ABSTRACT
A preventive human immunodeficiency virus type 1 (HIV-1) vaccine is an essential part of the strategy to eradicate AIDS. A critical question is whether antibodies that do not neutralize primary isolate (tier 2) HIV-1 strains can protect from infection. In this study, we investigated the ability of an attenuated poxvirus vector (NYVAC) prime-envelope gp120 boost to elicit potentially protective antibody responses in a rhesus macaque model of mucosal simian-human immunodeficiency virus (SHIV) infection. NYVAC vector delivery of a group M consensus envelope, trivalent mosaic envelopes, or a natural clade B isolate B.1059 envelope elicited antibodies that mediated neutralization of tier 1 viruses, cellular cytotoxicity, and phagocytosis. None of the macaques made neutralizing antibodies against the tier 2 SHIV SF162P3 used for mucosal challenge. Significant protection from infection was not observed for the three groups of vaccinated macaques compared to unvaccinated macaques, although binding antibody to HIV-1 Env correlated with decreased viremia after challenge. Thus, NYVAC Env prime-gp120 boost vaccination elicited polyfunctional, nonneutralizing antibody responses with minimal protective activity against tier 2 SHIV mucosal challenge.
IMPORTANCE The antibody responses that confer protection against HIV-1 infection remain unknown. Polyfunctional antibody responses correlated with time to infection in previous macaque studies. Determining the ability of vaccines to induce these types of responses is critical for understanding how to improve upon the one efficacious human HIV-1 vaccine trial completed thus far. We characterized the antibody responses induced by a NYVAC-protein vaccine and determined the protective capacity of polyfunctional antibody responses in an R5, tier 2 mucosal SHIV infection model.
KEYWORDS: HIV, HIV vaccine, neutralizing antibodies, simian-human immunodeficiency virus
INTRODUCTION
A human immunodeficiency virus type 1 (HIV-1) vaccine continues to be needed to halt the spread of the HIV-1 pandemic (1). The poxvirus-Env gp120 vaccination regimen of the RV144 trial exhibited an estimated vaccine efficacy of 31.2% (2). Efforts to increase the efficacy of RV144 have led to the development and testing of other poxvirus prime-Env gp120 boost regimens (3). NYVAC vectors are one of the poxvirus vectors that have been tested in human clinical trials (4–6). NYVAC vectors are devoid of genes associated with poxvirus virulence and pathogenicity (7), do not disseminate (7, 8), and induce low immune activation (9). Thus, NYVAC vectors represent a strategy for improving the protective efficacy of HIV-1 vaccines.
One hypothesis for improving HIV-1 vaccine efficacy is to elicit both T and B cell responses to HIV-1. NYVAC vectors alone or in combination with DNA have previously been shown to elicit robust T cell responses against HIV and simian immunodeficiency virus (SIV) (5, 10–12). In general, DNA prime followed by NYVAC boost elicits better cellular immune responses than NYVAC alone (5, 11). However, it is unclear if DNA-NYVAC is a particularly effective vaccine regimen for inducing antibody responses, and antibody responses may be key components of the HIV vaccine-induced immune response since various antibody effector functions and neutralizing antibodies have inversely correlated with infection risk in human and nonhuman primate vaccine trials (13–19). Protein immunizations could be critical for eliciting antibody responses (20), especially given that the RV144 trial included poxvirus and protein immunogens (2). In particular for NYVAC immunizations, recombinant Env protein boosts enhance antibody responses (12, 21). Thus, a strategy for vaccine induction of protective antibody responses could include NYVAC-protein immunizations. Previous NYVAC vaccines have successfully protected against intravenous challenge with nonpathogenic HIV-2 (22, 23) and mucosal challenge with SIV mac251 (24). Neutralizing antibodies generally do not appear to be responsible for the protection or reduction in viral load after immunization (22, 24, 25). Thus, antibody effector functions may be key for understanding NYVAC vaccine-mediated protection from infection.
The Env sequences delivered in the vaccine also influence the elicitation of protective antibody responses. Consensus and mosaic immunogens aim to induce immunity against diverse global HIV isolates (26, 27). In nonhuman primates, mosaic Env immunogens have elicited enhanced cellular and humoral responses compared to those elicited by natural sequence immunogens (28, 29). Similarly, in nonhuman primates, consensus Envs derived from group M HIV-1 sequences elicited broader recognition of T cell epitopes (30). Immunization of guinea pigs with the group M consensus Envs induced broader binding antibodies than natural Envs and neutralizing antibodies against a subset of difficult-to-neutralize HIV-1 strains (31, 32). Mosaic Envs have been shown to induce protective immune responses that correlate with antibody responses (33, 34). Thus, the use of consensus and mosaic Env immunogens may be another strategy for enhancing the protective efficacy of a poxvirus-protein vaccine.
In this study, we compared the immunogenicities of natural, consensus, and mosaic Env delivered by NYVAC followed by Env gp120 boosts in rhesus macaques. We determined the protective efficacy of the NYVAC prime-Env gp120 boost vaccine regimen with a repeat, low-dose mucosal simian-human immunodeficiency virus (SHIV) challenge model. All three immunogens elicited polyfunctional antibody responses but induced no neutralizing antibodies against the challenge virus, SHIV SF162P3. Ultimately, none of the vaccines provided significant protection from SHIV transmission with mucosal challenge.
RESULTS
NYVAC-gp120 viral vectors elicited antibody responses that were boosted by recombinant protein.
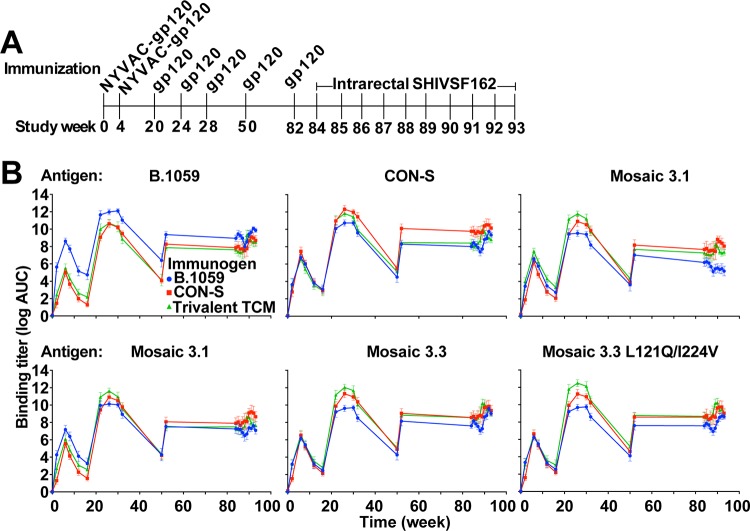
Three groups of 6 macaques each were administered intramuscularly (i.m.) at weeks 0 and 4 recombinant NYVAC vectors expressing different forms of gp120 (Fig. 1A). Purified recombinant gp120s autologous to the Envs delivered by the NYVAC vectors were administered i.m. to macaques at weeks 20, 24, 28, 50, and 82 (Fig. 1A). All immunizations were well tolerated based on the absence of visible change at the injection site and the absence of a change in body weight. In the immunization protocol, each group of macaques received as priming either wild-type transmitted/founder clade B (B.1059), group M consensus (CON-S), or three T cell mosaic Envs encoded by NYVAC, and as a booster they received the same gp120 immunogens used as recombinant protein. We measured the enzyme-linked immunosorbent assay (ELISA) binding titers to all Envs 2 weeks after the first and second NYVAC immunizations. For each antigen examined, there was an increase in binding antibody titers from the first NYVAC immunization to the second NYVAC immunization (Fig. 1B). The binding titers for each group were comparable for each antigen, except the B.1059 group, which showed preferential binding to the autologous B.1059 gp120. Across all groups the binding titers declined by 50% over the subsequent 10 weeks after the second NYVAC immunizations. Each group of macaques was boosted at week 20 with the recombinant gp120 protein that corresponded to the NYVAC encoded Env. The binding titers to the gp120 immunogens were boosted higher than after the two NYVAC immunizations. In general, the binding titer in response to the autologous Env was higher than those in response to the heterologous Envs, but the differences were not significant. Two additional boosts with gp120 elicited small changes in the binding titers. As quickly as 4 weeks after the third protein boost, the Env-specific binding antibody titers had begun to wane in all 3 groups (Fig. 1B). The macaques were given an immunologic rest period of 22 weeks, and at week 50, all macaques had low but detectable binding antibody responses. Interestingly, boosting with recombinant proteins at week 50 elicited binding titers that were lower at week 52 than after the first gp120 boost (week 22). The macaques were boosted again with gp120 after an additional 30-week interval. Again, the week 84 titers were not as high as after the first gp120 boost. The binding titers for all of the groups remained high once the macaques were challenged weekly with SHIV SF162P3 (Fig. 1B).
FIG 1.
Binding plasma IgG titers primed by NYVAC vector immunization are boosted by protein immunization. (A) Immunization and challenge schedule for Indian origin rhesus macaques. Macaques were immunized with 108 PFU of NYVAC encoding gp120 and subsequently boosted with recombinant gp120 protein. Macaques were challenged weekly with an intrarectal administration of a 1:400 dilution of tier 2 CCR5-tropic SHIV SF162P3. (B) Binding plasma IgG against each vaccine immunogen was analyzed over time in the vaccinated macaques. The binding antibody titers are depicted as log area under the curve (AUC). The values are the means and SDs for all six animals. Each graph shows the plasma IgG binding titer for all three groups (blue, red, and green lines) to the antigen listed.
CON-S gp120 vaccination induced high levels of CD4 binding site blocking antibodies and V3-glycan blocking antibodies.
Plasma blocking of the binding of a panel of monoclonal antibodies, both broadly neutralizing and nonneutralizing, was used to determine the epitopes that were affected by the antibodies elicited by vaccination. We note that blocking of a broadly neutralizing antibody (bnAb) does not constitute proof of the presence of the bnAb since antibodies can sterically hinder or allosterically modulate access to bnAb epitopes. Comparison across multiple antibodies showed that the peak of plasma blocking activity occurred at the same time point. Blocking activity was not detectable after either NYVAC immunization and peaked after two gp120 immunizations. Similar to total plasma binding antibodies, three protein immunizations 4 weeks apart generated optimal blocking antibodies.
We have previously observed that CON-S gp140CFI immunization over 4 years elicited antibodies capable of blocking 2G12 and PGT128 binding to Env (35). CON-S immunization in the present study also elicited 2G12-blocking antibodies (Fig. 2) (35). Interestingly, immunization with B.1059 or the group of three T cell mosaics did not elicit 2G12-blocking activity. In previous studies we have shown that 2G12 blocking is more specific for indicating the presence of Env glycan antibodies (35). Immunization with CON-S also elicited the highest titers of CD4 binding site blocking antibodies (Fig. 2) (exact Wilcoxon test, false discovery rate [FDR] corrected P < 0.05).
FIG 2.
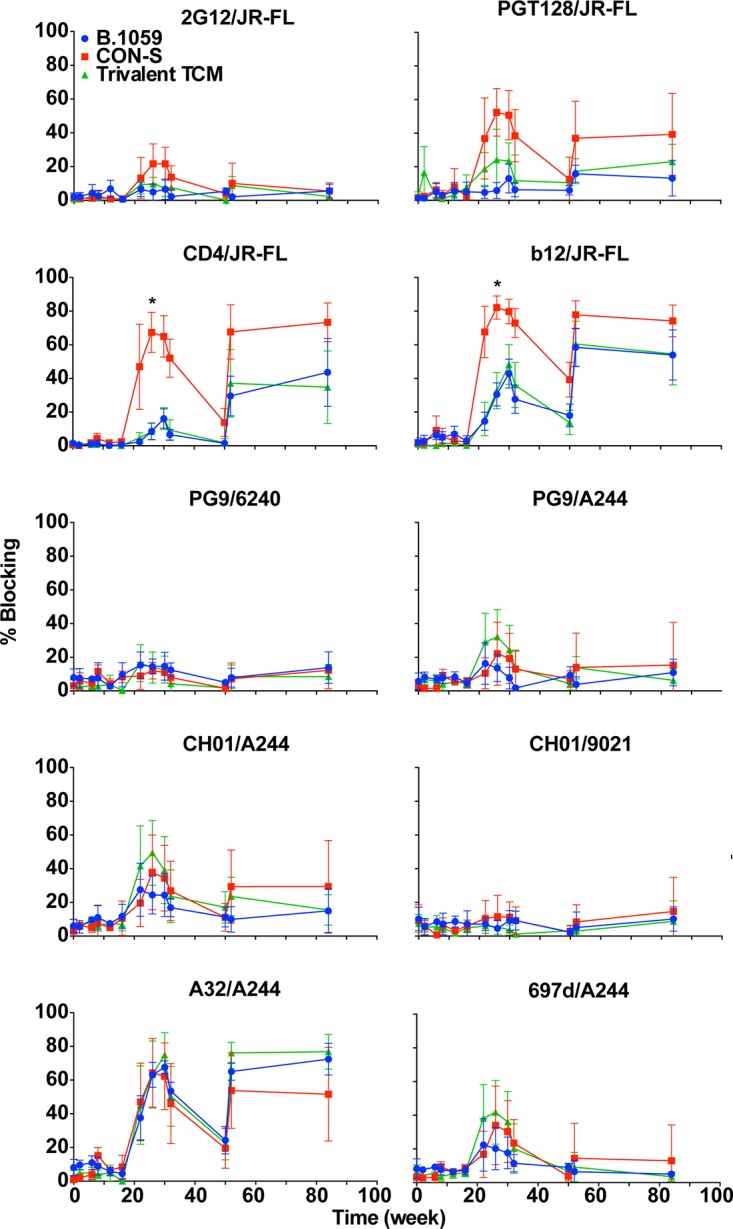
CON-S vaccination induces higher plasma antibody blocking of the V3-glycan and CD4 binding site on HIV-1 Env. Plasma competition ELISAs for various broadly neutralizing and nonneutralizing monoclonal antibodies that bind to various sites on HIV-1 Env were performed. The antibody and Env used for competition are indicated above each graph. Values are the means and SDs for all six animals. Values for each macaque were measured in triplicate. Twenty percent is the cutoff for positive blocking responses. *, exact Wilcoxon test, FDR corrected, P < 0.05.
Vaccination elicits heterologous tier 1 neutralizing antibodies and autologous tier 2 neutralizing antibodies.
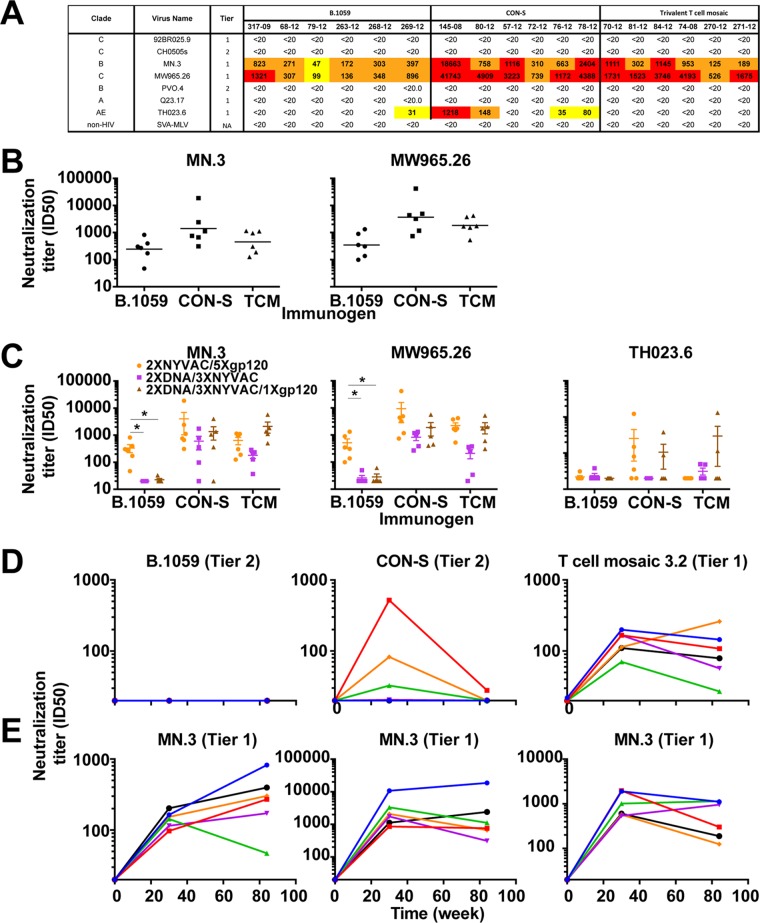
The neutralizing antibody responses were compared for each group at the end of the vaccination regimen using the TZM-bl cell assay. All three groups possessed heterologous tier 1, but not heterologous tier 2, neutralizing antibodies (Fig. 3A and B). CON-S vaccination elicited the highest neutralizing titers against the tier 1 viruses (Fig. 3B).
FIG 3.
Protein boosts maintain heterologous tier 1 plasma neutralizing antibodies but not autologous tier 2 plasma neutralizing antibodies. (A) Week 84 (2× NYVAC/5× gp120) plasma neutralization activity against a panel of tier 1 and 2 viruses by each vaccinated macaque in each group. The immunization group is indicated in the column header. The neutralization titer is shown as reciprocal plasma dilution that yields 50% inhibition of virus replication (ID50). Neutralization titers are color-coded based on neutralization potency as follows: white, <20; yellow, 20 to 99; orange, 100 to 999; and red, >1,000. (B) Comparison of neutralization titers (ID50) elicited by the three immunogens against MN.3 (left) and MW965.26 (right). (C) Comparison of tier 1 neutralization titers (ID50) elicited by NYVAC-protein (orange), DNA-NYVAC (purple), and DNA-NYVAC-protein (brown) vaccination regimens. Immunogens used for the vaccination regimen are listed on the x axis. (D and E) Week 30 and 84 plasma neutralization of autologous tier 1 and 2 viruses (D) and heterologous tier 1 virus MN.3 (E) by each macaque vaccinated with B.1059, CON-S, and T cell mosaic envelopes. Neutralization was tested before vaccination (week 0), after two NYVAC primes and 3 protein boosts (week 30), and after two NYVAC primes and 5 protein boosts (week 84). Each symbol represents the neutralization titer (ID50) of an individual macaque.
We compared the neutralizing antibody responses elicited in this study to those elicited in our previous studies in which macaques were immunized with DNA-NYVAC or DNA-NYVAC-protein vaccines. When B.1059 was the immunogen, NYVAC prime-protein boost elicited higher neutralizing antibody titers than the other two vaccine regimens (exact Wilcoxon test corrected P = 0.006 [Fig. 3C]).
In the absence of heterologous tier 2 neutralization, it is possible to elicit autologous tier 2 neutralizing antibodies (36–38). Autologous tier 2 neutralizing antibodies did not arise in the B.1059-immunized group, although heterologous tier 1 antibodies were elicited (Fig. 3D and E). In contrast, CON-S-immunized macaques possessed autologous tier 2 neutralizing activity in plasma. The neutralizing activity was not boosted with subsequent immunizations, suggesting immunologic restraints on the neutralizing B cell clones. Tier 1 neutralizing antibodies, however, did persist throughout the vaccination regimen (Fig. 3D and E). T cell mosaic 3.2 virus (tier 1A virus) was neutralized by the autologous plasma at weeks 30 and 84 but to a lower titer than MN.3 (Fig. 3D and E).
Detection of antibody effector functions, but not neutralizing antibodies against SHIV SF162P3.
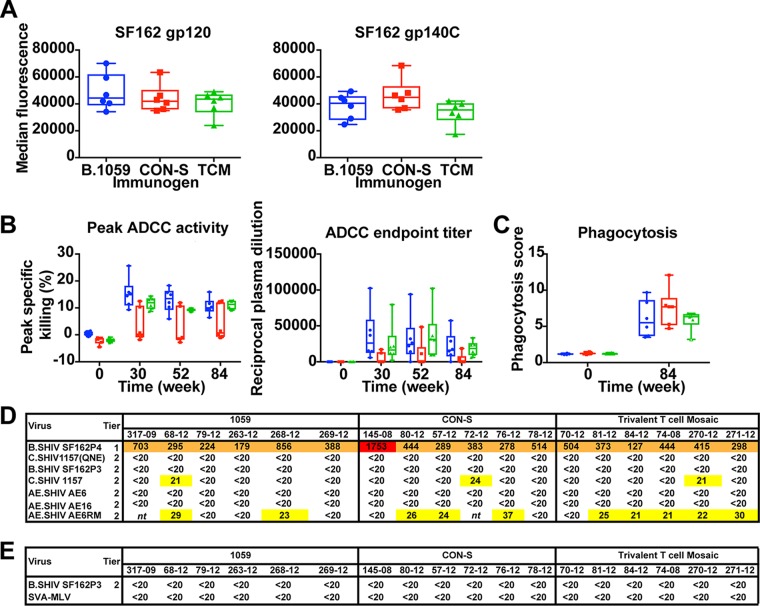
After the final immunization, all macaques possessed binding antibodies to SF162 gp120 and SF162 gp140 (Fig. 4A). The median titers of binding antibodies to both SF162 gp120 and gp140 were comparable among the three groups, and the differences were not significant.
FIG 4.
Vaccination-induced antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent phagocytosis (ADCP) activity but not neutralization of the challenge strain prior to challenge. (A) Plasma IgG BAMA binding titers in response to monomeric SF162 gp120 protein or oligomeric SF162 gp140C protein after two NYVAC and five protein immunizations (week 84). (B) The plasma of B.1059 and trivalent T cell mosaic (TCM)-vaccinated macaques mediates ADCC against cells coated with SF162 gp120. The magnitude (left) and endpoint titer (right) of ADCC against SF162 gp120-coated cells are shown. Plasma from after the third (week 30), fourth (week 52), and fifth (week 84) protein boosts were assessed for activity. The box-and-whisker plots show the median, interquartile range, minimum, and maximum. The symbols represent values for individual macaques. Intrarectal challenges were initiated at week 84. (C) Antibody-mediated phagocytosis of SHIV SF162P3 gp120-coated fluorescent beads. Plasma samples prevaccination (week 0) and after the fifth (week 84) protein boost were examined for activity. Filled symbols show positive values and open symbols show negative values at week 84. (D) Week 52 plasma neutralization of tier 1 and tier 2 SHIVs, including SHIV SF162P3 used for the challenge. The neutralization titer is shown as reciprocal plasma dilution that yields 50% inhibition of virus replication (ID50). Neutralization titers are color-coded based on neutralization potency as follows: white, <20; yellow, 20 to 99; orange, 100 to 999; and red >1,000. nt, not tested. (E) Plasma neutralization of the challenge virus SHIV SF162P3 on the day of challenge (week 84).
Next we examined antibody-dependent cellular cytotoxicity (ADCC) against SF162 gp120-coated cells. After three gp120 boosts, the B.1059 group possessed the highest magnitude of peak specific killing and the CON-S group had the lowest magnitude (Fig. 4B, left). The peak specific killing by the B.1059 group was reduced after each of the two subsequent gp120 boosts down to the mean activity of the T cell mosaic group. Both the B.1059 and the T cell mosaic groups had higher peak specific killing than the CON-S group at all three time points examined. Similarly, when ADCC activity was measured as the ADCC endpoint titer, the B.1059 group waned over the last three gp120 boosts (Fig. 4B, right). Thus, comparable ADCC endpoint titers were present in all groups at the time of SHIV SF162P3 challenge, but the titers tended toward being higher in the B.1059 group at the peak of immunogenicity. ADCC peak specific killing or endpoint titer at week 84 did not significantly correlate with the number of weeks protected from SHIV infection.
We examined the magnitude of a second antibody effector function, antibody-mediated phagocytosis (ADCP), before vaccination and on the day of SHIV mucosal challenge. NYVAC-protein vaccination elicited positive ADCP responses against SHIV SF162P3 gp120-coated beads in all macaques except one in the T cell mosaic group (Fig. 4C). CON-S-immunized macaques tended to have the highest median magnitude of response, but overall the magnitudes were not significantly different among the groups at the time of challenge (Fig. 4C).
Neutralizing antibodies can protect against SHIV challenge (39–41). Therefore, we examined plasma neutralization against the challenge stock SHIV SF162P3 and a panel of other SHIVs after the last two protein boosts. After 4 gp120 boosts, all of the vaccinated macaques exhibited neutralization of the tier 1 SHIV SF162P4 (Fig. 4D). Weak neutralization against five tier 2 SHIVs occurred in multiple groups, but none of the macaque plasma neutralized the SHIV SF162P3 challenge virus (Fig. 4D). Additionally, after the last immunization none of the vaccinated macaque plasma neutralized SHIV SF162P3 (Fig. 4E). SHIV SF162P3 and SF162P4 are 97% and 99% similar to HIV-1 SF162, respectively. Many of the differences are in their variable loop sequences. These differences may be why SHIV SF162P3 is more difficult to neutralize than HIV-1 SF162 and SHIV SF162P4. Nonetheless, after the last two boosts there was no in vitro plasma neutralization of the challenge virus.
V1V2-specific IgG binding responses.
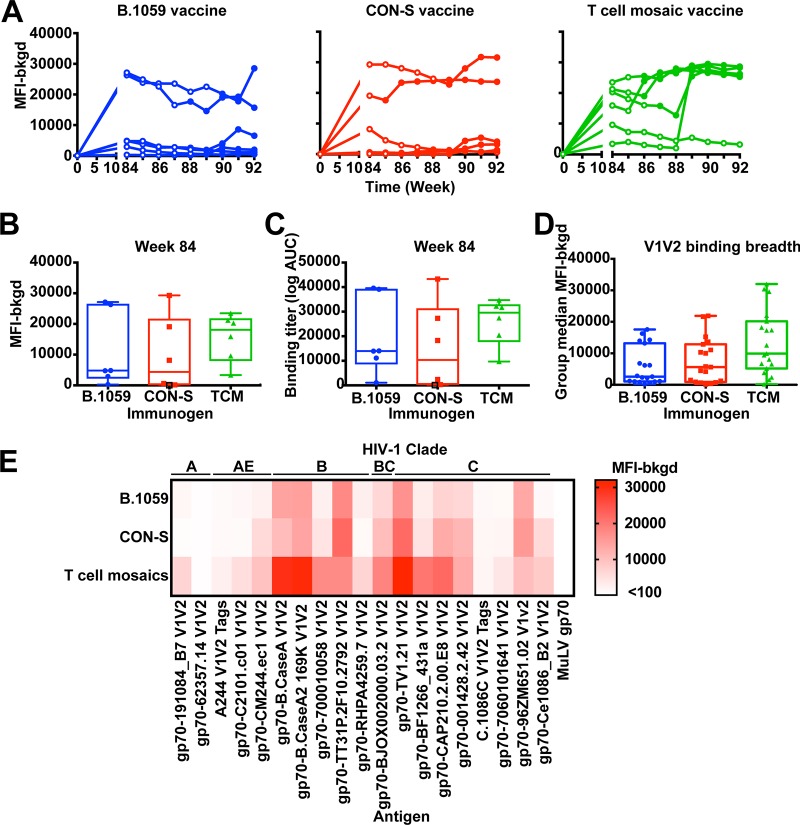
V1V2-specific IgG responses correlated with decreased infection risk against HIV-1 infection in the RV144 trial (15, 19). We examined whether plasma IgG was elicited against B.CaseA2 V1V2 prior to and after SHIV challenge (Fig. 5). Interestingly, prior to infection, each group of macaques had a subset of very high responders and a subset of very low responders, resulting in a large interquartile range of binding titer (Fig. 5B and C). Except in two T cell mosaic-immunized macaques, macaques had similar titers before and after SHIV challenge (Fig. 5A). Plasma IgG B.CaseA2 V1V2 was elicited to similar binding titers among all of the groups (Fig. 5B and C). We examined the correlation between plasma IgG B.CaseA2 V1V2 binding titers and time to infection across vaccine groups. A Cox hazard analysis did not find a correlation between time to infection and plasma IgG B.CaseA2 V1V2 binding titers. We also examined the breadth of V1V2-specific plasma IgG elicited by the vaccine regimen. Each of the vaccines induced V1V2 antibodies capable of binding numerous V1V2 sequences (Fig. 5D and E). Among the 19 V1V2 proteins (54), B.CaseA2 V1V2 was among the most strongly bound V1V2 sequences. The T cell mosaic-immunized macaques possessed very strong binding (median fluorescence intensity of >20,000) to 5 of the 19 V1V2 constructs (Fig. 5D and E). Thus, the median binding titer for all 19 proteins was highest in this group, although the difference was not significantly different from the other groups.
FIG 5.
Vaccine induction of broad V1V2 binding antibody responses. (A) Macaque plasma IgG binding to gp70 B.CaseA2 V1V2 was examined before vaccination (week 0) or from the weeks of SHIV challenge using the BAMA. SHIV challenges occurred weekly beginning at week 84. Results for each vaccinated group are shown in individual graphs, with each curve representing an individual macaque. Open circles and filled circles represent time points where the macaque was uninfected and infected, respectively. (B and C) Comparison of plasma IgG binding magnitude as background subtracted median fluorescence intensity (B) or area under the curve (C) for response to gp70 B.CaseA2 V1V2 among the three groups. Each column represents one vaccinated group, and individual macaques are shown by each symbol. Nonresponders are shown by open black symbols; animals were considered nonresponders if their responses were below 100 and less than three times background. There were no significant differences observed among vaccinated groups. (D) Group median fluorescence intensity (median fluorescence intensity minus background) for week 84 plasma IgG binding to 19 V1V2 constructs. Each symbol represents the group median fluorescence for a unique V1V2 construct. The middle line of the box shows the median binding for all 19 constructs, and the whiskers represent the maximum and minimum median values. (E) Comparison of the magnitudes of binding by week 84 plasma IgG to the 19 V1V2 constructs listed across the top. Each row shows the binding magnitudes for the immunogen shown on the left. Each column shows a different V1V2 antigen. The squares are the median binding magnitude (median fluorescence intensity minus background) for each V1V2 construct for an entire group. The binding titers are color-coded as indicated on the right.
Vaccine induction of gp120-specific IgA binding responses.
We examined plasma IgA binding to eight HIV-1 gp120 antigens. Two gp120 antigens were bound by plasma IgA, including the vaccine strain B.1059 and HIV-1 SF162, from which the challenge strain was derived. We also detected low plasma IgA binding to the C1 region that was a correlate of increased HIV-1 risk in the RV144 trial (15). Overall, Env binding IgA antibodies were relatively low compared to Env-specific IgG antibodies (Fig. 6), with only one macaque generating positive responses against all four antigens. Positive IgA responses were highest against the HIV-1 SF162 gp120, with half of the B.1059 group and one macaque from the T cell mosaic group generating detectable responses (Fig. 6). However, IgA responses against HIV-1 SF162 gp120 were very low; therefore, differences among groups were likely not meaningful.
FIG 6.

B.1059 immunization increases the frequency and magnitude of plasma IgA responses. Plasma IgA responses against HIV-1 envelope antigens were examined by the BAMA. Plasma after two NYVAC and five protein immunizations was tested at a 1:40 dilution. Values greater than 100 and 3-fold over baseline were considered positive responses. Nonresponders are represented by open symbols. The box-and-whisker plots show the median, interquartile range, minimum, and maximum. No detectable binding was measured for antigens A1.con.env03 gp140CF, CON-S gp140CFI, SF162.LS gp140C, and 00MSA 4076 gp140.
Vaccination does not protect macaques from repeat low-dose, mucosal challenge with a tier 2 SHIV.
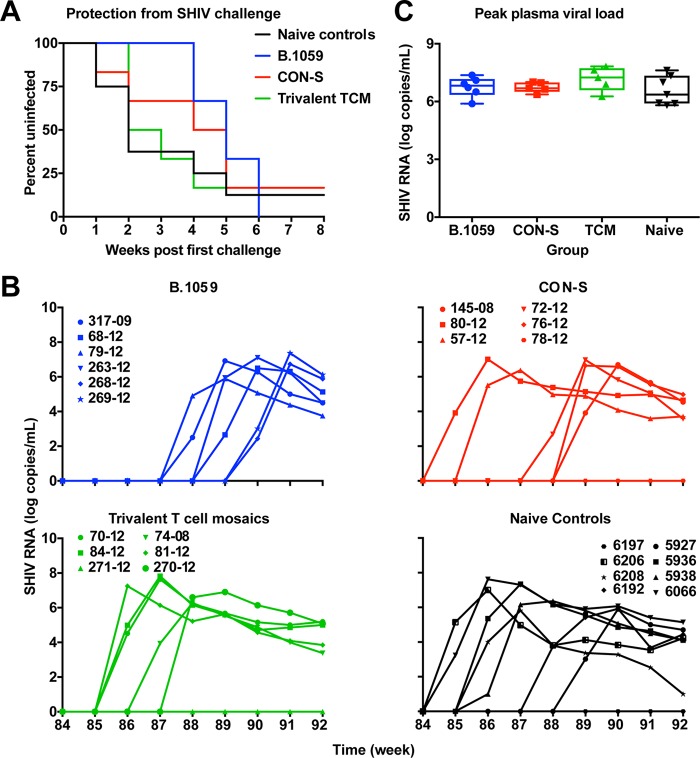
To assess whether the immune responses were protective, the vaccinated macaques were challenged weekly with tier 2 SHIV SF162P3 2 weeks after the fifth protein boost (Fig. 1A). The heterologous challenge virus and vaccine immunogen gp120s were 75.7% to 83.0% similar in amino acid sequence (Table 1). Eight unvaccinated macaques were used as naive controls for infection. Plasma viremia was used to determine infection. After 8 low-dose intrarectal challenges, none of the vaccine groups were significantly protected compared to naive unvaccinated control macaques (Fig. 7A; log rank test). The vaccine efficacy was approximately 29% for B.1059 and CON-S immunogens and 11% for the trivalent T cell mosaics (Table 2). Four, one, and one SHIV challenge were required for the first macaque to be infected in the B.1059, CON-S, and T cell mosaic groups, respectively. The peak viral loads among the groups were not significantly different (Fig. 7C).
TABLE 1.
Amino acid similarity between the gp160 and gp120 of the vaccine immunogens and the challenge virus SHIV SF162P3
| Immunogen | % similarity to SHIV SF162P3 proteina,b |
|
|---|---|---|
| gp120 | gp160 | |
| B.1059 | 80.8 | 84.0 |
| CON-S | 81.0 | 82.9 |
| T cell mosaic 3.1 | 75.7 | 79.6 |
| T cell mosaic 3.2 | 83.0 | 84.9 |
| T cell mosaic 3.3 | 77.8 | 77.6 |
gp120 and gp160 sequences without the signal peptide were compared among Envs. Values represent amino acid similarity calculated with Thermo Fisher AlignX.
The SHIV SF162P3 envelope sequence GenBank accession number is CCE66371.
FIG 7.
Lack of protection from tier 2 SHIV SF162P3 low-dose, repetitive intrarectal challenge. (A) Log rank tests for the Kaplan-Meier curve for infection during the repetitive low-dose intrarectal challenge of macaques vaccinated with all three Envs. The first challenge occurred 2 weeks after the fifth protein boost. Naive animals were included as positive controls for challenge. Vaccine efficacy statistics are presented in Table 2. (B) Plasma viral load of the challenged animals over time. The limit of detection is 100 copies/ml. Each line represents an individual macaque. Each group immunogen is listed above the panel, and the lines are color-coded as indicated in panel A. (C) Comparison of peak vial load across vaccinated and control groups. Each column represents one group, and individual macaques are shown by each symbol. TCM, T cell mosaic.
TABLE 2.
Vaccine efficacy statistics for protection against SHIV SF162P3 mucosal challenge
| Immunogen | Median exposurea | Log rank test P value vs controlb |
Vaccine efficacy (%) | Per-exposure risk (%) |
|---|---|---|---|---|
| B.1059 | 5.0 | 0.25 | 29 | 20 |
| CON-S | 4.5 | 0.47 | 29 | 20 |
| T cell mosaics | 2.5 | 0.90 | 11 | 25 |
| Control | 2.0 | 28 |
Group median for the number of challenges required to infect the macaques in that group.
Numbers of uninfected macaques were compared between vaccine groups and the unvaccinated control macaques.
Plasma viremia correlates with gp120 binding IgG titers in post hoc analyses.
In a post hoc analysis of viremia, we assessed whether any of 5 measured antibody functions correlated with viral load at the time of infection across the vaccine groups. We observed that IgG binding titers in response to B.1059 gp120 and SF162 gp120 on the day of challenge inversely correlated with the magnitude of viremia at the first time point that viremia was detected (Kendall's tau = −0.55 and −0.50, respectively [Fig. 8]). SF162 gp120 and B.1059 gp120 binding titers were highly correlated and likely described the same antibody response. Conversely, ADCC, tier 1 neutralization, phagocytosis, and plasma blocking of CD4 binding to gp140 did not correlate with viral load magnitude at the first time point that viremia was detected. As the responses evaluated were a subset of all antibody features measured, we provide the tau correlation coefficient to indicate the strength of the correlation but do not provide a P value.
FIG 8.
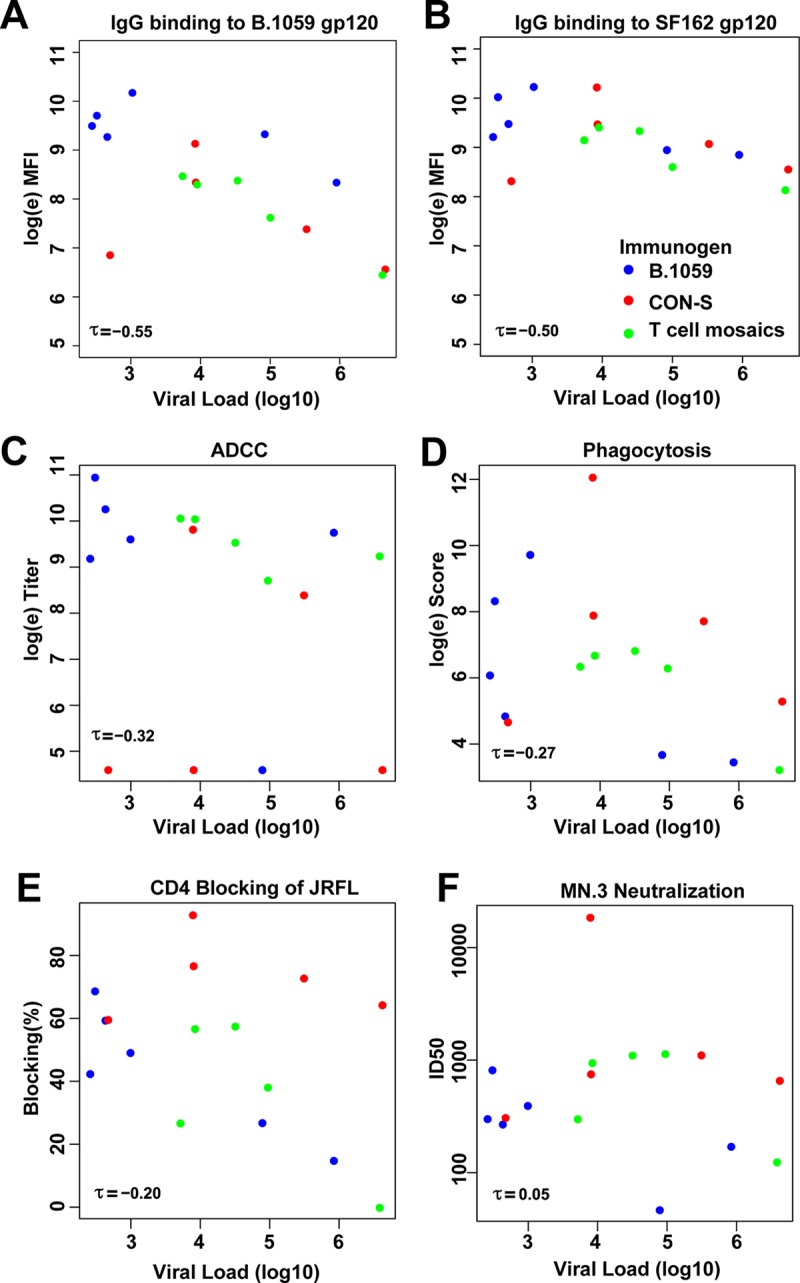
IgG gp120 binding titers inversely correlate with viral load magnitude when viremia is first detected. Kendall's tau is shown for BAMA plasma IgG binding titer in response to B.1059 gp120 (A), BAMA plasma Ig binding titer in response to SF162 gp120 (B), ADCC (C), phagocytosis (D), plasma blocking of CD4 binding to JRFL gp140CF (E), and tier 1 HIV-1 MN.3 neutralization (F). Each vaccine group is shown as colored circles as indicated in panel B. Uninfected animals were omitted from the correlation analysis between antibody function and viral load.
DISCUSSION
We describe here elicitation of humoral immune responses in nonhuman primates by an NYVAC Env gp120 prime, Env gp120 protein boost immunization regimen. The Env immunogens administered in this study were significant because they were used as DNAs in the HVTN106 human clinical trial (NCT02296541). We challenged the macaques in this study with a difficult-to-neutralize tier 2 CCR5-tropic SHIV, SF162P3. While macaque plasma had both binding and tier 1 neutralizing antibodies, all of the animals lacked plasma neutralization of the tier 2 SHIV SF162P3. Thus, we assessed nonneutralizing antibodies as possible mediators of protection. In a previous NYVAC immunization study that used intrarectal simian immunodeficiency virus (SIV) challenge, 5 of 11 macaques were protected from infection (24). Our study included groups of 6 macaques, which resulted in less power to detect significant protection. In the previous study, vaccine-induced protection did not correlate with SIV neutralization titer (24), suggesting that antibody effector functions may have played a role in the protection from infection. In our study, we elicited nonneutralizing antibodies but did not observe significant protection from SHIV transmission. The difference in outcome could be because the study of Benson et al. challenged once with SIV mac251, whereas we repetitively challenged the macaques with SHIV SF162P3 (24). Also, the previous study used SIV instead of HIV immunogens and analyzed antibody responses with different metrics; thus, it is unclear how similar the antibody responses were between the two studies. While nonneutralizing antibodies elicited by this regimen may not have been sufficient for protection against this particular SHIV, multiple studies have indicated that high levels of binding antibodies against the immunogen or against the viral challenge Env correlate with time to infection (33, 42, 43).
The analysis of the immune correlates of decreased infection risk in the RV144 trial revealed V1V2 antibodies and ADCC, in the presence of low IgA, as immune correlates of decreased infection risk in certain immune settings (15, 18, 19). Furthermore, ADCC has correlated with vaccine-mediated protection from SIV and SHIV infection in macaques (33, 44). This NYVAC-based vaccine elicited ADCC responses, but the titer of the response waned during the protein boosting. Increasing the intervals between gp120 boosts from 1 month to 6 months did not enhance ADCC responses. Although an immunologic rest period may be still be beneficial, the rest period may need to be longer than 6 months (18). Thus, the outcome observed in this study could be because the ADCC-mediating antibodies were at insufficient titers at the time of SHIV challenge to be protective.
IgA can compete with IgG for binding to the same epitope leading to diminished anti-HIV-1 IgG effector functions in vitro (16). Indeed, ADCC inversely correlated with infection risk in the RV144 trial when in the presence of low IgA. The Env-specific IgA titers were very low overall and low compared to Env-specific IgG titers, which was similar to previous NYVAC vaccine studies (3). Considering the results of the RV144 trial (15), vaccines, like the ones studied here, that elicit low IgA responses may be desirable. Whether the level of IgA antibody responses elicited in nonhuman primate vaccine studies play a role in the outcome of the challenge study warrants further study.
The ultimate goal of vaccination is to prevent infection. In cases where this does not occur, it may be possible for the vaccine to reduce the viral load. Reduction in viral load is usually attributed to cytolytic T cell responses (45). However, here we show that gp120 binding responses inversely correlated with viral load at the first time point of detectable viremia. This result raises a hypothesis for future studies that antibodies may mediate viral load control by nonneutralizing mechanisms.
In summary, recent SIV vaccine studies used systems serology to show that the best protection from infection was correlated with a polyfunctional antibody response (33, 44). Here we have demonstrated that NYVAC-gp120 vaccines can induce a polyfunctional antibody response in primates, including tier 1 neutralizing antibodies, phagocytosis, and ADCC.
MATERIALS AND METHODS
Macaque immunizations.
Twenty-six adult Indian rhesus macaques (Macaca mulatta) were selected for this study. Each macaque was verified by a PCR-based assay to not express the rhesus monkey major histocompatibility complex (MHC) class I allele Mamu-A*01. The monkeys were cared for in the AAALAC-accredited New England Primate Research Center in Southborough, MA. The monkeys were treated in accordance with National Institutes of Health and Harvard Medical School guidelines, and all procedures were approved by the New England Primate Research Center IACUC. Eighteen rhesus macaques were assigned to one of the three vaccine groups (B.1059, CON-S, and trivalent T cell mosaics; n = 6 per group). The remaining 8 macaques were assigned to the unvaccinated infection control group (naive controls). At weeks 0 and 4, the macaques in the vaccine groups received intramuscular immunizations using 108 PFU of NYVAC expressing the HIV-1 Env gp120 corresponding to their group assignment. NYVAC vectors were constructed as described below. At weeks 20, 24, 28, 50, and 82, the macaques were administered the autologous recombinant gp120 protein formulated in a Toll-like receptor (TLR) 7, 8, and 9 agonist, STR8-C (46). This adjuvant is an oil-in-water emulsion with oligoCpG and R848. Blood samples were drawn immediately before and 2 weeks after each immunization.
Low-dose intrarectal challenge and viral load measurements.
Two weeks after the final protein boost, all of the macaques were challenged with the tier 2 simian-human immunodeficiency virus (SHIV) SF162P3. While under general anesthesia the macaques were administered intrarectally a single 1:400 dilution of virus each week for 8 weeks. The acquisition of infection was measured by the presence of SIV gag RNA in the plasma. SIV gag RNA was measured by a real-time PCR-based assay as previously described (47).
NYVAC vector construction.
The different NYVAC transfer vectors were constructed as previously described (12). Briefly, the gp120 sequences from different HIV group assignments were PCR amplified from plZAW1 and cloned into pCyA20. The plasmid pCyA20 was used for the engineering of the recombinant NYVAC viruses. It contains thymidine kinase (TK) left and right flanking sequences, a short TK left arm repeat, and a vaccinia virus E3L promoter-driven β-galactosidase (β-Gal) expression cassette. Between the two TK flanking sequences, there is a vaccinia virus synthetic early/late (E/L) promoter driving the expression of the gp120 genes. This plasmid directs the insertion of the gp120 expression cassette into the NYVAC genome via recombination within the TK gene locus.
To recombine the gp120 expression cassette into the NYVAC genome, 3 million monkey BSC-40 cells were infected with wild-type NYVAC and 1 h later transfected with the gp120-containing pCyA20 plasmid transfer vector. At 72 h postinfection, the cells were harvested, lysed by freeze-thaw cycling, sonicated, and used for recombinant virus screening. Recombinant NYVAC viruses containing gp120 genes and transiently coexpressing the β-Gal marker gene were selected by consecutive rounds of plaque purification in BSC-40 cells stained with 5-bromo-4-chloro-3-indolyl β-galactoside. In the following rounds, recombinant NYVAC viruses containing gp120 genes and having deletions of the β-Gal marker gene were isolated by additional consecutive rounds of plaque purification screening for nonstaining viral foci in chicken embryo fibroblasts (CEFs). The final plaque was amplified to generate a P2 stock in CEFs. The titer of the P2 stock was determined by plaque immune staining assay, and antigen expression was confirmed as described previously (12). The final vector was propagated in large cultures of BHK-21 cells, followed by virus purification through two 36% (wt/vol) sucrose cushions. The NYVAC vector encoding T cell mosaic 3.3 was found to contain L121Q and I224V mutations; therefore, antibody binding was also assessed for this Env.
Direct ELISAs.
Nuncsorp 384-well plates were coated with 30 ng (2 μg/ml) of antigen in 0.1 M sodium bicarbonate overnight at 4°C. Plates were washed once with SuperWash and blocked with 40 μl of SuperBlock for 1 h at room temperature. Plasma was diluted 1:30 in SuperBlock and diluted serially 11 times. The plate was washed twice with SuperWash, and 10 μl of the plasma or antibody dilution series was incubated in the plate for 90 min. The plate was washed twice, and 10 μl of a 1:8,000 dilution of anti-human IgG (Rockland) or a 1:8,000 dilution of anti-rhesus IgG conjugated to horseradish peroxidase (HRP) was incubated in the plate for 1 h. The plate was washed four times with SuperWash, and 20 μl of tetramethylbenzidine (TMB; KPL) was incubated in the plate for 15 min. The reaction was stopped with 20 μl of 1% HCl. The absorbance at 450 nm of each well was read with a SpectraMax plate reader (Molecular Devices), and SoftMax Pro v5.3 (Molecular Devices) was used to calculate log area under the curve (AUC).
Plasma competition ELISAs.
Plasma competition assays were performed as described previously (12, 35). In brief, Nuncsorp plates were coated with HIV-1 envelope, washed, and blocked as described above for direct ELISAs. After blocking was complete, nonhuman primate plasma was diluted in SuperBlock at a 1:50 dilution and incubated in triplicate wells for 90 min. Nonbiotinylated monoclonal antibodies were incubated with the Env in triplicate as positive controls for blocking. To determine relative binding, no plasma or no antibody was added to a group of wells scattered throughout the plate. After 90 min, the nonbiotinylated antibody or plasma was washed away and biotinylated monoclonal antibodies were incubated in the wells for 1 h at subsaturating concentrations. For CD4 blocking assays, soluble CD4 was added, followed by biotinylated anti-CD4 monoclonal antibody. Each well was washed, and binding of biotinylated monoclonal antibodies was determined with a 1:30,000 dilution of HRP-conjugated streptavidin. HRP was detected with tetramethylbenzidine and stopped with 1% HCl. The absorbance at 450 nm of each well was read with a Spectramax plate reader (Molecular Devices). Binding of the biotinylated monoclonal antibody to HIV-1 envelope in the absence of plasma was compared to that in the presence of plasma to calculate percent inhibition of binding. Based on historical negative controls, assays were considered valid if the positive-control antibodies blocked greater than 20% of the biotinylated antibody binding.
In vitro HIV-1 neutralization.
Neutralizing antibody activity was measured in 96-well culture plates using Tat-regulated luciferase (Luc) reporter gene expression to quantify reductions in virus infection in TZM-bl cells as described previously (48). TZM-bl cells were obtained from the NIH AIDS Research and Reference Reagent Program, as contributed by John Kappes and Xiaoyun Wu. Serum samples were heat inactivated at 56°C for 30 min prior to being serially diluted in cell culture medium. The diluted serum was preincubated with virus (∼150,000 relative light unit equivalents) for 1 h at 37°C, and TZM-bl cells were subsequently added. Following 48 h of incubation, cells were lysed and Luc activity was determined using a microtiter plate luminometer and BriteLite Plus reagent (PerkinElmer). Neutralization titers are the reciprocal plasma or serum dilution at which relative luminescence units (RLU) were reduced by 50% compared to RLU in virus control wells after subtraction of background RLU in cell control wells.
Binding antibody multiplex assay (BAMA).
Antibody responses to HIV-1 antigens were determined as previously described (49, 50). IgA responses to both the subtype AE RV144 C1 peptide (Bio-KKKMQEDVISLWDQSLKPCVKLTPLCV) and the subtype BC C1 peptide (Bio-KKKMHEDIISLWDQSLKPCVKLTPLCV) were measured as previously described (15). N-hydroxysuccinimide polystyrene microsphere beads conjugated to protein were counted under a hemocytometer to ensure the presence of beads after conjugation. For IgG and IgA responses, antigen-coated Luminex beads were incubated with plasma or IgG-depleted plasma, followed by biotinylated anti-IgG or anti-IgA and streptavidin-phycoerythrin (PE), respectively. Fluorescence intensity values are blank well subtracted and negative-control antigen subtracted. For experiments where titrations were performed, the binding titer is shown as log AUC.
ADCC.
The antibody-dependent cellular cytotoxicity (ADCC) assay was carried out as described previously (51). Briefly, CEM.NKRCCR5 cells were coated with 10 μg/ml of recombinant SF162 gp120 for 75 min. After 75 min of incubation of target cells with gp120 alone, viability dye and TFL4 cell marker were added to the wells and the incubation was continued for an additional 15 min. Peripheral blood mononuclear cells (PBMCs), including natural killer cells, were used as effector cells, at an effector-to-target ratio of 30:1. The cells were mixed with a granzyme B (GrB) substrate, followed by the addition of plasma samples. After 15 min of incubation, the cells were pelleted in the assay plates by centrifugation and incubated for an additional hour. After the cells were washed, the fluorescent signal arising from cleavage of the GrB substrate was analyzed by flow cytometry. The percent GrB-positive viable cells (minus the background) was calculated for each dilution. The titer of the ADCC-mediating antibodies was then calculated by interpolation of the dilution exhibiting a GrB activity matching the cutoff value for positivity of 8%.
Antibody-dependent phagocytosis.
Phagocytosis was measured as stated previously (52, 53). Briefly, recombinant SHIV SF162P3 gp120 was incubated with 1 μm fluorescent beads overnight at 4°C while rotating. The beads were subsequently washed twice with 0.1% bovine serum albumin–phosphate-buffered saline (BSA-PBS) to remove unbound gp120. gp120-coated beads were incubated with prevaccination and postvaccination plasma IgG for 2 h at 37°C. As a positive control, HIVIG was incubated with the beads. As a negative control, PG9 was incubated with the beads since this antibody does not bind SHIV SF162P3 gp120. THP-1 cells were incubated with anti-CD4 antibody SK3 (Biolegend) and then added to the bead-plasma IgG mixture. The mixture was spinoculated for 1 h at 4°C, followed by another 1 h of incubation at 37°C. The cells were washed and fixed, and fluorescence due to bead internalization was measured by flow cytometry. Positive phagocytosis was determined by two criteria. First, values at week 84 were considered positive if they were greater than the 95th percentile of the mean phagocytosis score of prevaccination samples from the all macaques. Second, values obtained at week 84 had to be >3-fold higher than the prevaccination sample from the same macaque.
Recombinant antibody expression.
Expi293 cells (Life Technologies) were cultured in Expi293 medium at less than 5 million cells per ml. On the day of transfection, cells were diluted to 2.5 million cells/ml in Expi293 medium. 293i cells were cotransfected with 400 μg of heavy chain plasmid and light chain plasmid using Expifectamine. Five days after transfection, cell culture medium was cleared of cells by centrifugation and filtration (0.8-μm-pore-size filter). The cell-free supernatant was incubated with protein A beads (Thermo Fisher) overnight at 4°C. The protein A beads were centrifuged and cell culture supernatant was removed. The beads were washed with 25 ml of PBS with 340 mM NaCl. Thirty milliliters of 10 mM glycine (pH 2.4)–150 mM NaCl was used to elute the antibody off the beads. The pH of the antibody solution was increased to neutral pH by adding 1 M Tris (pH 8.0). The neutral pH eluate was buffer exchanged into PBS with successive rounds of centrifugation, filtered, and stored at −80°C.
Recombinant Env expression.
One milligram of plasmid DNA per 1 liter of cells was diluted in Dulbecco modified Eagle medium (DMEM) and mixed with polyethyleneimine (PEI). PEI-DNA mixtures were added to cells for 4 h. 293F (Invitrogen) cells were subsequently washed and diluted to 1.25 million cells/ml in Freestyle293 medium (Invitrogen). Five days after transfection, the cell culture medium was centrifuged and filtered through a 0.8-μm-pore-size filter to eliminate cells. The cell culture was concentrated with a 10-kDa-molecular-mass-cutoff Vivaflow 50. The concentrated cell culture supernatant was rotated with Galanthus nivalis lectin beads (Vistar Labs) overnight at 4°C. The beads were pelleted by centrifugation the next day and washed twice with morpholineethanesulfonic acid (MES) buffer. The protein was eluted with methyl-α-pyranoside, buffer exchanged into PBS, and stored at −80°C.
Statistical analyses.
To evaluate protection, the first time point with detectable viral load was used as the indicator of infection. Between-group differences in protection were assessed using a log rank test. The three vaccine groups were also combined and compared to the naive unvaccinated group (n = 18 vaccinated and n = 8 naive controls). Vaccine efficacy was calculated as previously described (5). Our primary immune correlate was B.CaseA2 V1V2 binding assessed by a Cox proportional hazard model as a time-varying covariate on the combined vaccinated macaques. The P values in the primary correlate analysis of B.CaseA2 V1V2 binding were not corrected because this immune measure was prespecified in a statistical analysis plan. Spearman correlations were performed for ADCC responses at week 84 and time to infection across all vaccine groups. Between-group differences in ADCC, binding IgG, binding IgA, neutralization titers, and plasma blocking were tested using Wilcoxon exact tests, and all of the P values reported were corrected for multiple comparisons using the Benjamini-Hochberg false discovery rate method. Post hoc, we examined the correlation between first detectable viral load measurement and antibody functions using Kendall's tau. Macaques without detectable plasma viral loads were omitted for the analyses of Kendall's tau correlation. Since not all possible correlations were examined and the analysis was hypothesis generating, P values were not reported for Kendall's tau correlation analyses. All statistical analysis was performed using SAS v9.4 (SAS Institute, Inc.).
ACKNOWLEDGMENTS
We thank Krissey E. Lloyd, Charles Beck, Melissa Zinter, Christine Arocena, and R. Glenn Overman for technical assistance. We thank Meng Chen for statistical analyses. We thank Abe Pinter for gp70V1V2 antigens and Xiaoying Shen for the Env peptide design.
This work was supported by NIAID extramural project grant R01-AI120801 (K.O.S.) and NIH, NIAID, Division of AIDS, UM1 grant AI100645 for the Center for HIV/AIDS Vaccine Immunology-Immunogen Discovery (CHAVI-ID; B.F.H.), and the Duke University Center for AIDS Research (AI064518).
The funders had no role in data collection and interpretation or the decision to submit the work for publication.
B.K., B.F.H., and H.-X.L. have filed International Patent Application PCT/US2004/030397 and National Stage Applications directed to CON-S and its use as an immunogen.
REFERENCES
- 1.Fauci AS, Marston HD. 2014. Ending AIDS—is an HIV vaccine necessary? N Engl J Med 370:495–498. doi: 10.1056/NEJMp1313771. [DOI] [PubMed] [Google Scholar]
- 2.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, MOPH-TAVEG Investigators . 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.García-Arriaza J, Perdiguero B, Heeney J, Seaman M, Montefiori DC, Labranche C, Yates NL, Shen X, Tomaras GD, Ferrari G, Foulds KE, McDermott A, Kao SF, Roederer M, Hawkins N, Self S, Yao J, Farrell P, Phogat S, Tartaglia J, Barnett SW, Burke B, Cristillo A, Weiss D, Lee C, Kibler K, Jacobs B, Asbach B, Wagner R, Ding S, Pantaleo G, Esteban M. 2015. Head-to-head comparison of poxvirus NYVAC and ALVAC vectors expressing identical HIV-1 clade C immunogens in prime-boost combination with Env protein in nonhuman primates. J Virol 89:8525–8539. doi: 10.1128/JVI.01265-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bart PA, Goodall R, Barber T, Harari A, Guimaraes-Walker A, Khonkarly M, Sheppard NC, Bangala Y, Frachette MJ, Wagner R, Liljestrom P, Kraehenbuhl JP, Girard M, Goudsmit J, Esteban M, Heeney J, Sattentau Q, McCormack S, Babiker A, Pantaleo G, Weber J, EuroVacc Consortium. 2008. EV01: a phase I trial in healthy HIV negative volunteers to evaluate a clade C HIV vaccine, NYVAC-C undertaken by the EuroVacc Consortium. Vaccine 26:3153–3161. doi: 10.1016/j.vaccine.2008.03.083. [DOI] [PubMed] [Google Scholar]
- 5.Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, Burnet S, Cellerai C, Erlwein O, Barber T, Moog C, Liljestrom P, Wagner R, Wolf H, Kraehenbuhl JP, Esteban M, Heeney J, Frachette MJ, Tartaglia J, McCormack S, Babiker A, Weber J, Pantaleo G. 2008. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med 205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormack S, Stohr W, Barber T, Bart PA, Harari A, Moog C, Ciuffreda D, Cellerai C, Cowen M, Gamboni R, Burnet S, Legg K, Brodnicki E, Wolf H, Wagner R, Heeney J, Frachette MJ, Tartaglia J, Babiker A, Pantaleo G, Weber J. 2008. EV02: a phase I trial to compare the safety and immunogenicity of HIV DNA-C prime-NYVAC-C boost to NYVAC-C alone. Vaccine 26:3162–3174. doi: 10.1016/j.vaccine.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 7.Tartaglia J, Perkus ME, Taylor J, Norton EK, Audonnet JC, Cox WI, Davis SW, van der Hoeven J, Meignier B, Riviere M, Languet B, Paoletti E. 1992. NYVAC: a highly attenuated strain of vaccinia virus. Virology 188:217–232. [DOI] [PubMed] [Google Scholar]
- 8.Pincus S, Tartaglia J, Paoletti E. 1995. Poxvirus-based vectors as vaccine candidates. Biologicals 23:159–164. [DOI] [PubMed] [Google Scholar]
- 9.Guerra S, Najera JL, Gonzalez JM, Lopez-Fernandez LA, Climent N, Gatell JM, Gallart T, Esteban M. 2007. Distinct gene expression profiling after infection of immature human monocyte-derived dendritic cells by the attenuated poxvirus vectors MVA and NYVAC. J Virol 81:8707–8721. doi: 10.1128/JVI.00444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harari A, Rozot V, Cavassini M, Bellutti Enders F, Vigano S, Tapia G, Castro E, Burnet S, Lange J, Moog C, Garin D, Costagliola D, Autran B, Pantaleo G, Bart PA. 2012. NYVAC immunization induces polyfunctional HIV-specific T-cell responses in chronically-infected, ART-treated HIV patients. Eur J Immunol 42:3038–3048. doi: 10.1002/eji.201242696. [DOI] [PubMed] [Google Scholar]
- 11.Hel Z, Tsai WP, Thornton A, Nacsa J, Giuliani L, Tryniszewska E, Poudyal M, Venzon D, Wang X, Altman J, Watkins DI, Lu W, von Gegerfelt A, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. 2001. Potentiation of simian immunodeficiency virus (SIV)-specific CD4(+) and CD8(+) T cell responses by a DNA-SIV and NYVAC-SIV prime/boost regimen. J Immunol 167:7180–7191. doi: 10.4049/jimmunol.167.12.7180. [DOI] [PubMed] [Google Scholar]
- 12.Hulot SL, Korber B, Giorgi EE, Vandergrift N, Saunders KO, Balachandran H, Mach LV, Lifton MA, Pantaleo G, Tartaglia J, Phogat S, Jacobs B, Kibler K, Perdiguero B, Gomez CE, Esteban M, Rosati M, Felber BK, Pavlakis GN, Parks R, Lloyd K, Sutherland L, Scearce R, Letvin NL, Seaman MS, Alam SM, Montefiori D, Liao HX, Haynes BF, Santra S. 2015. Comparison of immunogenicity in rhesus macaques of transmitted-founder, HIV-1 group M consensus, and trivalent mosaic envelope vaccines formulated as a DNA prime, NYVAC, and envelope protein boost. J Virol 89:6462–6480. doi: 10.1128/JVI.00383-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, Dunphy LJ, Suscovich TJ, Frahm N, Linde C, Mahan AE, Hoffner M, Streeck H, Ackerman ME, McElrath MJ, Schuitemaker H, Pau MG, Baden LR, Kim JH, Michael NL, Barouch DH, Lauffenburger DA, Alter G. 2015. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell 163:988–998. doi: 10.1016/j.cell.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, Wenschuh H, Zerweck J, Greene K, Gao H, Berman PW, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Robb ML, Michael NL, Kim JH, Zolla-Pazner S, Haynes BF, Mascola JR, Self S, Gilbert P, Montefiori DC. 2013. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. 2013. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomaras GD, Plotkin SA. 2017. Complex immune correlates of protection in HIV-1 vaccine efficacy trials. Immunol Rev 275:245–261. doi: 10.1111/imr.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. 2014. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 6:228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zolla-Pazner S, deCamp A, Gilbert PB, Williams C, Yates NL, Williams WT, Howington R, Fong Y, Morris DE, Soderberg KA, Irene C, Reichman C, Pinter A, Parks R, Pitisuttithum P, Kaewkungwal J, Rerks-Ngarm S, Nitayaphan S, Andrews C, O'Connell RJ, Yang ZY, Nabel GJ, Kim JH, Michael NL, Montefiori DC, Liao HX, Haynes BF, Tomaras GD. 2014. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 9:e87572. doi: 10.1371/journal.pone.0087572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalah R, Kulkarni V, Patel V, Rosati M, Alicea C, Bear J, Yu L, Guan Y, Shen X, Tomaras GD, LaBranche C, Montefiori DC, Prattipati R, Pinter A, Bess J Jr, Lifson JD, Reed SG, Sardesai NY, Venzon DJ, Valentin A, Pavlakis GN, Felber BK. 2014. DNA and protein co-immunization improves the magnitude and longevity of humoral immune responses in macaques. PLoS One 9:e91550. doi: 10.1371/journal.pone.0091550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asbach B, Kliche A, Kostler J, Perdiguero B, Esteban M, Jacobs BL, Montefiori DC, LaBranche CC, Yates NL, Tomaras GD, Ferrari G, Foulds KE, Roederer M, Landucci G, Forthal DN, Seaman MS, Hawkins N, Self SG, Sato A, Gottardo R, Phogat S, Tartaglia J, Barnett SW, Burke B, Cristillo AD, Weiss DE, Francis J, Galmin L, Ding S, Heeney JL, Pantaleo G, Wagner R. 2016. Potential to streamline heterologous DNA prime and NYVAC/protein boost HIV vaccine regimens in rhesus macaques by employing improved antigens. J Virol 90:4133–4149. doi: 10.1128/JVI.03135-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abimiku AG, Franchini G, Tartaglia J, Aldrich K, Myagkikh M, Markham PD, Chong P, Klein M, Kieny MP, Paoletti E, Gallo RC, Robert-Guroff M. 1995. HIV-1 recombinant poxvirus vaccine induces cross-protection against HIV-2 challenge in rhesus macaques. Nat Med 1:321–329. doi: 10.1038/nm0495-321. [DOI] [PubMed] [Google Scholar]
- 23.Patterson LJ, Peng B, Abimiku AG, Aldrich K, Murty L, Markham PD, Kalyanaraman VS, Alvord WG, Tartaglia J, Franchini G, Robert-Guroff M. 2000. Cross-protection in NYVAC-HIV-1-immunized/HIV-2-challenged but not in NYVAC-HIV-2-immunized/SHIV-challenged rhesus macaques. AIDS 14:2445–2455. doi: 10.1097/00002030-200011100-00005. [DOI] [PubMed] [Google Scholar]
- 24.Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo RC, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV(mac251): dependence on route of challenge exposure. J Virol 72:4170–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, Montefiori DC, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J Immunol 169:4778–4787. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]
- 26.Fischer W, Perkins S, Theiler J, Bhattacharya T, Yusim K, Funkhouser R, Kuiken C, Haynes B, Letvin NL, Walker BD, Hahn BH, Korber BT. 2007. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med 13:100–106. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- 27.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, Novitsky V, Haynes B, Hahn BH, Bhattacharya T, Korber B. 2002. Diversity considerations in HIV-1 vaccine selection. Science 296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 28.Barouch DH, O'Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, Sun YH, La Porte A, Riggs AM, Lynch DM, Clark SL, Backus K, Perry JR, Seaman MS, Carville A, Mansfield KG, Szinger JJ, Fischer W, Muldoon M, Korber B. 2010. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med 16:319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santra S, Liao HX, Zhang R, Muldoon M, Watson S, Fischer W, Theiler J, Szinger J, Balachandran H, Buzby A, Quinn D, Parks RJ, Tsao CY, Carville A, Mansfield KG, Pavlakis GN, Felber BK, Haynes BF, Korber BT, Letvin NL. 2010. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med 16:324–328. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santra S, Korber BT, Muldoon M, Barouch DH, Nabel GJ, Gao F, Hahn BH, Haynes BF, Letvin NL. 2008. A centralized gene-based HIV-1 vaccine elicits broad cross-clade cellular immune responses in rhesus monkeys. Proc Natl Acad Sci U S A 105:10489–10494. doi: 10.1073/pnas.0803352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao F, Weaver EA, Lu Z, Li Y, Liao HX, Ma B, Alam SM, Scearce RM, Sutherland LL, Yu JS, Decker JM, Shaw GM, Montefiori DC, Korber BT, Hahn BH, Haynes BF. 2005. Antigenicity and immunogenicity of a synthetic human immunodeficiency virus type 1 group M consensus envelope glycoprotein. J Virol 79:1154–1163. doi: 10.1128/JVI.79.2.1154-1163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao HX, Sutherland LL, Xia SM, Brock ME, Scearce RM, Vanleeuwen S, Alam SM, McAdams M, Weaver EA, Camacho Z, Ma BJ, Li Y, Decker JM, Nabel GJ, Montefiori DC, Hahn BH, Korber BT, Gao F, Haynes BF. 2006. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology 353:268–282. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, Shields J, Parenteau L, Whitney JB, Abbink P, Ng‘ang'a DM, Seaman MS, Lavine CL, Perry JR, Li W, Colantonio AD, Lewis MG, Chen B, Wenschuh H, Reimer U, Piatak M, Lifson JD, Handley SA, Virgin HW, Koutsoukos M, Lorin C, Voss G, Weijtens M, Pau MG, Schuitemaker H. 2015. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 349:320–324. doi: 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, Dugast AS, Alter G, Ferguson M, Li W, Earl PL, Moss B, Giorgi EE, Szinger JJ, Eller LA, Billings EA, Rao M, Tovanabutra S, Sanders-Buell E, Weijtens M, Pau MG, Schuitemaker H, Robb ML, Kim JH, Korber BT, Michael NL. 2013. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155:531–539. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saunders KO, Nicely NI, Wiehe K, Bonsignori M, Meyerhoff RR, Parks R, Walkowicz WE, Aussedat B, Wu NR, Cai F, Vohra Y, Park PK, Eaton A, Go EP, Sutherland LL, Scearce RM, Barouch DH, Zhang R, Von Holle T, Overman RG, Anasti K, Sanders RW, Moody MA, Kepler TB, Korber B, Desaire H, Santra S, Letvin NL, Nabel GJ, Montefiori DC, Tomaras GD, Liao HX, Alam SM, Danishefsky SJ, Haynes BF. 2017. Vaccine elicitation of high mannose-dependent neutralizing antibodies against the V3-glycan broadly neutralizing epitope in nonhuman primates. Cell Rep 18:2175–2188. doi: 10.1016/j.celrep.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradley T, Fera D, Bhiman J, Eslamizar L, Lu X, Anasti K, Zhang R, Sutherland LL, Scearce RM, Bowman CM, Stolarchuk C, Lloyd KE, Parks R, Eaton A, Foulger A, Nie X, Karim SS, Barnett S, Kelsoe G, Kepler TB, Alam SM, Montefiori DC, Moody MA, Liao HX, Morris L, Santra S, Harrison SC, Haynes BF. 2016. Structural constraints of vaccine-induced tier-2 autologous HIV neutralizing antibodies targeting the receptor-binding site. Cell Rep 14:43–54. doi: 10.1016/j.celrep.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hessell AJ, Malherbe DC, Pissani F, McBurney S, Krebs SJ, Gomes M, Pandey S, Sutton WF, Burwitz BJ, Gray M, Robins H, Park BS, Sacha JB, LaBranche CC, Fuller DH, Montefiori DC, Stamatatos L, Sather DN, Haigwood NL. 2016. Achieving potent autologous neutralizing antibody responses against tier 2 HIV-1 viruses by strategic selection of envelope immunogens. J Immunol 196:3064–3078. doi: 10.4049/jimmunol.1500527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, Arendt H, Kim HJ, Lee JH, Pugach P, Williams M, Debnath G, Moldt B, van Breemen MJ, Isik G, Medina-Ramirez M, Back JW, Koff WC, Julien JP, Rakasz EG, Seaman MS, Guttman M, Lee KK, Klasse PJ, LaBranche C, Schief WR, Wilson IA, Overbaugh J, Burton DR, Ward AB, Montefiori DC, Dean H, Moore JP. 2015. HIV-1 vaccines. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349:aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 40.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol 73:4009–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol 75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, Labranche C, Foulds KE, Louder MK, Yang ZY, Todd JP, Buzby AP, Mach LV, Shen L, Seaton KE, Ward BM, Bailer RT, Gottardo R, Gu W, Ferrari G, Alam SM, Denny TN, Montefiori DC, Tomaras GD, Korber BT, Nason MC, Seder RA, Koup RA, Letvin NL, Rao SS, Nabel GJ, Mascola JR. 2014. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature 505:502–508. doi: 10.1038/nature12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradley T, Pollara J, Santra S, Vandergrift N, Pittala S, Bailey-Kellogg C, Shen X, Parks R, Goodman D, Eaton A, Balachandran H, Mach LV, Saunders KO, Weiner JA, Scearce R, Sutherland LL, Phogat S, Tartaglia J, Reed SG, Hu SL, Theis JF, Pinter A, Montefiori DC, Kepler TB, Peachman KK, Rao M, Michael NL, Suscovich TJ, Alter G, Ackerman ME, Moody MA, Liao HX, Tomaras G, Ferrari G, Korber BT, Haynes BF. 2017. Pentavalent HIV-1 vaccine protects against simian-human immunodeficiency virus challenge. Nat Commun 8:15711. doi: 10.1038/ncomms15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson S, Eller M, Teigler JE, Maloveste SM, Schultz BT, Soghoian DZ, Lu R, Oster AF, Chenine AL, Alter G, Dittmer U, Marovich M, Robb ML, Michael NL, Bolton D, Streeck H. 2015. Cooperativity of HIV-specific cytolytic CD4 T cells and CD8 T cells in control of HIV viremia. J Virol 89:7494–7505. doi: 10.1128/JVI.00438-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moody MA, Santra S, Vandergrift NA, Sutherland LL, Gurley TC, Drinker MS, Allen AA, Xia SM, Meyerhoff RR, Parks R, Lloyd KE, Easterhoff D, Alam SM, Liao HX, Ward BM, Ferrari G, Montefiori DC, Tomaras GD, Seder RA, Letvin NL, Haynes BF. 2014. Toll-like receptor 7/8 (TLR7/8) and TLR9 agonists cooperate to enhance HIV-1 envelope antibody responses in rhesus macaques. J Virol 88:3329–3339. doi: 10.1128/JVI.03309-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saunders KO, Pegu A, Georgiev IS, Zeng M, Joyce MG, Yang ZY, Ko SY, Chen X, Schmidt SD, Haase AT, Todd JP, Bao S, Kwong PD, Rao SS, Mascola JR, Nabel GJ. 2015. Sustained delivery of a broadly neutralizing antibody in non-human primates confers long-term protection against simian/human immunodeficiency virus infection. J Virol 89:5895–5903. doi: 10.1128/JVI.00210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen X, Basu R, Sawant S, Beaumont D, Kwa SF, LaBranche C, Seaton KE, Yates NL, Montefiori DC, Ferrari G, Wyatt LS, Moss B, Alam SM, Haynes BF, Tomaras GD, Robinson HL. 11 October 2017. HIV-1 gp120 protein and MVAgp140 boost immunogens increase immunogenicity of a DNA/MVA HIV-1 vaccine. J Virol doi: 10.1128/JVI.01077-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. 2011. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 79:603–612. doi: 10.1002/cyto.a.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, Alter G. 2011. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods 366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tay MZ, Liu P, Williams LD, McRaven MD, Sawant S, Gurley TC, Xu TT, Dennison SM, Liao HX, Chenine AL, Alam SM, Moody MA, Hope TJ, Haynes BF, Tomaras GD. 2016. Antibody-mediated internalization of infectious HIV-1 virions differs among antibody isotypes and subclasses. PLoS Pathog 12:e1005817. doi: 10.1371/journal.ppat.1005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yates NL, deCamp AC, Korber BT, Liao H-X, Irene C, Pinter A, Peacock J, Harris LJ, Sawant S, Hraber P, Shen X, Rerks-Ngarm S, Pitisuttithum P, Nitayapan S, Berman PW, Robb ML, Pantaleo G, Zolla-Pazner S, Haynes BF , Alam SM, Montefiori DC, Tomaras GD. 2018. HIV-1 envelope glycoproteins from diverse clades differentiate antibody responses and durability among vaccinees. J Virol 92:e01843-17. doi: 10.1128/JVI.01843-17. [DOI] [PMC free article] [PubMed] [Google Scholar]