer prognosis in comparison to operable cases (5). According to the report, the median tumor diameter was 7.24 cm (range 2.4-20.0). We herein report the case of a 73-year-old woman with a 1.7-cm primary hepatic angiosarcoma that was diagnosed at the time of surgery.
Case Report
A 73-year-old woman had been diagnosed with diabetes eleven years previously. She underwent abdominal ultrasonography for screening purposes, and the examination indicated the presence of a liver tumor. Additional blood biochemistry revealed increased tumor marker levels. She was admitted to our hospital for a detailed investigation of her liver tumor.
The results of the blood tests on admission are shown in the Table. The levels of γ-glutamyl transpeptidase and creatinine were elevated. The blood tests also revealed the following findings: carcinoembryonic antigen, 2.7 ng/mL; carbohydrate antigen 19-9, 50.5 U/mL; alpha fetoprotein, 2.6 ng/mL; protein induced by the absence of vitamin K or antagonist II, 19 mAU/mL; s-pancreas-1 antigen, 40.4 U/mL; and Duke pancreatic monoclonal antigen type-2, 163 U/mL. A serological analysis revealed that the patient was negative for hepatitis B virus antigen and hepatitis C virus antibodies.
Table.
The Patient’s Laboratory Data.
| Parameter, units | Patient | Reference values |
|---|---|---|
| Blood counts | ||
| WBC, ×1,000/μL | 5.9 | 3.5-8.5 |
| RBC, ×10,000/μL | 405 | 370-490 |
| Hb, g/dL | 12.3 | 11.5-15.0 |
| Platelets, ×10,000/μL | 16.5 | 15.0-35.0 |
| Biochemistry and coagulation | ||
| Alb, g/dL | 4.3 | 3.7-5.5 |
| AST, U/L | 32 | 13-33 |
| ALT, U/L | 27 | 6-27 |
| LDH, U/L | 200 | 119-229 |
| ALP, U/L | 346 | 115-359 |
| γ-GTP, U/L | 202 | 10-47 |
| T-bil, mg/dL | 0.32 | 0.30-1.20 |
| BUN, mg/dL | 13.7 | 8.0-22.0 |
| Cr, mg/dL | 0.73 | 0.40-0.70 |
| CRP, mg/dL | 0.16 | 0.00-0.30 |
| PT, % | 100 | 80-120 |
| Tumor markers | ||
| CEA, ng/mL | 2.7 | 0.0-5.0 |
| CA19-9, U/mL | 50.5 | 0.0-37.0 |
| AFP, ng/mL | 2.6 | 0.0-10.0 |
| PIVKA-II, mAU/mL | 19 | 0.0-40.0 |
| Span-1, U/mL | 40.4 | 0.0-30.0 |
| DUPAN-2, U/mL | 163 | 0-150 |
CRP: C-reactive protein, PT: prothrombin time, CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9, AFP: alpha-fetoprotein, PIVKA II: protein induced by vitamin K absence or antagonist II, Span-1: s-pancreas-1 antigen, DUPAN-2: Duke pancreatic monoclonal antigen type-2
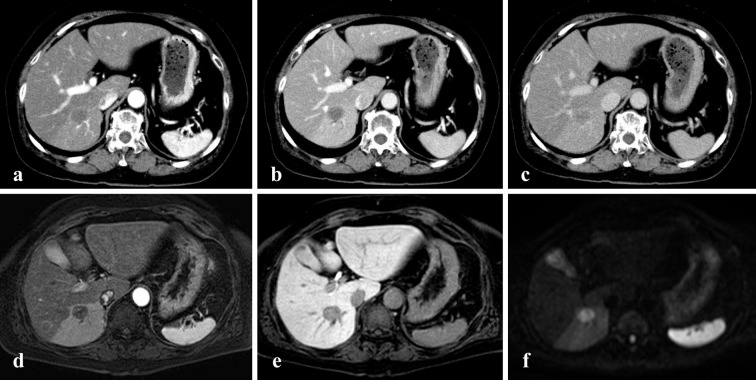
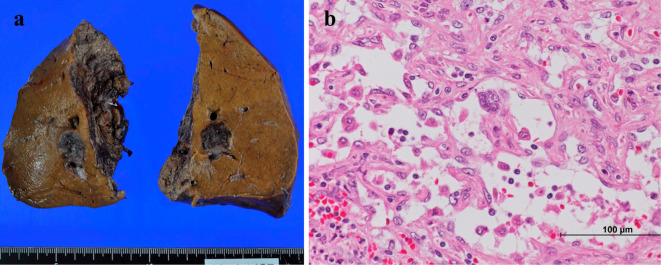
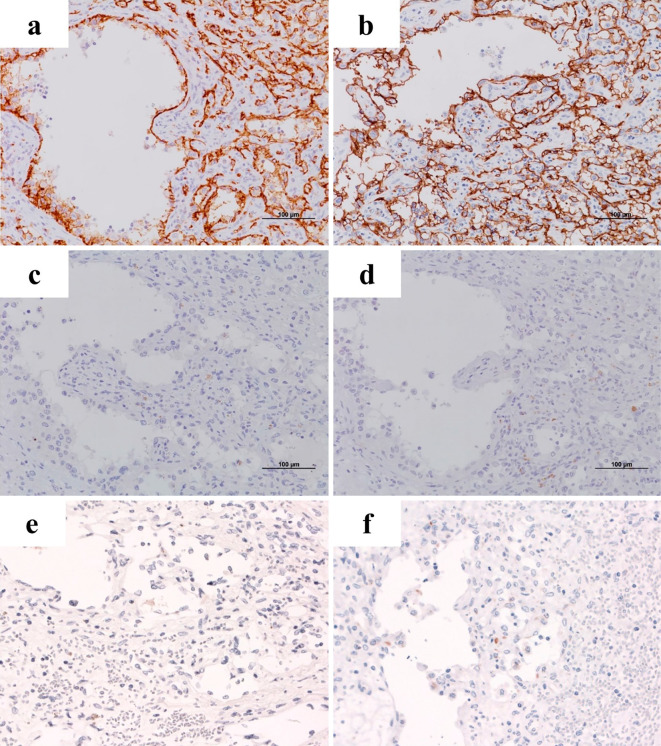
A hypoechoic mass measuring 18 mm in diameter was detected in the right lobe on abdominal ultrasound. On contrast-enhanced ultrasound, the tumor appeared as an unenhanced area between the vascular and the post vascular phases. Unenhanced and three-phase contrast-enhanced computed tomography images were obtained. The images revealed a hepatic tumor of approximately 20 mm in size in the right lobe without enhancement from the arterial phase to the delayed phase (Fig. 1). Magnetic resonance imaging revealed that the tumor showed a low intensity signal on T1-WI, a medium high intensity signal on T2-WI, and a high signal intensity on diffusion weighted imaging. The dynamic study also revealed a single non-enhanced tumor, 2 cm in diameter, in the right hepatic lobe (Fig. 1). These imaging findings indicated no metastatic disease. As the differential diagnoses of this non-enhanced mass, we considered intrahepatic cholangiocarcinoma, poorly differentiated hepatocellular carcinoma, a metastatic liver tumor, and a rare malignant tumor. Esophagogastroduodenoscopy and total colonoscopy revealed no malignant findings. Resection was performed in an attempt to preserve the liver function. The macroscopic examination revealed a dark red-brown nodular lesion of 1.7×1.7×1.7 cm in size. The microscopic findings revealed disordered atypical vessels and spindle-shaped neoplastic cells with marked nuclear atypicality (Fig. 2). The atypical vessels and spindle-shaped cells were positive for CD31 and CD34, supporting the diagnosis of a vascular tumor (Fig. 3). They were also negative for Hep-par 1, glypican 3, AE1/AE3, CK7, and CAM5.2; thus, intrahepatic cholangiocarcinoma and hepatocellular carcinoma were excluded. Factor VIII was partially expressed, and the Ki-67 proliferative index was almost 15%. Based on the pathological findings we diagnosed the patient with primary hepatic angiosarcoma.
Figure 1.
Computed tomography and magnetic resonance imaging (MRI) of the abdomen (transverse scan). The lesion showed no enhancement in the arterial phase (a), portal vein phase (b), or delayed phase (c). Dynamic MRI also revealed a single non-enhanced tumor in the arterial phase (d) and hepatocellular phase (e). The tumor showed a high signal intensity on diffusion weighted imaging (f).
Figure 2.
The macroscopic and histological findings. A macroscopic image of the 1.7-cm sized tumor of the right lobe of the liver (a) and the histological features (b) of the tumor (Hematoxylin and Eosin staining; original magnification, ×400).
Figure 3.
Immunohistochemical staining. The hepatic angiosarcoma components were positive for CD31 (a) and CD34 (b), negative for Hep-par 1 (c), glypican 3 (d), AE1/AE3 (e), and CK7 (f) (original magnification, ×200).
Discussion
Primary hepatic angiosarcoma is a rare malignant tumor that accounts for approximately 1% of all primary liver tumors (1,2). Patients with primary hepatic angiosarcoma often show non-specific symptoms, such as weight loss, fatigue, abdominal pain, and fever (2,4). Most primary hepatic angiosarcomas are detected at an advanced stage, and patients may be asymptomatic at the early stages, such as in our case.
Primary hepatic angiosarcoma is diagnosed based on the results of a histopathological examination. Histologically, primary hepatic angiosarcoma is composed of spindle-shaped and polyhedral cells, which demonstrate various patterns of vascular channels. Immunohistochemical markers such as CD31, CD34, and Factor VIII are used in making a diagnosis of angiosarcoma (6-8). In this case, the mass expressed CD31, CD34, and partially expressed Factor VIII. Most primary hepatic angiosarcomas show multiple nodules and dominant masses with necrosis and hemorrhage on computed tomography and magnetic resonance imaging. Contrast-enhanced dynamic studies have a primary role in the differential diagnosis of liver lesions. Most primary hepatic angiosarcomas show hypoattenuation, and some tumors show irregularities or ring enhancement (6,9,10). The imaging findings include necrosis and hemorrhage in the tumor complexes. In this case, the tumor showed a relatively uniform lesion on computed tomography and magnetic resonance imaging. A dynamic study also revealed a single non-enhanced tumor. There were no findings indicating necrosis or hemorrhage, which may have been due to the small size of the tumor. It is difficult to diagnose hepatic angiosarcoma accurately due to the lack of specific imaging findings. Although we considered more than one type of malignant tumor in the differential diagnoses, the examinations that were performed before surgery did not confirm the diagnosis. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma are common primary liver malignancies (11,12). Regarding the differential diagnoses of small hepatic nodules, Byrnes et al. reported that interval growth and rim enhancement of arterially enhancing nodules of <2 cm in size were associated with malignancy, and that most arterially enhancing nodules were benign (13). However, there was a possibility that the hypovascular tumor in this case was intrahepatic cholangiocarcinoma, which is usually fatal due to the lack of an effective non-surgical therapy. In our case, the patient made satisfactory progress after surgical treatment.
The clinical course of primary hepatic angiosarcoma is aggressive and the prognosis is poor in inoperable cases. Zheng summarized 25 articles from January 2000 to December 2012 and found that the median survival time in 64 cases of hepatic angiosarcoma was only 5 months (14).
No effective chemotherapy regimen has been established to date, and complete surgical resection of the tumor is considered to be the only curative treatment; unresectable cases are usually fatal (14,15). Some reports have described an improved prognosis after surgical resection (5,16,17). Huang et al. large patient populations and demonstrated a significant survival benefit of surgical treatment for stage I hepatic angiosarcoma. They reported that the prognosis of patients who underwent surgical treatment (1- and 3-year survival rates: 80.0±10.3% and 65.5±12.6%, respectively) was significantly better than that of patients who received non-operative treatment (1- and 3-year survival rates: 50.0±15.8% and 10.0±9.5%, respectively) in the early stages of the disease (17). However, most primary hepatic angiosarcomas are unresectable at the time of the diagnosis, and they are of considerable size, even in resectable cases. While some studies have reported the surgical treatment of primary hepatic angiosarcoma of 2.4-20.0 cm in size, few reports have described surgery for tumors of <2 cm in size (5,6,17-20). The present patient survived for more than eight months after the diagnosis without recurrence. In conclusion, we reported the surgical treatment of a 1.7 cm tumor, which is one of the smallest primary hepatic angiosarcomas described in the literature.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Kim HR, Rha SY, Cheon SH, Roh JK, Park YN, Yoo NC. Clinical features and treatment outcomes of advanced stage primary hepatic angiosarcoma. Ann Oncol 20: 780-787, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Molina E, Hernandez A. Clinical manifestations of primary hepatic angiosarcoma. Dig Dis Sci 48: 677-682, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bolt HM. Vinyl chloride-a classical industrial toxicant of new interest. Crit Rev Toxicol 35: 307-323, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Locker GY, Doroshow JH, Zwelling LA, Chabner BA. The clinical features of hepatic angiosarcoma: a report of four cases and a review of the English literature. Medicine (Baltimore) 58: 48-64, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Huang IH, Wu YY, Huang TC, Chang WK, Chen JH. Statistics and outlook of primary hepatic angiosarcoma based on clinical stage. Oncol Lett 11: 3218-3222, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu YP, Chen YM, Matro E, et al. Primary hepatic angiosarcoma: A report of two cases and literature review. World J Gastroenterol 21: 6088-6096, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bioulac-Sage P, Laumonier H, Laurent C, Blanc JF, Balabaud C. Benign and malignant vascular tumors of the liver in adults. Semin Liver Dis 28: 302-314, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Huang NC, Wann SR, Chang HT, Lin SL, Wang JS, Guo HR. Arsenic, vinyl chloride, viral hepatitis, and hepatic angiosarcoma: a hospital-based study and review of literature in Taiwan. BMC Gastroenterol 11: 142, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyama T, Fletcher JG, Johnson CD, Kuo MS, Notohara K, Burgart LJ. Primary hepatic angiosarcoma: findings at CT and MR imaging. Radiology 222: 667-673, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Bruegel M, Muenzel D, Waldt S, Specht K, Rummeny EJ. Hepatic angiosarcoma: cross-sectional imaging findings in seven patients with emphasis on dynamic contrast-enhanced and diffusion-weighted MRI. Abdom Imaging 38: 745-754, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 362: 1907-1917, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet 366: 1303-1314, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Byrnes V, Shi H, Kiryu S, Rofsky NM, Afdhal NH. The clinical outcome of small (<20 mm) arterially enhancing nodules on MRI in the cirrhotic liver. Am J Gastroenterol 102: 1654-1659, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Zheng YW, Zhang XW, Zhang JL, et al. Primary hepatic angiosarcoma and potential treatment options. J Gastroenterol Hepatol 29: 906-911, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Almogy G, Lieberman S, Gips M, et al. Clinical outcomes of surgical resections for primary liver sarcoma in adults: results from a single centre. Eur J Surg Oncol 30: 421-427, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Duan XF, Li Q. Primary hepatic angiosarcoma: a retrospective analysis of 6 cases. J Dig Dis 13: 381-385, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Huang NC, Kuo YC, Chiang JC, et al. Hepatic angiosarcoma may have fair survival nowadays. Medicine (Baltimore) 94: e816, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthaei H, Krieg A, Schmelzle M, et al. Long-term survival after surgery for primary hepatic sarcoma in adults. Arch Surg 144: 339-344; discussion 44, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Okano A, Sonoyama H, Masano Y, et al. The natural history of a hepatic angiosarcoma that was difficult to differentiate from cavernous hemangioma. Intern Med 51: 2899-2904, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YM, Li B, Yin ZM, et al. Results of hepatic resection for primary hepatic angiosarcoma in adults. Med Sci Monit 16: CR61-CR66, 2010. [PubMed] [Google Scholar]