Abstract
AIM:
The purpose of this review is to represent acids that can be used as surface etchant before adhesive luting of ceramic restorations, placement of orthodontic brackets or repair of chipped porcelain restorations. Chemical reactions, application protocol, and etching effect are presented as well.
STUDY SELECTION:
Available scientific articles published in PubMed and Scopus literature databases, scientific reports and manufacturers’ instructions and product information from internet websites, written in English, using following search terms: “acid etching, ceramic surface treatment, hydrofluoric acid, acidulated phosphate fluoride, ammonium hydrogen bifluoride”, have been reviewed.
RESULTS:
There are several acids with fluoride ion in their composition that can be used as ceramic surface etchants. The etching effect depends on the acid type and its concentration, etching time, as well as ceramic type. The most effective etching pattern is achieved when using hydrofluoric acid; the numerous micropores and channels of different sizes, honeycomb-like appearance, extruded crystals or scattered irregular ceramic particles, depending on the ceramic type, have been detected on the etched surfaces.
CONCLUSION:
Acid etching of the bonding surface of glass - ceramic restorations is considered as the most effective treatment method that provides a reliable bond with composite cement. Selective removing of the glassy matrix of silicate ceramics results in a micromorphological three-dimensional porous surface that allows micromechanical interlocking of the luting composite.
Keywords: Acid etching, Ceramic surface treatment, Hydrofluoric acid, Acidulated phosphate fluoride, Ammonium hydrogen bifluoride
Introduction
The increasing use of ceramic materials in the fabrication of indirect restorations as well as placement of orthodontic brackets on teeth restored with ceramic crowns imposed a need for improvement of cements that enable bonding [1] [2]. The most appropriate are luting composites [3] [4] which not only provide the strongest bond but can increase the fracture resistance of the restored tooth and indirect ceramic restoration as well [3] [4] [5] [6] [7].
However, to ensure a long-term bond between ceramic material and tooth structures, orthodontic brackets or composite material used for repair, treatment of ceramic bonding surface is required [8]. There are various methods for the stated purpose. According to the mechanism of action, they could be grouped into three categories: methods of mechanical, chemical or chemo-mechanical surface treatments. The mechanical alteration can be achieved by surface acid etching, airborne particle abrasion or grinding with diamond rotary instruments [8] [9]. The chemical treatment is performed using universal or ceramic primers, i.e. silanization [10] [11], while the method used for chemo-mechanical alteration of the bonding surface is tribochemical silica - coating, i.e. silicatization [12] [14]. Numerous alternative treatments are proposed for treatment of restorations made up from zirconia such as: selective infiltration etching (SIE) [15] [16] followed by application of various silane-based zirconia primers [17] [18], gas - phase chloro -silane pretreatment [19], gas - plasma, argon - ion bombardment, alumina or zirconia sandblasting [20], [21], non - termal plasma treatment [22], nano -structure alumina coating [23] or aluminium nitride coating by reactive magnetron sputtering [24].
Which surface conditioning method will be selected depends on the chemical composition of the ceramic restoration [8] [25] [26]. Ceramics the matrix of which is based on silicon dioxide (“conventional” or glass - ceramics) belong to the group in which acid etching is the recommended surface treatment [26]. These include feldspar - based, leucite-reinforced, lithium disilicate [25] [26] and zirconia-reinforced lithium silicate ceramics [26] as well as fluorapatite ceramics. Airborne particle abrasion (sandblasting) can be used for surface treatment of all types of ceramics [9] [14] [20] [21] [26]. Universal or ceramic primers having reactive radicals in their molecule change the chemical composition of the ceramic surface, thus making it much more reactive for binding with composite cement [8] [10] [11] [26]. Tribochemical silica - coating is primarily used for the treatment of aluminium trioxide and zirconium dioxide ceramics [14] [16]; acid etching will not have any impact on their surface morphology, as these materials don’t contain silicon in their composition [9].
Acid etching as a method for mechanical alteration of glass - ceramic surface, application protocol and the effect on surface micromorphology are presented in this review paper.
Etching Acids
The acid etching of silica-based dental ceramics was first introduced in 1983 by Simonsen and Calamia [27]. Although acids represent chemical agents, they are not included in the group of agents for a chemical but in those for mechanical alteration, because they cause a mechanical type of changes onto the ceramic surface.
The acids that are used as ceramic etchants are the hydrofluoric acid (HF), the acidulated phosphate fluoride (APF), and the ammonium hydrogen difluoride.
The hydrofluoric acid is the most frequently used acid, which when applied onto the ceramic surface reacts with the silica matrix creating silicon tetrafluoride and molecules of water that are released [8] [28]:
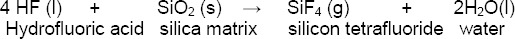
The silicon tetrafluoride reacts with other molecules of hydrofluoric acid forming a soluble complex ion, hexafluourosilicate:
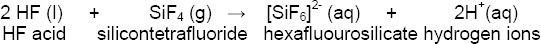
Further on, the hydrogen ions react with the hexafluorosilicate complex forming a fluorosilicic acid that can be rinsed off:
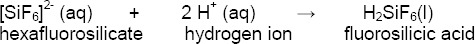
By dissolving and removing the surface layer of the glassy matrix containing silica (SiO2), silicates (SiO44-) and leucite crystals (K2O•Al2O3•4SiO2), the surface becomes porous with a pore size of 3 - 4 μm.
The acidulated phosphate fluoride contains 1.23% fluoride ions originated from sodium fluoride and hydrofluoric acid, acidified with 0.1 M phosphoric acid [8].
The ammonium hydrogen difluoride, NH4HF2, in reaction with silica matrix creates silicon tetrafluoride and ammonium fluoride:
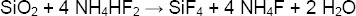
This acid may be used as a glass etchant or as an intermediate for the production of hydrofluoric acid [8].
There are different products that may be used for ceramic etching. It should be noted that, besides their efficiency, these products are forbidden in some countries because of their highly toxic potential.
Products for Acid Etching
IPS Ceramic Etching Gel, Ivoclar Vivadent [29], is an aqueous solution of 4% hydrofluoric acid in the form of a gel, unlike VITA CERAMICS ETCH, VITA Zahnfabrik [30], which is an alcoholic solution of 5% hydrofluoric acid and 10% sulfuric acid in the form of a gel. Porcelain Etch, Ultradent [31], is 9% hydrofluoric acid gel; mainly because of its high viscosity, according to the manufacturer’s instructions, it may be used for intraoral repairs of fractured porcelain in porcelain – fused – to - metal or all-ceramic restorations, as well as for the porcelain surface treatment before placing an orthodontic brackets. PORCELAIN ETCHANT, Bisco [32] [33], can be supplied in two different concentrations, like 4% and 9.5% of hydrofluoric acid gel (for intra - or extra - oral application). Mirage offers two different Porcelain Etchants [34]: 9.6% gel of HF acid and 4% Acidulated Phosphate Fluoride gel. APF - gel may be used intra -orally and is indicated for luting of orthodontic brackets to porcelain crowns.
Application Protocol
After the application, which is performed with a disposable micro brush, IPS Ceramic Etching Gel or VITA CERAMICS ETCH are left to react for 20 s when treating lithium disilicate and 30 s for zirconia-reinforced lithium silicate ceramic, 60 s for feldspar -based or leucite-reinforced and 120 seconds for treatment of fluorapatite ceramics. Then, HF acid should be thoroughly rinsed off from the treated surface with water spray into a polyethylene cup. The diluted solution should be neutralised with a neutralising powder composed of sodium - and calcium - carbonate (Na2CO3 and Ca2CO3; IPS Neutralizing Powder, Ivoclar Vivadent) in 5 minutes [35]. Eventually, the restoration is recommended to be placed in an ultrasonic bath. To remove the porcelain salts and debris formed by treatment with Porcelain Etch (90 s - etching time), Ultradent recommends a subsequent use of 35% phosphoric acid, Ultra-Etch, for 5 seconds; then, the treated restoration should be rinsed with water and dried [31].
Etching Effect
The effect of acid etching depends on the acid type and its concentration, the etching time and the ceramic type being treated. The most effective is hydrofluoric (HF) acid [8]; increasing the concentration and the etching time [36], increases the etching effect. Surface roughness and etching pattern depend on the ceramic type as well; acids do not affect polycrystalline ceramics that do not contain glassy matrix [9][14]. Contrary, the silica matrix and leucite crystals are selectively dissolved in glass-ceramics, generating three-dimensional porous structure [9][37]. The effect is multiplied: cleanses the bonding surface by removing the unwanted oxides, debris and grease [38], increases the roughness thus increasing the bonding area and wettability of the ceramic surface [39], and creates micro retention (micropores, grooves and channels) that can be easily infiltrated with uncured flowable composite cement [40][41], all together significantly increasing the resin - ceramic bond strength.
Acid type: The expressiveness of the surface micromorphology after acid etching depends on the acid type. HF - etching results in higher ceramic surface roughness, compared to other acids that may be used for this purpose [42][43][44][45]. A scanning electron microscope analysis of Della Bona and Anusavice [43] has shown that ceramic surface etched with 4% acidulated phosphate fluoride for 2 min was covered with precipitates, grooves could be detected after using ammonium bifluoride (1 min), while irregular etching pattern with pores as characteristic topographic feature was observed when the surface was treated with 9.6% hydrofluoric acid for 2 minutes. Etching the feldspathic porcelain with APF for 10 min resulted in shallow patterns, compared to deep channels, pores and precipitates formed by HF etching for 1 min; larger and deeper channels have been created when the HF - etching time was increased up to 4 min [44]. An insignificant effect of APF may be explained by low concentrations of hydrofluoric acid and available fluoride ions [43][45]. However, when the application time of 1.23% APF gel (clinically used as a topical fluoride for reduction of the incidence and progress of caries) was prolonged up to 60 min, increasing in surface roughness was detected, with numerous pores and deposits of particles in the form of precipitate or degradation material in the glassy matrix [46].
According to Kato et al. [47] the strongest immediate bond was achieved by etching of the feldspathic porcelain surface with HF acid (23.7 MPa); when using a solution of sulfuric and hydrofluoric acid (SHF) the bond strength was 21.3 MPa, while it was significantly lower when etching with ammonium hydrogen bifluoride (18.4 MPa). The weakest bond was built with phosphoric acid used as an etching agent. Significant reduction in bond strength after thermocycling was observed for all acids, although the HF - and SHF - treated groups exhibited bond strengths greater than 15 MPa [47]. When the etching time with 1.23% APF gel of the leucite - reinforced ceramic was prolonged up to 10 min, shear bond strength value was not significantly different to that after etching with 9.6% HF acid for 4 minutes (17.33 MPa and 17.64 MPa respectively) [48].
Acid concentration: An effect of acid etching depends not only on the acid type, but on its concentration as well. An increase in HF acid concentration increases the microroughness of the ceramic surface [49]. Uniform crystal structure is observed as a result of a dissolving of the glassy phase when the treatment is performed by HF acid with higher concentration (52%), whereas an amorphous structure expressing large porosity can be detected when etching with lower concentration (20%), when more of the crystalline phase has been dissolved [50].
Ceramic’s bi-axial flexure strength after surface etching depends on the HF acid concentration. When HF etching of low fusing feldspathic porcelain was conducted during 45 or 90 s, the mean value for bi-axial flexure strength was not significantly reduced when increasing HF acid concentration from 5 to 10%. Increasing the acid concentration to 20% resulted in a significant decrease in the strength for the both etching periods. However, an acid concentration (5, 10 or 20%) had no significant effect on reduction of the bi-axial flexure strength when the etching period was prolonged to 180 s [49].
Etching time: The etching time of hydrofluoric acid affects the expressiveness of micro - morphological changes and microroughness of the ceramic surface [49]: extending the etching time, increases the depth of the pores.
According to Wolf et al. [51], the longer the action of the acid is, the rougher the ceramic surface is; anyway, etching longer than 60 s increases the occurrence of cohesive failures in the ceramic material. A SEM analysis confirmed minor changes in the structure when etching the ceramic surface between 20 and 60 s; prolonged acid etching between 90 and 180 s resulted in a more pronounced surface morphology with protruded lithium disilicate crystals [52]. Bajraktarova Valjakova [36] had found more pronounced etching pattern when the etching time was prolonged from 20 up to 120 seconds. A difference in micro-morphology was not observed when the acid etching time was extended from 20 to 30 s; prolonged etching up to 90 and 120 s caused a visible change and emphasising of the surface micromorphology. The pores and grooves in feldspar - based and leucite-reinforced ceramics became deeper; lithium disilicate - and lithium silicate - crystals became more protruded as a result of increased dissolving of the surrounding silica matrix. Prolonged etching of polymer - infiltrated ceramic network material resulted in the significant dissolving of the ceramic network [36].
Increased surface roughness and resin -ceramic bond strength were noted when increasing the etching time up to 120 s when treating feldspar -based ceramic Vita Mark II; however, prolonged etching of 180 s hurt the bond strength [53]. Nagayassu et al. [54] detected the negative effect of long-term (4 min) HF etching over the resin -ceramic bond. According to Zogheib et al. [52] prolonging the etching time increases the average roughness of the treated surface, but at the same time slightly reduces the flexural strength of the IPS e.max CAD ceramic. However, when the etching time was increased from 45 to 90 and 180 s, bi - axial flexure strength values of feldspathic porcelain were not significantly different when using 5% HF acid [49].
Ceramic type: Different ceramics expressed different surface pattern when acid - etched under the same conditions; surface etching of feldspar-based ceramic for 5 min produces pores of 5 - 7 μm depth, while 5 min etching of the glass ceramic increases the pores depth up to 10 μm [28]. According to Kim et al. [55] the effect of the surface treatment primarily depends on the chemical composition of the ceramic; hydrofluoric acid etching is the most appropriate treatment of a lithium disilicate ceramic, while tribochemical silica coating has a positive impact on the bond of luting composite to aluminum oxide and zirconium oxide ceramics. According to Valandro et al. [56], HF acid is not useful in the treatment of ceramics with a polycrystalline structure. The surface of the leucite-reinforced ceramic, after HF etching, gets a honeycomb appearance, while exposure of crystals is observed after etching of the lithium disilicate ceramic [9].
The effect of hydrofluoric acid etching on the micro-morphology of various types of glassy ceramics is not the same and depends not only on their chemical composition but the structural molecular arrangement as well. Feldspathic VITA Mark II compared to leucite-reinforced IPS Empress CAD, as well as lithium disilicate IPS e.max CAD and zirconia-reinforced lithium silicate Celtra Duo, have a similar chemical composition and percentage contribution of components, but surfaces get different etching pattern as a result of the different molecular distribution. According to manufacturers’ official documents, silica and alumina contribution (in the abovementioned ceramics) is as follows: SiO2: 56 -64%, 60 - 65%, 57 - 80%, 56 - 64%; Al2O3: 20 - 23%, 16 - 20%, 5%, and 4% respectively. It may be noted that all glassy ceramics have almost identical content of silicon dioxide - about 60%, while the presence of aluminium oxide is similar between feldspar - based and leucite-reinforced ceramics - about 20%, and between lithium disilicate and zirconia-reinforced lithium silicate - about 4%. However, the specific internal structure and presence of other oxides, influence the effect of HF acid etching. According to Bajraktarova-Valjakova et al. [36] numerous micropores and channels of different sizes with irregular ceramic particles can be observed on the surface of VITA Mark II; the etching surface of IPS Empress CAD gets honeycomb-like appearance, while numerous elongated or bean-like crystals have been extruded as a result of silica - matrix dissolving after etching of IPS e.max CAD and Celtra Duo respectively. HF etching of VITA Enamic causes dissolving of the superficial ceramic network, so that acrylic polymer network became visible with scattered irregular ceramic particles [36].
Hydrofluoric acid etching has no effect on the so-called polycrystalline ceramics; such are aluminium trioxide [57] [58] and zirconium dioxide partially stabilized with yttrium oxide [59]. Increase in mechanical strength by increasing the number of crystals and reducing the content of glass (silicon dioxide), leads to the creation of acid - resistant ceramics (non - etchable). Treatment with any acid will not induce any satisfactory (microretentive) changes to the surface micro - morphology to ensure proper bonding of composite cement [9] [25] [45] [60] [61] [62] [63]. Surface treatment of such ceramic restorations is performed using other methods: tribochemical silica coating [12] [13] [16], chemical treatment with methacryloyloxydecyl dihydrogen phosphate (MDP) - containing primers [13], or alternative methods [15] [16] [17] [18] [19] [20].
In conclusion, there are various methods that can be used for the treatment of ceramic bonding surface when adhesive luting is recommended. Undoubtedly, the most appropriate treatment method for silica-based ceramics is acid etching. Expressed microroughness, and microretentive pattern that is achieved by etching with hydrofluoric acid, ensure long-term bond between luting composites and ceramic materials.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Pameijer CH. A review of luting agents. Int J Dent 2012. 2012:752861. doi: 10.1155/2012/752861. https://doi.org/10.1155/2012/752861. PMid:22505909. PMCid:PMC3296365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diaz-Arnold A, Vargas MA, Haselton DR. Current status of luting agents for fixed prosthodontics. J Prosthet Dent. 1999;81:135–41. doi: 10.1016/s0022-3913(99)70240-4. https://doi.org/10.1016/S0022-3913(99)70240-4. [DOI] [PubMed] [Google Scholar]
- 3.Krämer N, Lohbauer U, Frankenberger R. Adhesive luting of indirect restorations. Am J Dent. 2000;13:60D–76D. PMid:11763920. [PubMed] [Google Scholar]
- 4.Chen L, In Suh B. Bonding of resin materials to all-ceramics: a review. Current research in dentistry. 2012;3:7–17. https://doi.org/10.3844/crdsp.2012.7.17. [Google Scholar]
- 5.Blatz MB, Sadan A, Kern M. Resin-ceramic bonding: a review of the literature. J Prosthet Dent. 2003;89:268–74. doi: 10.1067/mpr.2003.50. https://doi.org/10.1067/mpr.2003.50. PMid:12644802. [DOI] [PubMed] [Google Scholar]
- 6.Vargas MA, Bergeron C, Diaz-Arnold A. Cementing all-ceramic restorations. JADA. 2011;142:20S–4S. doi: 10.14219/jada.archive.2011.0339. https://doi.org/10.14219/jada.archive.2011.0339. PMid:21454837. [DOI] [PubMed] [Google Scholar]
- 7.Burke FJ, Fleming GJ, Nathanson D, Marquis PM. Are adhesive technologies needed to support ceramics? An assessment of the current evidence. J Adhes Dent. 2002;4:7–22. PMid:12071631. [PubMed] [Google Scholar]
- 8.Ho GW, Matinlinna JP. Insights on ceramics as dental materials. Part II: chemical surface treatments. Silicon. 2011;3:117–23. https://doi.org/10.1007/s12633-011-9079-6. [Google Scholar]
- 9.Borges GA, Sophr AM, de Goes MF, Sobrinho C, Chan DCN. Effect of etching and airborne particle abrasion on the microstructure of different dental ceramics. J Prosthet Dent. 2003;89:479–88. doi: 10.1016/s0022-3913(02)52704-9. https://doi.org/10.1016/S0022-3913(02)52704-9. [DOI] [PubMed] [Google Scholar]
- 10.Matinlinna JP, Lassila LV, Özcan M, Yli-Urpo A, Vallittu PK. An introduction to silanes and their clinical applications in dentistry. Int J Prosthodont. 2004;17:155–64. PMid:15119865. [PubMed] [Google Scholar]
- 11.Lung CYK, Matinlinna JP. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent Mater. 2012;28:467–77. doi: 10.1016/j.dental.2012.02.009. https://doi.org/10.1016/j.dental.2012.02.009. PMid:22425571. [DOI] [PubMed] [Google Scholar]
- 12.Lung CYK, Kukk E, Hägerth T, Matinlinna JP. Surface modification of silica-coated zirconia by chemical treatments. Appl Surf Sci. 2010;257:1228–35. https://doi.org/10.1016/j.apsusc.2010.08.029. [Google Scholar]
- 13.Inokoshi M, Kameyama A, De Munck J, Minakuchi S, Van Meerbeek B. Durable bonding to mechanically and/or chemically pre-treated dental zirconia. J Dent. 2013;41:170–9. doi: 10.1016/j.jdent.2012.10.017. https://doi.org/10.1016/j.jdent.2012.10.017. PMid:23137995. [DOI] [PubMed] [Google Scholar]
- 14.Bottino MC, Özcan M, Coelho PG, Valandro LF, Bressiani JC, Bressiani AHA. Micro-morphological changes prior to adhesive bonding: high-alumina and glassy-matrix ceramics. Braz Oral Res. 2008;22:158–63. doi: 10.1590/s1806-83242008000200011. https://doi.org/10.1590/S1806-83242008000200011. PMid:18622486. [DOI] [PubMed] [Google Scholar]
- 15.Aboushelib MN, Kleverlaan CJ, Feilzer AJ. Selective infiltration-etching technique for a strong and durable bond of resin cements to zirconia-based materials. J Prosthet Dent. 2007;98:379–88. doi: 10.1016/S0022-3913(07)60123-1. https://doi.org/10.1016/S0022-3913(07)60123-1. [DOI] [PubMed] [Google Scholar]
- 16.Casucci A, Mazzitelli C, Monticelli F, Toledano M, Osorio R, Osorio E, Papacchini F, Ferrari M. Morphologycal analysis of three zirconium oxide ceramics: Effect of surface treatments. Dent Mater. 2010;26:751–60. doi: 10.1016/j.dental.2010.03.020. https://doi.org/10.1016/j.dental.2010.03.020. PMid:20471073. [DOI] [PubMed] [Google Scholar]
- 17.Aboushelib MN, Matinlinna JP, Salameh Z, Ounsi H. Innovations in bonding to zirconia-based materials: part I. Dent Mater. 2008;24:1268–72. doi: 10.1016/j.dental.2008.02.010. https://doi.org/10.1016/j.dental.2008.02.010. PMid:18417204. [DOI] [PubMed] [Google Scholar]
- 18.Aboushelib MN, Feilzer AJ, Kleverlaan CJ. Bonding to zirconia using a new surface treatment. J Prosthodont. 2010;19:340–6. doi: 10.1111/j.1532-849X.2010.00575.x. https://doi.org/10.1111/j.1532-849X.2010.00575.x. PMid:20202104. [DOI] [PubMed] [Google Scholar]
- 19.Piascik JR, Swift EJ, Thompson JY, Grego S, Stoner BR. Surface modification for enhanced silanation of zirconia ceramics. Dent Mater. 2009;25:1116–21. doi: 10.1016/j.dental.2009.03.008. https://doi.org/10.1016/j.dental.2009.03.008. PMid:19376572. PMCid:PMC2720441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallmann L, Ulmer P, Wille S, Polonskyi O, Köbel S, Trottenberg T, Bornholdt S, Haase F, Kersten H, Kern M. Effect of surface treatments on the properties and morphological change of dental zirconia. J Prosthet Dent. 2016;115:341–9. doi: 10.1016/j.prosdent.2015.09.007. https://doi.org/10.1016/j.prosdent.2015.09.007. PMid:26581661. [DOI] [PubMed] [Google Scholar]
- 21.Hallmann L, Ulmer P, Lehmann F, Wille S, Polonskyi O, Johannes M, Köbel S, Trottenberg T, Bornholdt S, Haase F, Kersten H, Kern M. Effect of surface modifications on the bond strength of zirconia ceramic with resin cement resin. Dent Mater. 2016;32:631–9. doi: 10.1016/j.dental.2016.02.001. https://doi.org/10.1016/j.dental.2016.02.001 PMid:26898723. [DOI] [PubMed] [Google Scholar]
- 22.Valverde GB, Coelho PG, Janal MN, Lorenzoni FC, Carvalho RM, Thompson VP, et al. Surface characterization and bonding of Y-TZP following non-thermal plasma treatment. J Dent. 2013;41(1):51–9. doi: 10.1016/j.jdent.2012.10.002. https://doi.org/10.1016/j.jdent.2012.10.002. PMid:23044388. [DOI] [PubMed] [Google Scholar]
- 23.Jevnikar P, Krnel K, Kocjan A, Funduk N, Kosmac T. The effect of nano-structured alumina coating on resin-bond strength to zirconia ceramics. Dent Mater. 2010;26(7):688–96. doi: 10.1016/j.dental.2010.03.013. https://doi.org/10.1016/j.dental.2010.03.013. PMid:20381854. [DOI] [PubMed] [Google Scholar]
- 24.Külünk T, Külünk Ş, Baba S, Öztürk Ö, Danişman Ş, Savaş S. The effect of alumina and aluminium nitride coating by reactive magnetron sputtering on the resin bond strength to zirconia core. J Adv Prosthodont. 2013;5(4):382–7. doi: 10.4047/jap.2013.5.4.382. https://doi.org/10.4047/jap.2013.5.4.382. PMid:24353874. PMCid:PMC3865191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Özcan M, Vallittu PK. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent Mater. 2003;19:725–31. doi: 10.1016/s0109-5641(03)00019-8. https://doi.org/10.1016/S0109-5641(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 26.Peumans M, Valjakova EB, De Munck J, Mishevska CB, Van Meerbeek B. Bonding effectiveness of luting composites to different CAD/CAM materials. J Adhes Dent. 2016;18(4):289–302. doi: 10.3290/j.jad.a36155. PMid:27222889. [DOI] [PubMed] [Google Scholar]
- 27.Simonsen RJ, Calamia JR. Tensile bond strength of etched porcelain. J Dent Res. 1983;62:297. Abstr No. 1154. [Google Scholar]
- 28.Yen TY, Blackman RB, Baez RJ. J. Effect of acid etching on the flexural strength of a feldspathic porcelain and a castable glass ceramic. J Prosthet Dent. 1993;70:224–33. doi: 10.1016/0022-3913(93)90056-t. https://doi.org/10.1016/0022-3913(93)90056-T. [DOI] [PubMed] [Google Scholar]
- 29. [Accessed May 1 2016]. http://www.ivoclarvivadent.us/zoolu-website/media/document/4176/IPS+Ceramic+Etching+gel+ (document last reviewed on 04/14/2016)
- 30. [Accessed May 1 2016]. https://www.vita-zahnfabrik.com/en/VITA-CERAMICS-ETCH-26710,27568.html .
- 31. [Accessed May 1 2016]. https://www.ultradent.com/en-us/Dental-Products-Supplies/Bond-Etch/Etchants/ Hydrofluoric-Acid-Gel/Ultradent-Porcelain-Etch-and-Silane-Ceramic-Etchant-and-Silane-Solution/Pages/default.aspx .
- 32. [Accessed May 1 2016]. https://www.bisco.com/instructions/%284%29Porcelain%20Etchant_English.pdf .
- 33. [Accessed May 1 2016]. https://www.bisco.com/instructions/%289.5%29Porcelain%20Etchant_English.pdf .
- 34. [Accessed May 1 2016]. http://www.miragecdp.com/por_etch.htm .
- 35. [Accessed May 1 2016]. http://www.ivoclarvivadent.us/zoolu-website/media/document/16985/ IPS+Ceramic+Etching +gel+
- 36.Bajraktarova Valjakova E. [Evaluation of the bonding effectiveness of luting composites to different ceramic CAD/CAM materials –in vitro study] [dissertation] Faculty of Dentistry, University “Ss Cyril and Methodius”; 2014 Macedonian. [Google Scholar]
- 37.Bajraktarova Valjakova E, De Munck J, Yoshihara K, Misevska C, Grozdanov A, Peumans M, Van Meerbeek B. Micro-morphological changes of various CAD-CAM blocks after different surface treatments. 47th Meeting of CED-IADR, Antalya, Turkey; 2015 Abstr No. 0576. [Google Scholar]
- 38.Scheller-Sheridan C. Acid etchant, bonding agents and fissure sealants. In: Scheller-Sheridan C, editor. Basic guide to dental materials. 1st ed. Oxford, UK: Wiley-Blackwell; 2010. pp. 63–79. [Google Scholar]
- 39.Della Bona A. Characterizing ceramics and the interfacial adhesion to resin: II- the relationship of surface treatment, bond strength, interfacial toughness and fractography. J Appl Oral Sci. 2005;13:101–9. doi: 10.1590/s1678-77572005000200002. https://doi.org/10.1590/S1678-77572005000200002. PMid:20924531. [DOI] [PubMed] [Google Scholar]
- 40.Matinlinna JP, Vallittu PK. Bonding of resin composites to etchable ceramic surfaces - an insight review of the chemical aspects on surface conditioning. J Oral Rehabil. 2007;34(8):622–30. doi: 10.1111/j.1365-2842.2005.01569.x. https://doi.org/10.1111/j.1365-2842.2005.01569.x. PMid:17650173. [DOI] [PubMed] [Google Scholar]
- 41.Van Noort R. All-ceramic restorations: resin-bonded ceramics. In: Van Noort R, editor. Introduction to dental materials. 3rd ed. Philadelphia, PA: Elsevier; 2007. pp. 261–9. [Google Scholar]
- 42.Aida M, Hayakawa T, Mizukawa K. Adhesion of composite porcelain with various surface conditions. J Prosthet Dent. 1995;73(5):464–70. doi: 10.1016/s0022-3913(05)80076-9. https://doi.org/10.1016/S0022-3913(05)80076-9. [DOI] [PubMed] [Google Scholar]
- 43.Della Bona A, Anusavice KJ. Microstructure, composition, and etching topography of dental ceramics. Int J Prosthodont. 2002;15(2):159–67. PMid:11951806. [PubMed] [Google Scholar]
- 44.Canay Ş, Hersek N, Ertan A. Effect of different acid treatments on a porcelain surface. J Oral Rehabil. 2001;28(1):95–101. doi: 10.1046/j.1365-2842.2001.00626.x. https://doi.org/10.1046/j.1365-2842.2001.00626.x. PMid:11298915. [DOI] [PubMed] [Google Scholar]
- 45.Della Bona A, Anusavice KJ, Hood JA. Effect of ceramic surface treatment on tensile bond strength to a resin cement. Int J Prosthodont. 2002;15(3):248–53. PMid:12066487. [PubMed] [Google Scholar]
- 46.Ccahuana VZS, Özcan M, Mesquita AMM, Nishioka RS, Kimpara ET, Bottino MA. Surface degradation of glass ceramics after exposure to acidulated phosphate fluoride. J Appl Oral Sci. 2010;18(2):155–65. doi: 10.1590/S1678-77572010000200010. https://doi.org/10.1590/S1678-77572010000200010. PMid:20485927. PMCid:PMC5349752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato H, Matsumura H, Atsuta M. Effect of etching and sandblasting on bond strength to sintered porcelain of unfilled resin. J Oral Rehabil. 2000;27(2):103–10. doi: 10.1046/j.1365-2842.2000.00489.x. https://doi.org/10.1046/j.1365-2842.2000.00489.x. PMid:10672145. [DOI] [PubMed] [Google Scholar]
- 48.Kukiattrakoon B, Thammasitboon K. The effect of different etching times of acidulated phosphate fluoride gel on the shear bond strength of high-leucite ceramics bonded to composite resin. J Prosthet Dent. 2007;98(1):17–23. doi: 10.1016/S0022-3913(07)60033-X. https://doi.org/10.1016/S0022-3913(07)60033-X. [DOI] [PubMed] [Google Scholar]
- 49.Addison O, Marquis PM, Fleming GJP. The impact of hydrofluoric acid surface treatments on the performance of a porcelain laminate restorative material. Dent Mater. 2007;23(4):461–8. doi: 10.1016/j.dental.2006.03.002. https://doi.org/10.1016/j.dental.2006.03.002. PMid:16620948. [DOI] [PubMed] [Google Scholar]
- 50.Stangel I, Nathanon D, Hsu CS. Shear strength of the composite bond to etched porcelain. J Dent Res. 1987;66(9):1460–5. doi: 10.1177/00220345870660091001. https://doi.org/10.1177/00220345870660091001. PMid:3305639. [DOI] [PubMed] [Google Scholar]
- 51.Wolf DM, Powers JM, O’Keefe KL. Bond strength of composite to etched and sandblasted porcelain. Am J Dent. 1993;6(3):155–8. PMid:8240779. [PubMed] [Google Scholar]
- 52.Zogheib LV, Della Bona A, Kimpara ET, McCabe JF. Effect of hydrofluoric acid etching duration on the roughness and flexural strength of a lithium disilicate-based glass ceramic. Braz Dent J. 2011;22(1):45–50. doi: 10.1590/s0103-64402011000100008. https://doi.org/10.1590/S0103-64402011000100008. PMid:21519648. [DOI] [PubMed] [Google Scholar]
- 53.Chen JH, Matsumura H, Atsuta M. Effect of different etching periods on the bond strength of a composite resin to a machinable porcelain. J Dent. 1998;26(1):53–8. doi: 10.1016/s0300-5712(96)00078-4. https://doi.org/10.1016/S0300-5712(96)00078-4. [DOI] [PubMed] [Google Scholar]
- 54.Nagayassu MP, Shintome LK, Uemura ES, de Araujo JEJ. Effect of surface treatment on the shear bond strength of a resin-based cement to porcelain. Braz Dent J. 2006;17(4):290–5. doi: 10.1590/s0103-64402006000400005. https://doi.org/10.1590/S0103-64402006000400005. PMid:17262141. [DOI] [PubMed] [Google Scholar]
- 55.Kim B-K, Bae H E-K, Shim J-S, Lee K-W. The influence of ceramic surface treatments on the tensile bond strength of composite resin to all-ceramic coping materials. J Prosthet Dent. 2005;94(4):357–62. doi: 10.1016/j.prosdent.2005.08.012. https://doi.org/10.1016/j.prosdent.2005.08.012. PMid:16198173. [DOI] [PubMed] [Google Scholar]
- 56.Valandro LF, Della Bona A, Antonio Bottino M, Neisser MP. The effect of ceramic surface treatment on bonding to densely sintered alumina ceramic. J Prosthet Dent. 2005;93(3):253–9. doi: 10.1016/j.prosdent.2004.12.002. https://doi.org/10.1016/j.prosdent.2004.12.002. PMid:15775926. [DOI] [PubMed] [Google Scholar]
- 57.Lu YC, Tseng H, Shin YH, Lee SY. Effects of surface treatments on bond strength of glass-infiltrated ceramic. J Oral Rehabil. 2001;28(9):805–13. doi: 10.1046/j.1365-2842.2001.00735.x. https://doi.org/10.1046/j.1365-2842.2001.00735.x. [DOI] [PubMed] [Google Scholar]
- 58.Özcan M, Alkumru HN, Gemalmaz D. The effect of surface treatment on the shear bond strength of luting cement to a glass-infiltrated alumina ceramic. Int J Prosthodont. 2001;14(4):335–9. PMid:11508088. [PubMed] [Google Scholar]
- 59.Kern M, Wegner SM. Bonding to zirconia ceramic: Ahesion methods and their durability. Dent Mater. 1998;14(1):64–71. doi: 10.1016/s0109-5641(98)00011-6. https://doi.org/10.1016/S0109-5641(98)00011-6. [DOI] [PubMed] [Google Scholar]
- 60.Derand P, Derand T. Bond strength of luting cements to zirconium oxide ceramics. Int J Prosthodont. 2000;13(2):131–5. PMid:11203621. [PubMed] [Google Scholar]
- 61.Della Bona A, Anusavice KJ, Shen C. Microtensile strength of composite bonded to hot-pressed ceramics. J Adhes Dent. 2000;2(4):305–13. PMid:11317377. [PubMed] [Google Scholar]
- 62.Madani M, Chu FCS, McDonalds AV, Smales RJ. Effects of surface treatments on shear bond strengths between a resin cement and an alumina core. J Prosthet Dent. 2000;83(6):644–7. https://doi.org/10.1067/mpr.2000.107337. PMid:10842131. [PubMed] [Google Scholar]


