Abstract
Alzheimer’s disease (AD) is the most common type of dementia among individuals 65 or older. There are more than 5 million diagnosed cases in the US alone and this number is expected to triple by 2050. Therefore, AD has reached epidemic proportions with significant socioeconomic implications. While aging in general is the greatest risk factor for AD, several additional demographic factors that have contributed to the rise in AD in the US are under study. One such factor is associated with the relatively fast growth of the Latino population. Several reports indicate that AD is more prevalent among blacks and Latinos. However, the reason for AD disparity among different ethnic groups is still poorly understood and highly controversial. The Latino population is composed of different groups based on nationality, namely South and Central America, Mexico, and Caribbean Hispanics. This diversity among the Latino population represents an additional challenge since there are distinct characteristics associated with AD and comorbidities. In this review, we aim to bring attention to the intersection between social determinants of health and genetic factors associated with AD within the Latino community. We argue that understanding the interplay between identified social determinants of health, co-morbidities, and genetic factors could lead to community empowerment and inclusiveness in research and healthcare services, contributing to improved diagnosis and treatment of AD patients. Lastly, we propose that inserting a neuroethics perspective could help understand key challenges that influence healthcare disparities and contribute to increased risk of AD among Latinos.
Keywords: Alzheimer’s disease, health disparities, healthcare, Latinos, social determinants of health
INTRODUCTION
Alzheimer’s disease (AD) is a debilitating disease characterized by loss of memory and executive functions [1–4]. The progressive cognitive impairment interferes with daily living tasks, which increase the burden on caregivers and the healthcare system [1]. Currently, available treatments of AD manage some of the symptoms but do not halt or reverse disease pathophysiology [5]. In addition, home and institutional care of AD patients represents an economic challenge, since many families struggle to afford the necessary care [6]. Therefore, the burden of disease that AD represents goes beyond the patient, since it indirectly affects caregivers’ health and their economic stability [6, 7]. Based on these aspects, the quality of AD care and treatment is influenced by social disparities within the US population, especially when the prevalence of AD is higher among Blacks and Latinos.
Latinos/Hispanics are the fastest growing population in the US [8, 9]. Unfortunately, twelve percent of older adults in the Latino/Hispanics population are diagnosed with AD, the highest proportion among different ethnic groups in the US [6, 7, 10, 11]. Therefore, there is a need to understand and develop strategies to address the Latino/Hispanics health disparity in AD. The first important aspect is to define the Latino/Hispanics population, recognizing its diversity. The terms Latino and Hispanics are interchangeable use in the literature, which generates confusion and makes the search of information more complicated. As illustrated in Table 1, when the terms Latino and/or Hispanics are used either in PubMed or Google Scholar searches, it results in a different amount of published articles, illustrating the interchangeably used of these terms. However, these terms denote different aspects of the population. The term Hispanics is used to denote all those who come from a country that speaks Spanish, including Spain and Latin-America (Central and South America and the Caribbean) but excluding Brazil, Guyana, Suriname, and French Guiana. Latino, however, is a geographical term used to identify those with origin or heritage from Latin-America (including Brazil, but excluding Guyana, Suriname, and French Guiana) and the Caribbean (i.e., Cuba, Dominic Republic, and Puerto Rico). In addition, the term Caribbean Hispanics was adopted to identify Latino islanders from Puerto Rico, Dominican Republic, and Cuba, distinguishing them from other Latinos [12]. Based on the definition of these terms, we chose to use Latino to refer to the collective of individuals in this review.
Table 1.
Number of published articles on dementia and the Latino/Hispanic population based on specific terms in PubMed and Google Scholar
| # Articles
|
||
|---|---|---|
| Term | PubMed | Google Scholar |
| Hispanics | 32702 | 268000 |
| Latinos | 29802 | 304000 |
| Dementia | 167524 | 1710000 |
| Hispanics and dementia | 395 | 21800 |
| Latinos and dementia | 364 | 19800 |
| Hispanics and Latinos and dementia | 356 | 16300 |
| Alzheimer’s disease | 122832 | 1110000 |
| Hispanics and Alzheimer’s disease | 277 | 20300 |
| Latinos and Alzheimer’s disease | 251 | 16700 |
| Hispanics and Latinos and Alzheimer’s disease | 243 | 18000 |
| Hispanics and Alzheimer’s disease and mutations | 22 | 8240 |
| Latinos and Alzheimer’s disease and mutations | 17 | 8240 |
| Hispanics and Latinos and Alzheimer’s disease and mutations | 17 | 6700 |
| Hispanics and Alzheimer’s disease and genes | 51 | 16900 |
| Latinos and Alzheimer’s disease and genes | 40 | 17200 |
| Hispanics and Latinos and Alzheimer’s disease and genes | 40 | 16700 |
| Hispanics and education and Alzheimer’s disease | 92 | 18800 |
| Latinos and education and Alzheimer’s disease | 86 | 17200 |
| Hispanics and Latinos and education and Alzheimer’s disease | 83 | 17200 |
| Hispanics and Alzheimer’s disease and socioeconomic status | 11 | 13600 |
| Latinos and Alzheimer’s disease and socioeconomic status | 10 | 13600 |
| Hispanics and Latinos and Alzheimer’s disease and socioeconomic status | 9 | 12400 |
| Hispanics and Latinos and metabolic syndrome | 389 | 20500 |
| Hispanics and obesity | 2768 | 64800 |
| Hispanics and Latinos and socioeconomic status | 2947 | 61400 |
| Hispanics and Latinos and education | 6923 | 192000 |
It is crucial to understand the diversity of the Latino population living in the US, which should not be studied as a homogenous ethnic group. Latinos can be divided into four major groups based on the nationality that they identify with, namely Mexican, Central American, South American, and Caribbean [8, 9]. Geographically, these groups show a gradient distribution across the US. The Caribbean Hispanics population is concentrated throughout the east of the US, from Massachusetts to Florida. Mexicans are by far the largest Latino group in the US [8]. The Mexican population is distributed across the US, but it is more concentrated in southwest and south of the country. Similarly, Latinos from Central and South America also follow a population distribution that emanate from the southwest to the rest of the US [8, 9]. Based on this geographical distribution, the Latino cohorts used in AD research have different compositions or ratios of representatives from these culturally and genetically diverse subgroups with different socioeconomic status and, consequently, different social determinants of health. Therefore, it is important to define the groups under study to identify intrinsic differences and characteristics associated with Latinos at risk or afflicted by AD.
Differences in symptomatology and prevalence of AD and related dementias among different Latino cohorts have been reported [11–20]. The annual average incidence of dementia among the Mexican population in Sacramento, CA was estimated at 0.8%, while Caribbean Hispanics in North Manhattan ranged from 2.3 to 5.3% based on different studies [12–15]. Notably, a study conducted in Philadelphia showed that Latinos have more severe symptomatology associated with AD than any other ethnic group studied [16]. This cohort (87% Puerto Rican) had a significant lower age of onset (68 years of age), lower Mini-Mental State Examination (MMSE) score, and more depression and cognitive decline than Blacks and White Non-Hispanics [16]. Similarly, a study in Texas showed that Mexicans in Fort Worth had a lower age of onset, MMSE score, and years of formal education and more depressive symptoms than White Non-Hispanics [18]. These results contrast to those obtained in Puerto Rico and Mexico. Studies of Puerto Ricans, living on the island of Puerto Rico, indicated that the mortality rate associated with AD is higher than their counterparts living in mainland US, but the age of onset is later and they have higher formal education than the cohorts from Philadelphia and North Manhattan [15, 20–23]. In contrast, studies in Mexico indicate that the prevalence of dementia among individuals aged 60 or older is 8% and there are over 800,000 diagnosed cases of AD [24–28]. Despite these differences, it is vastly accepted that, as a group, the Latino community displays an earlier age of onset of AD and that ethnicity is significantly associated with this younger age of onset in comparison to White Non-Hispanics [6, 7]. The unanswered question is: Are ethnic, social, and economic factors potential contributors of the disparity in AD prevalence? In this review, we discuss the differences among the Latino population in the US and those important factors that may contribute to the ethnic health disparities associated with AD. We aim to bring attention to important social determinants of health, co-morbidities and genetic characteristics that may contribute to the higher prevalence of AD among Latinos, in order to improve diagnosis and promote effective intervention strategies to reduce the risk of developing AD.
DEMOGRAPHIC AND SOCIOECONOMIC STATUS OF THE LATINO POPULATION IN THE US
The Latino population in the US is over 55 million, of which the majority are of Mexican descent (34.6 million), followed by Puerto Ricans (5.1 million) and other Latino islanders (Caribbean Hispanics) from Cuba and the Dominic Republic [8, 9]. Importantly, there are more Puerto Ricans living in US than Puerto Rico (3.6 million) due, in part, to a recent increase in migration forced by the dreadful economic situation on the island [8]. However, in contrast to immigrants from Mexico and other countries of Latin America, Puerto Ricans are US citizens at birth, even if they are born on the island of Puerto Rico. Mexican and Caribbean Hispanics comprised over 80% of the Latino population of the US [8]. The other members of the Latino community are those of Central and South America nationality. Latinos also constitute one of the fastest-growing racial/ethnic groups in the US. Based on the 2000 and 2010 census reports, the number of Latinos increased nearly 60% during that decade [9, 10]. If this rapid, 2–3% annual growth rate remains stable, it is predicted that the Latino population will reach over 97 million and account for one-quarter of the U.S. population by 2050 [9, 10]. Even though most of the Latino population is concentrated in the southern and western regions of the US, the highest percentage change in the Latino population from 2000 to 2010 took place mostly in the southeastern half of the USA [9, 10, 29, 30]. For examples, states such as South Carolina, Alabama, Tennessee, Kentucky, Arkansas, North Carolina, Mississippi, and Maryland experienced over 100% increase in their Latino population during that period [29]. This growth rate of the Latino population and its geographical distribution represent important factors to consider when designing strategies to study AD among the Latino population and the allocation of resources for caregiving. Moreover, it is important to define the cohort under study based on origin or heritage to detect differences intrinsic to each Latino subgroup.
An important demographic aspect of the Latino community is its socioeconomic status. As an under-represented group in many aspects of the social structure in the US, Latino income and socioeconomic status are significantly lower than that of White Non-Hispanics. Currently, 25% of the Latino population lives in poverty [30, 31]. As of 2015, the median income of White Non-Hispanics was $62,950, while the median family income for Latinos was $45,148 [30, 31]. Even though the Latino community has shown a steady increase in median income, more than any other ethnic group, there has not been a significant change in income inequality. In terms of income, there are also differences among the two major Latino subgroups. The median annual personal income for Mexicans aged 16 or older is $20,000, in contrast to $25,000 median annual personal income for Puerto Ricans in the same age group [31]. Although the personal income for Puerto Ricans is higher than for all Latinos in US (median annual income of $21,900), it is still lower than the median earnings of the US population ($30,000). However, we cannot assume that all Latinos are in the same income class. Proctor et al. reported that 38.6% of Latinos had a household income between $50,000 and $149,999, in comparison to 45.6% White Non-Hispanics and 33.6% Blacks [31]. Additionally, 6.9% of Latino households reported an income of $200,000 or more, in comparison to 14% of White Non-Hispanic and 5.1% of Black households [31]. It is also important to point out that, in most cases, the Latino cohorts selected for AD studies have fewer years of education than White Non-Hispanics [7, 11–24]. Therefore, household income is an important factor that should be used to define the cohort under study since low median income is associated with lower educational attainment and other risk factors associated with AD [32–37].
Low educational attainment is associated with individual health outcomes, including higher mortality rates and dementia [32–37]. The level of educational attainment and risk of developing AD or related dementias is still controversial since there are other factors that could explain the association between them. Low education level is associated with increased risk-factor exposures in adult life and differential brain reserve [38, 39]. In contrast, high educational attainment has been associated with cognitive resiliency in the elderly, suggesting that it could serve as a neuroprotective factor or, at least, increase the tolerance of pathological insults that lead to more detected hippocampal atrophy and less clinical severity [reviewed in 40]. Langa et al. reported that the reduction in the prevalence of dementia in the US from 2000 to 2012 was associated, in part, to an increase in educational attainment [37]. However, a 2013 report indicated that 22% of Latino adults (25 years and over) had earned an associate degree or higher, compared to Asians (60%), White Non-Hispanics (46%), and Blacks (31%) [41]. Despite a documented increase in reading and math proficiency within the Latino student community, this group continues to lag behind other ethnic groups, as shown by relatively lower scores on the SAT and ACT [41]. This lag is also reflected in the lower percentage of Masters (7%) and Doctorate (less than 1%) degrees conferred to Latinos in the US [41]. The lower educational attainment is illustrated in the Caribbean Hispanics cohort in Philadelphia and North Manhattan that had a mean year of education of 5.8 and 6.0, respectively [11–14, 16, 17]. These cohorts showed a significant lower age of onset in comparison to Blacks and White Non-Hispanics. This is a similar profile that the one reported for Mexican Americans [18]. In contrast, profiling of Puerto Ricans living in Puerto Rico diagnosed with AD had a mean years of education of 12.7 and very similar age of onset (77.9) to that reported for White Non-Hispanics (76.9) [20]. This observation is consistent with the reported association between reduced cognitive decline and higher education at early stages of AD, and perhaps it suggests that ethnicity is a confounding factor and not the cause for the health disparity observed. Thus, to avoid selection bias and to determine the effect that socioeconomic status and education has in the prevalence of AD among Latinos, the cohort under study should have representation of different levels of educational attainment and, consequently, socioeconomic status.
Another important component associated with the older Latino community is a reduced number of individuals who are proficient in English [41]. This represents an important barrier for Latino access to healthcare, especially for elders and the cognitively impaired. The barriers to Health Care Access Survey reported that older Latinos perceive personal beliefs, language proficiency, and economic status to be the most prevalent barriers to healthcare [42]. Effective patient-physician communication is central to the process of healthcare delivery, since language barriers may result in excessive orders for medical tests, lack of understanding of medication side effects and provider instructions, decreased use of primary care, increased use of the emergency department, and inadequate follow-up [33]. While close to 90% of the Latino population between ages 5 and 17 speaks English only or speaks the language very well, only about 40% of Latinos 69 years of age are fluent [41]. Moreover, a study in Sacramento found that older Latinos who spoke primarily Spanish had lower educational attainment than English speakers. 83% of the primarily Spanish speakers had 8 years or less of formal education [43]. This suggests that the population at higher risk of developing AD within the Latino population is less educated and less proficient in English. Based on these facts, healthcare providers have invested in cultural-sensitivity training and translators to facilitate the patient-physician communication [44]. However, only those Latinos who engage the healthcare system will benefit from these services and it may be too late for preventive healthcare. Thus, the lack of English proficiency in the population of Latinos at greater risk for AD represents an important demographic factor that needs to be taken in consideration in prevention and treatment strategies designed to reach out to the Latino population.
EPIDEMIOLOGY OF AD IN THE LATINO POPULATION
The Latino community is estimated to be 1.5 times more prone to develop AD than White Non-Hispanics. Today, there are approximately four million Latinos over the age of 65 living in the US, which is twice as many as those in 2000 [8, 9]. Since Latinos are the fastest growing population, it is expected that number of those over the age 65 will more than double by year 2030 [9]. In 2012, the number of Latinos diagnosed with AD was determined to be 379,000 [10]. However, particular cultural perspectives and a lack of accurate information among Latinos about normal aging, AD, its diagnosis, and medical and psychosocial interventions, could lead to a significant number of undiagnosed or underestimated AD cases among the Latino population. Nevertheless, it is estimated that the number of Latinos with AD will increase nine-fold by 2060, reaching 3.5 million [10]. These numbers are of epidemic proportions and represent an expected high social and economic burden.
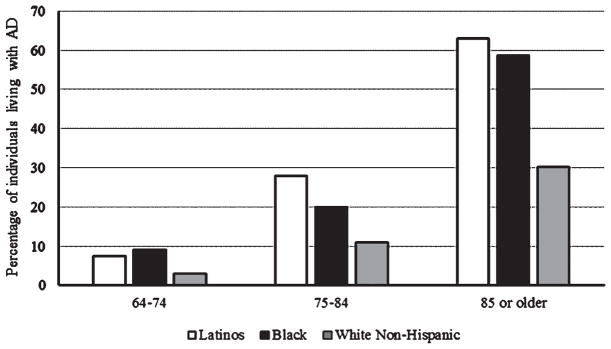
The distribution of the total number of Latinos living with AD or related dementias based on age illustrates a significant health disparity among the different ethnic groups. For the group of AD cases between 64 and 74 years of age, Latinos comprise 7.5% of the cases, whereas Blacks comprise 9.1% and White Non-Hispanic comprise 2.9% (Fig. 1). Between 75–84 years of age, Latinos living with AD are 27.9% of the total, in comparison to 19.9% Blacks and 10.9% White non-Hispanics (Fig. 1) [10, 22]. Most AD cases in the Latino population are in the group of 85 or older with 62.9% of the cases, which is distinctly higher to the 58.6% of Blacks and 30.2% of White non-Hispanics (Fig. 1) [10, 22]. Therefore, interventions directed to reduce the risk of AD within the Latino community need to address the disparate issues related to healthy aging among different ethnic groups.
Fig. 1.
Percentage of individuals living with AD. The graph illustrates the percentage of individuals diagnosed with AD per age group of the total cases in each ethnic background.
AD is currently the sixth leading cause of death in the US [45]. Previously, we found that the mortality rate associated with AD of Puerto Ricans living in Puerto Rico is higher than that for Puerto Ricans living in the US [15]. Geographical differences in mortality rate in Puerto Rico were identified, where some regions had lower or comparable rates to that reported in the US, while other regions showed significantly higher mortality rates associated with AD [15]. The underlying causes of the differential mortality rates and clinical profiles of AD patients in Puerto Rico are still unknown. A similar differential distribution of AD mortality in the US has been reported, even after correcting for age, race, and sex [46]. Two factors that correlated with higher mortality rate were age and educational attainment [46]. Interestingly, however, the leading causes of death changed when analyzed by race [45]. AD is the sixth leading cause of death among White Non-Hispanics, but eighth among Latinos and tenth among Blacks [45]. The factors that contribute to these differences are still poorly understood. However, factors ranging from different social determinants of health to the relative incidence of metabolic and vascular disorders and genetics may contribute to these differences.
Metabolic and vascular disorders in the Latino population
Are health disparities in AD associated with prevalence rates of diseases identified as risk factors? Approximately one-third of the adult U.S. population has metabolic syndrome [47, 48]. Metabolic syndrome affects cognitive performance, including memory, executive function, processing speed, and overall intellectual capabilities [23, 49–52]. The adverse effect of metabolic syndrome on cognitive function suggests that it may be associated with an increased risk of developing AD [50–52]. Notably, the prevalence of metabolic syndrome is the highest among Latino adults, suggesting that it could be a risk factor that may contribute to health disparities in AD. The overall prevalence of metabolic syndrome in the Latino community is highest among Puerto Ricans (37%) and lowest among South Americans (27%) [53]. This syndrome comprises a group of conditions such as diabetes, stroke, hyperlipidemia, and obesity, among others, which by themselves are prevalent in the Latino community and recognized as risk factors of AD and related dementias [48] (Table 2). Obesity and diabetes have been found to be prevalent in Latinos at alarming rates [54, 55]. Studies involving a Latino cohort in California indicated that 96% of women and 73% of men with metabolic syndrome had abdominal obesity using the conventional 102/88 cm threshold [53]. Abdominal adiposity predominates in Latino women. Reports from the National Center for Health Statistics indicated that 40% of Mexican men and 52% of Mexican women are overweight, in comparison to 34% of male and 33% of female White Non-Hispanics [56, 57].
Table 2.
Differences in the prevalence of risk factors among Latino subgroups
| Risk factor | Latinos (all) | Mexican | Puerto Rican | Cuban | Dominican | South American | Central American |
|---|---|---|---|---|---|---|---|
| Metabolic Syndrome | 35% | 35% | 37.1% | 34.8% | 31.5% | 27.3% | 35.8% |
| Diabetes | 17% | 18.3% | 18.1% | 13.4% | 18.1% | 10.2% | 17.7% |
| Obesity | 40.1% (men) | 35.6% (men) | 40.9% (men) | – | – | – | – |
| 44.4% (women) | 44.3% (women) | 51.4% (women) | |||||
| Hypertension | 25.4% | 17.2% | 24% | 25% | 22% | 12% | 12% |
Differences among Latino subgroups have also been reported [53]. Mexicans have higher proportions of overweight men and women than Puerto Ricans and Cubans, whereas a greater percentage of Puerto Rican women are overweight compared to Cuban women, and a greater percentage of Cuban men are overweight compared to Puerto Rican men [53]. The high prevalence of obesity, which is a risk factor for diabetes and cardiovascular disease, suggests that the Latino population is more prone to develop AD and related dementias due to a higher prevalence of these chronic, but preventable, diseases.
In the case of diabetes, the estimated percentage of Latinos with comorbid dementia and diabetes fluctuates from 46% to 24%, depending of the composition of the cohort [17, 18, 20, 23, 43]. The total prevalence of diabetes among Latinos is about 17% for both male and females [58]. However, again there are differences among Latino subgroups. The prevalence varied from 18.3% for Mexicans to 10.2% for people from South America [53]. It has also been shown that the prevalence among Dominicans and Puerto Ricans is 18.1%, while 17.7% of Central Americans and 13.4% of Cubans had type 2 diabetes [53]. Other metabolic factors such as high cholesterola and triglycerides, which also contribute to high blood pressure, are higher among Latinos in comparison to White Non-Hispanics [49–53]. Thus, modifiable and controllable social and cultural factors associated with these health conditions may explain the higher incidence of dementias among Latinos. The development and implementation of effective and culturally sensitive disease prevention strategies and healthcare programs could play an important role in reducing Latinos risk of developing dementias [59].
Physiologically, insufficient blood flow leads to tissue damage in the heart and brain [58, 60, 61]. Vascular diseases are also considered risk factors for AD and stroke-related dementia [62, 63]. In this regard, vascular dementia is the second leading cause of dementia. There are three major forms of vascular dementia, based on the pattern of the vascular brain lesion, namely multi-infarct dementia, strategic infarct dementia and subcortical vascular encephalopathy [62]. The location and morphology of vascular brain lesions dictate the clinical presentation. However, despite the establishment of consensus criteria for the diagnosis of vascular dementia, in many cases it goes undiagnosed making its prevalence very difficult to determine, with reported rates ranging from 4.5% to 39% [62]. Among Latino adults, Mexicans showed a significantly higher incidence of stroke than White Non-Hispanics [58]. Increased rates of stroke have been also reported among Caribbean Hispanics in comparison to White Non-Hispanics where self-reported stroke prevalence is highest in Dominican men and Puerto Rican women [58]. The prevalence of stroke is also different among US-born (6.6%) and foreign-born (4.5%) Latinos aged 60 or older. Importantly, cerebrovascular disease represents the fourth leading cause of death among Latinos, while AD is the seventh leading cause [45]. The cause for the high mortality rate associated with cerebrovascular disease among Latinos could be due to the high prevalence of modifiable cardiovascular disease risk factors such as total cholesterol and HDL cholesterol, body mass index, management of high blood pressure, diabetes and smoking [60]. The prevalence of these risk factors among Latinos represents a major reason for increased screening and interventions to prevent strokes. Based on these facts, vascular dementia should be suspected during the diagnosis process of Latinos with cognitive impairment complaints.
Familial AD among the Latino population
APOE ε4 inheritance has the strongest association with AD than any other identified genetic risk factor. In general, the APOE ε4 allele increases the risk of developing AD by 50% [64]. However, in the Latino population APOE ε4 does not seem to be associated with risk or age-of-onset of AD [13, 16, 17, 65]. Previous studies showed that Caribbean Hispanics have an increased frequency of AD compared to White Non-Hispanics, regardless of APOE genotype [13, 16, 17]. Several other studies suggest that the risk for early onset AD associated with APOE ε4 genotype is significantly lower in older Mexicans and Caribbean Hispanics compared to White Non-Hispanics [12, 18, 19, 66, 67]. In contrast, other studies found that APOE ε4 allele is associated with AD in the Latino population [68–70]. The reason for this lack of association between APOE ε4 and AD among Latinos is not understood. The disparity among reports suggests that different factors, including recruitment bias, sample size, or population stratification, could influence the results. However, it was reported that Mexicans are less likely to carry the APOE ε4 allele than White Non-Hispanics [71]. Although APOE ε4 allele frequency in other Latino subgroups is not known, this finding could explain the lack of correlation of APOE ε4 with the incidence of AD among Latinos. Alternatively, as the cumulative disadvantage theory suggests [72], a life course of differential exposure to previously unidentified genetic or biopsychosocial risk factors (e.g., acculturation, distress, depression, stigma, racism/prejudice) may be contributing factors to the increased prevalence and early onset of AD among the Latino population. The identification and association of disease associated SNPs in the Latino population have been described and reviewed elsewhere (reviewed in [18, 66, 67]). Here, we will highlight mutations found in familial cases of AD and those having a founder effect among Latinos.
Specific mutations associated with inherited AD have been found in the Latino population. A novel G206A mutation in Presenilin 1 (PSEN1) was identified in a cohort of Caribbean Hispanics in New York and the Dominic Republic [14]. The same mutation was found in a Latino cohort of Puerto Ricans in Philadelphia [17]. Most recently, the same mutation was also identified among Puerto Ricans living in Florida [73]. Based on DNA microsatellite marker analyses, the PSEN1 G206A is a founder mutation that causes familial AD among Puerto Ricans. In addition, a L174M mutation in PSEN1 was identified in a Cuban family with an early age of onset of AD [74]. Targeted sequencing of known genetic risk factors identified two missense mutations associated with late onset AD among Caribbean Hispanics [75]. A P460L mutation in Ephrin type-A receptor 1 (EPHA1) was found in one family from Dominic Republic with four affected members [75]. In the same study was also identified a mutation (K358R) in BIN1 (Bridging Integrator 1) segregating only in Caribbean Hispanic families, but the nationality of the families was not disclosed [75]. BIN1 has been associated directly to the pathophysiology of AD, affecting both tau and Aβ pathogenesis, indicating that this gene plays a crucial role in the pathogenesis of AD [76, 77]. Mutations in PSEN2 and APP have also been found in Caribbean Hispanics families, mainly of Puerto Rican and Dominican heritage (Table 3) [78]. Interestingly, specific mutations in MAPT and GRN (progranulin) genes (Table 3) were also associated with AD within Caribbean Hispanic families [78]. These results suggest that the presence of unidentified mutant carriers in the population could influence the early age of onset registered in cohorts composed of Caribbean Hispanic families.
Table 3.
Specific mutations identified in the Latino community
| Latino subgroup | Gene | Mutations | Reference |
|---|---|---|---|
| Mexican | PSEN1 | L171P, A431E, | [79–81] |
| MTOC II | A8027G, G7603A, A8003C, T8082C, C8301T | [82] | |
| Puerto Rican | PSEN1 | G206A | [17, 72, 73] |
| Cuban | PSEN1 | L174M | [74] |
| Dominican Republican | EPHA1 | P460L | [75] |
| Caribbean Hispanics (mostly Puerto Rican and Dominican) | PSEN1 | E318G | [78] |
| PSEN2 | A344V, I235F, P301A | [78] | |
| APP | V340M | [78] | |
| MAPT | S318L | [78] | |
| GRN | V519M, C222Y | [78] | |
| BIN1 | K358R | [75] | |
| Colombian | PSEN1 | E280A | [83] |
APP, amyloid precursor protein; BIN1, Bridging Integrator 1; EPHA1, Ephrin type-A receptor 1; GRN, progranulin; MAPT, microtubule-associated protein tau; MTOC II, mitochondrial cytochrome c-oxidase gene II; PSEN1, presenilin-1; PSEN1, presenilin-2.
In those of Mexican origin, two different PSEN1 mutations have been identified [79–81]. The L171P mutation was the first PSEN1 mutation identified in four Mexican families [79]. Almost a decade later, the A431E mutation in PSEN1 was identified in other Mexican families [80, 81]. This A431E mutation originated in the State of Jalisco in western Mexico, but 12 out of the 15 families identified live in Southern California [81]. Moreover, different missense mutations were found in the mitochondrial cytochrome c-oxidase gene II (MTCO II) in a Mexican cohort from Jalisco, Mexico. The most frequent (12%) mutation found was A8027G in four cases out of 33 patients with probable AD. Three of the identified cases had family history and late onset of AD, but one was diagnosed as early onset AD (50 years old). Four additional mutations in MTCO II were found (G7603A, A8003C, T8082C, C8301T), all of them in early onset AD cases, ranging from 44 to 57 years of age at the time of diagnosis [82]. Although MTCO II represents a new gene associated with familial AD, the role that this mitochondrial gene plays in the etiology of AD remains to be demonstrated. Among Colombian families an E280A mutation in PSEN1 was identified [83]. Interestingly, this mutation has a founder effect within the population with West European origin in the region of Antioquia Colombia, excluding those with African or Amerindian origin [83]. It is tempting to speculate that the younger age of onset among Latinos could be influenced by the inclusion of undetected inherited AD cases. Therefore, the identification of these mutations exclusive to subgroups of Latinos should also be taken into consideration by scientists during the establishment of cohorts and by physicians during clinical evaluations for AD.
FUTURE DIRECTIONS
Based on social determinants of health, comorbidities, and genetics factors mentioned here, it is plausible to hypothesize that the intersection of these factors contributes to the higher incidence of AD and related dementias among Latinos. More research is required to determine the direct role that these factors have on the etiology of AD and its prevalence among different ethnic groups. Unfortunately, the persistent healthcare disparities among underrepresented communities contrast with our effort to increase their participation in research projects where, in most instances, the results of the project and/or expected benefits are not communicated back to the community effectively. In recognition of health disparities in the US, the Committee on Community-Based Solutions to Promote Health Equity sought to address this issue by emphasizing solutions that could be implemented at the local and national levels [84]. The Committee detailed their findings and recommendations in a book titled, “Communities in Action: Pathways to Health Equity”, which states that health equity is a notion embedded in our nation’s values, and that we should work hard to achieve it [84]. Additionally, they indicated that health inequity has consequences for the US economy, national security, business viability, and public finances [84]. To address this issue, the Committee made a series of important recommendations, including the support of education and enforcement related to civil rights laws, the need for community-based research in low-income and minority communities, and the need to enhance inclusion of community members in health services practices and knowledge-based empowerment of the community, among others [84]. Taking these recommendations into consideration will contribute to the better understanding of health disparities in AD.
Understanding the diversity of the Latino community will help to differentiate the roles that genetic factors and social determinants of health play in the prevalence of AD. Even though Latinos tend to be considered as one group, there are significant differences among its subgroups [33]. These differences need to be studied and taken into consideration when planning research projects within the Latino community. In addition, noting the differential prevalence of co-morbidities among Latino subgroups (Table 2), it is equally important to broaden the recruitment of Latinos beyond the traditionally targeted inner city neighborhoods. This approach introduces recruitment and racial biases, depicting a narrow view of the Latino community and generating stigmas that could become embedded in the healthcare system [43]. A systematic way to tackle these issues is through community-based participatory research, where members of the Latino community are involved in all components of the research endeavor, from design and recruitment to data collection and interpretation [84]. Community leaders could also help identify members within different socioeconomic and educational levels in order to obtain a representative sample of the entire community. Additionally, fostering research collaborations between researchers in different areas of the US and their counterparts in Puerto Rico and Mexico, as well as other Latin American countries will help identify factors that contribute to the development of effective intervention strategies to reduce risk, improve diagnostic criteria, and lead to better treatment and care of AD patients. However, to develop and implement these research approaches successfully, it is important to address the social and cultural barriers that lead to mistrust of research and the healthcare system within the Latino community [42].
Health disparities in AD: A neuroethics perspective
There are several ethical and social implications of neuroscientific research and translation of that research in the clinical setting and public domain. Any complete model for understanding health disparities in AD, particularly in underrepresented communities, must include an understanding of the “agent in context” capturing the complex interactions of genetics, social contexts, and cultural differences. All of this calls for a practical and holistic approach responsive to the wide range of issues and stakeholders at play. The field of neuroethics focuses on the “study of the ethical, legal, and social questions that arise when scientific findings about the brain are carried into medical practice, legal interpretations, and health and social policy,” including health care disparities, and unequal access to the benefits of such advances [85]. Thus neuroethics provides an analytic and pragmatic framework to meaningfully discuss the ethical and social implications of health disparities and research in AD. A pragmatic neuroethics framework seeks to identify starting points and alternatives to resolve difficult ethical challenges through a negotiated scientific and social process, acquire empiric evidence to understand those ethical challenges and deliver practical solutions in the form of recommendations and guidelines [86]. Another neuroethics approach has been used in other settings to discuss the unique and intersecting challenges of ethically translating research into clinical care and the public domain, for example in the case of children and neonates with neurodevelopmental disorders [87] or in exploring the impact and interplay of medicalization with a First Nation’s knowledge and approaches to wellness in relation to early onset familial AD [88].
In the case of AD in the Latino community, a pragmatic neuroethics approach will help us identify and discuss challenges related to health care disparities in AD, decision-making when there are different cultural understandings at stake, consenting to the use of emerging treatments to modify disease progression and diagnostic procedures as well as challenges connected to participating in research [89]. Such an approach will also help us to address questions related to human values, integrity and respect for persons in the context of Latino communities, and the multidimensional and multidirectional nature of knowledge and communication about diagnosis, treatments and nature of AD [86]. In this way, a cross-cultural neuroethics approach can help identify and overcome potential misunderstandings that might arise when the worldview of researchers and clinicians differs from that of the community they are investigating or treating [90]. For example, frequently documented cultural differences distinguishing the Latino population include greater spirituality, a stronger sense of duty to family, and a lack of recognition of the early symptoms of dementia, as these are usually attributed to old age [91–93].
Neuroethics acknowledges the importance of individuals’ subjective experiences and of patient empowerment to ensure the delivery of culturally appropriate information about risk factors and treatments options, as well as to create diagnostic instruments that are sensitive to cultural differences and strategies to mitigate potential bias in the clinical diagnosis of AD in Latino populations. Another potential barrier in healthcare, which neuroethics can shed light on, is connected to mistrust towards the health care system. From a social justice perspective, neuroethics can help in identifying and creating strategies to address current barriers limiting access to health care and fostering health disparities. In sum, neuroethics can align community and health system priorities and perspectives in a cultural appropriate manner.
CONCLUSION
The epidemic of AD among the Latino population is real and represents an eminent social and economic issue. In order to tackle this epidemic, the diverse Latino population has specific characteristics that need to be considered based on the idiosyncrasies of the different subgroups. The specific characteristics include differences in traditions, socioeconomics, education, migration status, and others. These factors influence the proficiency of individuals to seek appropriate healthcare and navigate the health-care system, contributing to healthcare disparities that lead to increased risk for the development of chronic diseases, such as diabetes and cardiovascular diseases. Thus, it is important to consider the impact that social determinant factors have on the higher risk of AD among the Latino population. Additionally, the identification of endemic genetic factors that contribute to higher risk and familial AD among Latinos indicates that ethnicity also plays an important role. Research should be directed to understand the penetrance of the identified founder mutations as well as to the identification of genetic factors that are linked to increased AD risk within the Latino population. Lastly, to establish effective health promotion and disease prevention strategies, it is important to understand the influence of social determinants of health among the specific populations or ethnic groups that are being targeted. Using neuroethical approaches will help to address these issues in a manner that honors the values and social complexities of the Latino community and delivers culturally appropriate information about risk factors, diagnosis, and treatment options. Ultimately, these efforts will improve Latino engagement with healthcare services and lead to earlier interventions that lower the likelihood for dementia. After all, addressing healthcare and health disparities is a matter of social justice.
Acknowledgments
This work was support, in part, by MSU S3 Collaborative Grant Program, Michigan Alzheimer’s Disease Core Center (P30AG053760) and AG014449.
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-1261r2).
References
- 1.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza LC, Stern Y, Visser PJ, Scheltens P. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D, Mandelkow E, Holtzman D. Deciphering Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a011460. doi: 10.1101/cshperspect.a011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings J, Aisen PS, DuBois B, Frölich L, Jack CR, Jr, Jones RW, Morris JC, Raskin J, Dowsett SA, Scheltens P. Drug development in Alzheimer’s disease: The path to 2025. Alzheimers Res Ther. 2016;8:39. doi: 10.1186/s13195-016-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in US race/ethnic populations. Alzheimers Dement. 2017;13:72–83. doi: 10.1016/j.jalz.2016.06.2360. [DOI] [PubMed] [Google Scholar]
- 8.US Census Bureau. [Accessed on November 25, 2016]; https://www.census.gov/topics/population/data.html.
- 9.Krogstad JN. Fact Tank, news in the numbers. Pew Research Center; [Accessed on November 12, 2016]. Key facts about how the U.S. Hispanic population is changing. http://www.pewresearch.org/fact-tank/2016/09/08/key-facts-about-how-the-u-s-hispanic-population-is-changing/ [Google Scholar]
- 10.Wu S, Vega WA, Resendez J, Jin H. Latinos and Alzheimer’s disease: New numbers behind the crisis. [Accessed December 3, 2016];Projection of the costs for US Latinos living with Alzheimer’s disease through 2060. http://www.usagainstalzheimers.org/sites/default/files/Latinos-and-AD_USC_UsA2-Impact-Report.pdf.
- 11.Chin AL, Negash S, Hamilton R. Diversity and disparities in dementia: The impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25:187–195. doi: 10.1097/WAD.0b013e318211c6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Olarte L, Schupf N, Lee JH, Tang MX, Santana V, Williamson J, Maramreddy F, Tycko B, Mayeux R. Apolipoprotein E epsilon4 and age at onset of sporadic and familial Alzheimer disease in Caribbean Hispanics. Arch Neurol. 2006;63:1586–1590. doi: 10.1001/archneur.63.11.1586. [DOI] [PubMed] [Google Scholar]
- 14.Athan ES, Williamson J, Ciappa A, Santana V, Romas SN, Lee JH, Rondon H, Lantigua RA, Medrano M, Torres M, Arawaka S, Rogaeva E, Song YQ, Sato C, Kawarai T, Fafel KC, Boss MA, Seltzer WK, Stern Y, St George-Hyslop P, Tycko B, Mayeux R. A founder mutation in presenilin 1 causing early-onset Alzheimer disease in unrelated Caribbean Hispanic families. JAMA. 2008;286:2257–2263. doi: 10.1001/jama.286.18.2257. [DOI] [PubMed] [Google Scholar]
- 15.Figueroa R, Steenland K, MacNeil JR, Levey AI, Vega IE. Geographical differences in the occurrence of Alzheimer’s disease mortality: United States versus Puerto Rico. Am J Alzheimers Dis Other Demen. 2008;23:462–469. doi: 10.1177/1533317508321909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livney MG, Clark CM, Karlawish JH, Cartmell S, Negrón M, Nuñez J, Xie SX, Entenza-Cabrera F, Vega IE, Arnold SE. Ethnoracial differences in the clinical characteristics of Alzheimer disease at initial presentation at an urban Alzheimer’s disease center. Am J Geriatr Psychiatry. 2011;19:430–439. doi: 10.1097/JGP.0b013e3181f7d881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold SE, Vega IE, Karlawish JH, Wolk DA, Nunez J, Negron M, Xie SX, Wang LS, Dubroff JG, McCarty-Wood E, Trojanowski JQ, Van Deerlin V. Frequency and clinicopathological characteristics of presenilin 1 Gly206Ala mutation in Puerto Rican Hispanics with dementia. J Alzheimers Dis. 2013;33:1089–1095. doi: 10.3233/JAD-2012-121570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Bryant SE, Johnson L, Balldin V, Edwards M, Barber R, Williams B, Devous M, Cushings B, Knebl J, Hall J. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2013;33:373–379. doi: 10.3233/JAD-2012-121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitten LJ, Ortiz F, Fairbanks L, Bartzokis G, Lu P, Klein E, Coppola G, Ringman J. Younger age of dementia diagnosis in a Hispanic population in southern California. Int J Geriatr Psychiatry. 2014;29:586–593. doi: 10.1002/gps.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camacho-Mercado CL, Figueroa R, Acosta H, Arnold SE, Vega IE. Profiling of Alzheimer’s disease patients in Puerto Rico: A comparison of two distinct socioeconomic areas. SAGE Open Med. 2016;29:4. doi: 10.1177/2050312115627826. 2050312115627826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, Mayeux R. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14:481–493. [PubMed] [Google Scholar]
- 22.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 23.Muller M, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement Geriatr Cogn Disord. 2007;24:185–192. doi: 10.1159/000105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haan MN, Miller JW, Aiello AE, Whitmer RA, Jagust WJ, Mungas DM, Allen LH, Green R. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: Results from the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2007;85:511–517. doi: 10.1093/ajcn/85.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prince M. The 10/66 Dementia Research Group. 10 years on. Indian J Psychiatry. 2009;51:8–15. [PMC free article] [PubMed] [Google Scholar]
- 26.Llibre Rodriguez JJ, Ferri CP, Acosta D, Guerra M, Huang Y, Jacob KS, Krishnamoorthy ES, Salas A, Sosa AL, Acosta I, Dewey ME, Gaona C, Jotheeswaran AT, Li S, Rodriguez D, Rodriguez G, Kumar PS, Valhuerdi A, Prince M 10/66 Dementia Research Group. Prevalence of dementia in Latin America, India, and China: A population-based cross-sectional survey (for the 10/66 Dementia Research Group) Lancet. 2008;372:464–474. doi: 10.1016/S0140-6736(08)61002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and meta analysis. Alzheimers Dement. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Snyder HM, del Cardenas-Aguayo MC, Alonso A, Bain L, Iqbal K, Carrillo MC. Alzheimer’s disease research in Ibero America. Alzheimers Dement. 2016;12:749–754. doi: 10.1016/j.jalz.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Stepler R, Lopez MH. U.S. Latino Population Growth and Dispersion Has Slowed Since Onset of the Great Recession. Pew Research Center; 2016. [Accessed on Octobe 30, 2016]. http://www.pewhispanic.org/2016/09/08/latino-population-growth-and-dispersion-has-slowed-since-the-onset-of-the-great-recession/ [Google Scholar]
- 30.Demographic and Economic Profiles of Hispanics by State and County. Pew Research Center; 2014. [Accessed on October 30, 2016]. http://www.pewhispanic.org/states/ [Google Scholar]
- 31.Proctor BD, Seemega JL, Kollar MA. Income and Poverty in the United States: 2015. US Census Bureau; [Accessed on November 12, 2016]. http://www.census.gov/library/publications/2016/demo/p60-256.html. [Google Scholar]
- 32.Mortimer JA, Graves AB. Education and other socioeconomic determinants of dementia and Alzheimer’s disease. Neurology Suppl. 1993;4:S39–S44. [Google Scholar]
- 33.Morales LS, Lara M, Kington RS, Valdez RO, Escarce JJ. Socioeconomic, cultural, and behavioral factors affecting Hispanic health outcomes. J Health Care Poor Underserved. 2002;13:477–503. doi: 10.1177/104920802237532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology. 2009;72:460–465. doi: 10.1212/01.wnl.0000341782.71418.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker DP, Leon J, Smith Greenaway EG, Collins J, Movit M. The education effect on population health: A reassessment. Popul Dev Rev. 2011;37:307–332. doi: 10.1111/j.1728-4457.2011.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. 2016;35:407–411. doi: 10.1037/hea0000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, Weir DR. A comparison of the prevalence of dementia in the united states in 2000 and 2012. JAMA Intern Med. 2017;177:51–58. doi: 10.1001/jamainternmed.2016.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17:502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang X, Varma VR, Miller MI, Carlson MC. Education is associated with sub-regions of the hippocampus and the amygdala vulnerable to neuropathologies of Alzheimer’s disease. Brain Struct Funct. 2016;222:1469–1479. doi: 10.1007/s00429-016-1287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santiago DA, Calderon-Galdeano E, Taylos M. [Accessed on November 2, 2016];The condition of Latinos in Education: 2015 Factbook, Excelencia in Education. 2015 http://www.nccpsafety.org/assets/files/library/The_Condition_of_Latinos_in_Education.pdf.
- 42.Ortiz F, Fitten LJ. Barriers to healthcare access for cognitively impaired older Hispanics. Alzheimer Dis Assoc Disord. 2000;14:141–150. doi: 10.1097/00002093-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: The influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 44.Polacek GN, Martinez R. Assessing cultural competence at a local hospital system in the United States. Health Care Manag (Frederick) 2009;28:98–110. doi: 10.1097/HCM.0b013e3181a2cb32. [DOI] [PubMed] [Google Scholar]
- 45.Heron M. Death: Leading causes for 2014. Natl Vital Stat Rep. 2016;65:1–96. [PubMed] [Google Scholar]
- 46.Steenland K, MacNeil J, Vega I, Levey A. Recent trends in Alzheimer disease mortality in the United States, 1999 to 2004. Alzheimer Dis Assoc Disord. 2009;23:165–170. doi: 10.1097/wad.0b013e3181902c3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agular M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 48.Prasad H, Ryan DA, Celzo MF, Stapleton D. Metabolic syndrome: Definition and therapeutic implications. Postgrad Med. 2012;124:21–30. doi: 10.3810/pgm.2012.01.2514. [DOI] [PubMed] [Google Scholar]
- 49.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: A selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32:2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Dijk G, van Heijningen S, Reijne AC, Nyakas C, van der Zee EA, Eisel UL. Integrative neurobiology of metabolic diseases, neuroinflammation, and neurodegeneration. Front Neurosci. 2015;9:173. doi: 10.3389/fnins.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campos-Peña V, Toral-Rios D, Becerril-Pérez F, Sánchez-Torres C, Delgado-Namorado Y, Torres-Ossorio E, Franco-Bocanegra D, Carvajal K. Metabolic syndrome as a risk factor for Alzheimer’s disease: Is Aβ a crucial factor in both pathologies? Antioxid Redox Signal. 2016;26:542–560. doi: 10.1089/ars.2016.6768. [DOI] [PubMed] [Google Scholar]
- 52.De Felice FG, Lourenco MV. Brain metabolic stress and neuroinflammation at the basis of cognitive impairment in Alzheimer’s disease. Front Aging Neurosci. 2015;7:94. doi: 10.3389/fnagi.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heiss G, Snyder ML, Teng Y, Schneiderman N, Llabre MM, Cowie C, Carnethon M, Kaplan R, Giachello A, Gallo L, Loehr L, Avilés-Santa L. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: The Hispanic Community Health Study/Study of Latinos. Diabetes Care. 2014;37:2391–2399. doi: 10.2337/dc13-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yracheta JM, Alfonso J, Lanaspa MA, Roncal-Jimenez C, Johnson SB, Sánchez-Lozada LG, Johnson RJ. Hispanic Americans living in the United States and their risk for obesity, diabetes and kidney disease: Genetic and environmental considerations. Postgrad Med. 2015;127:503–510. doi: 10.1080/00325481.2015.1021234. [DOI] [PubMed] [Google Scholar]
- 55.Wu S, Fisher-Hoch SP, Reininger B, McCormick JB. Recommended levels of physical activity are associated with reduced risk of the metabolic syndrome in Mexican-Americans. PLoS One. 2016;11:e0152896. doi: 10.1371/journal.pone.0152896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;219:1–8. [PubMed] [Google Scholar]
- 57.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balfour PC, Jr, Ruiz JM, Talavera GA, Allison MA, Rodriguez CJ. Cardiovascular Disease in Hispanics/Latinos in the United States. J Lat Psychol. 2016;4:98–113. doi: 10.1037/lat0000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grill JD, Galvin JE. Facilitating Alzheimer disease research recruitment. Alzheimer Dis Assoc Disord. 2014;28:1–8. doi: 10.1097/WAD.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Piña IL, Ramirez SM, Rodriguez B, Sims M American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular, Stroke Nursing. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: A science advisory from the American Heart Association. Circulation. 2014;130:593–625. doi: 10.1161/CIR.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Statistics Committee Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: A report from the American heart association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013;5:17. doi: 10.3389/fnagi.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vijayan M, Reddy PH. Stroke, vascular dementia, and Alzheimer’s disease: Molecular links. J Alzheimers Dis. 2016;54:427–443. doi: 10.3233/JAD-160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 65.Tycko B, Lee JH, Ciappa A, Saxena A, Li CM, Feng L, Arriaga A, Stern Y, Lantigua R, Shachter N, Mayeux R. APOE and APOC1 promoter polymorphisms and the risk of Alzheimer disease in African American and Caribbean Hispanic individuals. Arch Neurol. 2004;61:1434–1439. doi: 10.1001/archneur.61.9.1434. [DOI] [PubMed] [Google Scholar]
- 66.Reitz C, Mayeux R. Genetics of Alzheimer’s disease in Caribbean Hispanic and African American populations. Biol Psychiatry. 2014;75:534–541. doi: 10.1016/j.biopsych.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Bryant SE, Johnson L, Reisch J, Edwards M, Hall J, Barber R, Devous MD, Sr, Royall D, Singh M. Risk factors for mild cognitive impairment among Mexican Americans. Alzheimers Dement. 2013;9:622–631. doi: 10.1016/j.jalz.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rippon GA, Tang MX, Lee JH, Lantigua R, Medrano M, Mayeux R. Familial Alzheimer disease in Latinos: Interaction between APOE, stroke, and estrogen replacement. Neurology. 2006;66:35–40. doi: 10.1212/01.wnl.0000191300.38571.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romas SN, Santana V, Williamson J, Ciappa A, Lee JH, Rondon HZ, Estevez P, Lantigua R, Medrano M, Torres M, Stern Y, Tycko B, Mayeux R. Familial Alzheimer disease among Caribbean Hispanics: A reexamination of its association with APOE. Arch Neurol. 2002;59:87–91. doi: 10.1001/archneur.59.1.87. [DOI] [PubMed] [Google Scholar]
- 70.Lee JH, Barral S, Cheng R, Chacon I, Santana V, Williamson J, Lantigua R, Medrano M, Jimenez-Velazquez IZ, Stern Y, Tycko B, Rogaeva E, Wakutani Y, Kawarai T, St George-Hyslop P, Mayeux R. Age-at-onset linkage analysis in Caribbean Hispanics with familial late-onset Alzheimer’s disease. Neurogenetics. 2008;9:51–60. doi: 10.1007/s10048-007-0103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Campos M, Edland SD, Peavy GM. Exploratory study of apolipoprotein E ε4 genotype and risk of Alzheimer’s disease in Mexican Hispanics. J Am Geriatr Soc. 2013;61:1038–1040. doi: 10.1111/jgs.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dannefer D. Aging as intracohort differentiation: Accentuation, the Matthew effect, and the life course. Sociol Forum. 1997;2:211–236. [Google Scholar]
- 73.Ravenscroft TA, Pottier C, Murray ME, Baker M, Christopher E, Levitch D, Brown PH, Barker W, Duara R, Greig-Custo M, Betancourt A, English M, Sun X, Ertekin-Taner N, Graff-Radford NR, Dickson DW, Rademakers R. The presenilin 1 p. Gly206Ala mutation is a frequent cause of early-onset Alzheimer’s disease in Hispanics in Florida. Am J Neurodegener Dis. 2016;5:94–101. [PMC free article] [PubMed] [Google Scholar]
- 74.Bertoli Avella AM, Marcheco Teruel B, Llibre Rodriguez JJ, Gomez Viera N, Borrajero Martinez I, Severijnen EA, Joosse M, van Duijn CM, Heredero Baute L, Heutink P. A novel presenilin 1 mutation (L174 M) in a large Cuban family with early onset Alzheimer disease. Neurogenetics. 2002;4:97–104. doi: 10.1007/s10048-002-0136-6. [DOI] [PubMed] [Google Scholar]
- 75.Vardarajan BN, Ghani M, Kahn A, Sheikh S, Sato C, Barral S, Lee JH, Cheng R, Reitz C, Lantigua R, Reyes-Dumeyer D, Medrano M, Jimenez-Velazquez IZ, Rogaeva E, St George-Hyslop P, Mayeux R. Rare coding mutations identified by sequencing of Alzheimer disease genome-wide association studies loci. Ann Neurol. 2015;78:487–498. doi: 10.1002/ana.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calafate S, Flavin W, Verstreken P, Moechars D. Loss of Bin1 promotes the propagation of tau pathology. Cell Rep. 2016;17:931–940. doi: 10.1016/j.celrep.2016.09.063. [DOI] [PubMed] [Google Scholar]
- 77.Sannerud R, Annaert W. Bin1 and CD2AP polarize Aβ generation in neurons. EMBO Rep. 2017;18:5–7. doi: 10.15252/embr.201643634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JH, Kahn A, Cheng R, Reitz C, Vardarajan B, Lantigua R, Medrano M, Jiménez-Velázquez IZ, Williamson J, Nagy P, Mayeux R. Disease-related mutations among Caribbean Hispanics with familial dementia. Mol Genet Genomic Med. 2014;2:430–437. doi: 10.1002/mgg3.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramirez-Dueñas MG, Rogaeva EA, Leal CA, Lin C, Ramirez-Casillas GA, Hernandez-Romo JA, St George-Hyslop PH, Cantu JM. A novel Leu171Pro mutation in presenilin-1 gene in a Mexican family with early onset Alzheimer disease. Ann Genet. 1998;41:149–153. [PubMed] [Google Scholar]
- 80.Yescas P, Huertas-Vazquez A, Villarreal-Molina MT, Rasmussen A, Tusié-Luna MT, López M, Canizales-Quinteros S, Alonso ME. Founder effect for the Ala431Glu mutation of the presenilin 1 gene causing early-onset Alzheimer’s disease in Mexican families. Neurogenetics. 2006;7:195–200. doi: 10.1007/s10048-006-0043-3. [DOI] [PubMed] [Google Scholar]
- 81.Murrell J, Ghetti B, Cochran E, Macias-Islas MA, Medina L, Varpetian A, Cummings JL, Mendez MF, Kawas C, Chui H, Ringman JM. The A431E mutation in PSEN1 causing familial Alzheimer’s disease originating in Jalisco State, Mexico: An additional fifteen families. Neurogenetics. 2006;7:277–279. doi: 10.1007/s10048-006-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loera-Castañeda V, Sandoval-Ramírez L, Pacheco Moisés FP, Macías-Islas MÁ, Alatorre Jiménez MA, González-Renovato ED, Cortés-Enríquez F, Célis de la Rosa A, Velázquez-Brizuela IE, Ortiz GG. Novel point mutations and A8027G polymorphism in mitochondrial-DNA-encoded cytochrome c oxidase II gene in Mexican patients with probable Alzheimer disease. Int J Alzheimers Dis. 2014;2014:794530. doi: 10.1155/2014/794530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lalli MA, Cox HC, Arcila ML, Cadavid L, Moreno S, Garcia G, Madrigal L, Reiman EM, Arcos-Burgos M, Bedoya G, Brunkow ME, Glusman G, Roach JC, Hood L, Kosik KS, Lopera F. Origin of the PSEN1 E280A mutation causing early-onset Alzheimer’s disease. Alzheimers Dement. 2014;10(Suppl 5):S277–S283e10. doi: 10.1016/j.jalz.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.National Academies of Sciences, Engineering, and Medicine. Communities in action: Pathways to health equity. The National Academies Press; Washington, DC: 2017. doi:10.17226–24624. [PubMed] [Google Scholar]
- 85.Safire W. Neuroethics: Mapping the Field Conference Proceedings. Dana Press; New York: 2002. Visions for a new field of neuroethics. [Google Scholar]
- 86.Racine E. Pragmatic Neuroethics: Improving treatment and understanding of the Mind-Brain. The MIT Press; 2010. [Google Scholar]
- 87.Racine E, Bell E, Di Pietro NC, Wade L, Illes J. Evidence-based neuroethics for neurodevelopmental disorders. Semin Pediatr Neurol. 2011;18:21–25. doi: 10.1016/j.spen.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 88.Cabrera LY, Beattie BL, Dwosh E, Illes J. Converging approaches to understanding early onset familial Alzheimer disease: A First Nation study. SAGE Open Med. 2015;3:2050312115621766. doi: 10.1177/2050312115621766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gauthier S, Leuzy A, Racine E, Rosa-Neto P. Diagnosis and management of Alzheimer’s disease: Past, present and future ethical issues. Prog Neurobiol. 2013;110:102–113. doi: 10.1016/j.pneurobio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 90.Brief E, Illes J. Tangles of neurogenetics, neuroethics, and culture. Neuron. 2010;68:174–177. doi: 10.1016/j.neuron.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 91.Napoles AM, Chadiha L, Eversley R, Moreno-John G. Developing culturally sensitive dementia caregiver interventions: Are we there yet? Am J Alzheimers Dis Other Demen. 2010;25:389–406. doi: 10.1177/1533317510370957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ayalon L, Arean PA. Knowledge of Alzheimer’s disease in four ethnic groups of older adults. Int J Geriatr Psychiatry. 2004;19:51–57. doi: 10.1002/gps.1037. [DOI] [PubMed] [Google Scholar]
- 93.Gelman CR. Learning from recruitment challenges: Barriers to diagnosis, treatment, and research participation for Latinos with symptoms of Alzheimer’s disease. J Gerontol Social Work. 2010;53:94–113. doi: 10.1080/01634370903361847. [DOI] [PMC free article] [PubMed] [Google Scholar]