ABSTRACT
There is an urgent need for chemical-free and biological-free safe adjuvants to enhance the immunogenicity of vaccines against widespread viral pathogens, such as herpes simplex virus 2 (HSV-2), that infect a large proportion of the world human population. In the present study, we investigated the safety, immunogenicity, and protective efficacy of a laser adjuvant-assisted peptide (LAP) vaccine in the B6 mouse model of genital herpes. This LAP vaccine and its laser-free peptide (LFP) vaccine analog contain the immunodominant HSV-2 glycoprotein B CD8+ T cell epitope (HSV-gB498–505) covalently linked with the promiscuous glycoprotein D CD4+ T helper cell epitope (HSV-gD49–89). Prior to intradermal delivery of the LAP vaccine, the lower-flank shaved skin of B6 or CD11c/eYFP transgenic mice received a topical skin treatment with 5% imiquimod cream and then was exposed for 60 s to a laser, using the FDA-approved nonablative diode. Compared to the LFP vaccine, the LAP vaccine (i) triggered mobilization of dendritic cells (DCs) in the skin, which formed small spots along the laser-treated areas, (ii) induced phenotypic and functional maturation of DCs, (iii) stimulated long-lasting HSV-specific effector memory CD8+ T cells (TEM cells) and tissue-resident CD8+ T cells (TRM cells) locally in the vaginal mucocutaneous tissues (VM), and (iv) induced protective immunity against genital herpes infection and disease. As an alternative to currently used conventional adjuvants, the chemical- and biological-free laser adjuvant offers a well-tolerated, simple-to-produce method to enhance mass vaccination for widespread viral infections.
IMPORTANCE Herpes simplex viruses 1 and 2 (HSV-1 and HSV-2) infect a large proportion of the world population. There is an urgent need for chemical-free and biological-free safe adjuvants that would advance mass vaccination against the widespread herpes infections. The present study demonstrates that immunization with a laser-assisted herpes peptide vaccine triggered skin mobilization of dendritic cells (DCs) that stimulated strong and long-lasting HSV-specific effector memory CD8+ T cells (TEM cells) and tissue-resident CD8+ T cells (TRM cells) locally in the vaginal mucocutaneous tissues. The induced local CD8+ T cell response was associated with protection against genital herpes infection and disease. These results draw attention to chemical- and biological-free laser adjuvants as alternatives to currently used conventional adjuvants to enhance mass vaccination for widespread viral infections, such as those caused by HSV-1 and HSV-2.
KEYWORDS: genital herpes, laser adjuvant, CD8+ T cells, CD4+ T cells, dendritic cells, herpes simplex virus
INTRODUCTION
Safe, easy-to-handle, chemical- and biological-free immunoadjuvants are urgently needed for quick implementations of mass human vaccination protocols to protect from the emerging and established viral infectious diseases that affect large human populations (1–4). During the last decade, several chemical and biological adjuvants have been identified, providing renewed optimism that rapid and effective control of widespread infectious pathogens through vaccination might be feasible (2). Current traditional chemical and biological adjuvants often meet with numerous safety, logistical, and scientific obstacles. Conventional vaccines, which sometimes require multiple injections, necessitate medical personnel, training, and health care infrastructure, all of which hamper rapid and efficient mass vaccination campaigns. In addition, the cost of delivering conventional vaccines (i.e., cold-chain storage, sterile syringes and needles, medical infrastructure, well-trained medical personnel, and waste disposal requirements) are sometimes 6-fold more expensive than the cost of the vaccine itself. To date, aluminum salts (Alum) used in current vaccines have been the only adjuvant approved for widespread human use, but this adjuvant solution promotes mainly antibody responses, but not T cell responses (2). Thus, developing alternative, safe, and efficient chemical- and biological-free adjuvants that enhance both B and T cell responses to codelivered vaccines and replace the currently used traditional adjuvants remains a highly desirable goal (5).
The skin is rich in immune cells, including antigen-presenting cells (APCs) such as keratinocytes (KCs), macrophages (MΦ), and dendritic cells (DCs), as well as CD4+ T cells and CD8+ T cells, which are strategically positioned in the epidermis and dermis to be stimulated with various type of adjuvants (6). Skin exposure to various types of lasers has been successfully used to remove skin fine lines and wrinkles, and hence lasers represent excellent chemical- and biological-free adjuvants to enhance immune responses to vaccines (7–15). There are four different types of laser devices: (i) ultrashort pulsed lasers, (ii) nonpulsed lasers, (iii) nonablative fractional lasers, and (iv) ablative fractional lasers (13). We hypothesize that skin exposure to non-tissue-damaging FDA-approved nonablative fractional laser pulses would enhance immune responses to codelivered vaccines. The choice of nonablative fractional laser for vaccination is enhanced by the current availability of a safe, compact, and relatively simple low-voltage diode laser (PaloVia laser). This technology makes it possible to economically produce a portable (handheld) low-cost laser adjuvant device.
In this study, we report that skin exposure of B6 mice with the FDA-approved nonablative fractional diode laser (PaloVia laser) followed by an intradermal delivery of a herpes peptide vaccine safely induced potent and sustained HSV-specific CD8+ T cells, detected in both the draining lymph nodes (DLN) and in the vaginal mucosa (VM). More HSV-specific effector memory CD8+ T cells (TEM cells) and tissue-resident CD8+ T cells (TRM cells) were detected locally in the vaginal mucocutaneous tissues of laser-treated and peptide-vaccinated mice than in non-laser-treated and peptide-vaccinated mice. This was associated with lower virus titers and decreased overt signs of genital herpes disease following intravaginal HSV-2 challenge. No local or systemic side effects were noticed in vaccinated mice, besides an increase in DC infiltrates around the laser-treated skin area, suggesting involvement of activated skin-resident mature DCs in the immunogenicity of laser-assisted intradermal peptide vaccines. To the best of our knowledge, our study is the first to show that a laser-assisted adjuvant herpes peptide vaccine delivered intradermally induces a robust antiviral CD8+ T cell-dependent protective immunity against genital herpes. The findings underscore the potential of a chemical-free “laser-assisted adjuvant” as an attractive potential strategy to enhance immune responses against infectious pathogens, with greater safety than the currently used chemical- or biological-adjuvant-based vaccines. These findings have important implications for the development of efficient peptide immunization strategies against widespread viral pathogens, such as HSV-1 and HSV-2.
RESULTS
Brief exposure of skin to laser light triggers mobilization of local dendritic cells.
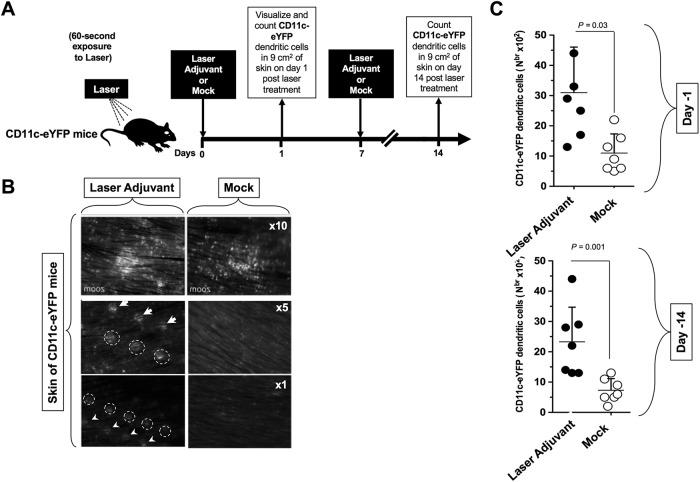
We first explored whether skin exposure to laser would affect mobilization of skin dendritic cells (DCs). To this end, we used CD11c/eYFP (where eYFP is enhanced yellow fluorescent protein) transgenic mice for in vivo tracking of skin-resident eYFP-labeled DCs (16, 17). Female CD11c/eYFP transgenic mice (n = 20) were shaved on the dorsal lower back area, over the area of the spinal cord and the dorsal root ganglia (DRG). The next day, all animals received a topical skin treatment with 5% imiquimod cream (here referred to as imiquimod cream 5%) over the shaved area and were then divided into two groups. The first group of mice (n = 10) were exposed to the FDA-approved nonablative diode laser (PaloVia laser) (“laser adjuvant” group) for 60 s, while the second group of control mice (n = 10) were left untreated (“mock” group) (Fig. 1A). As shown in Fig. 1B, formation of small DC infiltrates was detected in the skin of CD11c/eYFP transgenic mice along the laser-treated areas, as early as 24 h after laser exposure. Higher numbers of DCs were mobilized in laser-treated skin of CD11c/eYFP transgenic mice, on both day 1 and day 14 following laser exposure than in untreated skin (P = 0.03 and P = 0.001, respectively) (Fig. 1C). CD11c/eYFP DCs appeared to be colocalized or adjacent to CD8+ T cells in the skin of CD11c/eYFP mice that received LAP vaccine (not shown), suggesting that skin mobilization of DCs and CD8+ T cells may be occurring following skin exposure.
FIG 1.
Laser adjuvant mobilizes dendritic cells in the skin of CD11c/eYFP transgenic mice. Female CD11c/eYFP transgenic mice (n = 20) were shaved on the dorsal lower back area, over the area of the spinal cord and the dorsal root ganglia (DRG). (A) The next day, all animals received a topical skin treatment with imiquimod cream 5% over the shaved skin area and then divided in two groups. The first group of mice (n = 10) were exposed for 60 s to the FDA-approved nonablative diode laser (PaloVia Laser) (Laser Adjuvant). The second group of control mice (n = 10) were left untreated (Mock). (B) Pictures of skin of laser-treated and -untreated mice were taken 24 h later at various magnifications (×1, ×5, and ×10) to visualize CD11c/eYFP DCs in skin. Circles and arrows indicate the formation of spots of DC infiltrates along the laser-treated areas. (C) Skin of CD11c/eYFP transgenic mice was left untreated or exposed for 60 s to laser on day 1 and day 7. The DCs were quantified by FACS in a 9-cm2 skin tissue section harvested on day 1 (top) and on day 14 (bottom) following laser exposure. Results are representative of three independent experiments. The P values were determined by ANOVA test.
Together, these results indicate that a brief skin exposure to laser light drives a quick mobilization of local DCs along the laser-treated areas, as early as 24 h after laser exposure. We therefore asked whether laser exposure would also affect phenotypic and functional maturation of DCs.
Exposure of immature dendritic cells to laser promotes their phenotypic and functional maturation.
We next determined whether exposure of immature DC to laser would affect their phenotypic and functional maturation. For practical reasons, it was not feasible to obtain sufficient numbers of skin-derived immature DCs to conduct a systematic study of their maturation. Therefore, DC phenotype and function studies were performed in vitro using bone marrow-derived DCs, which contained a mixture of both myeloid and lymphoid DCs (18–21).
Immature bone marrow-derived DCs were derived from bone marrow stem cells of female B6 mice using interleukin-4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF), as described in Materials and Methods. Immature DCs were left untreated or were treated with laser for 10 min and then cultured for 24 h with 50 μg of herpes peptide vaccine, as illustrated in Fig. 2A and B. Laser-treated and untreated DCs were subsequently examined by flow cytometry for cell surface expression of major histocompatibility complex class I (MHC-I), MHC-II, and costimulatory molecules CD80 and CD86 markers, which indicate DC phenotypic maturation. Untreated immature DCs and lipopolysaccharide (LPS)-stimulated DCs were included as negative and positive controls, respectively. Figure 2A shows the average mean fluorescent intensities (MFI) of cell surface expression of MHC and costimulatory markers obtained in 10 B6 mice, with the horizontal bar representing the average ± standard deviation (SD). Laser-treated DCs displayed significant increases in cell surface expression of MHC-I, MHC-II, CD80, and CD86 molecules compared to untreated DCs (P < 0.005) (Fig. 2C). Laser exposure did not affect the surface expression of other DC markers that are unrelated to DC maturation, such as CD40 or CD11c (not shown), suggesting that DC maturation was not due to the global expression of cell surface proteins. As expected, the highest levels of expression of maturation markers were detected in LPS-stimulated DCs (not shown). Production of tumor necrosis factor alpha (TNF-α) and IL-12 was quantified by a sandwich enzyme-linked immunosorbent assay (ELISA) in DC supernatants taken 16 h after laser exposure, as described in Materials and Methods. Exposure of laser produced significantly more TNF-α and IL-12 in immature DCs than the levels observed in untreated immature DCs (P < 0.005) (Fig. 2D). As expected, the largest amount of proinflammatory cytokines was detected in LPS-stimulated DCs (not shown). These results indicate that exposure of immature DCs to laser triggered their phenotypic maturation and further stimulated their capacity to produce cytokines such as TNF-α and IL-12.
FIG 2.
Laser adjuvant induces phenotypic and functional maturation of dendritic cells and promotes production of proinflammatory cytokines. (A) Bone marrow cells were harvested from B6 mice, and immature DCs were generated using IL-4 and GM-CSCF as described in Materials and Methods. Immature DCs were then exposed to laser. The schematic shows the timing of 10-min laser exposure, a 24-h herpes peptide vaccine stimulation, and subsequent phenotypic and functional DC analyses 48 h later. Untreated DCs (Mock) and LPS (1 μg/ml)-treated DCs (LPS) were used as negative and positive controls, respectively. (B) Prototype of herpes peptide vaccine (LFP vaccine) used in the assay illustrated in panel A. (C) Expression levels of MHC-I, MHC-II, costimulatory molecules (CD80 and CD86), and CD40 on the surface of immature DCs that were exposed to laser and stimulated with LFP vaccine. Average mean fluorescent intensity (MFI) depicts the level of expression of MHC, CD80, CD86, and CD40 on the surface of laser-treated DCs (Laser Adjuvant, black circles) and untreated DCs (Mock, white circles). Each bar in panel C shows the averages ± SD from two independent experiments. (D) DCs were exposed to laser or left untreated, as described for panels A and C, and ELISAs were performed to determine the amount of proinflammatory cytokines IL-12 and TNF-α from culture supernatants collected at 24 h following laser exposure. The P values were determined using the ANOVA test. (E) Immature DCs exposed to laser demonstrated an increased ability to stimulate HSV-specific CD8+ T cells. DCs were exposed to laser or left untreated, as described for panels A, C, and D. Cells were then washed and treated with mitomycin C, and the indicated numbers of DCs were incubated in duplicate for 72 h with 105 autologous CD8+ T cells derived from HSV-2-infected B6 mice. The number of HSV-specific CD8+ T cells producing IFN-γ was determined by an ELISpot assay. Results show the averages ± SD from two independent experiments. The P values were determined using the ANOVA test.
We further explored whether the laser-mediated activation of phenotypic maturation of DCs would translate into a functional improvement in their ability to activate HSV-specific CD8+ T cells. Immature bone marrow DCs derived from B6 mice were either left untreated or treated with the laser for 10 min and then cultured for 16 h with 50 μg of herpes peptide vaccine, as shown in Fig. 2B, as described above. Increasing numbers of laser-treated DCs or untreated DCs were added to a constant number (105) of autologous HSV-specific CD8+ T cells isolated from the spleen of HSV-2-infected B6 mice, 35 days postinfection. The gamma interferon (IFN-γ)-producing CD8+ T cells were quantified by enzyme-linked immunospot (ELISpot) assay. The laser-treated DCs induced significantly more IFN-γ-producing CD8+ T cells than untreated DCs, and this occurred in a dose-dependent manner (P < 0.05) (Fig. 2E).
Collectively, these results demonstrate that exposure of immature DC to laser promotes their phenotypic and functional maturation.
A brief exposure of skin to laser adjuvant augments HSV-specific CD4+ and CD8+ T cell responses.
Since skin exposure to laser appeared to affect both mobilization and maturation of local DCs, we explored whether skin exposure would affect T cell responses to the herpes peptide vaccine in Fig. 2B, which contained the immunodominant HSV-2 glycoprotein B CD8+ T cell epitope (HSV-gB498–505) covalently linked with the promiscuous glycoprotein D CD4+ T cell epitope (HSV-gD49–89). We first performed a dose-response study in B6 mice of the herpes peptide vaccine using 50, 100, or 200 μg delivered intradermally (i.d.) in adjuvant-free saline on days 0 and 7. All three doses induced a similar magnitude of T cell responses in genital tract draining lymph nodes (GT-DLN) (data not shown). There were no obvious herpes peptide vaccine-related severe side effects at any of the three doses tested, as evaluated by lack of weight loss and lack of apparent skin lesions (data not shown). Accordingly, all subsequent experiments were carried out using the middle dose of 100 μg for the LAP vaccine.
Female B6 mice (n = 40) were shaved on the dorsal lower back area, over the area of the spinal cord and the dorsal root ganglia (DRG), the sites of herpes virus latent infection. The next day, all animals received a topical skin treatment with imiquimod cream 5% over the shaved area and were then divided in four groups (n = 10). The first group of mice were exposed to the FDA-approved nonablative diode laser (PaloVia laser) for 60 s and then immunized intradermally within the shaved area with 100 μg and 50 μg of herpes peptide vaccine on day 1 and day 7, respectively. This group was designated laser-assisted peptide vaccine or “LAP vaccine.” The second group of mice were not exposed to laser but were immunized i.d. with the same amount of herpes peptide vaccine. This group was designated laser-free peptide vaccine or “LFP vaccine.” The third group of control mice were exposed for 60 s to laser but were not immunized (“Laser alone”). The fourth group of mice (control) were mock treated and mock immunized (“Mock”) (Fig. 3A). A fifth group of mice (n = 10) were shaved on the dorsal lower back area (as described above); the next day, they received a sham topical skin treatment with phosphate-buffered saline (PBS) over the shaved area (instead of imiquimod cream) and laser exposure alone followed with i.d. immunization with the same amount of herpes peptide vaccine (i.e., laser control, in the absence of imiquimod treatment).
FIG 3.
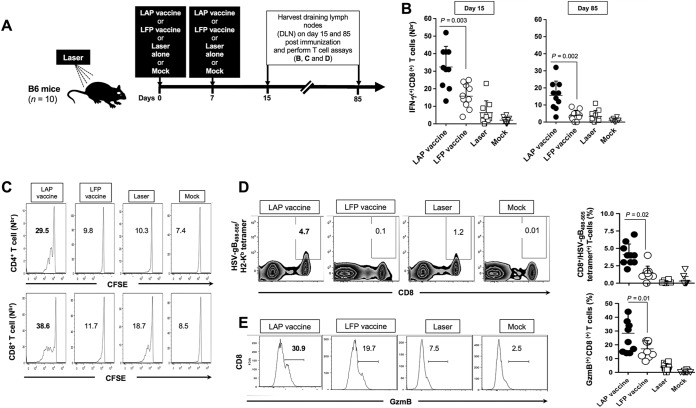
Potent and long-lasting CD4+ and CD8+ T cell responses detected in DLN following i.d. immunization with chemical-free laser adjuvant peptide vaccine (LAP vaccine). (A) Schedule of immunization with herpes peptide vaccine (see Fig. 2B) with (LAP vaccine) and without (LFP vaccine) laser adjuvant and timing of subsequent T cell assays. B6 mice, 5 to 6 weeks old, were treated with imiquimod and left untreated (LFP vaccine, n = 10) or treated with laser adjuvant (LAP vaccine, n = 10) and then immunized intradermally (i.d.) on days 0 and 7 with 100 μg of HSV peptide vaccine. Draining lymph nodes (DLN) were collected on days 15 and 85 following the first immunization. DLN cell suspensions were stimulated with DC pulsed with HSV-gB498–505 (for CD8+ T cells) or with the promiscuous HSV-gD49–89 peptide. (B) Cells collected on day 15 and day 85 after the first immunization and stimulated with HSV-gB498–505 peptide were double stained with anti-mouse CD8 MAb and IFN-γ. The percentages of IFN-γ-producing CD8+ T cells were analyzed by FACS. (C) Draining lymph nodes cells collected on day 15 after the first of immunization and stimulated with HSV-gB498–505 or HSV-gD49–89 peptides were double stained with anti-mouse CD4 or CD8 MAb and carboxyfluorescein succinimidyl ester (CFSE). The percentages of proliferating CFSE(+) CD4+ cells and CFSE(+) CD8+ cells were determined by FACS. (D and E) HSV-gB498–505 peptide-stimulated cells were triple stained with anti-mouse CD8 MAb, HSV-gB498–505/tetramers, and anti-mouse GzmB MAb. Representative percentages of HSV-gB498–505-epitope-specific CD8+ T cells expressing GzmB were determined by FACS (left panels). The averages ± SD of percentages of HSV-gB498–505-epitope-specific CD8+ T cells expressing GzmB detected in 10 mice are shown (right panels). The results are from two independent experiments, each done in duplicate. The P values were determined using the ANOVA test.
CD4+ and CD8+ T cell responses were analyzed in draining lymph nodes, using several immunological parameters, on day 15 and day 85 (i.e., during the memory phase) after the first immunization. Compared to the LFP vaccine, the LAP vaccine induced significantly more IFN-γ-producing CD8+ T cells, and this occurred both on day 15 and day 85 after the first immunization (P = 0.003) (Fig. 3B). When assessed on day 85, only the LAP-vaccinated mice had significant IFN-γ-producing CD8+ T cell responses compared to mock-immunized mice (P = 0.002). However, during this memory phase, no significant IFN-γ-producing CD8+ T cell responses were detected in the LFP-vaccinated mice (P = 0.002). As expected, no significant T cell responses were detected in LAP-vaccinated mice or in LFP-vaccinated mice against the irrelevant OVA257–264 target peptide, demonstrating the gB495–505 specificity of the CD8+ T cell responses induced by either type of vaccine (data not shown).
Moreover, significantly higher percentages of proliferating carboxyfluorescein succinimidyl ester (CFSE)(+) CD4+ cells and CFSE(+) CD8+ cells were detected in the DLN of LAP vaccine mice 7 days after the final immunization than in the DLN of LFP vaccine mice, which did not receive the laser exposure (P < 0.005) (Fig. 3C). More-frequent HSV-gB498–505 epitope-specific CD8+ T cells (P = 0.02) (Fig. 3D) and GzmB(+) CD8+ T cells (P = 0.01) (Fig. 3E) were also detected in DLN of LAP vaccine mice than in DLN of LFP vaccine mice. As expected, no significant T cell responses were detected in the fifth group of mice, which received laser exposure together with i.d. immunization with the herpes peptide vaccine, but in the absence of imiquimod treatment (i.e., laser control, in the absence of imiquimod treatment) (data not shown). No local or systemic side effects were noticed in LAP vaccine mice, besides an increase in inflammatory cells, including DC infiltrates around the laser-treated skin area, suggesting involvement of activated skin-resident DCs in the immunogenicity of LAP vaccine.
Altogether, these results demonstrate that intradermal immunizations with the peptide vaccine together with imiquimod treatment alone, in the absence of laser (i.e., LFP vaccine control) or with laser exposure alone, in the absence of imiquimod, were both unable to significantly boost HSV gB498–505-specific CD8+ T cell responses. In contrast, intradermal immunization with peptide vaccine together with a combination of imiquimod and laser (i.e., the LAP vaccine) induced significant HSV gB498–505-specific CD8+ T cell responses. These results suggest an additive or a synergistic effect between the imiquimod and laser that boosted potent and long-lasting HSV-specific CD4+ and CD8+ T cell responses.
Immunization with laser-assisted peptide vaccine induces strong protection against genital herpes compared to immunization with its homologous peptide vaccine alone.
Since immunization with the LAP vaccine induced stronger HSV-specific CD4+ and CD8+ T cell responses than the homologous LAF vaccine, it was of interest to determine whether it could also better protect against genital herpes.
Four groups of 20 age-matched B6 mice (n = 20) were either (i) exposed to laser and immunized i.d. with the LFP vaccine (LAP vaccine), (ii) not exposed to laser but immunized i.d. with the LAF vaccine (LAF vaccine), (iii) exposed to laser but not immunized (Laser), or (iv) mock treated and mock immunized (Mock) (Fig. 4A). One week after the final immunization, all mice were treated with Depo-Provera to synchronize the ovarian cycle and increase susceptibility to herpes infection. The mice were divided into two groups of 10 and subsequently challenged intravaginally with either (i) 5 × 106 PFU (200 × 50% lethal dose [LD50] for survival analysis) or (ii) 5 × 104 PFU (for virus titer and disease analysis) of HSV-2 (strain 333), as we previously described (22).
FIG 4.
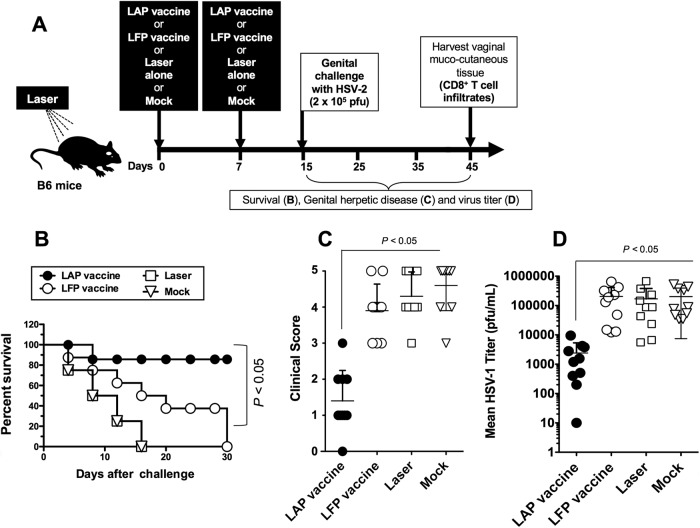
Intradermal immunization with chemical-free laser adjuvant peptide vaccine (LAP vaccine) confers protection against genital herpes infection and disease. (A) Time course for immunization, challenge, and protection analysis in B6 mice. Four groups of age-matched B6 female mice were left untreated and immunized i.d. with the herpes peptide vaccine (LFP vaccine) or treated with laser and then immunized i.d. with the herpes peptide vaccine (LAP vaccine) as described for Fig. 3. Control mice were untreated and not immunized (Mock) or laser treated but not immunized (Laser). One week after the final immunization, mice in each group were treated with Depo-Provera and were then challenged intradermally, on day 15, with 5 × 106 PFU (200× LD50 for survival analysis) or with 5 × 104 PFU (for virus titers and disease analysis) of HSV-2 (strain 333), as described in Materials and Methods. (B) Mice were observed daily from day 15 to day 45 postchallenge for mortality. (C) Mice were also observed daily for genital disease and clinically scored from 0 to 5. (D) The presence of infectious virus in vaginal washes was detected 7 days postchallenge. The data are expressed as means + SD of virus load (PFU/sample). The results are representative of two independent experiments. The P values were determined using the ANOVA test.
Mice immunized with the LAP vaccine showed 85% survival, in contrast to mice immunized with the homologous LAF vaccine, of whom only 40% survived, 25 days postchallenge (P < 0.05, one-way analysis of variance [ANOVA] test), and to mock-vaccinated mice, of whom 0% survived, 15 days postchallenge (P < 0.005, one-way ANOVA test) (Fig. 4B). The clinical scores observed in the LAP vaccine group were also much lower than those of all the other three groups (P < 0.05 for all, one-way ANOVA test) (Fig. 4C). Additionally, viral titers measured in the vaginal washes on day 7 postchallenge showed that the LAP vaccine group had significantly lower viral loads than the other three groups (P < 0.05) (Fig. 4D). In agreement with the protective efficacy of LAP vaccine, a fast virus clearance (within 3 to 4 days) was noticed in the VM of the LAP vaccine group, in contrast to much slower virus clearance in the LAF vaccine group and the mock-vaccinated group (not shown).
To assess the involvement of CD4+ and CD8+ T cell subsets in the protection induced by LAP vaccine, in vivo depletion of either CD4+ or CD8+ T cells was performed by administrating specific antibodies in immunized mice before virus challenge, as detailed in Materials and Methods. Depletion of CD8+ T cells, but not of CD4+ T cells, significantly abrogated the protection induced by immunization with the LAP vaccine (P < 0.005) (Table 1), suggesting that in this system, CD8+ T cells are required for protection against lethal genital herpes.
TABLE 1.
Laser-assisted LAP vaccine induced a CD8+ T cell protective immunity against genital herpes in micea
| Treatment of LAP-immunized mice | % of T cells in spleen |
No. protected/no. tested | % Survivalb | P valuec | |
|---|---|---|---|---|---|
| CD4+ | CD8+ | ||||
| No treatment | 17.2 | 5.9 | 17/20 | 85 | |
| Anti-CD4 MAb | 0.3 | 5.5 | 15/20 | 75 | <0.05 |
| Anti-CD8 MAb | 19.4 | 0.2 | 3/20 | 50 | 0.002 |
| Irrelevant IgG control | 20.1 | 5.7 | 16/20 | 85 | >0.05 |
LAP-vaccinated B6 mice were left untreated or depleted of CD4+ or CD8+ T cells following intraperitoneal injections of corresponding MAb as described in Materials and Methods. Control mice received intraperitoneal injections with an isotype control IgG.
Results are representative of two independent experiments.
P values comparing virus titration recorded in LAP-vaccinated untreated mice to the anti-CD4 MAb-, anti-CD8 MAb-, or IgG-treated mice as determined using ANOVA.
Altogether, these results indicate that immunization with LAP vaccine in the progestin-treated mouse model of genital herpes decreased virus replication at the site of infection and decreased overt signs of genital herpes disease. CD8+ T cell-mediated immunity was involved in the protection. The results suggest that laser adjuvant has a beneficial effect for the induction of an effective protective immunity against genital herpes.
Frequencies of CD8+ TEM and TRM cells in laser-assisted peptide-vaccinated mice correlated with protection from genital herpes.
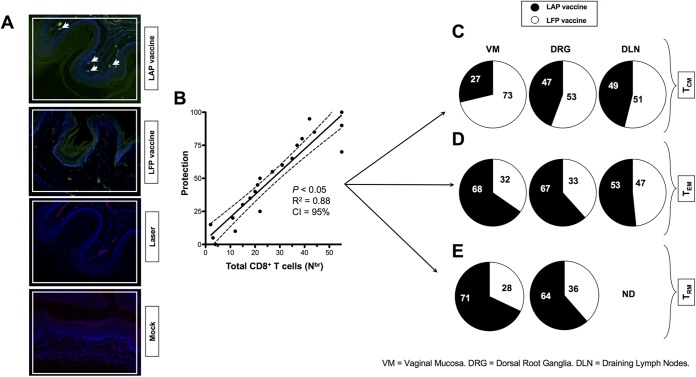
Vaginal mucocutaneous tissues were collected 10 days after the second immunization (i) from the LAP-immunized and infected mice (LAP vaccine), (ii) from the LFP-immunized and infected mice (LFP vaccine), (iii) from laser-treated nonvaccinated and infected mice (Laser), and (iv) from mock-immunized mice (Mock). Sections of vaginal mucocutaneous tissues from LAP-immunized and infected mice showed more infiltration of CD8+ T cells than did those from LFP-immunized and infected mice (Fig. 5A), detected by immunofluorescence microscopy, as described in Materials and Methods. The arrowheads in Fig. 5A indicate that more CD8+ T cells were accumulating at the vaginal epithelium of LAP-immunized and infected mice. Vaginal mucocutaneous tissues were collected from protected and nonprotected mice, and the percentages of central memory CD8+ T cells (TCM cells), TEM, and TRM cells were determined by fluorescence-activated cell sorter (FACS). Increased levels of protection correlated with increased numbers of CD8+ T cells detected in the vaginal mucosa of HSV-2-infected mice (Fig. 5B). To determine the subtypes of CD8+ T cells that are involved in protection, B6 mice (n = 10) that received either LAP vaccine or LAF vaccine were euthanized on day 10 postinfection, and single-cell suspensions from vaginal mucosa (VM), dorsal roots ganglia (DRG), and draining lymph nodes (DLN) were stained for the three major subsets of memory CD8+ cells (i.e., TCM, TEM, and TRM cells) and analyzed by FACS. Significantly higher frequencies of both CD44high CD62Llow CD8+ TEM cells and CD103high CCR7low CD62Llow CD8+ TRM cells were observed in the VM and DRG of LAP-vaccinated and protected mice than in LFP-vaccinated nonprotected mice (P < 0.05) (Fig. 5C and D). However, similar frequencies of CD44high CD62Lhigh CD8+ TCM cells were detected in the VM, DRG, and DLN from the LAP- and the LFP-vaccinated mice (Fig. 5E). These results indicate that, at least, frequent CD8+ TEM and TRM cells in the VM and DRG from the LAP-vaccinated mice were associated with a significant decrease in genital herpesvirus titers and severity of genital herpes lesions.
FIG 5.
High percentages of CD8+ TEM and TRM cells detected in the vaginal mucosa of LAP vaccine immunized and infected mice correlated with protection from genital herpes. (A) Vaginal mucocutaneous tissues were collected 10 days after the second immunization from the LAP vaccine-immunized and infected (LAP vaccine), LFP vaccine-immunized and infected (LFP vaccine), laser-treated, nonvaccinated, and infected mice (Laser), and mock-immunized mice. Sections of vaginal mucocutaneous tissues were stained with fluorescein isothiocyanate-conjugated anti-CD8 antibody (green), and the nuclei were visualized by staining with DAPI (blue). CD8+ T cells were detected by immunofluorescence microscopy, as described in Materials and Methods. Arrowheads indicate CD8+ T cells accumulating at the vaginal epithelium. (B) Vaginal mucocutaneous tissues were collected from protected and nonprotected mice, and the percentages of CD8+ TCM, TEM, and TRM cells were determined by FACS. Increased numbers of CD8+ T cells were detected in the vaginal mucosa of HSV-2-infected and protected mice compared to nonprotected mice. (C, D, and E) Increased numbers of CD8+ TEM and TRM cells were mobilized in the vaginal mucosa of LAP-vaccinated and protected compared to LFP-vaccinated unprotected mice. B6 mice (n = 10) that received either LAP vaccine or LFP vaccine were euthanized on day 10 postinfection, and single-cell suspensions from vaginal mucosa (VM), dorsal roots ganglia (DRG), and draining lymph nodes (DLN) were stained for the three major subsets of memory CD8+ cells (i.e., TCM, TEM, and TRM cells) and analyzed by FACS. (C) Percentages (pie charts) of the average frequencies of CD44high CD62Lhigh CD8+ TCM cells detected in the VM, DRG, and DLN from 10 LAP-vaccinated mice and 10 LFP-vaccinated mice. (D) Average frequencies of CD44high CD62Llow CD8+ TEM cells detected in the VM, DRG, and DLN from 10 LAP-vaccinated mice and 10 LFP-vaccinated mice. (E) Average frequencies of CD103high CCR7low CD62Llow CD8+ TRM cells detected in the VM, DRG, and DLN from 10 LAP-vaccinated mice and 10 LFP-vaccinated mice. Differences between the groups were identified by ANOVA multiple-comparison procedures. Results are representative of two independent experiments.
DISCUSSION
Herpes simplex virus 1 and 2 infections have been prevalent since the ancient Greek times. To this day, they still affect a staggering number of over 3.7 billion individuals worldwide. While prophylactic and therapeutic HSV vaccines have remained urgently needed for centuries, their development has been difficult. In the present study, we demonstrated a laser adjuvant system capable of improving antiviral CD8+ TEM and TRM cell responses associated with protective immunity against genital herpes infection and disease. The induced long-lasting HSV-specific CD8+ T cell responses persisted in the vaginal mucosa for up to 3 months postimmunization with the laser adjuvant-assisted herpes peptide vaccine. The findings underscore the potential of a safe and easy-to-use chemical- and biological-free laser vaccine adjuvant for inducing T cell immunity to protect against genital HSV-2 infection in mice. This approach could be useful against the widespread human HSV-1 and HSV-2 infections.
The lack of immunological adjuvants that are safe, easy to handle, and effective is an important obstacle for the development of mass vaccination against many infectious pathogens (11). No adjuvants have yet been approved for use with intradermal vaccines (11). Subunit vaccines usually fail to prime T cell responses unless they are delivered together with a potent chemical or biological adjuvant (23–25). Peptide-based vaccines have been emulsified with a variety of adjuvants, including Freund's (23–26), Montanide's ISA-51 and ISA-720 (20, 27–31), MF59 (32–34), and QS-21 (35) adjuvants. Others fail to reproduce in humans the results obtained in mice (reviewed in reference 36). Some of these limitations have been, in part, circumvented by chemical conjugation to viral (20) or bacterial (37) carrier proteins, by incorporation into liposomes (38, 39), proteasomes (40), or immunostimulating complexes (41), by synthesis of larger constructs such as multiple antigenic peptides (27), or by linkage to lipid moieties (20, 21, 27, 28, 30, 42–50). These different vaccine approaches often increase the immunogenicity of the antigen (Ag), at least with respect to antibody production, the main immunological parameter often used to assess their immunogenicity.
Many development stage chemical and biological adjuvants cause significant side effects. Some of these adjuvants tested in small laboratory animals have limitations due to toxicity. The ultimate goal of this study was to investigate an alternative to currently used chemical and biological adjuvants. We report, for the first time, that a brief (60-s) exposure of skin to laser light was sufficient to enhance protective T cell responses to herpes peptide vaccination in mice. However, this laser adjuvant requires coadministration of imiquimod cream 5% to induce a sufficiently increased immunological response. Nevertheless, we showed that non-tissue-damaging laser light given, in a brief (60-s) exposure, to small areas of the skin increased both CD4+ and CD8+ T cell responses to herpesvirus epitopes. This resulted in improved survival to a lethal challenge in the mouse model of genital herpes and led to a reduction in both genital herpes infection and disease. These provocative findings raise the following three important areas for further investigation, which we will address in future reports: (i) whether therapeutic LAP vaccine enhances long-term recall memory TEM and TRM cell responses to skin-based vaccination using our established herpes mouse models of induced reactivation; (ii) besides the involvement of laser in DC mobilization and maturation, reported here, identifying the underlying cellular and molecular mechanisms of action of the LAP vaccines; and (iii) the use of LAP vaccines in other routes, including mucosal routes. The translatability of this discovery is enhanced by the current availability of mature, safe, compact, and relatively simple low-voltage laser technology, making it possible to economically produce a portable (handheld) low-cost device for mass vaccination.
Despite the availability of many interventional strategies, such as sexual behavior education, barrier methods, and the costly guanine nucleoside antiviral drug therapies (e.g., acyclovir and derivatives), controlling the spread of genital herpes remains a challenge (1, 22, 51–55). The current medical opinion is that an effective clinical vaccine would constitute the best approach to protect the human populations from genital herpes (54, 56, 57). Such a vaccine would likely have a great impact in both developed and developing regions of the world (54, 56, 57). To date, however, no clinical vaccines for the prevention or treatment of genital herpes are available (58). Considering the limited success of recently tested clinical herpes vaccines using protein-in-chemical or biological adjuvants, the finding in the present study that a laser-assisted peptide vaccine induces CD8+ T cell-mediated protective immunity against genital herpes is rather interesting. T cell responses to HSV-2 antigens in the VM might be suboptimal due to the unique immune vaginal environment (59). Direct experiments in animal models (22, 37, 60) and indirect evidence in humans (61, 62) suggest that the successful control of herpes infection is associated with the induction of robust antiviral CD8+ T cells within the vaginal submucosal tissues (63). Thus, the present findings are encouraging, as they offer a safe and relatively low-cost strategy to provide a T-cell-based vaccine for clinical trials. Which T cell epitopes will be protective in humans have yet to be determined. Our inability to efficiently deliver Ags to stimulate strong local mucosal HSV-specific CD8+ T cell responses remains a critical roadblock in the development of an effective herpes vaccine. The present study is the first to show that a laser-assisted herpes peptide vaccine formulation administered i.d. induced robust, sustained CD8+ T cells locally in both DLN and the VM. The induced CD8+ T cells appeared to be mostly CD8+ TEM and TRM cells that produced cytotoxic activity and IFN-γ, two main effectors of immunity that are associated with a significant decrease in genital herpes simplex virus titers and lesions. This result, together with the observation of a high frequency of HSV-specific CD8+ T cells within healed human genital lesions (64) strongly suggests that local HSV-specific CD8+ T cells may be important immune effectors in the reduction or clearance of genital herpes.
Clearance of HSV-2 from recurrent genital lesions is associated with increased infiltration of both HSV-2-specific CD4+ and CD8+ T cells (61, 65) to the vaginal tissue (66, 67). CD4+ T cells specific to envelope and capsid proteins are thought to be among the main mediators of protective immunity during recurrent genital herpes (68–71). Following CD4+ T cells, HSV-2-specific CD8+ T cells persistently infiltrate healed genital herpes lesions (72). CD8+ T cells also a critical role in surveillance functions that limit virus reactivation and expansion (61, 65, 73–76). Furthermore, local infiltration of HSV-specific CD8+ T cells correlates with clearance of infectious virus particles in humans during recurrent genital herpes (61, 65). In mice, HSV-2-specific CD8+ T cells infiltrate acute and latently infected ganglia and mediate control over viral reactivation in an IFN-γ-dependent manner (77). Depletion of CD8+ T cells impaired clearance of virus from sensory neurons, whereas adoptive transfer of T cell receptor (TCR) transgenic CD8+ T cells, specific for gB498–505 epitope, into naive mice lacking other components of adaptive immunity results in viral clearance (78, 79). In agreement with the above-mentioned studies (78, 79), in the present study, depletion of CD8+ T cells, but not of CD4+ T cells, abrogated the protection induced following immunization with LAP vaccine (80).
Once the acute infection is cleared in the skin and vaginal mucosa, the sites of acute infection, the virus stays dormant in DRG, the site of latent infection. In LFP-vaccinated mice, we detected higher levels of viral replication in the vaginal mucosa and a higher level of latency in DRG than in LAP-vaccinated mice. The differences in viral load in the vaginal mucosa and DRG of LFP-vaccinated mice versus LAP-vaccinated mice might be associated with quantitative and qualitative differences in mobilization of memory CD8+ TRM cells. Since low numbers of neuronal cells are latently infected in DRG, fewer T cells are likely sufficient in the DRG compartment than in the vaginal mucosa compartment to reduce the level of latent viral load. Thus, although a lower frequency of CD8+ TRM cells was detected in the DRG than in the vaginal mucosa of LAP-vaccinated mice, these fewer cells seem to be sufficient to reduce the level of latent viral load in DRG. However, in the skin and vaginal mucosa, where there is an abundance of infected cells, more CD8+ TRM cells might be required to significantly reduce the level of viral load. Moreover, it is likely that laser exposure of skin might not be the only factor that operates in attracting TRM cells to the skin and vaginal mucosa. Besides laser exposure, imiquimod might also be involved in T-cell recruitment to the skin and vaginal mucosa. Hence, imiquimod might synergistically or additively operate with laser to attract TRM cells to the skin and vaginal mucosa.
The cellular and molecular mechanisms by which laser adjuvant delivered intradermally enhances protective T-cell responses remain to be fully elucidated. The success of the LAP vaccine is also highlighted by its ability to induce and facilitate the mobilization and establishment of effector memory CD8+ TEM cells and tissue-resident CD8+ TRM cells in the genital mucosal tissue (VM). Infiltrates of IFN-γ-producing CD8+ TEM and CD8+ TRM cells were detected in the VM of LAP-vaccinated and protected mice, but not of LFP-vaccinated unprotected mice, suggesting a preferential induction of memory CD8+ T cell subpopulations by the LAP vaccine. Several explanations are possible for the apparent differences in CD8+ TEM and TRM cell mobilization by LAP peptide versus LFP vaccines. LAP vaccine might be more efficient in inducing maturation and mobilization of DCs from the skin, which then migrate to the neighboring DLN and VM, where they present the gB498–505 peptide to CD8+ TEM and TRM cells, respectively. The size of the CD8+ TEM cell subpopulation expanded more prominently following LAP vaccine, but not following LFP vaccine, indicating the importance of laser adjuvant in the mobilization of memory CD8+ TEM cells. However, similar frequencies of CD8+ TCM cells were induced by LAP vaccine and LFP vaccine. CD8+ TEM cells likely migrate from the DLN to the vaginal mucosa, since the vaginal submucosa does not contain mucosa-associated lymphoid tissue (MALT) in the steady state (40). Generally, effector memory (TEM) cells circulate throughout the peripheral tissues, such as the VM, whereas central memory (TCM) cells reside in the secondary lymphoid tissues, such as DLN. Thus, regardless of the site of antigen encounter, HSV-specific memory CD8+ TEM cells must be found in various tissues, including the VM and DLN. The mucosa of vaginal canal is drained by several lymph nodes, including the common iliac, interiliac, external iliac, and inguinal femoral lymph nodes (in descending order, designated in this report DLN) (reviewed in reference 81). LAP vaccine likely induced tissue-resident CD8+ TRM cells locally in vaginal mucosa. Induction of both CD8+ TEM and TRM cells by LAP vaccine might occur through vaginal submucosal dendritic cells (DCs), which efficiently take up the LAP vaccine.
Another cellular mechanism behind the strong immunogenicity of laser-assisted peptide vaccine delivered intradermally appears to be associated with mobilization and maturation of skin-resident DCs. Dendritic cells are professional Ag-capturing and -presenting cells with the unique ability of priming naive T cells (1, 3). During recent years, it has become increasingly clear that manipulation of the immune system for vaccination purposes against intracellular viral, bacterial, and parasitic pathogens, as well as cancers, requires immunogenic formulations allowing targeting, Ag uptake, and maturation of dendritic cells at their site of uptake to cause immune induction (1, 3–5). Immature DCs reside in the periphery, where they serve as sentinels, and their interaction with foreign Ags and microbial molecules induces the so-called DC maturation program, which results in the expression of surface MHC and costimulatory molecules, production of proinflammatory cytokines, and migration to secondary lymphoid tissues, where potent interactions with T cells initiate cell-mediated immunity (6). Since all the mechanisms listed above are not mutually exclusive, it is possible that they all play a role in the T cell immunogenicity of the LAP vaccine, but additional experiments will be needed to assess the relative proportion of each mechanism.
In this study, using granulocyte/macrophage colony-stimulating factor (GM-CSF) and interleukin-4, we have derived dendritic cell (DC) lines from bone marrow of mice and demonstrated that they maintained phenotypes and functions characteristic of immature DCs. These results are in agreement with our previous report in which we showed that mouse bone marrow-derived DCs generated following treatment with GM-CSF plus IL-4 are generally immature (i.e., they do not express high levels of markers of maturation) (44, 45). Unlike peripheral blood-derived human DCs treated with GM-CSF plus IL-4, which appeared to maintain expression of MHC-I, MHC-II, CD80, and CD86 molecules (82), in our study bone marrow-derived mouse dendritic cells expressed low levels of these molecules. It is likely that there exist differences in the level of expression induced by GM-CSF plus IL-4 on human versus mouse dendritic cells. Regardless of the differences in the level of maturation between mouse and human DCs, it is striking that a short exposure of these DCs to laser was efficient in inducing their phenotypic maturation with the expression of high levels of MHC-I and -II, CD80, and CD86 molecules. The functional consequences of the short contact with laser resulted in an increase in the T cell-stimulatory capacity of DCs presenting HSV peptide to specific T cells. In ongoing experiments, we are now comparing the immunogenicity and protective efficacy of LAP vaccine to parenteral vaccination using a variety of adjuvant solutions. Results from those studies will be presented in future reports.
In conclusion, this study demonstrates that a short, 60-s exposure to a laser, using the FDA-approved nonablative diode, (i) triggered mobilization of dendritic cells in the skin, which formed small spots along the laser-treated areas and (ii) induced phenotypic and functional maturation of dendritic cells. Moreover, the study shows for the first time the ability of a novel laser-assisted genital herpes peptide vaccine to (iii) stimulate long-lasting HSV-specific effector memory CD8+ TEM cells and tissue-resident CD8+ TRM cells locally in the vaginal mucocutaneous tissues and (iv) induce protective immunity against genital herpes infection and disease. The findings underscore the potential of “laser vaccine adjuvant” to provide an accessible chemical-free and biological-free safe adjuvant for genital herpes vaccines and presumably for vaccines against other sexually transmitted diseases.
MATERIALS AND METHODS
Mice.
CD11c/eYFP transgenic mice originally developed by Michel C. Nussensweig (Rockefeller, University) were kindly provided to us by James T. Rosenbaum from Oregon Health & Science University. CD11c/eYFP transgenic mice have been successfully used for in vivo tracking of activated DCs (16, 17). C57BL/6 (B6) mice, 5 weeks old, were purchased from the Jackson Laboratory (Bar Harbor, ME). Animal studies conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. Mice were maintained at the University of California Irvine Animal Care Facility. The UCI Committee on Animal research approved all procedures (ID number 2002-2372).
Virus and cell lines.
Plaque-purified HSV-2 strain 333 was prepared as we described previously (22). Rabbit skin (RS) cells, used to prepare virus stocks and to culture virus from vaginal swabs, were grown in Eagle's minimum essential medium (EMEM) supplemented with 5% fetal calf serum (FCS; Invitrogen, Grand Island, NY). Heat-killed virus was made by heating the virus solution at 100°C for 5 min. HSV inactivation was confirmed by the inability to produce plaques when tested on Vero cells, as we described previously (83). The virus stock was aliquoted and stored at −80°C.
Laser adjuvant-assisted peptide vaccination.
The H2Kb SSIEFARL CD8+ cytotoxic T lymphocyte (CTL) target peptide (HSV-gB498–505) (84) and the promiscuous glycoprotein D CD4+ T helper cell epitope (HSV-gD49–89) QPPSLPITVYYAVLERACRSVLLNAPSEAPQIVR (85) were synthesized in tandem as Th-CTL chimeric epitopes (Fig. 1A), as we previously described (18, 85). This Th-CTL epitope chimera is designated laser-free peptide vaccine (LFP vaccine).
Female B6 mice were shaved on the dorsal lower back area, over the area of the spinal cord and the dorsal root ganglia (DRG), the sites of herpes latent infection. The next day, all animals received a topical skin treatment with imiquimod cream 5% over the shaved area (Fougera Pharmaceuticals Inc., Lexington, KY) and were then exposed for 60 s to the nonablative diode laser (PaloVia Laser) as shown in Fig. 1B. Mice were then immunized intradermally (i.d.) within the shaved area with 100 μg and 50 μg of herpes peptide vaccine, on day 1 and day 7, respectively.
Quantification of eYFP+ dendritic cells in the skin.
Female CD11c/eYFP transgenic mice were shaved on the dorsal lower back area, over the area of the spinal cord and the dorsal root ganglia (DRG). The next day, all animals received a topical skin treatment with imiquimod cream 5% over the shaved area and were then divided into two groups. One group of mice were exposed to the FDA-approved nonablative diode laser (PaloVia Laser) for 60 s, while the second group of mice (control) were left unexposed to laser (Fig. 1A). CD11c/eYFP mice were treated with calcium- and magnesium-free phosphate-buffered saline for whole-body perfusion prior to skin tissue harvest, as previously described (40, 86). The DCs were quantified by FACS in a 9-cm2 skin tissue section harvested on day 1 and on day 14 following laser exposure. Skin tissues were digested in 2 mg/ml collagenase D (Roche, Indianapolis, IN) and 0.5 mg/ml DNase (Roche) for 2 to 3 h at 37°C. Cell suspensions were triturated in 20 mM EDTA and then passed through a 70-μm filter (BD Falcon; Becton-Dickinson, Franklin Lakes, NJ), as described previously (86). Cells were incubated with Fc blocking antibody at 4°C in 0.5% bovine serum albumin (BSA). They were subsequently labeled with phycoerythrin (PE)-Cy7-conjugated CD11c (clone HL3; BD PharMingen) for 30 min in 0.5% BSA at 4°C in the dark. Aliquots were made in parallel for respective staining with the appropriate isotype control. All samples were washed and reconstituted in 0.5% BSA. Determination of the absolute numbers of eYFP-positive dendritic cells (eYFP+ CD11c+ cells) in the skin of laser-exposed and nonexposed individual mice was accomplished by extrapolation based on frequencies determined by FACS analysis.
Generation of bone marrow-derived dendritic cells.
Bone marrow-derived DCs were generated from B6 mice, as we previously described (19, 20). Briefly, single-cell suspensions of bone marrow cells were cultured in 100-mm petri dishes (Falcon 1029) at an initial density of 2 × 105 cells/ml in a final volume of 10 ml RPMI 1640 medium supplemented with 50 ng/ml murine GM-CSF and 50 ng/ml IL-4 (PeproTech Inc., NJ). After 8 to 10 days, over 90% of the nonadherent cells had acquired typical dendritic morphology and were at an immature stage. Routinely, ∼6 × 107 cells were obtained from one mouse. Differentiation of bone marrow cells into DCs that contained a mixture of both plasmacytoid DC and myeloid DC subsets (19, 20) was followed by flow cytometry analysis of different surface markers.
Immunohistochemistry.
Mice were euthanized, and the vaginal mucosal tissues were collected and fixed with 2% paraformaldehyde. After overnight fixation, the VM samples were cut into small longitudinal bands. Then, samples were blocked with anti-FcRg antibody (US Biological Inc., Swampscott, MA) at a dilution of 1:100 and in goat serum-PBS overnight. The anti-CD8 antibody conjugated to FITC at a dilution of 1:100 and 14.3 mM DAPI (4′,6-diamidino-2-phenylindole; Molecular Probes, Thermo Fisher Scientific, Waltham, MA) were applied overnight at 4°C. Then, samples were mounted in 50% glycerol-PBS. Confocal microscopy was performed with a laser confocal and multiphoton microscope system with a conventional laser confocal microscope (LSM 510 META; Zeiss, Jena, Germany) equipped with a femtosecond titanium laser (Chameleon; Coherent, CA).
Isolation of vaginal mucosal lymphocytes.
Lymphocytes were isolated from the female genital tract (GT) mucosal tissues. Mice were treated with calcium- and magnesium-free phosphate-buffered saline for whole-body perfusion prior to tissue harvest, as previously described (40). The female GT included the ovaries, fallopian tubes, uterus, and vagina (37). GT tissues were digested following a 2-h treatment with 2.5 μg/ml of collagenase type A (Roche catalogue number 1088 785) and 5 units/ml of DNase I (Roche catalogue number 104 159) suspended in RPMI 1640 with 5% fetal bovine serum, penicillin-streptomycin, and HEPES. Mucosal GT tissues were pooled from five mice per immunization group to provide sufficient cells to perform replicates of each experiment (IFN-γ ELISpot) and to allow for accurate measurement of immune responses in each immunization group. The average yield of cells per mouse was 4 × 106 cells/female reproductive tract.
Cytokine dosage by ELISA.
The supernatant was harvested from infected DCs at 24 h after peptide and/or LPS stimulation, and the concentrations of IL-12 and TNF-α cytokines were determined by ELISA as we previously described (20, 38, 39, 41).
IFN-γ ELISpot assay.
Genital tract draining lymph nodes (GT-DLN) and genital tract mucosal cells were cultured in 24-well plates for 5 days in a humidified 5% CO2 atmosphere with HSV-gB498–505 peptide alone (10 μg/ml), the irrelevant OVA257–264 CD8+ T-cell peptide (10 μg/ml), or autologous HSV-2 infected stimulator cells and subsequently analyzed in an IFN-γ-ELISpot assay. Functional T-cell recognition IFN-γ-ELISpot assays were performed with the mouse IFN-γ-ELISpot monoclonal antibody (MAb) pair (BD PharMingen, San Diego, CA). Briefly, on day 4, 96-well multiscreen immunoprecipitation (IP) plates were coated overnight at 4°C with 100 μl (1:250) of anti-IFN-γ capture MAb. Plates were then blocked with RPMI 1640 medium supplemented with 10% FCS for 2 h at room temperature. Cells (5 × 104/well) were added in triplicate to MAb-coated plates, and the plates were incubated for 18 to 24 h at 37°C, 5% CO2. Plates were then washed with PBS supplemented with a detection peroxidase-labeled antibody followed by a substrate according to the manufacturer's instructions. The developed spots were counted under a light microscope. Controls included cells cultured in medium in the absence of peptide stimulation. The frequency of IFN-γ spot-forming cells (SFCs) in control wells (typically <10 IFN-γ SFCs/106 cells) was subtracted from the frequency of IFN-γ SFCs detected in the peptide-stimulated cells for the calculation of antigen-specific IFN-γ responses.
Flow cytometry.
Standard flow cytometry was employed, as we previously described (20, 27, 28, 51), to assess surface expression of various markers using the following MAbs directly conjugated with either PE or FITC: FITC-CD4, FITC-CD8, and PE HSV-gB tetramer (PharMingen, San Diego, CA). IgG isotype-matched control Abs were used in all experiments. After staining, cells were washed and fixed in 1% buffered paraformaldehyde before being acquired on a Becton Dickinson FACSCalibur instrument (Mountain View, CA). Gating was on large granular cells, and for each sample, 20,000 events were acquired on a FACSCalibur and analyzed with CellQuest software, on an integrated Macintosh G4 (Becton Dickinson, San Jose, CA).
Tetramer assay.
gB498–505 tetramers were prepared and used as described previously (22). A total of 0.1 to 0.2 μg of phycoerythrin-labeled gB498–505 tetramer complexes and allophycocyanin-labeled anti-mouse CD8 (Ly-2; Caltag, South San Francisco, CA) monoclonal antibody was used to identify gB498–505-specific CD8+ T cells. Samples were analyzed with tetrameric gB498–505 tetramer complexes for the percentage of CD8+ T cells by two-color flow cytometry with a FACSCalibur (Becton Dickinson, Mountain View, CA) system.
Monitoring virus replication in vaginal tissue.
Two weeks after the final immunization, mice were treated with progesterone (Depo-Provera) to synchronize the ovarian cycle and increase susceptibility to herpes infection, after which they received an intravaginal HSV-2 challenge. The HSV-2 strain 333, described earlier (87), was used. The infection of the genital tract in the progesterone-treated mouse model appears to be similar to the initial infection in humans, the main difference being that the susceptible epithelial cells in mice are present in both vagina and cervix whereas in humans, they are mainly in the cervix (88). An inoculum of 5 × 104 PFU of HSV-2 or 5 × 106 PFU (= 200× LD50 for survival analysis) in 10 μl tissue culture medium was placed into the vaginal canal of immunized and control mice. To quantify vaginal HSV-2, the vaginal canal of immunized and control mice was swabbed once daily (days 1 to 10 postinfection) with a Dacron swab, and each swab was placed in a 75-mm culture tube containing 0.5 ml of RPMI medium. Aliquots (100 μl) of 10-fold serial dilutions were placed on confluent monolayer of RS cells in 6-well plates, incubated at 37°C for 1 h, and overlaid with medium containing 1% methylcellulose. The plates were incubated at 37°C for 3 days and stained with 1% crystal violet, and the viral plaques were counted.
In vivo depletion of CD4+ and CD8+ T cells.
In some experiments, beginning 7 days after the second dose of peptide vaccine, mice were intraperitoneally (i.p.) injected with six doses of 100 μl of PBS containing MAb GK1.5 (anti-CD4), a MAb 2.43 (anti-CD8), or hamster immunoglobulin-treated control (NCCC, Minneapolis, MN). Depletion of T cells was assessed by flow cytometric analysis of splenocytes at the end of the experiment.
Statistical analysis.
We examined the distribution of each immunological parameter. In the case of two-group comparisons, we used the parametric two-sample t test or the nonparametric Wilcoxon rank sum test, as appropriate. Differences between the groups were identified by ANOVA multiple-comparison procedures, as we previously described (22). Data are expressed as the means ± SD. Results were considered to be statistically significant at P values of <0.05. Flow cytometry data were analyzed with FlowJo software (TreeStar). For analysis, we used SAS v.9.4 (Statistical Analysis System, Cary, NC). Graphs were prepared with GraphPad Prism software (San Diego, CA). Data are expressed as the means + SD. Error bars show standard errors of the means (SEM).
ACKNOWLEDGMENTS
This work is dedicated to the memory of the late Steven L. Wechsler, “Steve” (1948–2016), whose numerous pioneering works on herpes infection and immunity laid the foundation to this line of research.
We thank Dale Long from the NIH Tetramer Facility (Emory University, Atlanta, GA) for providing the tetramers used in this study.
This work is supported by Public Health Service Research R01 grants EY026103, EY019896, and EY024618 from the National Eye Institute (NEI) and R21 grant AI110902 from the National Institute of Allergy and Infectious Diseases (NIAID) (to L.B.) and in part by a Discovery Center for Eye Research (DCER) and Research to Prevent Blindness (RPB) grant.
We declare that no conflict of interest exists.
REFERENCES
- 1.Gottlieb SL, Giersing BK, Hickling J, Jones R, Deal C, Kaslow DC, HSV Vaccine Expert Consultation Group. 2017. Meeting report: initial World Health Organization consultation on herpes simplex virus (HSV) vaccine preferred product characteristics, March 2017. Vaccine doi: 10.1016/j.vaccine.2017.10.084. [DOI] [PubMed] [Google Scholar]
- 2.Coffman RL, Sher A, Seder RA. 2010. Vaccine adjuvants: putting innate immunity to work. Immunity 33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee AS, Marrack P. 2017. Old and new adjuvants. Curr Opin Immunol 47:44–51. doi: 10.1016/j.coi.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corrie SR, Plebanski M. 2018. The emerging role of nanomaterials in immunological sensing—a brief review. Mol Immunol doi: 10.1016/j.molimm.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Wu MX. 2011. Laser vaccine adjuvant for cutaneous immunization. Expert Rev Vaccines 10:1397–1403. doi: 10.1586/erv.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. 2009. Skin immune sentinels in health and disease. Nat Rev Immunol 9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Shah D, Chen X, Anderson RR, Wu MX. 2014. A micro-sterile inflammation array as an adjuvant for influenza vaccines. Nat Commun 5:4447. doi: 10.1038/ncomms5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zerva I, Simitzi C, Siakouli-Galanopoulou A, Ranella A, Stratakis E, Fotakis C, Athanassakis I. 2015. Implantable vaccine development using in vitro antigen-pulsed macrophages absorbed on laser micro-structured Si scaffolds. Vaccine 33:3142–3149. doi: 10.1016/j.vaccine.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Scheiblhofer S, Strobl A, Hoepflinger V, Thalhamer T, Steiner M, Thalhamer J, Weiss R. 2017. Skin vaccination via fractional infrared laser ablation—optimization of laser-parameters and adjuvantation. Vaccine 35:1802–1809. doi: 10.1016/j.vaccine.2016.11.105. [DOI] [PubMed] [Google Scholar]
- 10.Terhorst D, Fossum E, Baranska A, Tamoutounour S, Malosse C, Garbani M, Braun R, Lechat E, Crameri R, Bogen B, Henri S, Malissen B. 2015. Laser-assisted intradermal delivery of adjuvant-free vaccines targeting XCR1+ dendritic cells induces potent antitumoral responses. J Immunol 194:5895–5902. doi: 10.4049/jimmunol.1500564. [DOI] [PubMed] [Google Scholar]
- 11.Morse K, Kimizuka Y, Chan MPK, Shibata M, Shimaoka Y, Takeuchi S, Forbes B, Nirschl C, Li B, Zeng Y, Bronson RT, Katagiri W, Shigeta A, Sirbulescu RF, Chen H, Tan RYY, Tsukada K, Brauns T, Gelfand J, Sluder A, Locascio JJ, Poznansky MC, Anandasabapathy N, Kashiwagi S. 2017. Near-infrared 1064 nm laser modulates migratory dendritic cells to augment the immune response to intradermal influenza vaccine. J Immunol 199:1319–1332. doi: 10.4049/jimmunol.1601873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimizuka Y, Callahan JJ, Huang Z, Morse K, Katagiri W, Shigeta A, Bronson R, Takeuchi S, Shimaoka Y, Chan MPK, Zeng Y, Li B, Chen H, Tan RYY, Dwyer C, Mulley T, Leblanc P, Goudie C, Gelfand J, Tsukada K, Brauns T, Poznansky MC, Bean D, Kashiwagi S. 2017. Semiconductor diode laser device adjuvanting intradermal vaccine. Vaccine 35:2404–2412. doi: 10.1016/j.vaccine.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashiwagi S, Brauns T, Poznansky MC. 2016. Classification of laser vaccine adjuvants. J Vaccines Vaccin 7(1):307. doi: 10.4172/2157-7560.1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashiwagi S, Brauns T, Gelfand J, Poznansky MC. 2014. Laser vaccine adjuvants. History, progress, and potential. Hum Vaccin Immunother 10:1892–1907. doi: 10.4161/hv.28840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Wang J, Shah D, Wu MX. 2013. An update on the use of laser technology in skin vaccination. Expert Rev Vaccines 12:1313–1323. doi: 10.1586/14760584.2013.844070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottfried-Blackmore A, Kaunzner UW, Idoyaga J, Felger JC, McEwen BS, Bulloch K. 2009. Acute in vivo exposure to interferon-gamma enables resident brain dendritic cells to become effective antigen presenting cells. Proc Natl Acad Sci U S A 106:20918–20923. doi: 10.1073/pnas.0911509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, Kaunzner UW, Liu K, Lindquist R, Nussenzweig MC, Steinman RM, McEwen BS. 2008. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol 508:687–710. doi: 10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. 2004. Lipopeptide epitopes extended by Ne-palmitoyl lysine moiety increases uptake and maturation of dendritic cell through a Toll-like receptor 2 pathway and triggers a Th1-dependent protective immunity. Eur J Immunol 34:1142–1149. doi: 10.1002/eji.200425166. [DOI] [PubMed] [Google Scholar]
- 19.BenMohamed L, Bertrand G, McNamara CD, Gras-Masse H, Hammer J, Wechsler SL, Nesburn AB. 2003. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J Virol 77:9463–9473. doi: 10.1128/JVI.77.17.9463-9473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.BenMohamed L, Belkaid Y, Loing E, Brahimi K, Gras-Masse H, Druilhe P. 2002. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur J Immunol 32:2274–2281. doi:. [DOI] [PubMed] [Google Scholar]
- 21.Bettahi I, Nesburn AB, Yoon S, Zhang X, Mohebbi A, Sue V, Vanderberg A, Wechsler SL, BenMohamed L. 2007. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Invest Ophthalmol Vis Sci 48:4643–4653. doi: 10.1167/iovs.07-0356. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. 2009. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol 2:129–143. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fayolle C, Deriaud E, Leclerc C. 1991. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol 147:4069–4073. [PubMed] [Google Scholar]
- 24.Kast WM, Melief CJ. 1991. In vivo efficacy of virus-derived peptides and virus-specific cytotoxic T lymphocytes. Immunol Lett 30:229–232. doi: 10.1016/0165-2478(91)90030-E. [DOI] [PubMed] [Google Scholar]
- 25.Schulz M, Zinkernagel RM, Hengartner H. 1991. Peptide-induced antiviral protection by cytotoxic T cells. Proc Natl Acad Sci U S A 88:991–993. doi: 10.1073/pnas.88.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kastrup IB, Stevanovic S, Arsequell G, Valencia G, Zeuthen J, Rammensee HG, Elliott T, Haurum JS. 2000. Lectin purified human class I MHC-derived peptides: evidence for presentation of glycopeptides in vivo. Tissue Antigens 56:129–135. doi: 10.1034/j.1399-0039.2000.560203.x. [DOI] [PubMed] [Google Scholar]
- 27.BenMohamed L, Krishnan R, Auge C, Primus JF, Diamond DJ. 2002. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology 106:113–121. doi: 10.1046/j.1365-2567.2002.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.BenMohamed L, Wechsler SL, Nesburn AB. 2002. Lipopeptide vaccines–yesterday, today, and tomorrow. Lancet Infect Dis 2:425–431. doi: 10.1016/S1473-3099(02)00318-3. [DOI] [PubMed] [Google Scholar]
- 29.BenMohamed L, Krishnan R, Longmate J, Auge C, Low L, Primus J, Diamond DJ. 2000. Induction of CTL response by a minimal epitope vaccine in HLA A*0201/DR1 transgenic mice: dependence on HLA class II restricted T(H) response. Hum Immunol 61:764–779. doi: 10.1016/S0198-8859(00)00139-7. [DOI] [PubMed] [Google Scholar]
- 30.Benmohamed L, Thomas A, Bossus M, Brahimi K, Wubben J, Gras-Masse H, Druilhe P. 2000. High immunogenicity in chimpanzees of peptides and lipopeptides derived from four new Plasmodium falciparum pre-erythrocytic molecules. Vaccine 18:2843–2855. doi: 10.1016/S0264-410X(00)00068-2. [DOI] [PubMed] [Google Scholar]
- 31.Daubersies P, Thomas AW, Millet P, Brahimi K, Langermans JA, Ollomo B, BenMohamed L, Slierendregt B, Eling W, Van Belkum A, Dubreuil G, Meis JF, Guerin-Marchand C, Cayphas S, Cohen J, Gras-Masse H, Druilhe P. 2000. Protection against Plasmodium falciparum malaria in chimpanzees by immunization with the conserved pre-erythrocytic liver-stage antigen 3. Nat Med 6:1258–1263. doi: 10.1038/81366. [DOI] [PubMed] [Google Scholar]
- 32.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. 1998. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology 252:200–209. doi: 10.1006/viro.1998.9454. [DOI] [PubMed] [Google Scholar]
- 33.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. 1998. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Invest Ophthalmol Vis Sci 39:1163–1170. [PubMed] [Google Scholar]
- 34.Nesburn AB, Slanina S, Burke RL, Ghiasi H, Bahri S, Wechsler SL. 1998. Local periocular vaccination protects against eye disease more effectively than systemic vaccination following primary ocular herpes simplex virus infection in rabbits. J Virol 72:7715–7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell MF, Eastman DJ, Lim A, Lucas C, Peterson M, Vennari J, Weissburg RP, Wrin T, Kensil CR, Newman MJ, Nunberg J, Cleland JL, Gregory TJ, Berman PW. 1995. Effect of adjuvants on immunogenicity of MN recombinant glycoprotein 120 in guinea pigs. AIDS Res Hum Retroviruses 11:203–209. doi: 10.1089/aid.1995.11.203. [DOI] [PubMed] [Google Scholar]
- 36.Gupta RK, Siber GR. 1995. Adjuvants for human vaccines—current status, problems and future prospects. Vaccine 13:1263–1276. doi: 10.1016/0264-410X(95)00011-O. [DOI] [PubMed] [Google Scholar]
- 37.King NJ, Parr EL, Parr MB. 1998. Migration of lymphoid cells from vaginal epithelium to iliac lymph nodes in relation to vaginal infection by herpes simplex virus type 2. J Immunol 160:1173–1180. [PubMed] [Google Scholar]
- 38.Pollara G, Speidel K, Samady L, Rajpopat M, McGrath Y, Ledermann J, Coffin RS, Katz DR, Chain B. 2003. Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J Infect Dis 187:165–178. doi: 10.1086/367675. [DOI] [PubMed] [Google Scholar]
- 39.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. 1999. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med 189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. 2008. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med 205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salio M, Cella M, Suter M, Lanzavecchia A. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol 29:3245–3253. doi:. [DOI] [PubMed] [Google Scholar]
- 42.BenMohamed L, Gras-Masse H, Tartar A, Daubersies P, Brahimi K, Bossus M, Thomas A, Druilhe P. 1997. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur J Immunol 27:1242–1253. doi: 10.1002/eji.1830270528. [DOI] [PubMed] [Google Scholar]
- 43.BenMohamed L, Thomas A, Druilhe P. 2004. Long-term multiepitopic cytotoxic-T-lymphocyte responses induced in chimpanzees by combinations of Plasmodium falciparum liver-stage peptides and lipopeptides. Infect Immun 72:4376–4384. doi: 10.1128/IAI.72.8.4376-4384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. 2004. Lipopeptide epitopes extended by an Nepsilon-palmitoyl-lysine moiety increase uptake and maturation of dendritic cells through a Toll-like receptor-2 pathway and trigger a Th1-dependent protective immunity. Eur J Immunol 34:3102–3114. doi: 10.1002/eji.200425166. [DOI] [PubMed] [Google Scholar]
- 45.Bettahi I, Zhang X, Afifi RE, BenMohamed L. 2006. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol 19:220–236. doi: 10.1089/vim.2006.19.220. [DOI] [PubMed] [Google Scholar]
- 46.Renaudet O, BenMohamed L, Dasgupta G, Bettahi I, Dumy P. 2008. Towards a self-adjuvanting multivalent B and T cell epitope containing synthetic glycolipopeptide cancer vaccine. ChemMedChem 3:737–741. doi: 10.1002/cmdc.200700315. [DOI] [PubMed] [Google Scholar]
- 47.Bettahi I, Dasgupta G, Renaudet O, Chentoufi AA, Zhang X, Carpenter D, Yoon S, Dumy P, BenMohamed L. 2009. Antitumor activity of a self-adjuvanting glyco-lipopeptide vaccine bearing B cell, CD4+ and CD8+ T cell epitopes. Cancer Immunol Immunother 58:187–200. doi: 10.1007/s00262-008-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chentoufi AA, Nesburn AB, BenMohamed L. 2009. Recent advances in multivalent self adjuvanting glycolipopeptide vaccine strategies against breast cancer. Arch Immunol Ther Exp (Warsz) 57:409–423. doi: 10.1007/s00005-009-0049-2. [DOI] [PubMed] [Google Scholar]
- 49.Renaudet O, Dasgupta G, Bettahi I, Shi A, Nesburn AB, Dumy P, BenMohamed L. 2010. Linear and branched glyco-lipopeptide vaccines follow distinct cross-presentation pathways and generate different magnitudes of antitumor immunity. PLoS One 5:e11216. doi: 10.1371/journal.pone.0011216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, Benmohamed L. 2012. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol 189:4496–4509. doi: 10.4049/jimmunol.1201121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dasgupta G, Nesburn AB, Wechsler SL, BenMohamed L. 2010. Developing an asymptomatic mucosal herpes vaccine: the present and the future. Future Microbiol 5:1–4. doi: 10.2217/fmb.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaishankar D, Shukla D. 2016. Genital herpes: insights into sexually transmitted infectious. Dis Microb Cell 3:438–450. doi: 10.15698/mic2016.09.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Castelli FA, Zhu X, Wu M, Maillere B, BenMohamed L. 2008. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol 15:1436–1449. doi: 10.1128/CVI.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dasgupta G, Chentoufi AA, Kalantari-Dehaghi M, Falatoonzadeh P, Chun S, Lim CH, Felgner PL, Davies DH, BenMohamed L. 2012. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays by serum antibodies from seropositive asymptomatic versus symptomatic individuals. J Virol 86:4358–4369. doi: 10.1128/JVI.07107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chentoufi AA, Kritzer E, Yu DM, Nesburn AB, BenMohamed L. 2012. Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown. Clin Dev Immunol 16:1466–1468. doi: 10.1155/2012/187585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalantari-Dehaghi M, Chun S, Chentoufi AA, Pablo J, Liang L, Dasgupta G, Molina DM, Jasinskas A, Nakajimi-sasaki R, Felgner J, Hermanson G, BenMohamed L, Felgner PL, Davies DH. 2012. Discovery of potential diagnostic and vaccine antigens in herpes simplex virus-1 and -2 by proteome-wide antibody profiling. J Virol 86:4328–4339. doi: 10.1128/JVI.05194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dervillez X, Wechsler S, Nesburn AB, BenMohamed L. 2012. Future of an “asymptomatic” T-cell epitope-based therapeutic herpes simplex vaccine. Future Virol 4:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen J. 2010. Immunology. Painful failure of promising genital herpes vaccine. Science 330:304. doi: 10.1126/science.330.6002.304. [DOI] [PubMed] [Google Scholar]
- 59.Mestecky J, Fultz PN. 1999. Mucosal immune system of the human genital tract. J Infect Dis 179(Suppl 3):S470–S474. doi: 10.1086/314806. [DOI] [PubMed] [Google Scholar]
- 60.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. 2003. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med 197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest 101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Posavad CM, Koelle DM, Shaughnessy MF, Corey L. 1997. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci U S A 94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jing L, Haas J, Chong TM, Bruckner JJ, Dann GC, Dong L, Marshak JO, McClurkan CL, Yamamoto TN, Bailer SM, Laing KJ, Wald A, Verjans GM, Koelle DM. 2012. Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. J Clin Invest 122:654–673. doi: 10.1172/JCI60556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koelle DM, Frank JM, Johnson ML, Kwok WW. 1998. Recognition of herpes simplex virus type 2 tegument proteins by CD4 T cells infiltrating human genital herpes lesions. J Virol 72:7476–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bassett I, Donovan B, Bodsworth NJ, Field PR, Ho DW, Jeansson S, Cunningham AL. 1994. Herpes simplex virus type 2 infection of heterosexual men attending a sexual health centre. Med J Aust 160:697–700. [PubMed] [Google Scholar]
- 67.Spruance SL, Overall JC Jr, Kern ER, Krueger GG, Pliam V, Miller W. 1977. The natural history of recurrent herpes simplex labialis: implications for antiviral therapy. N Engl J Med 297:69–75. doi: 10.1056/NEJM197707142970201. [DOI] [PubMed] [Google Scholar]
- 68.Mikloska Z, Cunningham AL. 1998. Herpes simplex virus type 1 glycoproteins gB, gC and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J Gen Virol 79(Part 2):353–361. [DOI] [PubMed] [Google Scholar]
- 69.Koelle DM, Benedetti J, Langenberg A, Corey L. 1992. Asymptomatic reactivation of herpes simplex virus in women after the first episode of genital herpes. Ann Intern Med 116:433–437. doi: 10.7326/0003-4819-116-6-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zarling JM, Moran PA, Brewer L, Ashley R, Corey L. 1988. Herpes simplex virus (HSV)-specific proliferative and cytotoxic T-cell responses in humans immunized with an HSV type 2 glycoprotein subunit vaccine. J Virol 62:4481–4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zarling JM, Moran PA, Burke RL, Pachl C, Berman PW, Lasky LA. 1986. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD. J Immunol 136:4669–4673. [PubMed] [Google Scholar]
- 72.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. 2005. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine 23:873–883. doi: 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 73.Koelle DM, Corey L, Burke RL, Eisenberg RJ, Cohen GH, Pichyangkura R, Triezenberg SJ. 1994. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol 68:2803–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koelle DM, Gonzalez JC, Johnson AS. 2005. Homing in on the cellular immune response to HSV-2 in humans. Am J Reprod Immunol 53:172–181. doi: 10.1111/j.1600-0897.2005.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koelle DM, Schomogyi M, Corey L. 2000. Antigen-specific T cells localize to the uterine cervix in women with genital herpes simplex virus type 2 infection. J Infect Dis 182:662–670. doi: 10.1086/315749. [DOI] [PubMed] [Google Scholar]
- 76.Koelle DM, Schomogyi M, McClurkan C, Reymond SN, Chen HB. 2000. CD4 T-cell responses to herpes simplex virus type 2 major capsid protein VP5: comparison with responses to tegument and envelope glycoproteins. J Virol 74:11422–11425. doi: 10.1128/JVI.74.23.11422-11425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khanna KM, Lepisto AJ, Hendricks RL. 2004. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol 25:230–234. doi: 10.1016/j.it.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 78.van Lint A, Ayers M, Brooks AG, Coles RM, Heath WR, Carbone FR. 2004. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J Immunol 172:392–397. doi: 10.4049/jimmunol.172.1.392. [DOI] [PubMed] [Google Scholar]
- 79.Simmons A, Tscharke DC. 1992. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med 175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blaney JE Jr, Nobusawa E, Brehm MA, Bonneau RH, Mylin LM, Fu TM, Kawaoka Y, Tevethia SS. 1998. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J Virol 72:9567–9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iwasaki A. 2010. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol 10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sallusto F, Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chentoufi AA, Dervillez X, Dasgupta G, Nguyen C, Kabbara WK, Jiang X, Nesburn AB, Wechsler SL, BenMohamed L. 2012. The herpes simplex virus type 1 latency associated transcript inhibits phenotypic and functional maturation of dendritic cells. Viral Immunol 25:204–215. doi: 10.1089/vim.2011.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berzofsky JA, Ahlers JD, Derby MA, Pendleton CD, Arichi T, Belyakov IM. 1999. Approaches to improve engineered vaccines for human immunodeficiency virus and other viruses that cause chronic infections. Immunol Rev 170:151–172. doi: 10.1111/j.1600-065X.1999.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 85.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. 2005. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nepsilon-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J Virol 79:15289–15301. doi: 10.1128/JVI.79.24.15289-15301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hattori T, Chauhan SK, Lee H, Ueno H, Dana R, Kaplan DH, Saban DR. 2011. Characterization of Langerin-expressing dendritic cell subsets in the normal cornea. Invest Ophthalmol Vis Sci 52:4598–4604. doi: 10.1167/iovs.10-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zwaagstra JC, Leung WC. 1987. The nucleotide sequence of herpes simplex virus type 2 (333) glycoprotein gB2 and analysis of predicted antigenic sites. Can J Microbiol 33:879–887. doi: 10.1139/m87-154. [DOI] [PubMed] [Google Scholar]
- 88.Parr MB, Parr EL. 1997. Protective immunity against HSV-2 in the mouse vagina. J Reprod Immunol 36:77–92. doi: 10.1016/S0165-0378(97)00055-7. [DOI] [PubMed] [Google Scholar]