ABSTRACT
A universal hepatitis C virus (HCV) vaccine should elicit multiantigenic, multigenotypic responses, which are more likely to protect against challenge with the range of genotypes and subtypes circulating in the community. A vaccine cocktail and vaccines encoding consensus HCV sequences are attractive approaches to achieve this goal. Consequently, in a series of mouse vaccination studies, we compared the immunogenicity of a DNA vaccine encoding a consensus HCV nonstructural 5B (NS5B) protein to that of a cocktail of DNA plasmids encoding the genotype 1b (Gt1b) and Gt3a NS5B proteins. To complement this study, we assessed responses to a multiantigenic cocktail regimen by comparing a DNA vaccine cocktail encoding Gt1b and Gt3a NS3, NS4, and NS5B proteins to a single-genotype NS3/4/5B DNA vaccine. To thoroughly evaluate in vivo cytotoxic T lymphocyte (CTL) and T helper (Th) cell responses against Gt1b and Gt3a HCV peptide-pulsed target cells, we exploited a novel fluorescent-target array (FTA). FTA and enzyme-linked immunosorbent spot (ELISpot) analyses collectively indicated that the cocktail regimens elicited higher responses to Gt1b and Gt3a NS5B proteins than those with the consensus vaccine, while the multiantigenic DNA cocktail significantly increased the responses to NS3 and NS5B compared to those elicited by the single-genotype vaccines. Thus, a DNA cocktail vaccination regimen is more effective than a consensus vaccine or a monovalent vaccine at increasing the breadth of multigenotypic T cell responses, which has implications for the development of vaccines for communities where multiple HCV genotypes circulate.
IMPORTANCE Despite the development of highly effective direct-acting antivirals (DAA), infections with hepatitis C virus (HCV) continue, particularly in countries where the supply of DAA is limited. Furthermore, patients who eliminate the virus as a result of DAA therapy can still be reinfected. Thus, a vaccine for HCV is urgently required, but the heterogeneity of HCV strains makes the development of a universal vaccine difficult. To address this, we developed a novel cytolytic DNA vaccine which elicits robust cell-mediated immunity (CMI) to the nonstructural (NS) proteins in vaccinated animals. We compared the immune responses against genotypes 1 and 3 that were elicited by a consensus DNA vaccine or a DNA vaccine cocktail and showed that the cocktail induced higher levels of CMI to the NS proteins of both genotypes. This study suggests that a universal HCV vaccine can most readily be achieved by use of a DNA vaccine cocktail.
KEYWORDS: HCV, NS5, vaccine, consensus, ELISpot, multiantigenic, DNA vaccines, hepatitis, multigenotypic
INTRODUCTION
The number of hepatitis C virus (HCV)-infected individuals continues to increase, as it is now estimated that there are 71 million individuals living with persistent HCV infection in the world, with 1.34 million deaths in 2015, a number comparable to that for tuberculosis and higher than that for HIV (1). Despite the fact that the new direct-acting antivirals (DAA) cure a large proportion of infected individuals, the therapy is not universally available due to its high cost, and consequently, individuals in developing countries with the greatest need are likely to remain untreated and represent a reservoir for continuing transmission. In 2015, there were more new HCV infections than patients who were started on treatment (1).
Furthermore, the treatment does not protect against reinfection, and with these limitations, DAA cannot end the HCV epidemic in the large population that is afflicted globally (2). Thus, an effective prophylactic vaccine is necessary to control the epidemic (3).
Although the HCV envelope glycoproteins (E1 and E2) are highly variable, neutralizing antibodies (NAb) to epitopes of the viral E2 glycoprotein can be protective and are associated with HCV clearance (4–8). Many broadly neutralizing Ab (bNAb) that target distinct epitopes on E1 and E2 and neutralize diverse HCV strains have been isolated from infected humans (9–12). Early induction of NAb contributes to control of viremia and resolution of infection in humans (13–15), while bNAb have been shown to be protective against HCV challenge in animal models (9, 10, 16, 17). NAb epitopes have been identified in E2, including hypervariable region 1 (HVR1) (18, 19) and a downstream region including the binding site for CD81 (9), one of several proteins involved in HCV binding and entry.
Novel humanized mouse models of HCV infection have reinforced the protective role of NAb; uPA-SCID mice repopulated with human liver cells were protected against homologous HCV challenge by immunoglobulin from infected individuals (20) and against heterologous challenge by use of a cross-neutralizing human monoclonal antibody (21). However, the virus continually escapes the NAb response by mutations which result in the appearance and selection of escape mutants (6, 22), making it difficult for a single bNAb to protect against a diverse HCV quasispecies challenge. Furthermore, E2 is heavily glycosylated, preventing access of NAb (23), while cell-cell transmission of the virus is common (24). The envelope HVR1 has been suggested to act as an immunological decoy to divert humoral responses toward variable regions of the virus envelope and to shield NAb epitopes (25, 26), and virus-associated lipids also protect against NAb activity (27, 28). Therefore, induction of multiple neutralizing monoclonal antibodies against envelope proteins may be necessary, with targeting of distinct epitopes, but to date, the most advantageous NAb combinations have not been identified.
Consequently, we (29, 30) and others (31–35) developed vaccines which are designed to elicit cell-mediated immunity (CMI) to the nonstructural (NS) proteins (e.g., NS3 and NS5), which are more conserved than the envelope proteins. Clearance of acute HCV infection is temporally associated with diverse, sustained, HCV-specific CD8+ and CD4+ T cell responses to multiple NS proteins, including NS3 and NS5 (36), and depletion of these cells leads to virus persistence in chimpanzees (37, 38). Furthermore, CMI appears to be responsible for the resultant immunity after clearance of a primary infection, resulting in reductions in the level and duration of viremia after reinfection (39, 40). Thus, a robust CMI to the NS proteins has the potential to restrict infection, eliminate virus-infected cells after challenge, and prevent persistent infection at the very least. As acute HCV infection is often subclinical and is not a serious disease, this is a highly acceptable outcome, and it has been suggested that a T cell vaccine is particularly suitable for HCV (41).
However, the limited protection against reinfection is often restricted to homologous virus strains, as reinfection with heterologous as opposed to homologous virus may lead to persistent infection (42, 43). Recent studies showed that HCV-specific T cells have limited cross-reactivity with proteins from different HCV genotypes (34, 44), while some cross-reactivity between epitope variants from homologous strains was detected (44). Consequently, the aim of a universal T cell-based vaccine should be to prevent the development of persistent infection resulting from exposure to multiple HCV genotypes. The design of a vaccine to achieve this aim should include conserved immunogens from multiple genotypes or a consensus sequence with the capacity to elicit multigenotypic T cell responses against NS proteins. Previous studies from our laboratory used cytolytic DNA vaccines in a unique bicistronic vector whereby the expression of the HCV immunogen and the cytolytic protein perforin (PRF) is controlled by the cytomegalovirus (CMV) and simian virus 40 (SV40) promoters, respectively. Following the expression of cytolytic PRF and a sufficient accumulation of the immunogen, necrotic cell death occurs in vaccine-targeted cells, resulting in significantly increased cross-presentation (45) and CMI (29, 30) and greater protection against viral challenge (46). Thus, the aim of this study was to examine multiantigenic, multigenotypic (genotype 1b [Gt1b] and Gt3a), HCV-specific T cell responses in mice after vaccination with cytolytic DNA encoding genotype-specific antigens, a global consensus sequence, or a Gt1b and Gt3a DNA cocktail.
RESULTS
DNA vaccines and vaccination regimens.
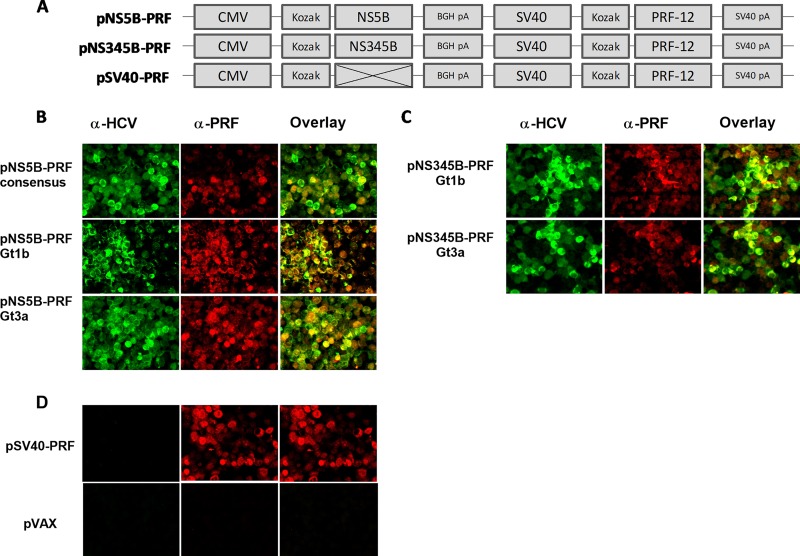
We previously described bicistronic, cytolytic DNA vaccines developed using the pVAX backbone (29, 30, 45–47). The premise of a cytolytic DNA vaccine is that necrotic cell death occurs in the vaccine antigen-positive cells after the accumulation of the antigen (30). This results in the release of the HCV immunogen and damage-associated molecular patterns (DAMPs), creating an adjuvant-rich milieu, and our studies have shown that the cytolytic DNA vaccine enhances the antigen-specific immune responses after immunization compared to those with canonical DNA (29, 30, 45, 46). For the current study, we generated different bicistronic DNA vaccines in which an NS5B global consensus or Gt1b or Gt3a protein was encoded downstream of the CMV promoter and the expression of cytolytic PRF was driven by a weaker SV40 promoter (Fig. 1A). In the same manner and as we described previously (29), NS3, NS4, and NS5B of Gt1b or Gt3a were encoded downstream of the CMV promoter, while a control vaccine (SV40-PRF) encoded only PRF (Fig. 1A). Following transient transfection of HEK293 cells and using an immunofluorescence assay, we confirmed that the HCV proteins and PRF colocalized in transfected HEK293 cells (Fig. 1B and C).
FIG 1.
DNA vaccines and detection of antigen expression. (A) Schematic diagram of the bicistronic DNA plasmid vaccines. pNS5B-PRF (7.3 kb) and pNS345B-PRF (9.8 kb) were based on the control plasmid pSV40-PRF (5.5 kb). Codon-optimized sequences in pNS5B-PRF that represent the HCV NS5B consensus sequence or Gt1b or Gt3a sequences were cloned into the multiple-cloning site with a Kozak sequence for enhanced mammalian expression under the control of the CMV promoter and flanked by the bovine growth hormone polyadenylation (BGH pA) site for termination of transcription. The expression of the cytolytic protein PRF was under the control of the weaker SV40 promoter and flanked by a downstream SV40 polyadenylation (pA) sequence. The pNS345B-PRF plasmid was modeled on pNS5B-PRF; the HCV genotype 1b or 3a NS3, NS4, and NS5B sequences were cloned into the multiple-cloning site as described previously (29). pSV40-PRF was used as a control plasmid, in which PRF was expressed from the SV40 promoter but no antigen was cloned under the control of the CMV promoter. (B to D) Photomicrographs of HEK 293T cells transfected with the different DNA vaccines (B and C) or vaccine controls (D) and stained with anti-HCV antibodies, anti-PRF, or a mixture of both (29, 30), showing colocalization of PRF and HCV antigens within transfected cells.
Immunogenicity of NS5B consensus and cocktail vaccination regimens as assessed by ELISpot assay.
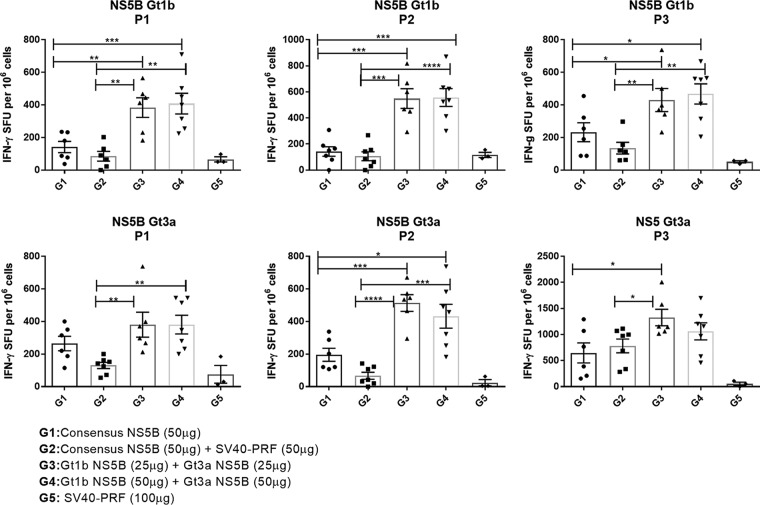
As previous studies showed that NS5B was highly immunogenic (35, 48), we examined whether multigenotypic responses were elicited more effectively by a global consensus NS5B vaccine or by a cocktail of two DNA vectors encoding NS5B proteins derived from Gt1b and Gt3a strains. BALB/c mice were split into groups (n = 7) and immunized with either (i) 50 μg of global consensus NS5B DNA vaccine (G1), (ii) a DNA cocktail comprising 25 μg of Gt1b NS5B and 25 μg of Gt3a NS5B (G3), (iii) a DNA cocktail comprising 50 μg of Gt1b NS5B and 50 μg of Gt3a NS5B (G4), or (iv) 100 μg of control vector (SV40-PRF) (G5). One group (G2) was vaccinated with a cocktail comprising the consensus NS5B vaccine (50 μg) and the control SV40-PRF (50 μg) vaccine to account for any effects resulting from expression of increased levels of PRF. Mice were vaccinated three times at 2-week intervals, and the magnitudes of the resultant T cell responses were determined 2 weeks after the last dose by a traditional enzyme-linked immunosorbent spot (ELISpot) assay following in vitro peptide stimulation. Splenocytes were stimulated with peptide pools derived from panels of overlapping 14- to 19-mer peptides spanning the entire NS5B proteins of strains J4L6S (genotype 1b) and K3a/650 (genotype 3a) (NIAID). For each genotype, three peptide NS5B pools were made (P1, P2, and P3), each containing 30 to 34 individual overlapping peptides. The results of this study showed that immunization with the cocktail of Gt1b and Gt3a NS5B proteins (G3 and G4) elicited significantly higher responses to all NS5B peptide pools than those with the global consensus NS5B vaccine (G1 and G2) (Fig. 2). Essentially, peptide stimulation of splenocytes from the G5 mice (vaccinated with the SV40-PRF control vaccine) resulted in mean background responses ranging from 10 to 100 spot-forming units (SFU), whereas the NS5B-specific responses for mice in G1 and G2 (vaccinated with the global consensus vaccine) ranged from 50 to 700 SFU and those for the cocktail-vaccinated mice (G3 and G4) ranged from 380 to 1,280 SFU (Fig. 2). There was no significant difference in the magnitudes of responses in G3 mice (vaccinated with 25 μg Gt1b plus 25 μg Gt3a DNA) and G4 mice (vaccinated with 50 μg Gt1b plus 50 μg Gt3a DNA, i.e., containing twice the amount of genetic material) (Fig. 2). While all NS5B DNA constructs proved to be immunogenic, the study clearly showed that significantly larger numbers of NS5B-specific T cells secreted gamma interferon (IFN-γ) in response to restimulation with Gt1b and Gt3a peptides for mice immunized with the lower cocktail dose (25 μg Gt1b and 25 μg Gt3a DNA) than for mice immunized with 50 μg of the global consensus NS5B vaccine. Responses to peptides matched to the global consensus NS5B sequence were not examined, as there is no likelihood of ever encountering a “consensus” HCV genotype in nature.
FIG 2.
Cell-mediated responses to DNA vaccines as determined by ELISpot analysis. Groups of BALB/c mice (n = 7 for G1 to G4 and n = 3 for G5) were vaccinated with DNA encoding the NS5B consensus sequence or the NS5B consensus sequence (25 μg) plus an equal dose of SV40-PRF (25 μg), a cocktail comprised of 25 μg each of Gt1b and Gt3a sequences encoding NS5B, a similar cocktail comprised of 50 μg each of Gt1b and Gt3a sequences, or a control (G5). At 14 days postvaccination, the splenocytes were harvested and stimulated with peptides representing the NS5B protein (4 μg), and IFN-γ-secreting cells were detected as described in Materials and Methods. The data are presented as mean (n = 7) ± SEM SFU per 1 × 106 splenocytes, and the unpaired nonparametric Mann-Whitney U test was used to analyze the statistical significance. Note that the scale of the y axis differs in different panels. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In vivo efficacy of NS5B consensus and cocktail DNA vaccination regimens.
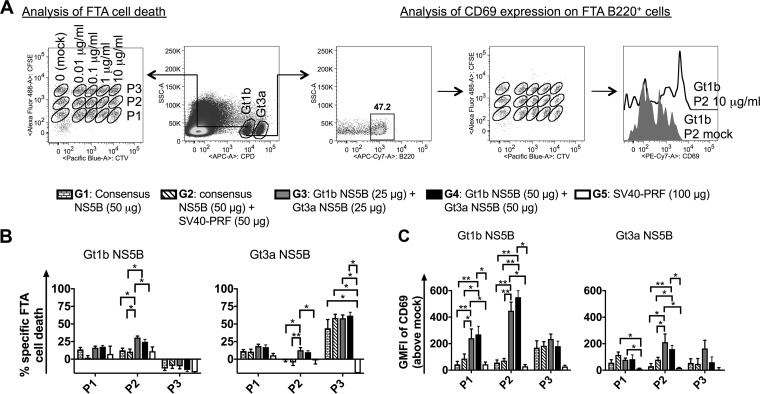
The ability to monitor T cell responses in vivo after immunization is of paramount importance for the development of efficacious vaccines. Therefore, we conducted fluorescent-target array (FTA) analysis to compare the in vivo responses 14 days after the final vaccination. FTA is a recently described technology (49, 50) which allows simultaneous measurement of in vivo cytotoxic T lymphocyte (CTL) (CD8) and Th (CD4) cell responses against numerous target cells pulsed with different peptides corresponding to vaccine antigens. It is based on the recovery of FTA target cells loaded with major histocompatibility complex (MHC)-binding peptides (and is hence a measure of cell killing by antigen-specific CTLs) and upregulation of CD69 on FTA B220+ target cells pulsed with MHC class II (MHC-II)-binding peptides (as a measure of antigen-specific CD4+ T cell help) following injection into vaccinated mice. The FTA is constructed using different concentrations and combinations of vital dyes, such as carboxyfluorescein succinimidyl ester (CFSE), cell trace violet (CTV), and cell proliferation dye efluor 670 (CPD), to delineate several populations of target cells by fluorescence assay. In this study, we generated an FTA comprised of splenocytes from naive mice pulsed with several concentrations of three different peptide pools (P1 to P3) of NS5B and injected these into mice vaccinated with the consensus NS5B vaccine or a cocktail encoding Gt1b and Gt3a NS5B proteins. The T cell responses were assessed by flow cytometry of FTA cells recovered from the spleens of vaccinated mice (Fig. 3A). The highest T cell responses in vivo resulted from target cells pulsed with 10 μg/ml of peptides (data not shown). Consequently, to clearly delineate the trends in the magnitude of T cell responses, we compared the genotype-specific responses to target cells pulsed with 10 μg/ml of NS5B peptide pools (P1, P2, and P3) (Fig. 3).
FIG 3.
FTA analysis of in vivo NS5B-specific T cell responses following consensus NS5B or cocktail vaccination. The Gt1b and Gt3a NS5B-specific in vivo CTL responses were examined following vaccination of mice with DNA encoding the consensus NS5B protein or a cocktail encoding Gt1b NS5B and Gt3a NS5B. Female BALB/c mice (n = 6 or 7 per group for G1 to G4 and n = 3 for G5) were vaccinated as described in Materials and Methods, and 13 days after the final vaccination, 1.3 × 106 peptide-pulsed cells (for each target cell cluster) or mock-pulsed target cells were transferred i.v. Fifteen hours after FTA challenge, splenocytes were harvested and analyzed by flow cytometry to determine the surviving number of peptide-pulsed target cells relative to the number of unpulsed target cells. (A) Flow diagram of the FTA analysis used in the experiments. Doubly discriminated lymphocytes were gated prior to analysis of the Gt1b and Gt3a targets based on CPD emission, as shown. For simplicity, the plots show an example in which Gt1b targets from a G4-vaccinated mouse were gated. All the peptide-pulsed cell targets were gated and analyzed for the percent recovery relative to that of mock-pulsed targets to determine the specific FTA cell loss, using the following equation: % cell loss = [(% mock-pulsed targets − % peptide-pulsed targets)/% mock-pulsed targets] × 100. For Th cell response analysis, B220+ cells from Gt1b or Gt3a targets were gated, and the geometric mean fluorescence intensity (GMFI) of CD69 was determined by flow cytometry. The values plotted in panel C reflect the GMFI of CD69 on peptide-pulsed B220+ targets minus the GMFI of CD69 on mock-pulsed B220+ targets. The representative histogram plot shows CD69 expression on B220+ cell targets pulsed with 10 μg/ml of Gt1b NS5B pool 2 (black line) or 0 μg/ml of Gt1b NS5B pool 2 (gray filled area). (B and C) Bar graphs showing the means and SEM of Gt1b- and Gt3a-specific CTL responses against target cells pulsed with 10 μg/ml of NS5B pools 1 to 3 (P1 to P3) (B) and Th cell responses for the same targets, based on the geometric mean fluorescence intensity of CD69 on FTA B220+ cells (C). The statistical significance of the data was determined as described in Materials and Methods. Note that only comparisons where at least one of the groups being compared had a statistically significant enhancement of the responses relative to those of the control group are shown. *, P < 0.05; **, P < 0.01.
The data demonstrate that significant in vivo CTL responses were generally detected against Gt3a NS5B P3, irrespective of whether the mice were vaccinated with the global consensus (G1 and G2) or cocktail (G3 and G4) vaccine, relative to those in the control mice (G5) (Fig. 3B). Most animals in G1 to G4 showed >50% killing of 10-μg/ml Gt3a NS5B P3-pulsed target cells (Fig. 3B), and statistical analysis showed no differences in G1 to G4. The negative percentages in Fig. 3B are indicative of no significant killing of peptide-pulsed target cells above that of the respective mock-pulsed targets injected into animals. However, only mice that were vaccinated with the cocktail regimens exhibited CTL responses to Gt1b and Gt3a NS5B P2, which were not detected in other groups (Fig. 3B), implying that cocktail vaccination increased the breadth of NS5B CTL responses. Examination of in vivo Th cell responses showed that mice vaccinated with the cocktail regimens elicited the greatest and significantly increased responses to Gt1b NS5B P1 and P2, as well as Gt3a NS5B P2 (Fig. 3C), compared to those with the consensus regimen. Statistically significant Th cell responses above those of the controls were not detected in any of the consensus NS5B-vaccinated groups, with the exception of G2 responses to Gt3a NS5B P1.
Although ELISpot assay does not differentiate between CD4+ and CD8+ T cell responses, the increased magnitude of cocktail responses in the ELISpot assay correlated with increased responses observed with the FTA, which clearly defines the CD8+ and CD4+ T cell contributions to the overall vaccine immunogenicity. Overall, the data suggest that the cocktail vaccination regimens increased the breadth and magnitude of CTL and Th cell responses in vivo more effectively than the global consensus vaccination regimens did.
A cocktail vaccine elicits more effective multiantigenic, multigenotypic immunity in vivo.
The above-mentioned studies suggest that the cocktail Gt1b and Gt3a DNA vaccine elicited multigenotypic, HCV-specific T cell responses to a single protein (NS5B) more effectively than a consensus vaccine did. However, an HCV vaccine should ideally elicit CMI to several antigens, and our previous study showed that a multiantigenic DNA vaccine encoding the HCV Gt3a NS3/4/5B polyprotein as an immunogen induced T cell responses to each of the encoded antigens without compromising the immunogenicity of the individual antigens (29).
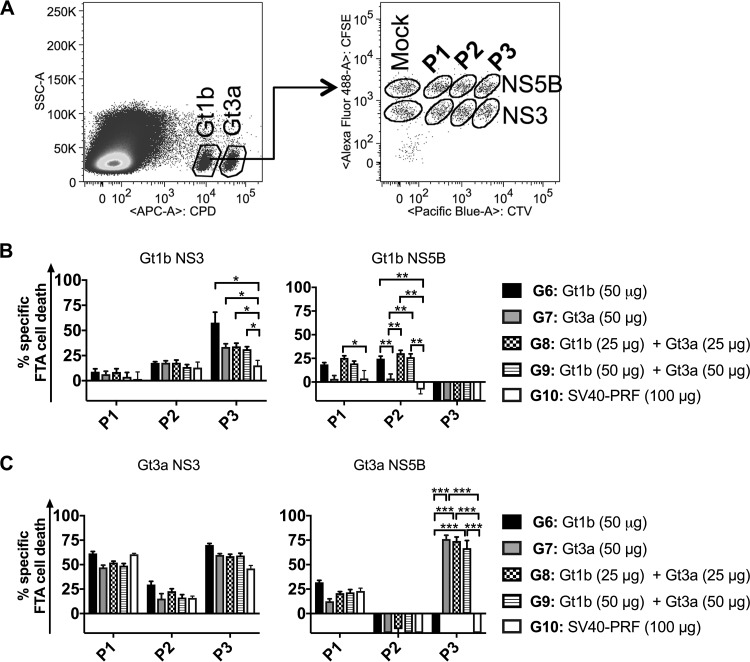
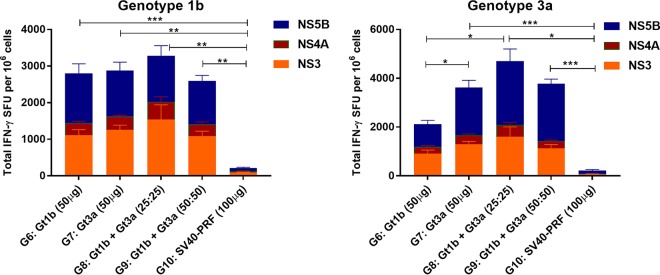
Consequently, to determine if a genotype cocktail can be used to elicit multigenotypic responses to multiple NS proteins, mice were vaccinated with cytolytic PRF-DNA vaccines encoding NS3/4/5B from Gt1b or Gt3a or with a cocktail (Gt1b and Gt3a), and the responses were evaluated by FTA and ELISpot assays. FTA analysis failed to detect CTL and Th cell responses to NS4A in vaccinated mice (data not shown), but our previous study showed that the inclusion of NS4A increased the immunogenicity of the NS3, NS4B, and NS5B proteins in the multiantigenic DNA vaccine (29). In vivo T cell responses were clearly observed for Gt1b and Gt3a NS3 and NS5B (3 peptide pools of each) in mice vaccinated with multiantigenic cytolytic DNA of Gt1b (G6), Gt3a (G7), or the Gt1b/Gt3a cocktail (G8 and G9) (Fig. 4 and 5). In this experiment, the FTA analysis was restricted to target cells pulsed with 10 μg/ml peptides, as these targets were most sensitive to CTL killing and upregulation of the activation marker CD69 on B220+ cells.
FIG 4.
In vivo multiantigenic and multigenotypic CTL responses resulting from monovalent and cocktail vaccinations. Female BALB/c mice (n = 6 or 7 per group for G6 to G9 and n = 4 for G10) were vaccinated as described in Materials and Methods. Thirteen days later, 6.5 × 105 peptide-pulsed cells (for each target cell cluster) or mock-pulsed target cells were injected i.v., and RBC-depleted splenocytes were harvested 15 h later and analyzed by flow cytometry to determine the percentage of surviving peptide-pulsed target cells. (A) FTA design for the experiment. For simplicity, the target cell matrix for the Gt1b population (CPDlo) is shown, but the same matrix was used to label the Gt3a population (CPDhi). (B and C) Means and SEM of Gt1b-specific (B) and Gt3a-specific (C) CTL responses. The statistical significance of the data was determined as described in Materials and Methods. Note that only comparisons where at least one of the groups being compared had a statistically significant enhancement of the responses relative to those of the control group are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 5.
In vivo multiantigenic and multigenotypic Th cell responses resulting from monovalent and cocktail vaccinations. The graphs show means and SEM representing the GMFI of CD69 (above the mock level) on FTA B220+ cells pulsed with 10 μg/ml peptides for the groups of mice described in the legend to Fig. 4. (A) Th cell responses to Gt1b NS3 (left) and NS5B (right) peptides. (B) Th cell responses to Gt3a NS3 (left) and NS5B (right) peptides. The statistical significance of the data was determined as described in Materials and Methods. Note that only comparisons where at least one of the groups being compared had a statistically significant enhancement of the responses relative to those of the control group are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Analysis of the CTL responses to Gt1b target cells revealed statistically significant killing responses above the control level (G10) for NS3 P3 and NS5B P1 and P2 in vaccinated mice (homologous G6 and cocktail G8 and G9) (Fig. 4A). Cocktail (Gt1b and Gt3a)-vaccinated mice (G8 and G9) elicited CTL responses against Gt1b NS3 P3 and NS5B P1 and P2 target cells at magnitudes similar to those for homologous Gt1b (G6)-vaccinated mice (Fig. 4A). The G7 mice (Gt3a vaccine) showed robust, cross-reactive CTL responses to Gt1b NS3 P3 but no cross-reactive responses to NS5B (Fig. 4A). In vivo CTL responses to Gt3a NS proteins were also evaluated (Fig. 4C). There was a trend for vaccinated mice in G6 to G9 to have higher killer T cell responses to Gt3a NS3 P3 than those of control mice, but this did not reach statistical significance. This is likely due to the size of the toxic effect resulting from pulsing with the peptides in Gt3a NS3 P3 being high, which was particularly evident for the control mice (G10) given that the recovery of Gt3a NS3 P1- and P3-pulsed targets following the FTA challenge was significantly lower than that of targets pulsed with other peptide pools for these mice (Fig. 3B and C). This is likely due to the toxicity associated with the Gt3a NS3 peptide pools, a phenomenon which we observed in previous experiments and which probably contributed to the high killing responses to NS3 P1 and NS3 P3 observed for the control group (Fig. 4C). Robust and statistically significant CTL responses to Gt3a targets were detected only against NS5B P3-pulsed targets in the Gt3a (G7) and cocktail (G8 and G9) vaccination groups (Fig. 4C). Furthermore, the CTL responses observed for the cocktail vaccinations (G8 and G9) showed magnitudes similar to those observed for the Gt3a homologous vaccine (G7). Overall, the CTL data suggest that the multiantigenic cocktail vaccine increased the breadth of multigenotypic CTL immunity in vivo compared to that with single-genotype vaccination.
The Th cell responses were evaluated in a manner similar to that described above. All four multiantigenic cytolytic DNA-vaccinated groups (G6 to G9) failed to generate significant Th cell responses to Gt1b NS3 P1 and P2 and Gt3a NS3 P1 and P2 and NS5B P1 and P3 (Fig. 5). However, robust and significant Th cell responses were detected against NS3 P3 and all three NS5B peptide pools for Gt1b targets (Fig. 5A). Significant Th cell responses were also detected for NS3 P3 and NS5B P2 Gt3a targets (Fig. 5B). The Gt3a vaccine (G7) induced Gt1b-cross-reactive NS3 P3- and NS5B P3-specific Th cell responses. However, cocktail immunization responses to Gt1b NS3 P3 and all three pools of Gt1b NS5B were detected, indicating that cocktail DNA immunization elicited greater multigenotypic immunity (Fig. 5A). Furthermore, mice vaccinated with the cocktail (G8 and G9) elicited Th cell responses to Gt1b and Gt3a target cells that were of the same magnitude as those obtained for homologous Gt1b (G6)- and Gt3a (G7)-vaccinated mice, respectively (Fig. 5). This confirms a common trend observed in this study, viz, that mice vaccinated with the cocktail regimens (G8 and G9) consistently generated cross-reactive, NS3- and NS5B-specific T cell responses, whereas the responses in G6 and G7 mice were directed mainly against homologous peptides, with minimal cross-reactivity.
ELISpot analysis confirms the superior cross-reactive immunity of the cocktail vaccine.
To complement the multiantigenic, multigenotypic FTA analysis, an IFN-γ ELISpot assay was conducted by in vitro restimulation of splenocytes with homologous or heterologous peptides. The results of this experiment showed a surprising degree of cross-reaction elicited by the single-genotype vaccines (G6 and G7) (Fig. 6), irrespective of whether the peptides were derived from NS3 or NS5B, although the responses to both Gt1b and Gt3a peptides were higher in splenocytes from the mice receiving the low-dose cocktail (G8; 25 μg Gt1b and 25 μg Gt3a) (Fig. 6). The responses for the high-dose cocktail group (G9; 50 μg Gt1b and 50 μg Gt3a) were essentially similar to homologous responses elicited by the individual genotype vaccines (G6 and G7). We then assessed the level of cross-reactivity: assuming that the homologous response is equivalent to 100%, stimulation of splenocytes from mice vaccinated with the Gt1b vaccine (G6) with the Gt3a peptides resulted in an SFU level equivalent to 75.6% (Table 1). Similarly, vaccination with the Gt3a vaccine (G7) followed by heterologous stimulation with the Gt1b peptides resulted in an SFU level equivalent to 79.5% (Table 1). In contrast, the low-dose cocktail DNA-vaccinated mice (G8) showed responses to the Gt1b and Gt3a peptides equivalent to 110% and 122.7%, while the high-dose cocktail vaccine (G9) responses were 90% and 105%, respectively (Table 1). It is important that the low-dose cocktail (G8; 25 μg Gt1b and 25 μg Gt3a) elicited broader and higher responses than those observed with 50 μg of the single-genotype vaccine or the high-dose cocktail (G9; 50 μg Gt1b and 50 μg Gt3a).
FIG 6.
Cumulative IFN-γ ELISpot response to the heterologous or cocktail vaccines. Groups of female BALB/c mice (n = 7) were vaccinated with DNA encoding HCV NS3/NS4/NS5B sequences of Gt1b (G6) or Gt3a (G7), a cocktail comprised of 25 μg each of Gt1b and Gt3a sequences (G8), a cocktail comprised of 50 μg each of Gt1b and Gt3a sequences (G9), or 50 μg of control SV40-PRF (G10). Fourteen days after the last dose of DNA vaccine, the splenocytes were harvested and stimulated with either Gt1b or Gt3a peptides representing the NS3, NS4A, or NS5B protein (4 μg), and IFN-γ-secreting cells were detected as described in Materials and Methods. The data are presented as mean (n = 7) and SEM SFU per 1 × 106 splenocytes, and the unpaired nonparametric Mann-Whitney U test was used to analyze the statistical significance of the data.
TABLE 1.
Total homologous and heterologous responses per vaccinea
Data which report homologous responses are unshaded and unmarked, while those for heterologous responses are shaded and those showing responses to the cocktail are shown in bold. The percentages in parentheses represent the percent SFU compared to the number of SFU generated by stimulation of splenocytes with peptides homologous to the vaccine.
In summary, vaccination with the cocktail DNA vaccine not only elicited antigen-specific IFN-γ responses to the Gt1b and Gt3a proteins, as determined by ELISpot assay, but also increased the responses compared to those elicited by the homologous vaccines, G6 and G7 (Fig. 6; Table 1).
DISCUSSION
An effective vaccine is needed to control the HCV epidemic. Whereas the correlates of protection in HCV are not precisely defined, a rational path to achieve this goal is to develop vaccines that elicit T cell-mediated immunity analogous to that resulting after recovery from acute HCV infection (3, 36), viz, targeting of multiple HCV antigens (51) and generation of robust CD4 and CD8 T cell responses (37, 40, 52). As DNA vaccines have historically failed to show efficacy in large-animal models and humans, we developed a novel DNA vaccine encoding an appropriate immunogen and a cytolytic protein, PRF, to increase the efficacy of vaccination. Vaccination with this DNA results in necrosis of vaccine-targeted cells after intradermal (i.d.) delivery, followed by release of the immunogen and damage-associated molecular patterns (DAMPs), thought to act as effective adjuvants. Dendritic cells (DC) exposed to necrotic or lytic cell environments mature and cross-present antigens to CD8+ T cells more efficiently than DC exposed to healthy or apoptotic cells (53–55), and we have shown that cytolytic PRF vaccination results in more efficient activation of DC to present vaccine-encoded antigens to CD8+ T cells in vivo than that with canonical DNA vaccination (45). This results in more robust CMI (29, 30) and increased protection against viral challenge compared to those with the canonical DNA vaccine counterpart (46).
This strategy mimics the effect of live attenuated viral vaccines, which although attenuated are still lytic, resulting in activation of cell death pathways that are considered important for generating robust immunity (56, 57). More recently, DNA vaccination followed by in vivo electroporation generated robust HCV-specific immune responses in mice (58), macaques (35), and humans (33). It has been suggested that electroporation results in an increase in the uptake of DNA and in cell injury, resulting in the release of DAMPs (44, 59). Indeed, cell death and release of cellular DNA are now recognized as mechanisms of action of alum (60).
HCV genotypes and subtypes differ by approximately 30% and 15 to 30%, respectively, at the nucleotide level (61). Thus, an effective vaccine should generate CMI to multiple HCV genotypes, particularly in regions of endemicity where multiple genotypes circulate. Studies of individuals with acute, self-limited infection suggest that robust Th cell and CTL responses which target multiple epitopes in NS3 and NS5 are important determinants of recovery (62, 63). Therefore, it is proposed that the success of a T cell-based vaccine in regions of endemicity where multiple HCV genotypes circulate will depend on the generation of T cells that have the ability to target multiple genotypes or viral proteins that are conserved between genotypes (64).
Consequently, we compared the efficacy of a consensus sequence-based DNA vaccination regimen to that of a genotypic DNA cocktail for eliciting multigenotypic CMI to NS5B and the ability of single-genotype vaccines encoding NS3, NS4, and NS5B to elicit cross-genotype reactions.
Whereas many vaccine studies evaluate immunogenicity by using in vitro peptide restimulation assays, such as ELISpot assay and intracellular flow cytometry, these may not always be reflective of immunogenic outcomes in vivo. Therefore, to complement the standard in vitro ELISpot analysis, which does not differentiate between CD8+ and CD4+ T cell responses, we used a highly versatile assay, the FTA assay (65). The FTA assay allows the simultaneous measurement of numerous CTL-mediated target cell killing events in vivo and, as such, comprehensively and clearly defines the contribution of CD8+ T cells to overall vaccine immunogenicity (49). In addition, in the FTA assay, the CD4+ T helper (Th) cell activity was measured based on the ability of Th cells to help activate cognate B cells directly (e.g., upregulation of CD69 expression on target B cells [B220+] pulsed with MHC class II-binding peptides). Thus, the FTA assay allows clear delineation of the individual contributions of CD8+ and CD4+ T cells to vaccine efficacy and immunogenicity in vivo following vaccination (50). ELISpot analysis of NS5B-specific responses showed that vaccination with the cocktail DNA increased the immunogenicity against all NS5B peptide pools for both Gt1b and Gt3a compared to responses obtained for the consensus vaccine. Notably, vaccination with the lower cocktail dose, consisting of 25 μg of each genotype (50 μg total), was capable of eliciting higher NS5B-specific immune responses than those observed with 50 μg of the consensus DNA vaccine.
In vivo, the consensus NS5B vaccine elicited CTL responses to Gt3a, but not Gt1b, whereas specific Th cell responses were detected against both genotypes. In contrast, the cocktail (Gt1b and Gt3a) vaccine elicited significantly higher CTL and Th cell responses against both genotypes and multiple peptide pools, suggesting an increase not only in the magnitude of the CTL and Th cell responses but also in the breadth of responses, although the consensus NS5B sequence has 88% and 85% amino acid sequence homology to the Gt1b and Gt3a NS5B sequences, respectively. As a result of this homology between the consensus and the Gt1b and Gt3a sequences, it is unlikely that one or two dominant epitopes are present in the Gt1b and Gt3a sequences, but not in the consensus, as an explanation for the differences in T cell induction. Although consensus sequences may be convenient for the development of universal vaccines against highly diverse pathogens, this study suggests that the magnitude and in vivo breadth of T cell responses to HCV epitopes are enhanced when a cocktail rather than a consensus vaccine is used.
As we had previously developed and characterized a cytolytic DNA vaccine encoding multiple NS proteins (NS3, NS4A, NS4B, and NS5B) from Gt3a that elicited CMI to each of the encoded proteins (29), we then examined the cross-reactivity against NS proteins from Gt1b, and vice versa, and compared this with the degree of cross-reactivity elicited by a Gt1b/Gt3a cocktail vaccine. The study revealed that the cocktail elicited strong in vivo CTL and Th cell responses to proteins from both genotypes, and the breadth of these multiantigenic, multigenotypic T cell responses was greater than that observed following vaccination with DNA encoding the genotype-specific proteins.
Previous studies evaluated the immunogenicity of pVAX DNA encoding consensus NS3/4A, NS4B, NS5A, or NS5B sequences from Gt1a and Gt1b strains in C57BL/6 mice and macaques (35, 48). These studies showed that the consensus regimen elicited broad CD4+ and CD8+ T cell responses both systemically and in the liver following in vitro peptide stimulation of T cells, but the analysis was restricted to consensus peptides representing the complete immunogen. Consequently, it is not clear whether the T cell responses observed were subtype specific, genotype specific, or multigenotypic.
A degree of T cell cross-reactivity between different HCV genotypes has been described in the past (66, 67), but a number of studies on patients with multiple infections have shown a lack of CD4+ T cell cross-reactivity (68, 69) and a lack of CD8+ T cell cross-reactivity (70) between immunodominant epitopes.
Other studies have suggested that chimpanzees and humans are more likely to clear subsequent infections if they have spontaneously cleared acute HCV infection (40, 42, 71), but the role of cross-reactive immunity in prevention of chronic infection after reinfection is not clear. Although some studies show clearance of heterologous HCV after reinfection (71), establishment of persistent infection with heterologous reinfection is also common (72).
It was previously shown that T cell targets in Gt1 and Gt3 infections are distinct in the setting of natural infection (67) and that cross-reactivity between circulating Gt1 and Gt3 viruses is limited. Most recently, the ability of T cells generated by prophylactic immunization with a chimpanzee adenovirus type 3 (ChAd3) prime followed by a boost with a modified vaccinia virus Ankara (MVA) vector, both encoding the NS3, NS4, NS5A, and NS5B proteins of HCV genotype 1b, to target genotypes 1a, 3a, and 4a was assessed in vaccinated humans (34). This highly immunogenic vaccine induced cross-reactive T cell responses between heterologous genotypes, but these were of a limited and reduced magnitude (34).
Consequently, the multiantigenic cocktail DNA vaccination regimen described in this study is an attractive strategy for developing universal HCV vaccines capable of inducing broad, HCV-specific T cell responses against multiple genotypes in vivo. The observed increase in the breadth of T cell responses is likely due to the presence of more T cell epitopes in the cocktail vaccine, but epitope mapping is beyond the scope of this study and represents the subject of further investigation.
In summary, the findings from the current study suggest that cytolytic DNA vaccines encoding an HCV antigen(s) and PRF can be administered as a cocktail to effectively elicit broad, HCV-specific Gt1b and Gt3a CD4+ and CD8+ T cell responses in vivo. These cytolytic DNA vaccines show exciting translational prospects for the development of effective pan-genotypic HCV vaccines designed to prevent the establishment of persistent infection.
MATERIALS AND METHODS
Vaccines.
The bicistronic DNA vaccines were constructed in pVAX (Life Technologies) as we described previously (29, 30). The sequence encoding a codon-optimized (Gene Art, Germany) consensus NS5B protein (73) or sequences encoding the respective HCV Gt1b or Gt3a NS5B protein (GenBank accession numbers AJ000009 and AF046866, respectively) were inserted downstream of the cytomegalovirus (CMV) promoter, and the truncated mouse PRF gene was inserted downstream of the simian virus 40 (SV40) promoter (Fig. 1A). The Gt3a multiantigenic vaccine, encoding codon-optimized NS3, NS4, and NS5B and truncated mouse PRF, was described previously (29), and the codon-optimized NS3/NS4/NS5B Gt1b vaccine was constructed in the same manner (derived from the GT1b sequence under GenBank accession number AJ000009) (Fig. 1A). All DNA vaccines were prepared using a Qiagen EndoFree plasmid gigakit with removal of bacterial endotoxins per the manufacturer's instructions (29, 30).
Immunofluorescence assay.
HEK293T cells were cultured in 96-well plates in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum and 1% penicillin-streptomycin at 37°C in 5% CO2 and transfected with 200 ng of DNA by use of Lipofectamine LTX (Life Technologies) according to the manufacturer's protocol. Antigen expression was detected as we described previously (29). Briefly, at 48 h posttransfection, transfected cells were fixed with 4% formalin (Sigma), permeabilized with methanol at −20°C, and then blocked in 2.5% bovine serum albumin (BSA) (Sigma) in phosphate-buffered saline (PBS) prior to overnight incubation at 4°C with primary antibodies. The primary antibodies were HCV-positive Gt3a or Gt1b pooled patient sera (kindly provided by Joseph Torresi, University of Melbourne, Australia) and rat anti-PRF (Abcam). Secondary antibodies were fluorescein isothiocyanate (FITC)-conjugated goat anti-human and Cy5-conjugated goat anti-rat antibodies (both from Life Technologies). Cells were visualized by fluorescence microscopy (Zeiss LSM-700 microscope).
Mice and immunizations.
All experiments/methods were performed in accordance with relevant regulations/guidelines and were approved by the University of Adelaide and SA Pathology Animal Ethics Committees. BALB/c mice (Animal Resource Centre, Australia) were bred under specific-pathogen-free conditions at the University of Adelaide Laboratory Animal Services and were housed at the Queen Elizabeth Hospital animal facility in individually ventilated HEPA-filtered cages (Aero 80; Tecniplast, Italy). During intradermal (i.d.) DNA vaccinations, age-matched (6 to 8 weeks) female BALB/c mice were anesthetized with isoflurane. The animals were vaccinated with 50 or 100 μg DNA on 3 occasions at 2-week intervals. In consensus NS5B vaccine experiments, a group of mice was vaccinated with a cocktail of the consensus NS5B vaccine and a vaccine lacking the NS genes (SV40-PRF) to control for any effects resulting from expression of increased levels of PRF.
Panels of overlapping 15- to 19-mer peptides spanning the entire NS3, NS4A, and NS5B proteins of strains J4L6S (genotype 1b) and K3a/650 (genotype 3a) were obtained from the National Institutes for Health Bio Defense and Emerging Infectious Research Resources Repository, NIAID, National Institutes of Health. Individual peptides overlap by 11 or 12 amino acids, and detailed information on their lengths and sequences is available from BEI Resources. The peptides were divided into three pools for NS3 and NS5B, each containing 30 to 34 individual peptides, while NS4A peptides comprised a single pool.
IFN-γ ELISpot assay.
A mouse IFN-γ ELISpot assay was performed on red blood cell (RBC)-depleted splenocytes from immunized mice, which were stimulated with NS3, NS4A, or NS5B peptide pools for 36 h at 37°C and 5% CO2, essentially as we described previously (29, 30). Developed spots were counted automatically by use of an ELISpot reader (AID Germany), and the number of spots for unstimulated splenocytes (<0 to 50) was subtracted from the number of spots for the peptide pool-stimulated splenocytes to generate the number of specific spot-forming units (SFU) per 106 cells. Data are presented as means ± standard errors of the means (SEM). Statistical analysis was performed using unpaired Mann-Whitney tests, with P values of ≤0.05 (*), ≤0.01 (**), and ≤0.001 (***) considered significant. Analysis was performed using GraphPad Prism, version 6.00, for Windows (GraphPad Software, La Jolla, CA).
FTA analysis.
Fluorescent-cell labeling and peptide pulsing were performed as described previously (49, 50). To generate the FTA used in the experiments described for Fig. 3, splenocytes from 14 naive mice were pooled, split evenly 5 ways, and labeled with either 22.95, 6.21, 1.67, 0.595, or 0 μM CTV. Subsequently, the cells were washed thrice, and the cells from each aliquot were split evenly 3 ways and labeled with either 5.17, 1.46, or 0.441 μM CFSE to result in 15 distinct populations of target cells (to delineate 5 concentrations of 3 sets of pooled peptides). The cells were washed again and split into two groups, designated for Gt1b and Gt3a peptide pools. The different populations were then pulsed with 10, 1, 0.1, 0.01, or 0 μg/ml of Gt1b or Gt3a NS5B peptide pools 1 to 3 (P1 to P3) for 4 h at 37°C and 5% CO2. The peptide-pulsed targets were washed, and all genotype-specific target cells were pooled and then labeled with 38.65 or 9.89 μM CPD to delineate the Gt1b or Gt3a peptide pools, respectively. The labeled cells were washed thrice and pooled for intravenous (i.v.) injection into immunized mice (4 × 107 cells [1.3 × 106 cells per target cell cluster] in 200 μl PBS/mouse). Fifteen hours later, the splenocytes were harvested, depleted of red blood cells, stained with B220 and CD69, and analyzed by flow cytometry (BD FACSCanto II flow cytometer) as described for Fig. 3.
To generate the FTA used in the experiments for Fig. 4, splenocytes from 12 naive mice were pooled, split evenly 4 ways, labeled with either 6.21, 1.67, 0.595, or 0 μM CTV, and washed as described above. The cells were then split into 2 aliquots and labeled with 5.17 or 1.46 μM CFSE to result in 8 populations. Each of these populations was subdivided into two more populations such that a total of 16 populations were present. Eight of these populations were designated to be pulsed with 10 μg/ml of Gt1b peptide pools (i.e., NS3 peptide pools 1 to 3 [P1 to P3], NS4A peptide pool, and NS5B peptide pools 1 to 3) or mock pulsed, similar to what we described above. The remaining populations were pulsed exactly in the same manner, but using peptide pools from an HCV Gt3a strain. All peptide-pulsed targets were washed as described above. The Gt3a targets were then pooled and labeled with 38.65 μM CPD, and the Gt1b targets were pooled and labeled with 9.89 μM CPD. The labeled cells were washed, pooled, and injected (6.5 × 105 cells per target cell cluster) into immunized mice prior to analysis of splenocytes as described above. FlowJo Tree Star (version 8.8.7) software was used to generate the flow cytometry plots.
The percentage of specific FTA loss (as a measure of CTL activity) was calculated using the following formula: % loss = [(mock-pulsed target value − peptide-pulsed target value)/mock-pulsed target value] × 100. GraphPad Prism 6 software was used to construct the graphs presented in this study. All statistical significance analyses were performed using IBM SPSS Statistics software (version 24). A Shapiro-Wilk test in the IBM SPSS Statistics software was used to test the normality of all the data pertinent to FTA analysis. A P value of >0.05 in this test is indicative of normally distributed data. In all FTA analyses, data found to be normally distributed were subjected to parametric Levene's test, and homogeneity of variance was assumed for data sets with P values of >0.05. For normally distributed and homoscedastic data, one-way analysis of variance (ANOVA) with Tukey's multiple-comparison test was used to determine the statistical significance of comparisons. For all heteroscedastic data sets, Welch ANOVA with the Games-Howell post hoc test was used to determine the statistical significance of comparisons. For nonnormally distributed data (P < 0.05), nonparametric Levene's test was performed for all data sets, and if homogeneity of variance was assumed (P > 0.05), the Kruskal-Wallis H test was used to determine the statistical significance of the data. IBM SPSS Statistics software (version 24) allows one to perform multiple comparisons between groups by using the Kruskal-Wallis H test as well.
ACKNOWLEDGMENTS
This work was supported by grants from The Hospital Research Foundation (THRF), the Australian Centre for Hepatitis and HIV Virology (ACH2), and the Australia-India Biotechnology Fund. Danushka K. Wijesundara is an early career fellow supported by THRF.
We have no conflicting interests.
REFERENCES
- 1.WHO. 2017. Global hepatitis report. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Agrawal B, Singh S, Gupta N, Li W, Vedi S, Kumar R. 2017. Unsolved puzzles surrounding HCV immunity: heterologous immunity adds another dimension. Int J Mol Sci 18:E1626. doi: 10.3390/ijms18081626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumert TF, Fauvelle C, Chen DY, Lauer GM. 2014. A prophylactic hepatitis C virus vaccine: a distant peak still worth climbing. J Hepatol 61:S34–S44. doi: 10.1016/j.jhep.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Yu MY, Bartosch B, Zhang P, Guo ZP, Renzi PM, Shen LM, Granier C, Feinstone SM, Cosset FL, Purcell RH. 2004. Neutralizing antibodies to hepatitis C virus (HCV) in immune globulins derived from anti-HCV-positive plasma. Proc Natl Acad Sci U S A 101:7705–7710. doi: 10.1073/pnas.0402458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broering TJ, Garrity KA, Boatright NK, Sloan SE, Sandor F, Thomas WD Jr, Szabo G, Finberg RW, Ambrosino DM, Babcock GJ. 2009. Identification and characterization of broadly neutralizing human monoclonal antibodies directed against the E2 envelope glycoprotein of hepatitis C virus. J Virol 83:12473–12482. doi: 10.1128/JVI.01138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. 2009. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology 136:2377–2386. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghuraman S, Park H, Osburn WO, Winkelstein E, Edlin BR, Rehermann B. 2012. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J Infect Dis 205:763–771. doi: 10.1093/infdis/jir835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meuleman P, Bukh J, Verhoye L, Farhoudi A, Vanwolleghem T, Wang RY, Desombere I, Alter H, Purcell RH, Leroux-Roels G. 2011. In vivo evaluation of the cross-genotype neutralizing activity of polyclonal antibodies against hepatitis C virus. Hepatology 53:755–762. doi: 10.1002/hep.24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, Ball JK, McKeating JA, Kneteman NM, Burton DR. 2008. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med 14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 10.Giang E, Dorner M, Prentoe JC, Dreux M, Evans MJ, Bukh J, Rice CM, Ploss A, Burton DR, Law M. 2012. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci U S A 109:6205–6210. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson DX, Voisset C, Tarr AW, Aung M, Ball JK, Dubuisson J, Persson MA. 2007. Human combinatorial libraries yield rare antibodies that broadly neutralize hepatitis C virus. Proc Natl Acad Sci U S A 104:16269–16274. doi: 10.1073/pnas.0705522104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong L, Giang E, Robbins JB, Stanfield RL, Burton DR, Wilson IA, Law M. 2012. Structural basis of hepatitis C virus neutralization by broadly neutralizing antibody HCV1. Proc Natl Acad Sci U S A 109:9499–9504. doi: 10.1073/pnas.1202924109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pestka JM, Zeisel MB, Blaser E, Schurmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S, Rispeter K, Blum HE, Roggendorf M, Baumert TF. 2007. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A 104:6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osburn WO, Snider AE, Wells BL, Latanich R, Bailey JR, Thomas DL, Cox AL, Ray SC. 2014. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 59:2140–2151. doi: 10.1002/hep.27013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlsen TH, Pedersen J, Prentoe JC, Giang E, Keck ZY, Mikkelsen LS, Law M, Foung SK, Bukh J. 2014. Breadth of neutralization and synergy of clinically relevant human monoclonal antibodies against HCV genotypes 1a, 1b, 2a, 2b, 2c, and 3a. Hepatology 60:1551–1562. doi: 10.1002/hep.27298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong YP, Dorner M, Mommersteeg MC, Xiao JW, Balazs AB, Robbins JB, Winer BY, Gerges S, Vega K, Labitt RN, Donovan BM, Giang E, Krishnan A, Chiriboga L, Charlton MR, Burton DR, Baltimore D, Law M, Rice CM, Ploss A. 2014. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci Transl Med 6:254ra129. doi: 10.1126/scitranslmed.3009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keck ZY, Wang Y, Lau P, Lund G, Rangarajan S, Fauvelle C, Liao GC, Holtsberg FW, Warfield KL, Aman MJ, Pierce BG, Fuerst TR, Bailey JR, Baumert TF, Mariuzza RA, Kneteman NM, Foung SK. 2016. Affinity maturation of a broadly neutralizing human monoclonal antibody that prevents acute hepatitis C virus infection in mice. Hepatology 64:1922–1933. doi: 10.1002/hep.28850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farci P, Alter HJ, Wong DC, Miller RH, Govindarajan S, Engle R, Shapiro M, Purcell RH. 1994. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci U S A 91:7792–7796. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JW, Kim K, Jung SH, Lee KJ, Choi EC, Sung YC, Kang CY. 1999. Identification of a domain containing B-cell epitopes in hepatitis C virus E2 glycoprotein by using mouse monoclonal antibodies. J Virol 73:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR, Tyrrell DL, Kneteman NM. 2001. Hepatitis C virus replication in mice with chimeric human livers. Nat Med 7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 21.Vanwolleghem T, Bukh J, Meuleman P, Desombere I, Meunier JC, Alter H, Purcell RH, Leroux-Roels G. 2008. Polyclonal immunoglobulins from a chronic hepatitis C virus patient protect human liver-chimeric mice from infection with a homologous hepatitis C virus strain. Hepatology 47:1846–1855. doi: 10.1002/hep.22244. [DOI] [PubMed] [Google Scholar]
- 22.von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, McKeating JA. 2007. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology 132:667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Helle F, Vieyres G, Elkrief L, Popescu CI, Wychowski C, Descamps V, Castelain S, Roingeard P, Duverlie G, Dubuisson J. 2010. Role of N-linked glycans in the functions of hepatitis C virus envelope proteins incorporated into infectious virions. J Virol 84:11905–11915. doi: 10.1128/JVI.01548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brimacombe CL, Grove J, Meredith LW, Hu K, Syder AJ, Flores MV, Timpe JM, Krieger SE, Baumert TF, Tellinghuisen TL, Wong-Staal F, Balfe P, McKeating JA. 2011. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J Virol 85:596–605. doi: 10.1128/JVI.01592-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bankwitz D, Steinmann E, Bitzegeio J, Ciesek S, Friesland M, Herrmann E, Zeisel MB, Baumert TF, Keck ZY, Foung SK, Pecheur EI, Pietschmann T. 2010. Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J Virol 84:5751–5763. doi: 10.1128/JVI.02200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prentoe J, Jensen TB, Meuleman P, Serre SB, Scheel TK, Leroux-Roels G, Gottwein JM, Bukh J. 2011. Hypervariable region 1 differentially impacts viability of hepatitis C virus strains of genotypes 1 to 6 and impairs virus neutralization. J Virol 85:2224–2234. doi: 10.1128/JVI.01594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grove J, Nielsen S, Zhong J, Bassendine MF, Drummer HE, Balfe P, McKeating JA. 2008. Identification of a residue in hepatitis C virus E2 glycoprotein that determines scavenger receptor BI and CD81 receptor dependency and sensitivity to neutralizing antibodies. J Virol 82:12020–12029. doi: 10.1128/JVI.01569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akazawa D, Morikawa K, Omi N, Takahashi H, Nakamura N, Mochizuki H, Date T, Ishii K, Suzuki T, Wakita T. 2011. Production and characterization of HCV particles from serum-free culture. Vaccine 29:4821–4828. doi: 10.1016/j.vaccine.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 29.Gummow J, Li Y, Yu W, Garrod T, Wijesundara D, Brennan AJ, Mullick R, Voskoboinik I, Grubor-Bauk B, Gowans EJ. 2015. A multiantigenic DNA vaccine that induces broad hepatitis C virus-specific T-cell responses in mice. J Virol 89:7991–8002. doi: 10.1128/JVI.00803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grubor-Bauk B, Yu W, Wijesundara D, Gummow J, Garrod T, Brennan AJ, Voskoboinik I, Gowans EJ. 2016. Intradermal delivery of DNA encoding HCV NS3 and perforin elicits robust cell-mediated immunity in mice and pigs. Gene Ther 23:26–37. doi: 10.1038/gt.2015.86. [DOI] [PubMed] [Google Scholar]
- 31.Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, Pezzanera M, Tafi R, Arcuri M, Fattori E, Lahm A, Luzzago A, Vitelli A, Colloca S, Cortese R, Nicosia A. 2006. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med 12:190–197. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 32.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, Brown A, Antrobus R, Ammendola V, Naddeo M, O'Hara G, Willberg C, Harrison A, Grazioli F, Esposito ML, Siani L, Traboni C, Oo Y, Adams D, Hill A, Colloca S, Nicosia A, Cortese R, Klenerman P. 2012. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med 4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiland O, Ahlen G, Diepolder H, Jung MC, Levander S, Fons M, Mathiesen I, Sardesai NY, Vahlne A, Frelin L, Sallberg M. 2013. Therapeutic DNA vaccination using in vivo electroporation followed by standard of care therapy in patients with genotype 1 chronic hepatitis C. Mol Ther 21:1796–1805. doi: 10.1038/mt.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swadling L, Capone S, Antrobus RD, Brown A, Richardson R, Newell EW, Halliday J, Kelly C, Bowen D, Fergusson J, Kurioka A, Ammendola V, Del Sorbo M, Grazioli F, Esposito ML, Siani L, Traboni C, Hill A, Colloca S, Davis M, Nicosia A, Cortese R, Folgori A, Klenerman P, Barnes E. 2014. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med 6:261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latimer B, Toporovski R, Yan J, Pankhong P, Morrow MP, Khan AS, Sardesai NY, Welles SL, Jacobson JM, Weiner DB, Kutzler MA. 2014. Strong HCV NS3/4a, NS4b, NS5a, NS5b-specific cellular immune responses induced in rhesus macaques by a novel HCV genotype 1a/1b consensus DNA vaccine. Hum Vaccin Immunother 10:2357–2365. doi: 10.4161/hv.29590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang TJ. 2013. Current progress in development of hepatitis C virus vaccines. Nat Med 19:869–878. doi: 10.1038/nm.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med 197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 39.Mehta SH, Cox A, Hoover DR, Wang XH, Mao Q, Ray S, Strathdee SA, Vlahov D, Thomas DL. 2002. Protection against persistence of hepatitis C. Lancet 359:1478–1483. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 40.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. 2010. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swadling L, Klenerman P, Barnes E. 2013. Ever closer to a prophylactic vaccine for HCV. Expert Opin Biol Ther 13:1109–1124. doi: 10.1517/14712598.2013.791277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prince AM, Brotman B, Lee DH, Pfahler W, Tricoche N, Andrus L, Shata MT. 2005. Protection against chronic hepatitis C virus infection after rechallenge with homologous, but not heterologous, genotypes in a chimpanzee model. J Infect Dis 192:1701–1709. doi: 10.1086/496889. [DOI] [PubMed] [Google Scholar]
- 43.Schulze Zur Wiesch J, Lauer GM, Timm J, Kuntzen T, Neukamm M, Berical A, Jones AM, Nolan BE, Longworth SA, Kasprowicz V, McMahon C, Wurcel A, Lohse AW, Lewis-Ximenez LL, Chung RT, Kim AY, Allen TM, Walker BD. 2007. Immunologic evidence for lack of heterologous protection following resolution of HCV in patients with non-genotype 1 infection. Blood 110:1559–1569. doi: 10.1182/blood-2007-01-069583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly C, Swadling L, Brown A, Capone S, Folgori A, Salio M, Klenerman P, Barnes E. 2015. Cross-reactivity of hepatitis C virus specific vaccine-induced T cells at immunodominant epitopes. Eur J Immunol 45:309–316. doi: 10.1002/eji.201444686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wijesundara DK, Yu WB, Quah BJC, Eldi P, Hayball JD, Diener KR, Voskoboinik I, Gowans EJ, Grubor-Bauk B. 2017. Cytolytic DNA vaccine encoding lytic perforin augments the maturation of—and antigen presentation by—dendritic cells in a time-dependent manner. Sci Rep 7:8530. doi: 10.1038/s41598-017-08063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gargett T, Grubor-Bauk B, Garrod TJ, Yu W, Miller D, Major L, Wesselingh S, Suhrbier A, Gowans EJ. 2014. Induction of antigen-positive cell death by the expression of perforin, but not DTa, from a DNA vaccine enhances the immune response. Immunol Cell Biol 92:359–367. doi: 10.1038/icb.2013.93. [DOI] [PubMed] [Google Scholar]
- 47.Gargett T, Grubor-Bauk B, Miller D, Garrod T, Yu S, Wesselingh S, Suhrbier A, Gowans EJ. 2014. Increase in DNA vaccine efficacy by virosome delivery and co-expression of a cytolytic protein. Clin Transl Immunol 3:e18. doi: 10.1038/cti.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang Kuhs KA, Toporovski R, Yan J, Ginsberg AA, Shedlock DJ, Weiner DB. 2012. Induction of intrahepatic HCV NS4B, NS5A and NS5B-specific cellular immune responses following peripheral immunization. PLoS One 7:e52165. doi: 10.1371/journal.pone.0052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quah BJ, Wijesundara DK, Ranasinghe C, Parish CR. 2012. Fluorescent target array killing assay: a multiplex cytotoxic T-cell assay to measure detailed T-cell antigen specificity and avidity in vivo. Cytometry A 81:679–690. doi: 10.1002/cyto.a.22084. [DOI] [PubMed] [Google Scholar]
- 50.Quah BJ, Wijesundara DK, Ranasinghe C, Parish CR. 2013. Fluorescent target array T helper assay: a multiplex flow cytometry assay to measure antigen-specific CD4+ T cell-mediated B cell help in vivo. J Immunol Methods 387:181–190. doi: 10.1016/j.jim.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Lauer GM, Barnes E, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, Robbins GK, Casson DR, Reiser M, Dusheiko G, Allen TM, Chung RT, Walker BD, Klenerman P. 2004. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology 127:924–936. doi: 10.1053/j.gastro.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. 2006. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology 44:126–139. [DOI] [PubMed] [Google Scholar]
- 53.Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. 1998. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med 4:581–587. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 54.Gallucci S, Lolkema M, Matzinger P. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat Med 5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 55.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. 2000. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 191:423–433. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bridle BW, Clouthier D, Zhang L, Pol J, Chen L, Lichty BD, Bramson JL, Wan Y. 2013. Oncolytic vesicular stomatitis virus quantitatively and qualitatively improves primary CD8+ T-cell responses to anticancer vaccines. Oncoimmunology 2:e26013. doi: 10.4161/onci.26013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parks CL, Picker LJ, King CR. 2013. Development of replication-competent viral vectors for HIV vaccine delivery. Curr Opin HIV AIDS 8:402–411. doi: 10.1097/COH.0b013e328363d389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fournillier A, Frelin L, Jacquier E, Ahlen G, Brass A, Gerossier E, Holmstrom F, Broderick KE, Sardesai NY, Bonnefoy JY, Inchauspe G, Sallberg M. 2013. A heterologous prime/boost vaccination strategy enhances the immunogenicity of therapeutic vaccines for hepatitis C virus. J Infect Dis 208:1008–1019. doi: 10.1093/infdis/jit267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roos AK, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, Brave A, Wahren B, Pisa P. 2009. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS One 4:e7226. doi: 10.1371/journal.pone.0007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P, Coban C, Akira S, Ishii KJ, Bureau F, Desmet CJ. 2011. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med 17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 61.Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. 2014. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology 59:318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tester I, Smyk-Pearson S, Wang P, Wertheimer A, Yao E, Lewinsohn DM, Tavis JE, Rosen HR. 2005. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J Exp Med 201:1725–1731. doi: 10.1084/jem.20042284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smyk-Pearson S, Tester IA, Klarquist J, Palmer BE, Pawlotsky JM, Golden-Mason L, Rosen HR. 2008. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J Virol 82:1827–1837. doi: 10.1128/JVI.01581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.von Delft A, Humphreys IS, Brown A, Pfafferott K, Lucas M, Klenerman P, Lauer GM, Cox AL, Gaudieri S, Barnes E. 2016. The broad assessment of HCV genotypes 1 and 3 antigenic targets reveals limited cross-reactivity with implications for vaccine design. Gut 65:112–123. doi: 10.1136/gutjnl-2014-308724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wijesundara DK, Ranasinghe C, Jackson RJ, Lidbury BA, Parish CR, Quah BJ. 2014. Use of an in vivo FTA assay to assess the magnitude, functional avidity and epitope variant cross-reactivity of T cell responses following HIV-1 recombinant poxvirus vaccination. PLoS One 9:e105366. doi: 10.1371/journal.pone.0105366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giugliano S, Oezkan F, Bedrejowski M, Kudla M, Reiser M, Viazov S, Scherbaum N, Roggendorf M, Timm J. 2009. Degree of cross-genotype reactivity of hepatitis C virus-specific CD8+ T cells directed against NS3. Hepatology 50:707–716. doi: 10.1002/hep.23096. [DOI] [PubMed] [Google Scholar]
- 67.Humphreys IS, von Delft A, Brown A, Hibbert L, Collier JD, Foster GR, Rahman M, Christian A, Klenerman P, Barnes E. 2012. HCV genotype-3a T cell immunity: specificity, function and impact of therapy. Gut 61:1589–1599. doi: 10.1136/gutjnl-2011-300650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harcourt GC, Lucas M, Godkin AJ, Kantzanou M, Phillips RE, Klenerman P. 2003. Evidence for lack of cross-genotype protection of CD4+ T cell responses during chronic hepatitis C virus infection. Clin Exp Immunol 131:122–129. doi: 10.1046/j.1365-2249.2003.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. 2003. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology 38:1437–1448. [DOI] [PubMed] [Google Scholar]
- 70.Fytili P, Dalekos GN, Schlaphoff V, Suneetha PV, Sarrazin C, Zauner W, Zachou K, Berg T, Manns MP, Klade CS, Cornberg M, Wedemeyer H. 2008. Cross-genotype-reactivity of the immunodominant HCV CD8 T-cell epitope NS3-1073. Vaccine 26:3818–3826. doi: 10.1016/j.vaccine.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 71.Lanford RE, Guerra B, Chavez D, Bigger C, Brasky KM, Wang XH, Ray SC, Thomas DL. 2004. Cross-genotype immunity to hepatitis C virus. J Virol 78:1575–1581. doi: 10.1128/JVI.78.3.1575-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bucci C, von Delft A, Christian A, Flemming VM, Harrison A, Halliday J, Collier J, Manganis C, Klenerman P, Irving W, Barnes E. 2013. ‘Favourable’ IL28B polymorphisms are associated with a marked increase in baseline viral load in hepatitis C virus subtype 3a infection and do not predict a sustained virological response after 24 weeks of therapy. J Gen Virol 94:1259–1265. doi: 10.1099/vir.0.051052-0. [DOI] [PubMed] [Google Scholar]
- 73.Waheed Y, Saeed U, Anjum S, Afzal MS, Ashraf M. 2012. Development of global consensus sequence and analysis of highly conserved domains of the HCV NS5B protein. Hepat Mon 12:e6142. doi: 10.5812/hepatmon.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]