ABSTRACT
Chronic wasting disease (CWD) is an emerging prion disease in North America. Recent identification of CWD in wild cervids from Norway raises the concern of the spread of CWD in Europe. CWD infectivity can enter the environment through live animal excreta and carcasses where it can bind to soil. Well-characterized hamster prion strains and CWD field isolates in unadsorbed or soil-adsorbed forms that were either hydrated or dehydrated were subjected to repeated rounds of freezing and thawing. We found that 500 cycles of repeated freezing and thawing of hydrated samples significantly decreased the abundance of PrPSc and reduced protein misfolding cyclic amplification (PMCA) seeding activity that could be rescued by binding to soil. Importantly, dehydration prior to freezing and thawing treatment largely protected PrPSc from degradation, and the samples maintained PMCA seeding activity. We hypothesize that redistribution of water molecules during the freezing and thawing process alters the stability of PrPSc aggregates. Overall, these results have significant implications for the assessment of prion persistence in the environment.
IMPORTANCE Prions excreted into the environment by infected animals, such as elk and deer infected with chronic wasting disease, persist for years and thus facilitate horizontal transmission of the disease. Understanding the fate of prions in the environment is essential to control prion disease transmission. The significance of our study is that it provides information on the possibility of prion degradation and inactivation under natural weathering processes. This information is significant for remediation of prion-contaminated environments and development of prion disease control strategies.
KEYWORDS: inactivation, prions
INTRODUCTION
Prion diseases are a group of neurodegenerative disorders affecting a number of mammalian species, including humans. These diseases include bovine spongiform encephalopathy (BSE) in cattle (1), chronic wasting disease (CWD) in mule deer, white-tailed deer, reindeer, elk, and moose (2–7), scrapie in sheep and goats (8, 9), and Creutzfeldt-Jakob disease (CJD) in humans (10, 11). The infectious agent of prion disease is comprised solely of PrPSc, a misfolded isoform of the cellular prion protein, PrPC. The two isoforms of the prion protein share the same primary amino acid sequence but have different tertiary and quaternary conformations. These structural changes can result in differences in resistance to proteolysis and solubility (12). Prion conversion in the central nervous system (CNS) can lead to spongiform degeneration and neurodegeneration (12, 13).
Unique among prion diseases, CWD affects both captive and free-ranging animals. CWD is of particular concern, as the disease has spread throughout the United States and has been found in 24 states since it was first identified in 1967 in Wyoming and northern Colorado (14). The identification of CWD in free-ranging reindeer and moose in Norway highlights the concern of CWD emergence in Europe (6). Efforts to control the spread of CWD to new areas and to decrease the prevalence of disease in areas where it is endemic have not succeeded.
Horizontal transmission of CWD and scrapie is facilitated by the environment. CWD and scrapie can persist in the environment, remain infectious (15–17), and be detected in environmental media such as soil and water (18, 19). Cervids and sheep exposed to landscapes where CWD and scrapie have been observed can become prion infected (15, 20). CWD can be transmitted through environments contaminated by excreta and decomposed carcasses (15, 21). Recent evidence suggests that prions bind to and are taken up by plants and can be an additional environmental source for prion transmission (22).
Soil is a significant environmental reservoir of prion infectivity. The prevalence of soil in the habitat of prion-infected host animals renders soil the largest environmental sink for prions shed from the host or from the decomposition of infected animal carcasses (15, 23–30). Additionally, prion-contaminated plants will decompose, contributing to the prion load of soil (22). After entering soil, prions can bind to a variety of soil and soil minerals and remain highly infectious (31–33). The high affinity of prions to solid surfaces effectively immobilizes prions (34–36), therefore they are primarily sustained in surface soils which are readily accessible to animals. Although the relative contribution of bioavailable soil-bound prions to the transmission of prion disease is unknown in natural prion disease, ingestion and inhalation of soil by animals indicates the significance of environmental soil-bound prions in disease transmission (7, 37–42).
The effects of environmental processes on the degradation and inactivation of soil-bound prions are poorly understood. Methods of sterilization, such as boiling, ionizing radiation, and UV radiation, which are effective on conventional pathogens, are largely ineffective on prions (43–45). The most effective inactivation methods for prions, such as treatments at high temperatures and harsh pH or chemical conditions, are not easily replicated in the natural environment (46–49). Recent work, however, suggests that natural processes can reduce prion infectivity. Microbial or enzymatic treatments or incubation of prions with intact lichens can degrade unbound PrPSc (50–54). Long-term incubation (>6 years) of prions with sewage wastewater can reduce prion infectivity but is strain dependent (55). Enzymatic degradation of soil-bound CWD PrPSc under environmentally relevant conditions (pH 7.4, 22°C) can occur (56), and repeated cycles of drying and wetting can degrade prions and reduce their infectivity (57). Importantly, prions bound to soil can have a significant influence on the susceptibility of prions to degradation (57). To further examine the role of natural environmental processes on prions, we determined the effects of repeated cycles of freezing and thawing on unbound and soil-bound prions in the presence and absence of water.
RESULTS
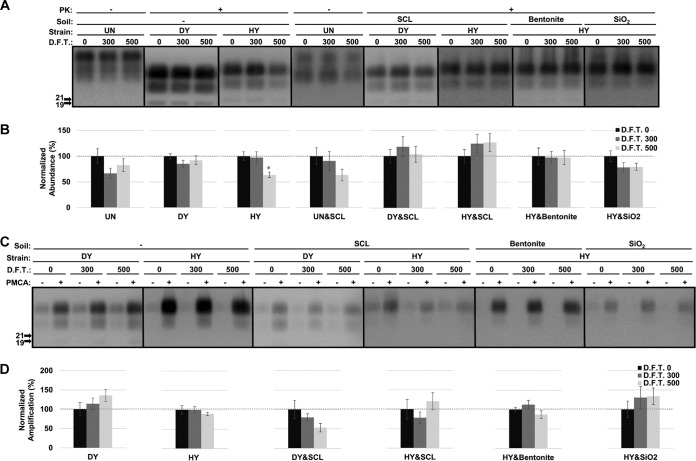
The abundance and protein misfolding cyclic amplification (PMCA) conversion capacity of hydrated hamster PrPSc are reduced after repeated cycles of freezing and thawing depending on strain and soil.
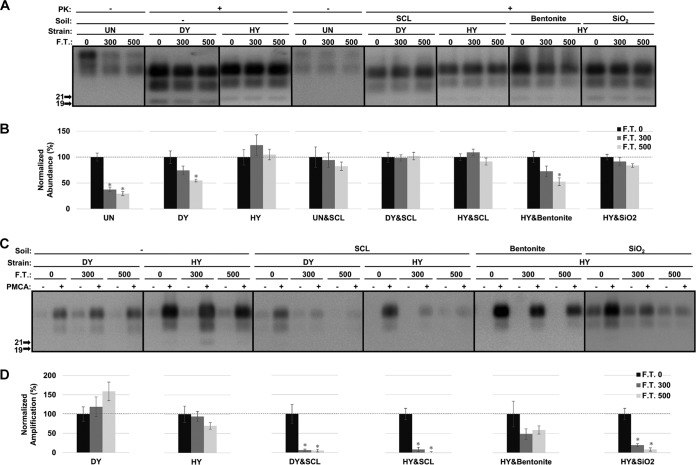
The abundance of cellular prion protein (PrPC) in uninfected hamster brain homogenate (UN) was significantly decreased (P value of <0.01) by 62% and 70% after 300 and 500 cycles of freezing and thawing (termed F.T. 300 and F.T. 500), respectively (Fig. 1A and B). Adsorption of PrPC to silty clay loam (SCL) protected PrPC from degradation by repeated freezing and thawing (Fig. 1A and B). A similar observation was found with PrPSc from the drowsy (DY) strain of transmissible mink encephalopathy (TME). Compared to the untreated sample, the abundance of DY PrPSc after proteinase K (PK) digestion did not significantly (P value of >0.01) differ after 300 cycles of freezing and thawing but was significantly reduced (P value of <0.01) by 45% after 500 cycles (Fig. 1A and B). Adsorption of DY PrPSc to SCL restored the resistance of DY PrPSc to PK digestion, and the abundance did not significantly differ (P value of >0.01) from that of untreated material after 500 cycles of freezing and thawing (Fig. 1A and B). Unlike UN and DY, repeated freezing and thawing did not significantly (P value of >0.01) decrease the resistance of unadsorbed PrPSc from the hyper (HY) strain of TME to PK digestion after 500 cycles compared to that of untreated material (Fig. 1A and B). The resistance of soil-adsorbed HY was not significantly changed (P value of >0.01) by repeated cycles of freezing and thawing except for bentonite-adsorbed HY PrPSc, for which a significant decrease (P value of <0.01) of PrPSc abundance of 47% was observed after 500 cycles (Fig. 1A and B).
FIG 1.
Abundance and PMCA conversion capacity of hydrated hamster PrPSc are reduced after repeated freezing and thawing. (A and B) Western blot analysis (A) and quantification (B) of hydrated brain homogenate from either uninfected hamsters (UN) or hamsters infected with HY or DY TME before (0) and after 300 or 500 cycles of F.T. in both unadsorbed and soil-adsorbed forms. UN&SCL, uninfected samples bound to SCL; DY&SCL, DY PrPSc bound to SCL; HY&SCL, HY PrPSc bound to SCL; HY&Bentonite, HY PrPSc bound to bentonite; HY&SiO2, HY PrPSc bound to SiO2. (C and D) Western blot analysis (C) and quantification (D) of PMCA reactions seeded with hydrated HY or DY before (0) and after 300 or 500 cycles of freezing and thawing in both unadsorbed and soil-adsorbed forms. Samples were subjected to one round of PMCA (two rounds for SCL-adsorbed DY and bentonite-adsorbed HY to obtain a detectable signal). The abundance and amplification of treated samples (F.T. 300 and 500) were normalized to the untreated control (F.T. 0). All samples were digested with PK except uninfected samples (UN and UN&SCL). Error bars represent standard errors of the means. Asterisks indicate significant difference between treated and untreated samples (n = 6; P value of <0.01).
PMCA conversion capacity of the HY and DY samples was determined. Three hundred and 500 cycles of freezing and thawing did not significantly (P value of >0.01) alter PMCA conversion capacity of unadsorbed DY and HY PrPSc (Fig. 1C and D). Binding to SCL significantly (P value of <0.01) reduced PMCA conversion capacity of DY and HY PrPSc by greater than 95% after 300 cycles of treatment (Fig. 1C and D). A significant (P value of <0.01) reduction in PMCA conversion capacity was also observed for HY PrPSc adsorbed to SiO2, with reductions of 80% and 92% after 300 and 500 cycles of treatment, respectively (Fig. 1C and D). PMCA conversion capacity for bentonite-adsorbed HY PrPSc was not significantly (P value of >0.01) changed after 500 cycles of treatment compared to that of the untreated controls (Fig. 1C and D).
Adsorption of PrPSc to either SCL or SiO2 protects it from digestion with PK; interestingly, PMCA conversion capacity is greatly reduced. Therefore, this treatment can disassociate PK resistance from PMCA conversion capacity.
PMCA conversion coefficient of hydrated hamster PrPSc is reduced after 500 cycles of freezing and thawing when bound to soil.
Repeated cycles of freezing and thawing can reduce the PMCA conversion capacity of PrPSc (Fig. 1). To further quantify the change of PrPSc seeding activity, we determined the PMCA conversion coefficient. After normalizing PrPSc abundance, selected samples treated with 0 or 500 cycles of freezing and thawing were subjected to 10-fold serial dilution followed by PMCA. PMCA conversion coefficient of unadsorbed DY and HY PrPSc did not significantly (<10-fold; P value of >0.01) differ after 500 cycles of freezing and thawing (0.2 and 1,000, respectively) compared to those of control samples (Fig. 2). Consistent with PMCA conversion capacity (Fig. 1C and D), binding of DY or HY PrPSc to SCL significantly (P value of <0.01) decreased the PMCA conversion coefficient following 500 cycles of treatment by 10-fold (from 0.1 to 0.01 for DY PrPSc and from 1 to 0.1 for HY PrPSc) compared to the untreated controls (Fig. 2). A significant (P value of <0.01) 10-fold reduction of PMCA conversion coefficient (from 10 to 1) for HY PrPSc was observed when binding to SiO2 (Fig. 2). Overall, PMCA conversion coefficient results (Fig. 2) were consistent with the conversion capacity of PrPSc (Fig. 1).
FIG 2.
PMCA conversion coefficient of hydrated hamster PrPSc is reduced after 500 cycles of freezing and thawing when bound to soil. Selected samples, untreated control (F.T. 0), and samples treated with 500 cycles of freezing and thawing (F.T. 500) were subjected to 10-fold serial dilutions followed by one round of PMCA (two rounds for SCL-adsorbed DY). The conversion coefficient of samples treated with 500 cycles of freezing and thawing was compared to that of untreated controls.
Limited influence of repeated cycles of freezing and thawing on the abundance and PMCA seeding activity of dehydrated hamster PrPSc.
To investigate the influence of hydration on changes to PrPSc mediated by freezing and thawing, samples were dehydrated prior to the treatment with repeated freezing and thawing. The abundance of UN PrPC remained unchanged (P value of >0.01) following 500 cycles of freezing and thawing in both unadsorbed and SCL-adsorbed forms compared to that of untreated controls (Fig. 3A and B). The abundance (Fig. 3A and B) and PMCA conversion capacity (Fig. 3C and D) of PrPSc were not significantly (P value of >0.01) changed in either unadsorbed or soil-adsorbed forms compared to those of the controls. The PMCA conversion coefficient of PrPSc remained unchanged (P value of >0.01) for all samples (Fig. 4). The only exception is for unadsorbed HY PrPSc, where 500 cycles of freezing and thawing resulted in a 36% reduction of PrPSc abundance (P value of >0.01) (Fig. 3A and B). That was restored by binding to SCL (Fig. 3A and B). Overall, in contrast to what was found with the hydrated samples (Fig. 1 and 2), PrPSc abundance and PMCA seeding activity were largely unchanged (Fig. 3 and 4).
FIG 3.
Limited influence of repeated cycles of freezing and thawing on the abundance and PMCA conversion capacity of dehydrated hamster PrPSc. (A and B) Western blot analysis (A) and quantification (B) of dehydrated brain homogenate from either uninfected hamsters (UN) or hamsters infected with hyper (HY) or drowsy (DY) before (0) or after 300 or 500 cycles of freezing and thawing (D.F.T.) in either unadsorbed or soil-adsorbed forms. (C and D) Western blot analysis (C) and quantification (D) of PMCA reactions seeded with dehydrated HY or DY before (0) and after 300 or 500 cycles of freezing and thawing in either unadsorbed or soil-adsorbed forms. The abundance and amplification of treated samples (D.F.T. 300 and 500) were normalized to those of the untreated control (D.F.T. 0). All samples were digested with proteinase K (PK) except uninfected samples (UN and UN&SCL). Error bars represent standard errors of the means. Asterisks indicate significant differences between treated and untreated samples (n = 6; P value of <0.01).
FIG 4.
PMCA conversion coefficient of dehydrated hamster PrPSc remains unchanged after repeated cycles of freezing and thawing. After one round of drying, dehydrated samples, untreated control (D.F.T. 0), or samples treated with 500 repeated cycles of freezing and thawing (D.F.T. 500) were subjected to 10-fold serial dilutions followed by one round of PMCA (two rounds for SCL-adsorbed DY). The conversion coefficient of samples treated with 500 cycles of freezing and thawing was compared to that of untreated controls.
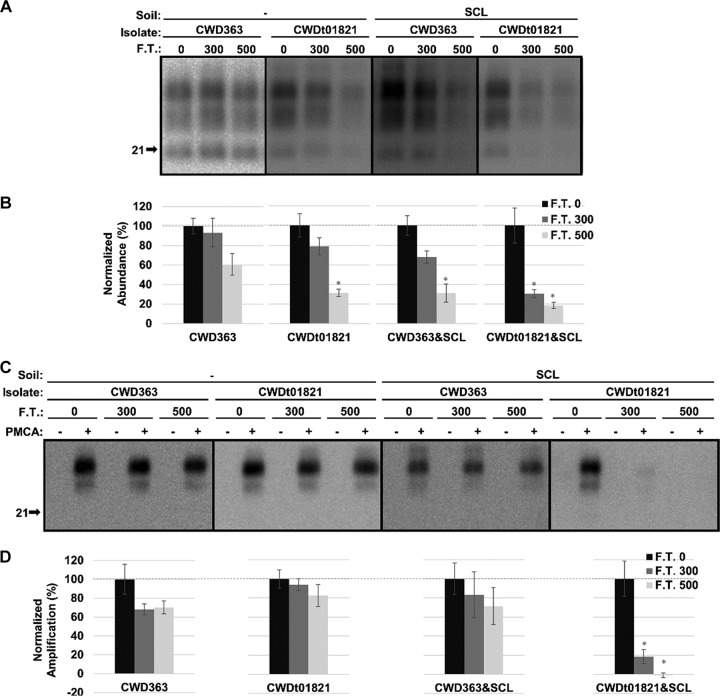
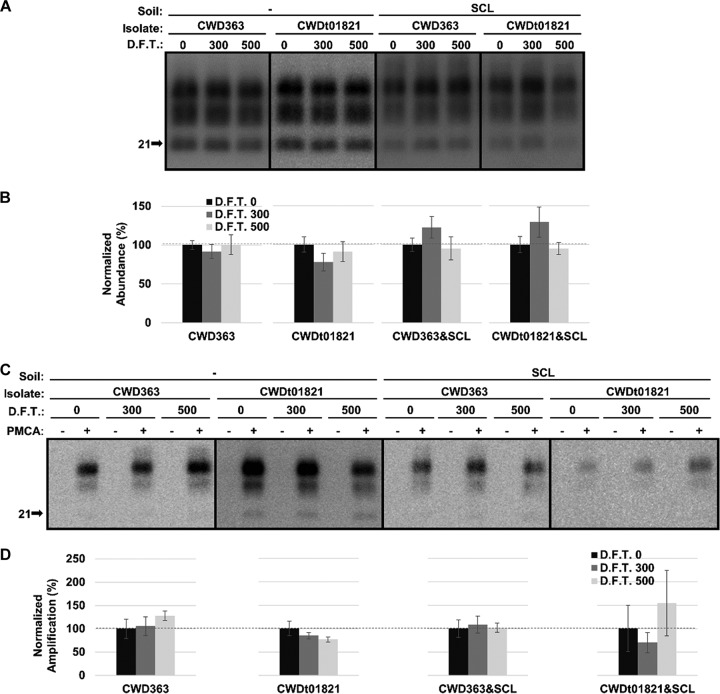
The abundance and PMCA conversion capacity of hydrated CWD PrPSc are reduced after 500 cycles of freezing and thawing depending on isolates.
After PK digestion, the abundance of unadsorbed CWD363 remained unchanged (P value of >0.01) after 500 cycles of freezing and thawing; however, the abundance of SCL-adsorbed CWD363 was significantly decreased (P value of <0.01) by 69% after 500 cycles of treatment (Fig. 5A and B). The abundance of unadsorbed CWDt01821 significantly (P value of <0.01) decreased by 69% after 500 cycles of freezing and thawing (Fig. 5A and B). Binding of CWDt01821 to SCL resulted in a significant decrease (P value of <0.01) in abundance of 70% after 300 cycles of treatment and 82% after 500 cycles of treatment (Fig. 5A and B).
FIG 5.
Abundance and PMCA conversion capacity of hydrated CWD PrPSc are reduced after repeated cycles of freezing and thawing. (A and B) Western blot analysis (A) and quantification (B) of hydrated brain homogenate from elk infected with CWD before (0) and after 300 or 500 cycles of F.T. in both unadsorbed and SCL-adsorbed forms. (C and D) Western blot analysis (C) and quantification (D) of PMCA reactions seeded with hydrated CWD PrPSc before (0) and after 300 or 500 cycles of F.T. in both unadsorbed and SCL-adsorbed forms. Samples were subjected to one round of PMCA (two rounds for SCL-adsorbed CWD). The abundance and amplification of treated samples (F.T. 300 and 500) were normalized to the untreated control (F.T. 0). All samples were digested with PK. Error bars represent standard errors of the means. Asterisks indicate significant difference between treated and untreated samples (n = 3; P value of <0.01).
PMCA conversion capacity of unadsorbed CWD363, SCL-adsorbed CWD363, and unadsorbed CWDt01821 did not significantly (P value of >0.01) differ after 500 cycles of freezing and thawing, although two of these samples demonstrated significant (P value of <0.01) reductions in PrPSc abundance (Fig. 5). PMCA conversion capacity of SCL-adsorbed CWDt01821 was significantly (P value of <0.01) reduced by 81% after 300 cycles of freezing and thawing and was undetected after 500 cycles of treatment (Fig. 5C and D).
Overall, repeated cycles of freezing and thawing can reduce the abundance and PMCA conversion capacity of hydrated CWD PrPSc depending on CWD isolates.
PMCA conversion coefficient of hydrated CWD PrPSc is reduced after 500 cycles of freezing and thawing when bound to soil.
After normalizing CWD PrPSc abundance, unadsorbed and SCL-adsorbed CWD treated with 0 or 500 cycles of freezing and thawing were subjected to 10-fold serial dilution followed by PMCA. The PMCA conversion coefficient of unadsorbed CWD363 and CWDt01821 did not significantly (<10-fold; P value of >0.01) differ after 500 cycles of freezing and thawing (1, and 0.017, respectively) compared to that of control samples (Fig. 6). Binding of CWD363 and CWDt01821 to SCL significantly (P value of <0.01) decreased the PMCA conversion coefficient following 500 cycles of treatment by at least 10-fold (from 0.03 to 0.003 or less for CWD363 and from 0.025 to 0.0025 or less for CWDt01821) compared to that of the untreated controls (Fig. 6). Overall, binding to soil renders these two CWD isolates more vulnerable to degradation/inactivation by repeated freezing and thawing in terms of PMCA seeding activity.
FIG 6.
PMCA conversion coefficient of hydrated CWD PrPSc is reduced after 500 cycles of freezing and thawing when bound to soil. Selected samples, untreated control samples (F.T. 0), and samples treated with 500 cycles of freezing and thawing (F.T. 500) were subjected to 10-fold serial dilutions, followed by one round of PMCA (two rounds for SCL-adsorbed CWD). The conversion coefficient of samples treated with 500 cycles of freezing and thawing was compared to that of untreated controls (n = 3; P value of <0.01).
The abundance and PMCA seeding activity of dehydrated CWD PrPSc remain unchanged after 500 cycles of freezing and thawing.
After dehydration, the abundance and PMCA conversion capacity of both unadsorbed and SCL-adsorbed CWD PrPSc was not significantly (P value of >0.01) changed after 500 cycles of freezing and thawing compared to those of the controls (Fig. 7). The PMCA conversion coefficient of unadsorbed CWD363 and CWDt01821 and SCL-adsorbed CWD363 did not significantly (P value of >0.01) differ after 500 cycles of treatment (<10-fold) (Fig. 8).
FIG 7.
Abundance and PMCA conversion capacity of dehydrated CWD PrPSc remain unchanged after repeated cycles of freezing and thawing. (A and B) Western blot analysis (A) and quantification (B) of dehydrated brain homogenate from elk infected with CWD before (0) and after 300 or 500 cycles of freezing and thawing (D.F.T.) in both unadsorbed and SCL-adsorbed forms. (C and D) Western blot analysis (C) and quantification (D) of PMCA reactions seeded with dehydrated CWD PrPSc before (0) and after 300 or 500 cycles of freezing and thawing (D.F.T.) in both unadsorbed and SCL-adsorbed forms. Samples were subjected to one round of PMCA (two rounds for SCL-adsorbed CWD). The abundance and amplification of treated samples (F.T. 300 and 500) were normalized to the untreated control (F.T. 0). All samples were digested with PK. Error bars represent standard errors of the means. Asterisks indicate significant differences between treated and untreated samples (n = 3; P value of <0.01).
FIG 8.
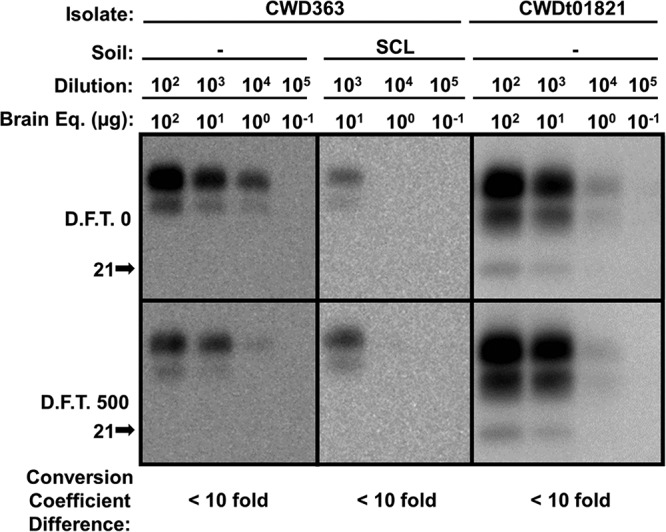
PMCA conversion coefficient of dehydrated CWD PrPSc remains unchanged after repeated cycles of freezing and thawing. After one round of drying, selected dehydrated samples, untreated control samples (D.F.T. 0), and samples treated with 500 cycles of freezing and thawing (D.F.T. 500) were subjected to 10-fold serial dilutions followed by one round of PMCA (two rounds for SCL-adsorbed CWD). The conversion coefficient of samples treated with 500 cycles of freezing and thawing was compared to that of untreated controls (n = 3; P value of <0.01).
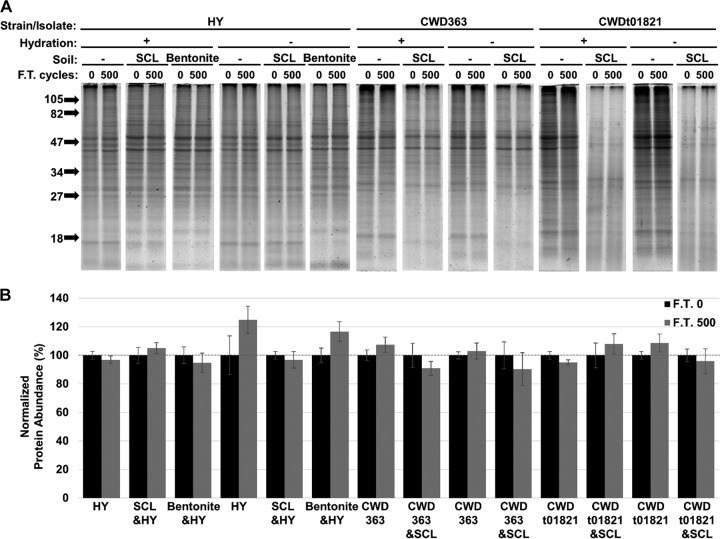
Total protein abundance remains unchanged after 500 cycles of freezing and thawing.
Hamster brain homogenate infected with HY transmissible mink encephalopathy (TME) and elk brain homogenate infected with CWD were adsorbed to SCL or bentonite or were left unadsorbed. These samples were either dehydrated or left hydrated prior to repeated cycles of freezing and thawing. The samples were size fractionated by gel electrophoresis and stained with Sypro ruby (Fig. 9A). Significant changes in total protein abundance were not observed (P value of >0.01) for all samples after 500 cycles of freezing and thawing compared to that of the untreated controls (Fig. 9B).
FIG 9.
Total protein abundance remain unchanged after 500 cycles of freezing and thawing. (A and B) Sypro ruby-stained gel (A) and quantification (B) of HY TME-infected brain homogenate adsorbed to SCL or bentonite or left unadsorbed (−) that was hydrated or dehydrated prior to 0 or 500 cycles of freezing and thawing (F.T.). The molecular mass markers (in kDa) are indicated on the left side of the gel. Sample size, n = 6. Error bars represent standard errors of the means. Hydration −, dehydrated; hydration +, hydrated.
DISCUSSION
Repeated cycles of freezing and thawing degraded both unadsorbed and soil-adsorbed hydrated prion protein in both cellular and infectious forms (Fig. 1 and 5). The mechanisms responsible for this observation are not well understood. It is probable that the process of freezing and thawing instead of the total time at the treatment temperature mainly leads to the change of prion protein properties, as PrPSc abundance could remain unchanged at room temperature for 30 days (58). Denaturation of proteins occurs under stresses such as cold temperature, ice formation, crystallization, and recrystallization during freezing and thawing (59–61).
The decrease in the detectable PrPC is likely due to the destruction of the epitope (amino acid residues 109 to 112), under repeated freezing and thawing, in cellular prion protein targeted by the primary antibody used in the study. The decrease in PrPSc abundance after PK digestion is possibly due to conformational change resulting in increased sensitivity of the C terminus, or more specifically, the primary antibody epitope, to PK digestion. Unadsorbed hydrated UN and DY exhibited similar levels of resistance to freezing and thawing degradation, but the result was different for HY (Fig. 1A), indicating higher conformational stability of HY PrPSc than PrPC or DY PrPSc under the stress of repeated freezing and thawing. The observed differences in the susceptibility of PrPSc to degradation from the two hydrated elk CWD samples (Fig. 5A and B) may be attributed to different PrPSc conformations in these field isolates.
Binding of different prion proteins (hydrated hamster PrPC, DY PrPSc, and elk CWD) to the same soil (SCL) (Fig. 1 and 5) and binding of the same prion protein (HY PrPSc) (Fig. 1) to different soil (SCL, SiO2, and bentonite) types resulted in varied responses of prion protein to freezing and thawing degradation. This is likely due to specific changes in soil-prion interactions among different soil-prion systems under cycles of freezing and thawing which are determined by specific soil and prion properties, such as surface charge, minerology, and PrPSc conformation. The changes induced by freezing and thawing can have either protective or destructive impacts on prion conformation depending on the soil-prion system, mechanisms of which are currently unknown.
Exposure of PrPSc to repeated cycles of freezing and thawing in a soil-associated environment decreased conversion efficiency compared to that of PrPSc in a soil-free matrix. The reduction is not necessarily related to the abundance of PrPSc, as PMCA seeding activity was reduced after 500 cycles of freezing and thawing either without a change in PrPSc abundance (Fig. 1 and 2) or with equalized PrPSc abundance (Fig. 5 and 6). This phenomenon suggests that freezing- and thawing-induced PrPSc conformational change, which is responsible for PK resistance, is independent of that responsible for prion conversion.
PMCA conversion coefficient is tightly associated with prion infectivity. Studies of the infectivity of SCL-adsorbed HY provide consistent correlation between reduced conversion coefficient of SCL-adsorbed HY and prolonged incubation period following intracranial inoculation (33, 57). In this study, we used PMCA to examine a large number of environment and strain combinations and identified alterations in the PrPSc conversion coefficient that suggest a reduction in infectivity.
Reductions of PrPSc abundance and PMCA seeding activity observed for hydrated samples were restored upon dehydration (Table 1). The absence of water results in an elimination of ice formation, crystallization, and recrystallization, which can change the conformation of proteins (60, 61). Moreover, without water molecules, the influence on PrPSc conformation contributed by mass transfer between solid and liquid phases and alteration of PrPSc-interacting soil surfaces under freezing and thawing (62) will not occur. The rescue of PrPSc through dehydration suggests the importance of water in degrading and inactivating PrPSc by repeated cycles of freezing and thawing. The exception observed for HY abundance might be because the PK epitope became accessible to digestion in the water-free environment after hundreds of cycles of freezing and thawing.
TABLE 1.
Repeated freezing and thawing decreased the abundance and PMCA seeding activity of hydrated hamster and elk CWD prions, depending on prion strain and soil type
| Sampled | Hydrated |
Dehydrated |
||||
|---|---|---|---|---|---|---|
| PrPC/PrPSc abundance | PMCA conversion capacity | PMCA extinction dilution factor | PrPC/PrPSc abundance | PMCA conversion capacity | PMCA extinction dilution factor | |
| UN | Decrease | NAc | NA | No change | NA | NA |
| DY | Decrease | No change | No changea | No change | No change | No change |
| HY | No change | No change | No change | Decrease | No change | No change |
| UN&SCL | No change | NA | NA | No change | NA | NA |
| DY&SCL | No change | Decrease | Decreaseb | No change | No change | No change |
| HY&SCL | No change | Decrease | Decrease | No change | No change | No change |
| HY&Bentonite | Decrease | No change | NA | No change | No change | NA |
| HY&SiO2 | No change | Decrease | Decrease | No change | No change | No change |
| CWD363 | No change | No change | No change | No change | No change | No change |
| CWD363&SCL | Decrease | No change | Decrease | No change | No change | No change |
| CWDt01821 | Decrease | No change | No change | No change | No change | No change |
| CWDt01821&SCL | Decrease | Decrease | Decrease | No change | No change | NA |
Difference of PMCA extinction dilution factor is less than 1 order of magnitude.
Difference of PMCA extinction dilution factor is 1 order of magnitude or greater.
NA, not applicable.
UN&SCL, uninfected samples bound to SCL; DY&SCL, DY PrPSc bound to SCL; HY&SCL, HY PrPSc bound to SCL; HY&Bentonite, HY PrPSc bound to bentonite; HY&SiO2, HY PrPSc bound to SiO2; CWD363&SCL, CWD363 bound to SCL; CWDt01821&SCL, CWDt01821 bound to SCL.
Hundreds of cycles of freezing and thawing are required to reach a level of PrPSc degradation equivalent to that which occurs by repeated cycles of wetting and drying. For example, a reduction of two orders of magnitude in SCL-adsorbed HY PrPSc conversion coefficient was achieved by 10 cycles of drying and wetting (57), while 500 cycles of freezing and thawing produced a reduction of one order of magnitude. We hypothesize that the major contributor to prion degradation and inactivation of these two simulated natural conditions is water movement. Dehydration diminishes the space between PrPSc and solids or other molecules, facilitating stacking of PrPSc, while rehydration expands the space between molecules, resulting in stacked compounds to be redissolved/redistributed into the liquid phase. We hypothesize that this shrinking and then expanding process has a greater impact on PrPSc conformation or the interaction between PrPSc and surroundings than freezing- and thawing-related water activities. Alternatively, we cannot exclude the possibility that the temperature differences between wetting and drying and freezing and thawing (40°C versus −20°C to ∼7°C) contributed to the observed differences in prion degradation/inactivation. However, we consider this to be less likely based on the thermostability of prions, which requires a much higher temperatures (>100°C) for inactivation (63).
Natural repeated cycles of freezing and thawing are common in places with significant temperature difference between day and night. For example, in Phantom Valley (lat 40.4°; long 105.9°), which is close to Rocky Mountain National Park, an area where CWD is endemic, temperature shifts from above 4°C to below 0°C occurred around 120 times in air and 20 times in soil to a depth of 0.05 m in a 1-year period (International Soil Moisture Network; https://ismn.geo.tuwien.ac.at/data-access/; accessed 11 June 2014 and 11 September 2017). Topsoil may experience more temperature shifts from above to below 0°C compared to subsoil, as it is completely exposed to air. Based on our findings, elk CWD adsorbed to SCL must be exposed to hundreds of cycles of freezing and thawing to reduce infectivity. However, the complexity of natural soil provides more opportunities for prion property alteration by introducing more components accessible to prions, such as nutrients and microbial populations, the fate of which is influenced by freezing and thawing (62, 64). Repeated cycles of freezing and thawing may be considered a long-term natural degradation process for soil-bound prions in the environment. Addition of drying and wetting processes may improve the degradation efficiency of freezing and thawing. However, the improvement may be hindered depending on the moisture content of soils. Modeling of prion transmission dynamics in the environment should take the influence of ambient environmental factors into account.
MATERIALS AND METHODS
Prion sources and tissue preparation.
Brain materials were collected from Syrian hamsters, either uninfected (UN) or infected with either the hyper (HY) or drowsy (DY) strain of transmissible mink encephalopathy (TME) and from elk naturally infected with CWD were used as described previously (65). Brains were homogenized to 10% (wt/vol) (20% [wt/vol] for elk CWDt01821) in Dulbecco's phosphate-buffered saline (DPBS) without Ca2+ or Mg2+ (Mediatech, Herndon, VA) using strain-dedicated Tenbroeck tissue grinders (Kontes, Vineland, NJ), aliquoted, and stored at −80°C. Uninfected Syrian hamster brain and uninfected Tg(CerPrP)1536 mouse brain, for use as the substrate for PMCA, were homogenized to 10% (wt/vol) in ice-cold PMCA conversion buffer (DPBS [pH 7.5] containing 5 mM EDTA, 1% [vol/vol] Triton X-100, and a complete protease inhibitor tablet [Roche Diagnostics, Mannheim, Germany]). The uninfected homogenate was centrifuged at 1,500 × g for 1 min, and the supernatant was collected and stored at −80°C.
Prion adsorption to soils and soil minerals.
Soil and soil minerals used in this study included sterile Rinda silty clay loam (SCL) soil (a VerticEpiaqualf), sodium bentonite clay (CETCO, Arlington Heights, IL), and silicon dioxide powder (Sigma-Aldrich, St. Louis, MO). Physicochemical properties of these soils and soil minerals have been described previously (32, 56). To obtain soil-adsorbed prions, 10% brain homogenate (BH) of UN, HY TME, or DY TME was added to soil. For each soil or soil mineral, the incubation time and prion-to-soil ratios were selected based on previous studies and are detailed in Table 2 (32, 65). The BH-soil mixture was rotated at 24 rpm (Mini Labroller, Edison, NJ) at room temperature. Samples were removed after incubation and centrifuged at 470 × g for 30 s, except for SiO2-adsorbed HY, which was at 1,500 × g for 1 min. The supernatant was removed and the pellets were washed five times with 1× DPBS. The soil pellets were resuspended in 1× DPBS at concentrations described in Table 2 and were stored at −80°C. HY TME and DY TME BH were used as unbound controls.
TABLE 2.
Preparation of soil-adsorbed HY TME, DY TME, and CWD PrPSca
| Soil/soil mineral | Incubation time (h) | Soil (mg soil/ml solution) | PrPSc (mg brain equivalent/ml solution) | Soil resuspension (mg soil/μl solution) | PMCA seed (mg brain equivalent/100 μl substrate) |
|---|---|---|---|---|---|
| Rinda SCL | 24 | 5 | 5b/10c | 0.1 | 0.1b/0.2c |
| Bentonite clay | 24 | 5 | 5 | 0.1 | 0.1 |
| SiO2 powder | 24 | 50 | 2.5 | 0.5 | 0.1 |
SCL-adsorbed DY TME and CWD were prepared with the same soil-to-brain ratio and under the same conditions as SCL-adsorbed HY TME.
Value for 10% brain homogenate of HY TME, DY TME, and CWD363.
Value for 20% brain homogenate of CWDt01821.
Freezing and thawing treatment.
Samples were placed in 0.2-ml capped PCR tubes (Thermo Scientific). Half of the samples were incubated uncapped at 40°C for 12 h before freezing and thawing treatment for dehydration. Both unpretreated (F.T.) and drying-pretreated (D.F.T.) samples were incubated in a freeze-thaw cycler (model H-3185; serial no. 061893; Logan Freeze-Thaw Mfg. Co.) equipped with a cold temperature control and a temperature recorder (model DTP; serial no. 289766; Dickson). One cycle consists of a linear change in temperature between −23°C and 7°C in 90 min. After the desired number of treatment cycles, samples were collected. Drying-pretreated samples were resuspended by adding 10 μl of ultrapure water and combined. All samples were stored at −80°C.
PMCA.
PMCA was performed as described previously (66). Sonication was performed with a QSonica sonicator (model Q700; serial no. 83914J-02-15) with amplitude set to level 17, generating an average output of around 170 W during sonication treatment. Samples were diluted 1:100 in PMCA substrate (10% [wt/vol] uninfected brain homogenate in PMCA conversion buffer) for the first round, and for subsequent rounds they were diluted 1:20 for HY TME and elk CWD and 1:1 for DY TME. Each round was performed at 37°C for 24 h and consisted of 144 cycles of 5 s of sonication followed by 9 min 55 s of incubation. Before each PMCA round, an aliquot was placed at −80°C as an unsonicated control. Samples containing unbound HY or DY TME diluted at 1:100 and only 10% (wt/vol) uninfected brain homogenate were included with each PMCA round as positive and negative controls, respectively. PMCA conversion capacity is defined as the ratio of the abundance of PrPSc of the amplified PMCA sample divided by the abundance of PrPSc prior to amplification (33). The PMCA conversion coefficient was determined as previously described (67). Briefly, selected samples either prior to or after 500 cycles of freezing and thawing treatment were normalized for PrPSc abundance and then serially diluted 10-fold prior to PMCA. The PMCA conversion coefficient was calculated as the reciprocal of the microgram equivalent of the last dilution of prion-infected brain homogenate (67).
Western blot analysis.
Western blot analysis was performed as described previously (68). Briefly, selected samples were incubated with 23.25 μg/ml (final concentration) proteinase K (PK; Roche Diagnostics Corporation, Indianapolis, IN) at 37°C for 30 min with constant agitation. The PK digestion was terminated by boiling in 1× SDS-PAGE sample buffer (final concentration). The samples were size fractionated with 12.5% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (NuPage; Invitrogen, Carlsbad, CA). The membrane was blocked with 5%, wt/vol, nonfat dry milk (Bio-Rad Laboratories, Hercules, CA) in 1× Tris-Tween-buffered saline for 30 min. Hamster samples were immunoblotted with monoclonal antibody (MAb) 3F4 (1:10,000; Chemicon, Temecula, CA). Elk CWD samples were immunoblotted with MAb 8H4 (1:2,500; Abcam, Cambridge, MA). The blots were developed with SuperSignal West Femto maximum sensitivity substrate according to the manufacturer's instructions (Pierce, Rockford, IL), imaged on a 4000R imaging station (Kodak, Rochester, NY), and analyzed using Kodak (New Haven, CT) molecular imaging software, V.5.0.1.27. Normalized PrPSc abundance (the net intensity divided by the area) was standardized to brain homogenate controls on the same gel to control for inter-gel variance. Statistical analysis (t test, two-tailed distribution, and two-sample equal variance) was performed using Student's t test (Microsoft Excel).
SYPRO ruby gel staining.
SDS-PAGE gels were stained with SUPRO ruby according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Briefly, the gel was placed in a clean plastic dish and incubated in undiluted SYPRO ruby staining solution for 16 h in the dark at room temperature. The gel was washed with 10% methanol and 7% acetic solution two times for 30 min and was imaged in a molecular imager (Typhoon 9410; serial no. 1493634) using a 610 BP 30 emission filter with 457-nm excitation and analyzed by ImageQuant, version 5.2. The protein abundance for each sample was compared and significance was analyzed with a two-tailed equal-variance Student's t test (Microsoft Excel).
ACKNOWLEDGMENTS
We thank Ronald Shikiya for technical assistance. We thank Michael Beard at Becton-Dickinson, Columbus, NE, for gamma irradiation of soils.
This study was supported by the National Science Foundation (award number CBET-1149424 to S.L.B.-H.) and the National Institutes of Health (grant numbers P20 RR0115635, C06 RR17417, and P01 2P01AI077774 to J.C.B.).
REFERENCES
- 1.Holt TA, Phillips J. 1988. Bovine spongiform encephalopathy. Br Med J 296:1581–1582. doi: 10.1136/bmj.296.6636.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spraker TR, Miller MW, Williams ES, Getzy DM, Adrian WJ, Schoonveld GG, Spowart RA, Orourke KI, Miller JM, Merz PA. 1997. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J Wildl Dis 33:1–6. doi: 10.7589/0090-3558-33.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Williams ES, Young S. 1980. Chronic wasting disease of captive mule deer–spongiform encephalopathy. J Wildl Dis 16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Williams ES, Young S. 1982. Spongiform encephalopathy of Rocky Mountain elk. J Wildl Dis 18:465–471. doi: 10.7589/0090-3558-18.4.465. [DOI] [PubMed] [Google Scholar]
- 5.Baeten LA, Powers BE, Jewell JE, Spraker TR, Miller MW. 2007. A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi). J Wildl Dis 43:309–314. doi: 10.7589/0090-3558-43.2.309. [DOI] [PubMed] [Google Scholar]
- 6.Benestad SL, Mitchell G, Simmons M, Ytrehus B, Vikoren T. 2016. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet Res 47:88. doi: 10.1186/s13567-016-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell GB, Sigurdarson CJ, O'Rourke KI, Algire J, Harrington NP, Walther I, Spraker TR, Balachandran A. 2012. Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PLoS One 7:e39055. doi: 10.1371/journal.pone.0039055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hourriga JL, Klingspo AL, McDaniel HA, Riemenschneider MN. 1969. Natural scrapie in a goat. J Am Vet Med Assoc 154:538–539. [PubMed] [Google Scholar]
- 9.Greig JR. 1950. Scrapie in sheep. J Comp Pathol Ther 60:263–266. doi: 10.1016/S0368-1742(50)80024-3. [DOI] [PubMed] [Google Scholar]
- 10.Stengel E, Wilson WEJ. 1946. Jakob-Creutzfeldt disease. J Mental Sci 92:370–378. [DOI] [PubMed] [Google Scholar]
- 11.Matthews WB. 1978. Creutzfeldt-Jakob disease. Postgrad Med J 54:591–594. doi: 10.1136/pgmj.54.635.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prusiner SB. 2004. An introduction to prion biology and diseases, p 1–88. In Prusiner SB. (ed), Prion biology and diseases, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 13.Prusiner SB. 1998. The prion diseases. Brain Pathol 8:499–513. doi: 10.1111/j.1750-3639.1998.tb00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartz JC, Kincaid AE, Bessen RA. 2002. Retrograde transport of transmissible mink encephalopathy within descending motor tracts. J Virol 76:5759–5768. doi: 10.1128/JVI.76.11.5759-5768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller MW, Williams ES, Hobbs NT, Wolfe LL. 2004. Environmental source of prion transmission in mule deer. Emerg Infect Dis 10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown P, Gajdusek DC. 1991. Survival of scrapie virus after 3 year's interment. Lancet 337:269–270. doi: 10.1016/0140-6736(91)90873-N. [DOI] [PubMed] [Google Scholar]
- 17.Georgsson G, Sigurdarson S, Brown P. 2006. Infectious agent of sheep may persist in the environment for at least 16 years. J Gen Virol 87:3737–3740. doi: 10.1099/vir.0.82011-0. [DOI] [PubMed] [Google Scholar]
- 18.Maddison BC, Baker CA, Terry LA, Bellworthy SJ, Thorne L, Rees HC, Gough KC. 2010. Environmental sources of scrapie prions. J Virol 84:11560–11562. doi: 10.1128/JVI.01133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols TA, Pulford B, Wyckoff AC, Meyerett C, Michel B, Gertig K, Hoover EA, Jewell JE, Telling GC, Zabel MD. 2009. Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion 3:171–183. doi: 10.4161/pri.3.3.9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greig JR. 1940. Scrapie: observation on the transmission of the disease by mediate contact. Vet J 96:203–206. [Google Scholar]
- 21.Miller MW, Williams ES. 2003. Horizontal prion transmission in mule deer. Nature 425:35–36. doi: 10.1038/425035a. [DOI] [PubMed] [Google Scholar]
- 22.Pritzkow S, Morales R, Moda F, Khan U, Telling GC, Hoover E, Soto C. 2015. Grass plants bind, retain, uptake, and transport infectious prions. Cell Rep 11:1168–1175. doi: 10.1016/j.celrep.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angers RC, Seward TS, Napier D, Green M, Hoover E, Spraker T, O'Rourke K, Balachandran A, Telling GC. 2009. Chronic wasting disease prions in elk antler velvet. Emerg Infect Dis 15:696–703. doi: 10.3201/eid1505.081458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC, Hoover EA. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133–135. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 25.Kariv-Inbal Z, Ben-Hur T, Grigoriadis NC, Engelstein R, Gabizon R. 2006. Urine from scrapie-infected hamsters comprises low levels of prion infectivity. Neurodegener Dis 3:123–128. doi: 10.1159/000094770. [DOI] [PubMed] [Google Scholar]
- 26.Murayama Y, Yoshioka M, Okada H, Takata M, Yokoyama T, Mohri S. 2007. Urinary excretion and blood level of prions in scrapie-infected hamsters. J Gen Virol 88:2890–2898. doi: 10.1099/vir.0.82786-0. [DOI] [PubMed] [Google Scholar]
- 27.Seeger H, Heikenwalder M, Zeller N, Kranich J, Schwarz P, Gaspert A, Seifert B, Miele G, Aguzzi A. 2005. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science 310:324–326. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- 28.Maluquer de Motes C, Grassi J, Simon S, Herva ME, Torres JM, Pumarola M, Girones R. 2008. Excretion of BSE and scrapie prions in stools from murine models. Vet Microbiol 131:205–211. doi: 10.1016/j.vetmic.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Safar JG, Lessard P, Tamguney G, Freyman Y, Deering C, Letessier F, DeArmond SJ, Prusiner SB. 2008. Transmission and detection of prions in feces. J Infect Dis 198:80–89. doi: 10.1086/588193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Race R, Jenny A, Sutton D. 1998. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J Infect Dis 178:949–953. doi: 10.1086/515669. [DOI] [PubMed] [Google Scholar]
- 31.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. 2006. Prions adhere to soil minerals and remain infectious. PLoS Pathog 2:e32. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunders SE, Bartz JC, Bartelt-Hunt SL. 2009. Prion protein adsorption to soil in a competitive matrix is slow and reduced. Environ Sci Technol 43:7728–7733. doi: 10.1021/es901385t. [DOI] [PubMed] [Google Scholar]
- 33.Saunders SE, Shikiya RA, Langenfeld K, Bartelt-Hunt SL, Bartz JC. 2011. Replication efficiency of soil-bound prions varies with soil type. J Virol 85:5476–5482. doi: 10.1128/JVI.00282-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson KT, Lee S, Somerville RA, McKenzie D, Benson CH, Pedersen JA. 2010. Transport of the pathogenic prion protein through soils. J Environ Qual 39:1145–1152. doi: 10.2134/jeq2009.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinckley GT, Johnson CJ, Jacobson KT, Bartholomay C, McMahon KD, McKenzie DI, Aiken JM, Pedersen JA. 2008. Persistence of pathogenic prion protein during simulated wastewater treatment processes. Environ Sci Technol 42:5254–5259. doi: 10.1021/es703186e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirchmayr R, Reichi HE, Schildorfer H, Braun R, Somerville RA. 2006. Prion protein: detection in “spiked” anaerobic sludge and degradation experiments under anaerobic conditions. Water Sci Technol 53:91–98. [DOI] [PubMed] [Google Scholar]
- 37.Arthur WJ, Alldredge AW. 1979. Soil ingestion by mule deer in northcentral Colorado. Rangeland Ecol Manag 32:67–71. doi: 10.2307/3897389. [DOI] [Google Scholar]
- 38.Beyer WN, Connor EE, Gerould S. 1994. Estimates of soil ingestion by wildlife. J Wildl Manag 58:375–382. doi: 10.2307/3809405. [DOI] [Google Scholar]
- 39.Kincaid AE, Bartz JC. 2007. The nasal cavity is a route for prion infection in hamsters. J Virol 81:4482–4491. doi: 10.1128/JVI.02649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O'Rourke KI, Hoover EA. 1999. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J Gen Virol 80:2757–2764. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 41.Balachandran A, Harrington NP, Algire J, Soutyrine A, Spraker TR, Jeffrey M, Gonzalez L, O'Rourke KI. 2010. Experimental oral transmission of chronic wasting disease to red deer (Cervus elaphus elaphus): early detection and late stage distribution of protease-resistant prion protein. Can Vet J 51:169–178. [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. 2007. Oral transmissibility of prion disease is enhanced by binding of soil particles. PLoS Pathog 3:e93. doi: 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibbs CJ, Gajdusek DC, Latarjet R. 1978. Unusual resistance to ionizing-radiation of the viruses of Kuru, Creutzfeldt-Jakob disease, and scrapie. Proc Natl Acad Sci U S A 75:6268–6270. doi: 10.1073/pnas.75.12.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor DM, Diprose MF. 1996. The response of the 22A strain of scrapie agent to microwave irradiation compared with boiling. Neuropathol Appl Neurobiol 22:256–258. [PubMed] [Google Scholar]
- 45.Bellinger-Kawahara C, Cleaver JE, Diener TO, Prusiner SB. 1987. Purified scrapie prions resist inactivation by UV irradiation. J Virol 61:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prusiner SB, Groth D, Serban A, Stahl N, Gabizon R. 1993. Attempts to restore scrapie prion infectivity after exposure to protein denaturants. Proc Natl Acad Sci U S A 90:2793–2797. doi: 10.1073/pnas.90.7.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Race RE, Raymond GJ. 2004. Inactivation of transmissible spongiform encephalopathy (prion) agents by Environ LpH. J Virol 78:2164–2165. doi: 10.1128/JVI.78.4.2164-2165.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peretz D, Supattapone S, Giles K, Vergara J, Freyman Y, Lessard P, Safar JG, Glidden DV, McCulloch C, Nguyen HB, Scott M, DeArmond SJ, Prusiner SB. 2006. Inactivation of prions by acidic sodium dodecyl sulfate. J Virol 80:322–331. doi: 10.1128/JVI.80.1.322-331.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown P, Rau EH, Lemieux P, Johnson BK, Bacote AE, Gajdusek DC. 2004. Infectivity studies of both ash and air emissions from simulated incineration of scrapie-contaminated tissues. Environ Sci Technol 38:6155–6160. doi: 10.1021/es040301z. [DOI] [PubMed] [Google Scholar]
- 50.Huang H, Spencer JL, Guan J. 2010. In vitro microbial degradation of abnormal prions in central nervous system from scrapie affected sheep. Open Vet Sci J 4:20–26. doi: 10.2174/1874318801004010020. [DOI] [Google Scholar]
- 51.Huang H, Spencer JL, Soutryine A, Guan J, Rendulich J, Balachandran A. 2007. Evidence of degradation of abnormal prion protein in tissues from sheep with scrapie during composting. Can J Vet Res 71:34–40. [PMC free article] [PubMed] [Google Scholar]
- 52.Scherbel C, Pichner R, Groschup MH, Mueller-Hellwig S, Scherer S, Dietrich R, Maertlbauer E, Gareis M. 2006. Degradation of scrapie associated prion protein (PrPSc) by the gastrointestinal microbiota of cattle. Vet Res 37:695–703. doi: 10.1051/vetres:2006024. [DOI] [PubMed] [Google Scholar]
- 53.Scherbel C, Pichner R, Groschup MH, Mueller-Hellwig S, Scherer S, Dietrich R, Maertlbauer E, Gareis M. 2007. Infectivity of scrapie prion protein PrPSc following in vitro digestion with bovine gastrointestinal microbiota. Zoonoses Public Health 54:185–190. doi: 10.1111/j.1863-2378.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- 54.Johnson CJ, Bennett JP, Biro SM, Duque-Velasquez JC, Rodriguez CM, Bessen RA, Rocke TE. 2011. Degradation of the disease-associated prion protein by a serine protease from lichens. PLoS One 6:e19836. doi: 10.1371/journal.pone.0019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marin-Moreno A, Espinosa JC, Fernandez-Borges N, Piquer J, Girones R, Andreoletti O, Torres JM. 2016. An assessment of the long-term persistence of prion infectivity in aquatic environments. Environ Res 151:587–594. doi: 10.1016/j.envres.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 56.Saunders SE, Bartz JC, Vercauteren KC, Bartelt-Hunt SL. 2010. Enzymatic digestion of chronic wasting disease prions bound to soil. Environ Sci Technol 44:4129–4135. doi: 10.1021/es903520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan Q, Eckland T, Telling GC, Bartz JC, Bartelt-Hunt SL. 2015. Mitigation of prion infectivity and conversion capacity by a simulated natural process–repeated cycles of drying and wetting. PLoS Pathog 11:e1004638. doi: 10.1371/journal.ppat.1004638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saunders SE, Yuan Q, Bartz JC, Bartelt-Hunt SL. 2011. Effects of solution chemistry and aging time on prion protein adsorption and replication of soil-bound prions. PLoS One 6:e18752. doi: 10.1371/journal.pone.0018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franks F. 1985. Biophysics and biochemistry at low temperature. Cambridge University Press, London, United Kingdom. [Google Scholar]
- 60.Cao EH, Chen YH, Cui ZF, Foster PR. 2003. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol Bioeng 82:684–690. doi: 10.1002/bit.10612. [DOI] [PubMed] [Google Scholar]
- 61.Pikal-Cleland KA, Rodriguez-Hornedo N, Amidon GL, Carpenter JF. 2000. Protein denaturation during freezing and thawing in phosphate buffer systems: monomeric and tetrameric β-galactosidase. Arch Biochem Biophys 384:398–406. doi: 10.1006/abbi.2000.2088. [DOI] [PubMed] [Google Scholar]
- 62.Edwards AC, Cresser MS. 1992. Freezing and its effect on chemical and biological properties of soil. Adv Soil Sci 18:59–79. [Google Scholar]
- 63.Brown P, Rohwer RG, Green EM, Gajdusek DC. 1982. Effect of chemicals, heat, and histopathological processing on high infectivity hamster-adapted scrapie virus. J Infect Dis 145:683–687. doi: 10.1093/infdis/145.2.683. [DOI] [PubMed] [Google Scholar]
- 64.Yu X, Zou Y, Jiang M, Lu X, Wang G. 2011. Response of soil constituents to freeze-thaw cycles in wetland soil solution. Soil Biol Biochem 43:1308–1320. doi: 10.1016/j.soilbio.2011.03.002. [DOI] [Google Scholar]
- 65.Saunders SE, Bartz JC, Bartelt-Hunt SL. 2009. Influence of prion strain on prion protein adsorption to soil in a competitive matrix. Environ Sci Technol 43:5242–5248. doi: 10.1021/es900502f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shikiya RA, Ayers JI, Schutt CR, Kincaid AE, Bartz JC. 2010. Coinfecting prion strains compete for a limiting cellular resource. J Virol 84:5706–5714. doi: 10.1128/JVI.00243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ayers JI, Schutt CR, Shikiya RA, Aguzzi A, Kincaid AE, Bartz JC. 2011. The strain-encoded relationship between PrPSc replication, stability and processing in neuron is predictive of the incubation period of disease. PLoS Pathog 7:e1001317. doi: 10.1371/journal.ppat.1001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartz JC, Kramer ML, Sheehan MH, Hutter JA, Ayers JI, Bessen RA, Kincaid AE. 2007. Prion interference is due to a reduction in strain-specific PrPSc levels. J Virol 81:689–697. doi: 10.1128/JVI.01751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]