Abstract
BACKGROUND:
Proton pump inhibitors (PPIs) represent the most widely prescribed antisecretory agents, but their prolonged use, may influence iron and vitamin B12 status, which could have important implications for clinical practice.
AIM:
We undertook this study aiming to investigate the association between PPIs use for 12 months and potential changes in iron and vitamin B12 status, as well as whether this potential association varies among four specific PPI drugs used in the study.
METHODS:
A total of 250 adult subjects were recruited into this study, of which 200 subjects were PPIs users while 50 subjects belonged to the control group. Serum iron, ferritin, vitamin B12, and homocysteine (Hcy) levels were measured before the start of the study and after 12 months. Mann - Whitney U test and Kruskal - Wallis test was used to compare the baseline characteristics of the study groups, while Wilcoxon test was used to analyse post - pre differences.
RESULTS:
Statistical analysis showed significant changes within PPIs group and specific PPIs subgroups between the two-time points in serum ferritin and vitamin B12 levels, respectively, while no significant changes in serum iron and homocysteine levels were shown. However, subsequent diagnosis of hypoferremia and hypovitaminosis B12 in the whole study sample at 12 months was established in only 3.8% and 2.9% of the subjects, respectively.
CONCLUSION:
PPIs use for 12 months did not result in clinically significant iron and/or vitamin B12 deficiency; thus, these findings argue routine screening under normal circumstances, although monitoring in elderly and malnourished may be of precious value.
Keywords: PPIs, Iron: Ferritin, Vitamin B12, Homocysteine
Introduction
Proton pump inhibitors (PPIs) represent the most widely prescribed antisecretory agents [1] Prolonged PPIs use is not without consequences, however [1] [2]. Concerns have been raised about a possible association between prolonged PPIs use and increased risk for vitamin and mineral deficiencies [3] [4]. It has been suggested that their prolonged use may influence iron and vitamin B12 status due to potent suppression of gastric acid secretion by parietal cells, which could have important implications for clinical practice [5] [6]. Relatively few studies have specifically investigated the association between PPIs use and iron status and/or risk of anemia while what is known about the association between PPIs use and vitamin B12 deficiency is largely based on case -reports or retrospective observational studies with considerable inconsistency in the findings [7] [8] [9] [10] [11] [12] [13] [14] [15] [16]. Moreover, they have failed to provide appropriate monitoring recommendations in this regard [17]. Most of the previous studies provided only the data comparing “treatment” with “no treatment”, we therefore undertook this study aiming to prospectively investigate the association between PPIs use for 12 months in new - users and potential changes in iron and vitamin B12 status, as well as whether this potential association varies among four specific PPI drugs used in the study. Also, the incidence of new-onset hypoferremia and hypovitaminosis B12 and hyperhomocysteinemia (HHcy) during the study was assessed.
Material and Methods
The methodology of this open - labelled prospective study is described in greater detail elsewhere [18] [19]. Briefly, the study population consisted of subjects aged 18 to 65 years with a confirmed diagnosis of osteoarthritis of small joints of the hands and on chronic NSAIDs that indicated to initiate gastroprotective maintenance therapy with PPIs. Control group consisted of 50 matched healthy participants and with no gastrointestinal or other risk factors present for iron and vitamin B12 deficiency. The participants belonging to the groups under treatment with PPIs were contacted every 3 months by telephone to assess the adherence to PPIs and the potential adverse effects, while participants in the control group were contacted by telephone after 12 months.
Subjects were enrolled in the study only if they had serum iron, ferritin and vitamin B12 levels greater than lower reference limit provided by the lab (Table 1). Subjects were not included in the study if they were using parenteral and/or oral supplements of iron, vitamin B12 and folic acid, respectively, as well as any of the antisecretory agents (including PPIs) during preceding 12 months. Also, subjects with known hypersensitivity to any drug were excluded. Subjects were also excluded from the study if they were blood donors, were on vegetarian diet, were chronic alcohol abusers, were using concomitantly drugs (namely metformin, thyroid hormone supplements, antiepileptic drugs, anticoagulant drugs, oral contraceptives, glucocorticoids) and/or had diseases that may affect iron and vitamin B12 status (namely dementia, acute inflammatory diseases, malabsorption diseases, abnormal uterine, gastrointestinal or urinary bleeding, patients with atrophic gastritis or gastrectomy, thyroid diseases, renal diseases, cardiovascular diseases, neoplastic diseases including leukemias and lymphomas). Subjects were not included if they were pregnant, lactating or planning a pregnancy. To enhance the validity of our findings all the potential study participants were screened for exclusion mentioned above criteria.
Table 1.
One-year changes in biochemical parameters according to study groups
| Biochemical parameters | PPI users (n=209) | p-value | PPI non-users (n=42) | p-value | ||
|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |||
| Homocysteine (5.5-15 µmol/l) | 14.22±3.3 | 14.5±4.3 | 0.32 | 14.7±4.3 | 13.8±3.2 | 0.08 |
| Vitamin B12 (191-663 pg/ml) | 433.6±122.5 | 393.5±139.4 | <0.001 | 418.9±127.3 | 416.2±115.6 | 0.66 |
| Ferritin (ng/ml)a | 135.2±111.2 | 94.1±76.2 | <0.001 | 105.8±87.4 | 87.6±82.8 | 0.21 |
| Iron (μmol/l)b | 17.6±5.23 | 16.9±5.4 | 0.049 | 19.0±4.9 | 19.6±4.9 | 0.14 |
p-values according to Wilcoxon signed-rank test;
♂: 30 - 400 ng/ml, ♀: 13 - 150 ng/ml;
♂: 10.6 - 28.3 μmol/l, ♀: 6.6 - 26.0 μmol/l.
A total of 250 adult subjects were recruited into this study, of which 200 subjects were PPIs users of orally taken dosage forms of PPIs while 50 subjects belonged to the control group (PPIs nonusers). PPIs users were divided into four matched groups, each of them consisting of 50 subjects: patients on omeprazole therapy (20 mg/day), patients on esomeprazole therapy (20 mg/day), patients on lansoprazole therapy (30 mg/day), and patients on pantoprazole therapy (40 mg/day).
All study participants provided written informed consent. Before the initiation of the study was obtained the approval of the study protocol from the local ethical committees of the University Clinical Center of Kosova and Faculty of Medicine, University of Prishtina.
Fasting blood samples were collected from the antecubital vein at 8 AM, and were centrifuged within the first hour to separate the serum. The measurements of serum iron, ferritin, vitamin B12 and homocysteine levels were done before the start of the study (at baseline) and after 12 months. Serum iron, ferritin and homocysteine (enzymatic test) concentrations were measured simultaneously using automated analyser, COBAS Integra 400 Plus (Roche Diagnostics, Switzerland), while serum vitamin B12 concentrations were measured by Electrochemiluminescence Immunoassay using Elecsys 2010 system (Roche Diagnostics, Switzerland). Hypoferremia was defined as serum levels of <10.6 μmol/l in male and < 6.6 μmol/l in female, hypoferritinemia was defined as serum levels of < 30 ng/ml in male and < 13 ng/ml in female, hypovitaminosis B12 was defined as serum levels of < 191 pg/ml, and hyperhomocysteinemia was defined as serum levels of >15 μmol/l.
All the study results are expressed as the mean ± standard deviation and as percentages, as appropriate. The baseline continuous characteristics of the study groups were compared by using Mann -Whitney U test and Kruskal-Wallis test, as appropriate, while baseline categorical characteristics were compared by using the chi-square test. The Wilcoxon signed-rank test was used to analyse post -pre differences in biochemical parameters. All statistical analyses were done with SPSS, version 20. A value of p < 0.05 was considered statistically significant.
Results
Out of a total of 250 participants that were initially recruited in the study, 209 participants completed 12 months of study. Thus only their data were analysed and presented here. The most common reasons for withdrawal from the study were non - compliance, changes in the place of residence and loss of follow - up, pregnancy and other personal reasons (Fig. 1). Most participants were female (74.6%), and the mean age at the beginning of the study was 50.59 ±10.61 years. Mann - Whitney U test and Kruskal - Wallis test showed no significant differences in the baseline study characteristics between groups and subgroups (data not shown).
Figure 1.
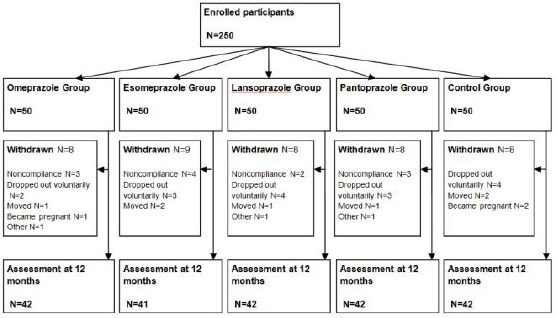
Study design [18]
The results of the Wilcoxon signed - rank test summarised in Table 1 showed the statistically significant difference between the baseline and 12 months’ serum ferritin and vitamin B12 levels within PPI group (p < 0.001 for both parameters). When specific PPI subgroups were analyzed (Table 2), the Wilcoxon signed - rank test showed that the most significant difference in mean serum ferritin levels was in pantoprazole group (p < 0.001), while the least, but still significant difference, was in omeprazole group (p = 0.01); as for serum vitamin B12 levels, the most significant difference was in lansoprazole group (p = 0.004).
Table 2.
One - year changes in biochemical parameters according to study subgroups
| Biochemical parameter | Pre-treatment | Post-treatment | p–value | |
|---|---|---|---|---|
| Omeprazole group (n=42) | Homocysteine | 14.3±3.9 | 14.5±3.0 | 0.48 |
| Vitamin B12 | 404.9±111.7 | 381.6±122.9 | 0.02 | |
| Ferritin | 133.1±101.1 | 99.5±87.9 | 0.01 | |
| Iron | 17.6±5.7 | 16.2±6.1 | 0.13 | |
| Esomeprazole group (n=41) | Homocysteine | 14.1±3.0 | 14.5±2.3 | 0.19 |
| Vitamin B12 | 439.4±128.4 | 395.6±156.6 | 0.02 | |
| Ferritin | 121.0±105.5 | 91.2±67.8 | 0.001 | |
| Iron | 17.8±4.4 | 17.4±4.3 | 0.29 | |
| Lansoprazole group (n=42) | Homocysteine | 14.9±3.4 | 15.1±2.7 | 0.79 |
| Vitamin B12 | 438.2±127.3 | 396.6±143.7 | 0.004 | |
| Ferritin | 138.7±114.7 | 93.3±76.7 | 0.001 | |
| Iron | 18.2±6.3 | 17.6±5.9 | 0.64 | |
| Pantoprazole group (n=42) | Homocysteine | 13.5±2.6 | 13.9±7.2 | 0.81 |
| Vitamin B12 | 452.1±121.4 | 399.9±137.2 | 0.01 | |
| Ferritin | 147.6±124.3 | 92.4±73.4 | <0.001 | |
| Iron | 16.9±4.6 | 16.8±5.2 | 0.30 | |
| Control group (n=42) | Homocysteine | 14.7±4.3 | 13.8±3.2 | 0.08 |
| Vitamin B12 | 418.9±127.3 | 416.2±115.6 | 0.66 | |
| Ferritin | 105.8±87.4 | 87.6±82.8 | 0.21 | |
| Iron | 19.0±4.9 | 19.6±4.9 | 0.15 |
p-values according to Wilcoxon signed-rank test; All the parameters are expressed as mean± standard deviation;
Although the statistical analysis did not show any statistically significant difference in the mean iron serum levels between specific PPI subgroups (Table 2), when all the study subjects under active treatment with PPIs were considered as a single group (Table 1), the difference reached a borderline statistical significance (p = 0.049).
No statistically significant difference in the mean homocysteine serum levels was noticed within PPI group and specific PPI subgroups; nevertheless, after 12 months, the incidence of the new - onset hyperhomocysteinemia in the whole study sample was 35.4 %, with sixty - six cases (39.5%) among PPIs users and eight cases (19.0%) among PPIs non-users (39.5% vs 19.0%, p = 0.013).
No statistically significant differences between PPIs users and non-users were noticed in the incidences of the new-onset of hypoferritinemia, hypoferremia and hypovitaminosis B12 (7.2% vs. 9.5%, p = 0.610, 4.8% vs. 0%, p = 0.148 and 3.0% vs. 2.4%, p = 0.832, respectively).
It is noteworthy that no statistically significant difference was shown in the control group in any of the biochemical parameters of the study.
Discussion
The results of the current study indicate a significant association between PPIs use for 12 months and reduction in iron body stores and vitamin B12 levels and higher prevalence of HHcy among PPI users. Our findings suggest that prolonged PPIs therapy affects iron and vitamin B12 status. Nevertheless, the subsequent diagnosis of hypoferremia and vitamin B12 deficiency at the end of the study was established in only 3.8% and 2.9% of the subjects, respectively, without significant differences between PPIs users and non - users.
Early studies that noticed the association between clinical conditions associated with achlorhydria or hypochlorhydria and diminished intestinal iron absorption raised interest about the potential effect of prolonged PPIs therapy on iron status [7] [20]. Years later, through mutations on proton pumps on rats, Krieg et al. [21] confirmed the necessity of gastric proton pump for iron absorption. Nevertheless, the number of studies that have specifically investigated the association between prolonged PPIs use and iron status is relatively small. Among the first studies to suggest such an association is a case report of two anemic patients [12], in whom no response to oral substitutive therapy with iron was noted when the patients continued to take PPIs, while their iron status improved after PPIs withdrawal; this made the authors to attribute the nonresponsiveness to iron therapy to PPIs. In another study on patients with hereditary hemochromatosis that require frequent phlebotomies [22], administration of PPIs (omeprazole or lansoprazole) for 7 days resulted in significant reduction in the amount of blood removed per annum to maintain adequate iron body stores (ferritin levels of ∼50 µg/l). A retrospective cohort study of Sarzynski et al. [13] on adult outpatients who have been on PPI therapy for at least one year, demonstrated a significant reduction in hematologic indices compared to baseline values. Similarly, in a recent retrospective study of Shikata et al. [23] proved that PPIs use was associated with anaemia in cardiovascular outpatients, thus reporting a high frequency of anaemia among PPIs users compared to non-users (51% vs 19%, p < 0.001).
The findings from this study indicate significant changes in iron body stores (serum ferritin levels) among PPIs and specific PPIs subgroups between the two time points, while no significant changes in serum iron levels (except for borderline significance when PPIs users as a single entity), and speak for pre-latent iron deficiency that is actually the initial phase in the pathogenesis of iron deficiency anemia.
In recent years there has been growing interest in the relationship of PPIs prolonged use and vitamin B12 deficiency; however, there is still considerable uncertainty about providing monitoring recommendations [10] [24] [25]. The potential mechanisms behind the increased risk of vitamin B12 deficiency in prolonged PPIs users are vitamin B12 malabsorption and bacterial overgrowth in the gut [3] [4].
Marcuard et al. [8] were among the first to prove dose-dependent reduction of absorption of cyanocobalamin in healthy volunteers on short-term therapy with omeprazole for two weeks; nevertheless, an increased risk for vitamin B12 deficiency due to long-term use of PPIs was first reported by Termanini et al. [9] in a prospective study performed on patients with Zollinger-Ellison syndrome. In the last few years, much more information has become available about the association between long-term use of PPIs and vitamin B12 deficiency [10] [11] [24] [26]. More recent studies of Lam et al. [14] and Lewis et al. [25] have also indicated the presence of vitamin B12 deficiency in patients exposed to chronic PPIs.
The findings from this study lend support to previous findings in the literature with data analysis indicating significant changes in vitamin B12 levels among PPIs users and specific PPIs subgroups at 12 months as well as significantly higher in-study incidence of HHcy among PPIs users. Nevertheless, Hcy serum levels in PPIs users resulted in a slight but non-significant increase, that might seem controversial at first because Hcy reflects the functional status of vitamin B12, but it can be explained by the fact that higher levels of Hcy are more prevalent in lower levels of vitamin B12, and vitamin B12 deficiency was established in only 2.9 % of the subjects.
On the other hand, we should mention that there are studies that do not support our findings of the association between long-term use of PPIs and vitamin B12 deficiency [7] [27] [28]. The main reason(s) for this inconsistency in findings is still not completely clear.
We are aware that our study might have certain limitations. The first might be sample size as it was arbitrarily chosen. Another one is the inability to assess dietary intake of iron and vitamin B12. Unfortunately, we were unable to measure folic acid serum levels and methylmalonic acid serum levels, a specific marker of vitamin B12 status, which might have also influenced the results.
In conclusion, in spite of the fact that significant changes on ferritin and vitamin B12 serum levels among PPIs users were shown, PPIs use for 12 months did not result in clinically significant iron and/or vitamin B12 deficiency. These findings argue routine screening under normal circumstances; however, considerable attention must be paid when PPIs are prescribed in elderly and malnourished, where monitoring may be of precious value.
Acknowledgement
This research was partly sponsored by a grant from the Ministry of Education, Science and Technology of Kosovo.
Footnotes
Funding: This research was partly sponsored by a grant from the Ministry of Education, Science and Technology of Kosovo
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Mössner J. The Indications, Applications, and Risks of Proton Pump Inhibitors. Dtsch Arzteblatt Int. 2016;113:477–83. doi: 10.3238/arztebl.2016.0477. PMid:27476707. PMCid:PMC4973002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scarpignato C, Gatta L, Zullo A, Blandizzi C SIF-AIG O-FIMMG Group, Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14:179. doi: 10.1186/s12916-016-0718-z. https://doi.org/10.1186/s12916-016-0718-z. PMid:27825371. PMCid:PMC5101793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931–50. doi: 10.1007/s10620-010-1560-3. https://doi.org/10.1007/s10620-010-1560-3. PMid:21365243. [DOI] [PubMed] [Google Scholar]
- 4.Heidelbaugh JJ. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Ther Adv Drug Saf. 2013;4:125–33. doi: 10.1177/2042098613482484. https://doi.org/10.1177/2042098613482484. PMid:25083257. PMCid:PMC4110863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013;6:443–51. doi: 10.1586/17512433.2013.811206. https://doi.org/10.1586/17512433.2013.811206. PMid:23927671. [DOI] [PubMed] [Google Scholar]
- 6.Schnoll-Sussman F, Katz PO. Clinical Implications of Emerging Data on the Safety of Proton Pump Inhibitors. Curr Treat Options Gastroenterol. 2017;15:1–9. doi: 10.1007/s11938-017-0115-5. https://doi.org/10.1007/s11938-017-0115-5. PMid:28130652. [DOI] [PubMed] [Google Scholar]
- 7.Koop H. Review article: metabolic consequences of long-term inhibition of acid secretion by omeprazole. Aliment Pharmacol Ther. 1992;6:399–406. doi: 10.1111/j.1365-2036.1992.tb00553.x. https://doi.org/10.1111/j.1365-2036.1992.tb00553.x. PMid:1420733. [DOI] [PubMed] [Google Scholar]
- 8.Marcuard SP, Albernaz L, Khazanie PG. Omeprazole therapy causes malabsorption of cyanocobalamin (vitamin B12) Ann Intern Med. 1994;120:211–5. doi: 10.7326/0003-4819-120-3-199402010-00006. https://doi.org/10.7326/0003-4819-120-3-199402010-00006. PMid:8273984. [DOI] [PubMed] [Google Scholar]
- 9.Termanini B, Gibril F, Sutliff VE, Yu F, Venzon DJ, Jensen RT. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am J Med. 1998;104:422–30. doi: 10.1016/s0002-9343(98)00087-4. https://doi.org/10.1016/S0002-9343(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 10.Force RW, Meeker AD, Cady PS, Culbertson VL, Force WS, Kelley CM. Ambulatory care increased vitamin B12 requirement associated with chronic acid suppression therapy. Ann Pharmacother. 2003;37:490–3. doi: 10.1345/aph.1C037. https://doi.org/10.1345/aph.1C037. PMid:12659601. [DOI] [PubMed] [Google Scholar]
- 11.Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol. 2004;57:422–8. doi: 10.1016/j.jclinepi.2003.08.015. https://doi.org/10.1016/j.jclinepi.2003.08.015. PMid:15135846. [DOI] [PubMed] [Google Scholar]
- 12.Sharma VR, Brannon MA, Carloss EA. Effect of omeprazole on oral iron replacement in patients with iron deficiency anemia. South Med J. 2004;97:887–9. doi: 10.1097/01.SMJ.0000110405.63179.69. https://doi.org/10.1097/01.SMJ.0000110405.63179.69. PMid:15455980. [DOI] [PubMed] [Google Scholar]
- 13.Sarzynski E, Puttarajappa C, Xie Y, Grover M, Laird-Fick H. Association between proton pump inhibitor use and anemia: a retrospective cohort study. Dig Dis Sci. 2011;56:2349–53. doi: 10.1007/s10620-011-1589-y. https://doi.org/10.1007/s10620-011-1589-y. PMid:21318590. [DOI] [PubMed] [Google Scholar]
- 14.Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310:2435–42. doi: 10.1001/jama.2013.280490. https://doi.org/10.1001/jama.2013.280490. PMid:24327038. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto R, Matsuda T, Chonan A. Iron-deficiency anemia caused by a proton pump inhibitor. Intern Med Tokyo Jpn. 2014;53:2297–9. doi: 10.2169/internalmedicine.53.2743. https://doi.org/10.2169/internalmedicine.53.2743. [DOI] [PubMed] [Google Scholar]
- 16.Dado DN, Loesch EB, Jaganathan SP. A Case of Severe Iron Deficiency Anemia Associated with Long-Term Proton Pump Inhibitor Use. Curr Ther Res. 2017:2017. doi: 10.1016/j.curtheres.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linder L, Tamboue C, Clements JN. Drug-Induced Vitamin B12 Deficiency: A Focus on Proton Pump Inhibitors and Histamine-2 Antagonists. J Pharm Pract. 2016 doi: 10.1177/0897190016663092. PMid:27520327. [DOI] [PubMed] [Google Scholar]
- 18.Bahtiri E, Islami H, Hoxha R, Qorraj-Bytyqi H, Rexhepi S, Hoti K, et al. Esomeprazole use is independently associated with significant reduction of BMD: 1-year prospective comparative safety study of four proton pump inhibitors. J Bone Miner Metab. 2016;34:571–9. doi: 10.1007/s00774-015-0699-6. https://doi.org/10.1007/s00774-015-0699-6. PMid:26209167. [DOI] [PubMed] [Google Scholar]
- 19.Bahtiri E, Islami H, Hoxha R, Gashi A, Thaçi K, Karakulak Ç, et al. Proton pump inhibitor use for 12 months is not associated with changes in serum magnesium levels: a prospective open label comparative study. Turk J Gastroenterol. 2017;28:104–9. doi: 10.5152/tjg.2016.0284. https://doi.org/10.5152/tjg.2016.0284. PMid:28082254. [DOI] [PubMed] [Google Scholar]
- 20.Annibale B, Capurso G, Delle Fave G. The stomach and iron deficiency anaemia: a forgotten link. Dig Liver Dis. 2003;35:288–95. doi: 10.1016/s1590-8658(03)00067-7. https://doi.org/10.1016/S1590-8658(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 21.Krieg L, Milstein O, Krebs P, Xia Y, Beutler B, Du X. Mutation of the gastric hydrogen-potassium ATPase alpha subunit causes iron-deficiency anemia in mice. Blood. 2011;118:6418–25. doi: 10.1182/blood-2011-04-350082. https://doi.org/10.1182/blood-2011-04-350082. PMid:21976678. PMCid:PMC3236123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchinson C, Geissler CA, Powell JJ, Bomford A. Proton pump inhibitors suppress absorption of dietary non-haem iron in hereditary haemochromatosis. Gut. 2007;56:1291–5. doi: 10.1136/gut.2006.108613. https://doi.org/10.1136/gut.2006.108613. PMid:17344278. PMCid:PMC1954964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shikata T, Sasaki N, Ueda M, Kimura T, Itohara K, Sugahara M, et al. Use of proton pump inhibitors is associated with anemia in cardiovascular outpatients. Circ J Off J Jpn Circ Soc. 2015;79:193–200. doi: 10.1253/circj.CJ-14-0582. [DOI] [PubMed] [Google Scholar]
- 24.Dharmarajan TS, Kanagala MR, Murakonda P, Lebelt AS, Norkus EP. Do acid-lowering agents affect vitamin B12 status in older adults? J Am Med Dir Assoc. 2008;9:162–7. doi: 10.1016/j.jamda.2007.10.004. https://doi.org/10.1016/j.jamda.2007.10.004. PMid:18294598. [DOI] [PubMed] [Google Scholar]
- 25.Lewis JR, Barre D, Zhu K, Ivey KL, Lim EM, Hughes J, et al. Long-term proton pump inhibitor therapy and falls and fractures in elderly women: a prospective cohort study. J Bone Miner Res. 2014;29:2489–97. doi: 10.1002/jbmr.2279. https://doi.org/10.1002/jbmr.2279. PMid:24825180. [DOI] [PubMed] [Google Scholar]
- 26.Rozgony NR, Fang C, Kuczmarski MF, Bob H. Vitamin B(12) deficiency is linked with long-term use of proton pump inhibitors in institutionalized older adults: could a cyanocobalamin nasal spray be beneficial? J Nutr Elder. 2010;29:87–99. doi: 10.1080/01639360903574734. https://doi.org/10.1080/01639360903574734. PMid:20391044. [DOI] [PubMed] [Google Scholar]
- 27.Schenk BE, Festen HP, Kuipers EJ, Klinkenberg-Knol EC, Meuwissen SG. Effect of short- and long-term treatment with omeprazole on the absorption and serum levels of cobalamin. Aliment Pharmacol Ther. 1996;10:541–5. doi: 10.1046/j.1365-2036.1996.27169000.x. https://doi.org/10.1046/j.1365-2036.1996.27169000.x. PMid:8853757. [DOI] [PubMed] [Google Scholar]
- 28.den Elzen WPJ, Groeneveld Y, de Ruijter W, Souverijn JHM, le Cessie S, Assendelft WJJ, et al. Long-term use of proton pump inhibitors and vitamin B12 status in elderly individuals. Aliment Pharmacol Ther. 2008;15(27):491–7. doi: 10.1111/j.1365-2036.2008.03601.x. https://doi.org/10.1111/j.1365-2036.2008.03601.x. PMid:18194503. [DOI] [PubMed] [Google Scholar]


