ABSTRACT
HIV-1 transmission occurs mainly through mucosal tissues. During mucosal transmission, HIV-1 preferentially infects α4β7+ gut-homing CCR7− CD4+ effector/effector memory T cells (TEM) and results in massive depletion of these cells and other subsets of TEM in gut-associated lymphoid tissues. However, besides being eliminated by HIV-1, the role of TEM during the early stage of infection remains inconclusive. Here, using in vitro-induced α4β7+ gut-homing TEM (α4β7+ TEM), we found that α4β7+ TEM differentiated into CCR7+ CD4+ central memory T cells (TCM). This differentiation was HIV-1 independent but was inhibited by SB431542, a specific transforming growth factor β (TGF-β) receptor I kinase inhibitor. Consistently, TEM-to-TCM differentiation was observed in α4β7+ TEM stimulated with TGF-β1 (TGF-β). The TCM properties of the TGF-β-induced TEM-derived TCM (α4β7+ TCM) were confirmed by their enhanced CCL19 chemotaxis and the downregulation of surface CCR7 upon T cell activation in vitro. Importantly, the effect of TGF-β on TCM differentiation also held in TEM directly isolated from peripheral blood. To investigate the significance of the TGF-β-dependent TEM-to-TCM differentiation in HIV/AIDS pathogenesis, we observed that both productively and latently infected α4β7+ TCM could differentiate from α4β7+ TEM in the presence of TGF-β during HIV-1 infection. Collectively, this study not only provides a new insight for the plasticity of TEM but also suggests that the TGF-β-dependent TEM-to-TCM differentiation is a previously unrecognized mechanism for the formation of latently infected TCM after HIV-1 infection.
IMPORTANCE HIV-1 is the causative agent of HIV/AIDS, which has led to millions of deaths in the past 30 years. Although the implementation of highly active antiretroviral therapy has remarkably reduced the HIV-1-related morbidity and mortality, HIV-1 is not eradicated in treated patients due to the presence of latent reservoirs. Besides, the pathogenesis in CD4 T cells early after infection still remains elusive. Immediately after HIV-1 mucosal infection, CD4 T cells are preferentially infected and depleted. However, in addition to being depleted, the other roles of the CD4 T cells, especially the effector/effector memory T cells (TEM), in disease progression are not completely understood. The significance of this study is in revealing a novel mechanism for the formation of latently HIV-1-infected central memory CD4 T cells, a major latent reservoir from CD4 TEM after infection. Our findings suggest previously unrecognized roles of CD4 TEM in HIV-1 pathogenesis.
KEYWORDS: CCR7, CD4 T cells, HIV-1, HIV-1 latent infection, central memory CD4 T cells, effector/effector memory CD4 T cells
INTRODUCTION
In cervicovaginal tissues, which serve as the portal of human immunodeficiency virus type 1 (HIV-1) entry, more than 90% of CD4 T cells are CCR7− CD4+ effector/effector memory T cells (TEM) (1). Due to the high expression of CCR5 and integrin α4β7 (α4β7), TEM, especially those residing within the cervicovaginal tissues, are highly susceptible to HIV-1 infection (1–3). In fact, TEM are preferentially depleted in the human gastrointestinal tract during infection (4, 5). Under these circumstances, TEM might be among the first target cells infected by HIV-1 after heterosexual transmission and might play an important role in determining the disease progression.
TEM and CCR7+ CD4+ central memory T cells (TCM) represent the two major CD4 memory T cell subsets in humans (6). The expression of CCR7 not only distinguishes TCM from TEM but also guides T cell exit from peripheral tissues to lymphatics (7, 8). Although the phenotype and migration pattern of TCM and TEM have been characterized, the lineage relationship of TCM and TEM has yet to be resolved. A previous study indicated that T cell receptor (TCR) stimulation results in CCR7 downregulation and TEM differentiation from human TCM (6). The TCM-to-TEM transition has also been observed in rhesus macaques under interleukin-15 (IL-15) therapy (9). In contrast, two independent studies suggested that CCR7 is expressed on anti-CD3/CD28-stimulated human TEM in vitro (10, 11). However, other mechanisms that can regulate the expression of CCR7 on TEM remain largely unknown.
The major obstacle for HIV-1 eradication is the presence of a small reservoir of latently infected cells (12). These cells contain a transcriptionally silent provirus and produce new infectious progeny upon cellular activations (13, 14) and treatment interruption (15). TCM have been recognized as the major HIV-1 latent reservoir (16). However, it is not clear how these latently infected TCM are established. A recent in vitro study using DuoFluo I, a dual-reporter pseudovirus, suggested that HIV-1 latency can establish in both resting and activated CD4 T cells immediately after infection (17). In addition, an in vivo macaque study has further demonstrated that latent reservoirs can be established within a few days after simian immunodeficiency virus (SIV) infection (18). Given that TEM are preferentially infected by HIV-1, we hypothesize that the differentiation of mucosal TEM to TCM through induction of CCR7 expression may represent a possible mechanism for the formation of latently infected TCM early after infection.
Transforming growth factor β1 (TGF-β1; TGF-β) is an immunosuppressive cytokine which suppresses T cell proliferation and induces regulatory T cell differentiation (19, 20). Recent studies have revealed that TGF-β also plays important roles in promoting proinflammatory T helper 17 (Th17) and T helper 9 (Th9) differentiation (21, 22). Moreover, TGF-β promotes survival of activated T cells and regulates T cell homing (23, 24). In regard to CCR7, TGF-β has been found to increase CCR7 expression on antigen-activated memory CD8 T cells and breast cancer cells that are undergoing epithelial-mesenchymal transition (25, 26). Interestingly, a recent study in rhesus macaques revealed that the genes downstream of the TGF-β signaling pathway are upregulated in SIV RNA-positive tissues as early as 1 day after infection (27). This result suggests that TGF-β might be produced immediately after HIV-1 infection and might subsequently regulate the differentiation and migration of TEM through regulating CCR7 expression.
Gut-homing α4β7+ CD4 T cells, the highly HIV-1-susceptible cells readily found in the gut and mucosal tissues, could be induced by retinoic acid (RA) in vitro (2, 28). In this study, we developed an allogeneic T cell activation method to generate gut-homing α4β7+ TEM (α4β7+ TEM) that were susceptible to HIV-1 infection for investigating the role of these cells in HIV-1 infection. Using this model, we discovered that a proportion of HIV-1-infected α4β7+ TEM could upregulate CCR7 and become TCM through TGF-β stimulation. This mechanism was also observed in TEM directly isolated from peripheral blood. This study suggests the previously unrecognized roles of α4β7+ TEM in the establishment of latently infected TCM after HIV-1 infection.
RESULTS
Characterization of α4β7+ MEMT and α4β7+ TEM.
Previous studies demonstrated that gut-homing α4β7+ CD4 T cells could be induced in vitro by stimulating T cells with anti-CD3 antibody and phytohemagglutinin (PHA) in the presence of RA (2, 28). Although both anti-CD3 antibody and PHA have been widely used to stimulate CD4 T cells for HIV-1 studies, the use of anti-CD3 antibody for large-scale T cell activation is not cost-effective and the cells activated by PHA might be functionally impaired (29). During sexual intercourse, T cells may be allogeneic activated and become susceptible to HIV-1 infection. We therefore developed an in vitro allogeneic T cell activation method to induce α4β7+ gut-homing memory CD4 T cells (α4β7+ MEMT) for investigating HIV-1 pathogenesis.
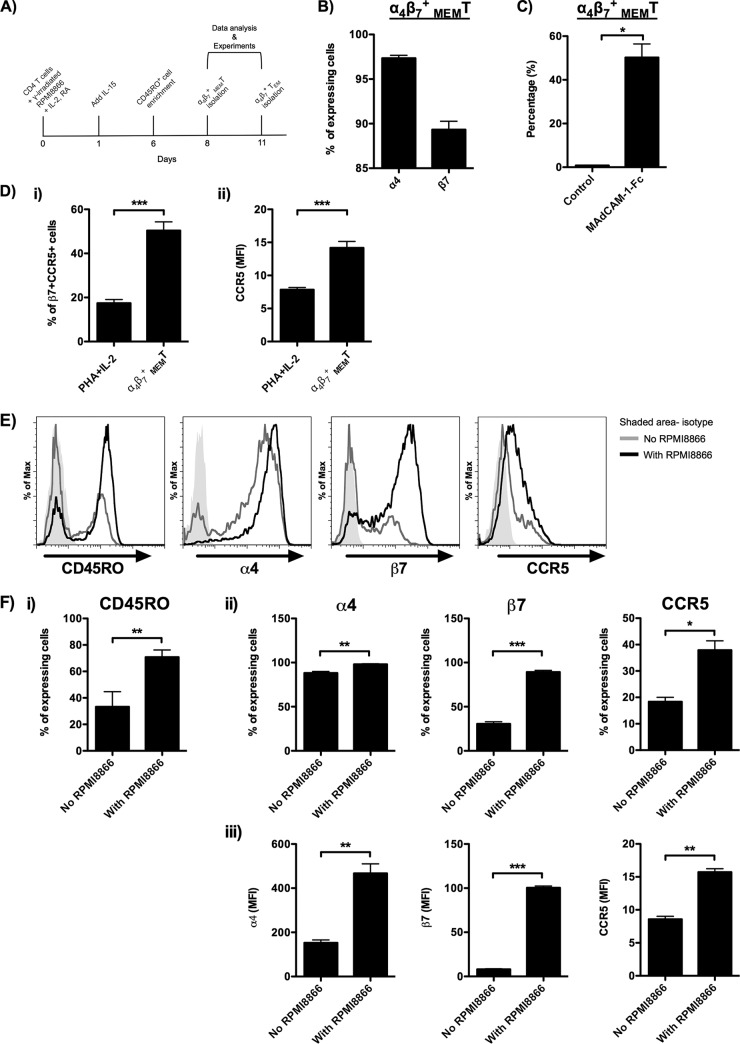
α4β7+ MEMT were generated by coculturing CD4 T cells with gamma-irradiated RPMI8866 cells in the presence of IL-2, IL-15, and RA followed by the enrichment of CD45RO+ cells. RPMI8866 is a human Epstein-Barr virus (EBV)-transformed B lymphoblastoid cell line which can significantly enhance the proliferation of cocultured T cells (30). A detailed cell preparation method is illustrated in Fig. 1A. By this method, about 95% (95.7% ± 0.4%) (data not shown) of CD4 T cells were CD45RO+ memory T cells on day 8. Integrin α4 (α4) and integrin β7 (β7) were expressed on 97.3% (97.3% ± 0.3%) and 89.3% (89.3% ± 0.9%) of CD45RO+ CD4 T cells, respectively (Fig. 1B). These results suggest that about 90% of the cells coexpressed α4 and β7. The function of α4β7 was further confirmed by its binding affinity toward its native ligand, mucosal vascular addressin cell adhesion molecule-1 (MAdCAM-1) (Fig. 1C). Besides, HIV-1 coreceptor CCR5 was expressed on 30 to 60% (50.4% ± 4.0%) of β7+ CD45RO+ cells. Compared with the conventional PHA plus IL-2 T cell stimulation method, our in vitro allogeneic activation method induced 2.9-fold more CCR5+ cells in the β7+ CD45RO+ cells (Fig. 1Di). In particular, the mean fluorescence intensity (MFI) of CCR5 was also significantly higher on the allogeneic activated CD45RO+ cells (Fig. 1Dii). The role of allogeneic stimulation in the generation of α4β7+ MEMT was also studied. In the presence of gamma-irradiated RPMI8866 cells, more CD45RO+ cells were induced on day 6 (Fig. 1E and Fi). Importantly, both the percentage and the MFI of α4, β7, and CCR5 expression on the CD45RO+ CD4 T cells were all increased in the allogeneic activated cells (Fig. 1E and Fii and iii). The significantly reduced MFI of α4 and β7 in the absence of gamma-irradiated RPMI8866 cells suggests that the allogeneic stimulation is necessary for the generation of α4β7+ MEMT (Fig. 1Fiii).
FIG 1.
Characteristics of α4β7+ MEMT generated from in vitro allogeneic T cell activation. (A) The schematic diagram illustrates the in vitro allogeneic T cell activation method used for the generation of α4β7+ MEMT and α4β7+ TEM. (B) Percentage of α4+ and β7+ α4β7+ MEMT on day 8 (donors = 14). (C) α4β7+ MEMT were incubated with 10 μg/ml MAdCAM-1–Fc for 30 min at 4°C. The percentage of MAdCAM-1–Fc-bound α4β7+ MEMT was quantified by flow cytometry (donors = 3). (D) The expression of β7 and CCR5 on the CD45RO+ CD4 T cells generated by either the conventional PHA–IL-2 method or the in vitro allogeneic T cell activation method mentioned above was measured by flow cytometry. (i) Percentage of β7+ CCR5+ cells in PHA–IL-2-activated CD4 T cells and α4β7+ MEMT (donors = 8). (ii) MFI of CCR5 expression on PHA–IL-2-activated CD4 T cells and α4β7+ MEMT (donors = 8). Purified CD4 T cells treated with IL-2, IL-15, and RA were cultured in the presence or absence of gamma-irradiated RPMI8866 cells for 6 days. The phenotypes of the cells were analyzed by flow cytometry before CD45RO+ cell enrichment. (E) Representative histograms show the expression of CD45RO on CD4 T cells and the expression of α4, β7, and CCR5 on CD45RO+ CD4 T cells (donors = 4). The shaded histogram represents the isotype control. (F) (i) Percentage of CD45RO+ CD4 T cells cultured in the presence or absence of gamma-irradiated RPMI8866 cells (donors = 4). (ii and iii) Percentage (ii) and MFI (iii) of α4, β7, and CCR5 on CD45RO+ CD4 T cells cultured in the presence or absence of gamma-irradiated RPMI8866 cells (donors = 4). Data are expressed as the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
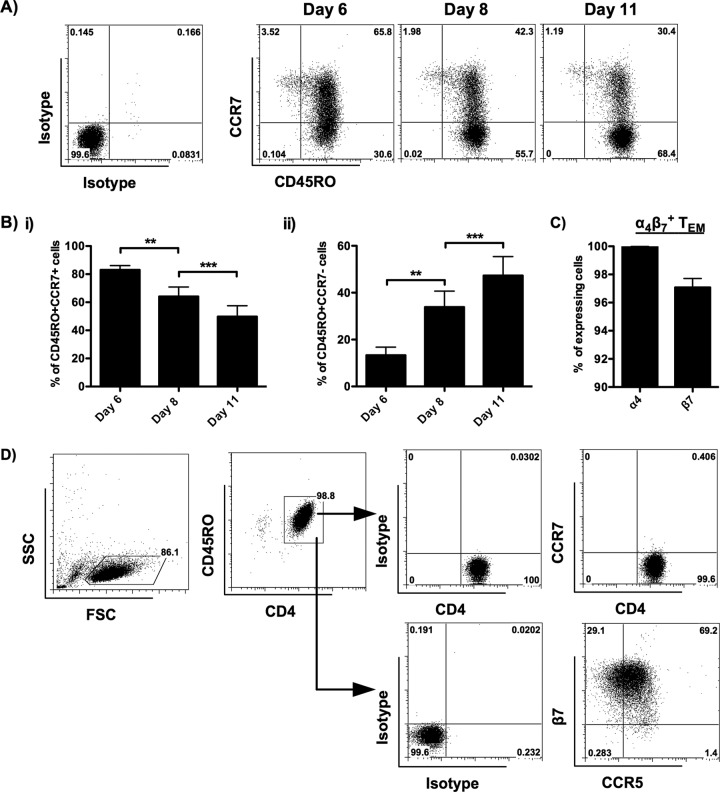
In order to increase the yield of TEM for our study, the kinetic changes of CCR7 expression in CD45RO+ CD4 T cells were examined. A time-dependent increase in the percentage of TEM was observed from day 6 to day 11 (Fig. 2A and Bi and ii). As a result, we determined to isolate the α4β7+ TEM from α4β7+ MEMT on day 11. To prepare the α4β7+ TEM for this study, CCR7− TEM were purified from α4β7+ MEMT by depleting the CCR7+ cells using negative selection. To avoid false-positive results due to CCR7+ TCM contamination, only TEM with a purity of greater than 99% (mean purity, 99.3%) were used. As shown in Fig. 2C, α4 and β7 were expressed on 100.0% (100.0% ± 0.02%) and 97.1% (97.1% ± 0.6%) of α4β7+ TEM, respectively. CCR5 was expressed on about 70% (72.5% ± 2.2%) of β7+ CD45RO+ cells (Fig. 2D). A representative result showing the expression of CD45RO, CCR7, β7, and CCR5 on α4β7+ TEM is illustrated in Fig. 2D. These results suggest that our method induces α4β7- and CCR5-coexpressing CD4 TEM.
FIG 2.
Characteristics of α4β7+ TEM isolated from α4β7+ MEMT. Purified CD4 T cells were cultured in the presence of gamma-irradiated RPMI8866 cells plus IL-2, IL-15, and RA. The phenotypes of the cells were analyzed by flow cytometry on day 6, day 8, and day 11 after CD45RO+ cell enrichment. (A) Representative flow cytometric analysis (donors = 8) shows the expression of CD45RO and CCR7 on allogeneic activated CD4 T cells. (B) Bar charts illustrate the kinetic changes in the percentage of CD45RO+ CCR7+ TCM (i) and CD45RO+ CCR7− TEM (ii) in allogeneic activated CD4 T cells (donors = 8). The phenotypes of α4β7+ TEM isolated from α4β7+ MEMT on day 11 were analyzed by flow cytometry. (C) Percentage of α4+ and β7+ α4β7+ TEM on day 11 (donors = 10). (D) Representative flow cytometric analysis (donors = 10) shows the expression of CD45RO, CCR7, integrin β7, and CCR5 on α4β7+ TEM. The positive cells were defined using the corresponding isotype controls. SSC, side scatter; FSC, forward scatter. Data are expressed as the mean ± SEM. **, P < 0.01; ***, P < 0.001.
α4β7+ TEM differentiate into p24+ TCM after HIV-1 infection.
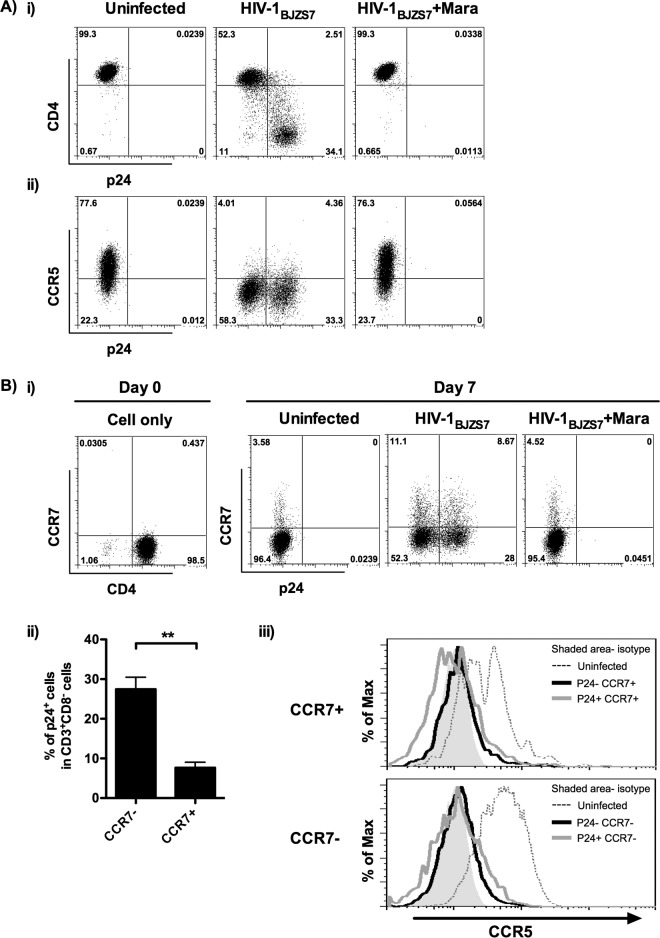
To investigate the phenotypic change of TEM after HIV-1 infection, α4β7+ TEM were challenged with live replicating CCR5-tropic HIV-1BJZS7. Within the CD3+ CD8− T cell population, we found that CD4 and CCR5 expression was reduced after infection (Fig. 3Ai and ii). The loss of these two receptors was mediated by HIV-1 because it was prevented by the entry inhibitor maraviroc. These findings demonstrate that α4β7+ TEM are susceptible to HIV-1 infection.
FIG 3.
Presence of p24+ α4β7+ TCM in HIV-1-infected α4β7+ TEM. α4β7+ TEM were infected with HIV-1BJZS7 in the presence or absence of 1 μM maraviroc (Mara). The phenotypes of the cells were analyzed by flow cytometry. (A) Representative results show the expression of p24 versus CD4 (i) or CCR5 (ii) on CD3+ CD8− T cells 6 days after infection (donors = 5). (B) (i) Representative flow cytometric analysis shows that CCR7 expression on uninfected and infected α4β7+ TEM was upregulated at 6 days after infection (donors = 5). (ii) Percentage of p24+ α4β7+ TEM and p24+ α4β7+ TCM in CD3+ CD8− T cells (donors = 5). (iii) Representative histograms show the expression of CCR5 on uninfected, p24−, and p24+ α4β7+ TCM (CCR7+) or α4β7+ TEM (CCR7−) (donors = 5). The shaded histogram represents the isotype control. Data are expressed as the mean ± SEM. **, P < 0.01.
A previous study demonstrated that HIV-1 downregulates CCR7 on TCM through its Vpu protein (31). However, the effect of HIV-1 infection on CCR7 expression in TEM remains undetermined. To study this, expression of CCR7 on CD3+ CD8− α4β7+ TEM was measured 6 days after HIV-1BJZS7 infection. Surprisingly, CCR7 was upregulated on both uninfected and infected α4β7+ TEM. The expression of CCR7 was HIV-1 independent because blockade of infection by maraviroc did not inhibit CCR7 expression (Fig. 3Bi). In addition to the CCR7− subset of α4β7+ TEM, p24 expression was also found in the CCR7+ subset, although the percentage of p24-positive (p24+) CCR7+ cells was significantly lower than that in TEM (Fig. 3Bi and ii). Phenotypically, like α4β7+ TEM, HIV-1-infected α4β7+ TCM, either p24+ or p24-negative (p24−) subsets, also showed a downregulation of surface CCR5 (Fig. 3Biii). These results not only demonstrate that α4β7+ TEM can differentiate into TCM (α4β7+ TCM) through CCR7 upregulation but also reveal that α4β7+ TCM can harbor replicating HIV-1 after infection.
Differentiation of α4β7+ TCM from α4β7+ TEM is TGF-β dependent.
CCR7 expression on CD4 TEM can be induced upon activation with anti-CD3/CD28 (10, 11). In comparison, there was no immediate anti-CD3/CD28 stimulation in our experimental setting, although the α4β7+ TEM were allogeneic activated. As a result, we speculated that mechanisms other than anti-CD3/CD28 stimulation might be responsible for the differentiation of α4β7+ TCM from α4β7+ TEM.
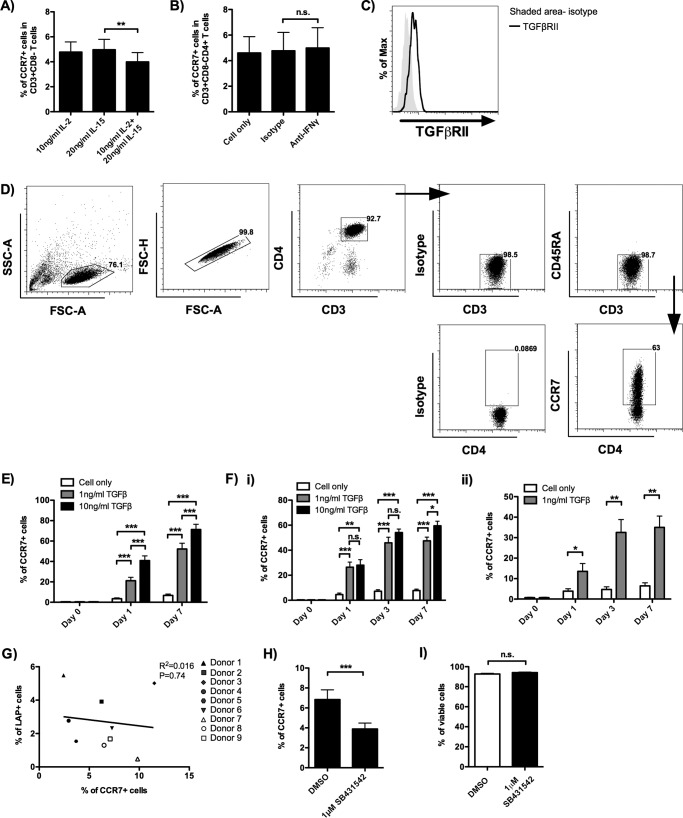
The mechanism underlying the upregulation of CCR7 on α4β7+ TEM in the absence of HIV-1 was examined. As α4β7+ TEM were cultured in the presence of IL-2 and IL-15, the effects of these two cytokines were first evaluated. By culturing the α4β7+ TEM in the presence of IL-2, IL-15, or both cytokines for 7 days, we found that neither IL-2 nor IL-15 was essential for CCR7 upregulation (Fig. 4A). As shown in Fig. 2D, about 70% of α4β7+ TEM coexpressed β7 and CCR5, suggesting that the majority of α4β7+ TEM were of the T helper 1 type, which produces interferon gamma (IFN-γ) (32). Indeed, IFN-γ was detectable in the α4β7+ TEM culture supernatants obtained on day 7 (concentration of IFN-γ, 2163 ± 533.5 pg/ml; donors = 4). Since IFN-γ has been reported to induce CCR7 expression in dendritic cells, we evaluated whether this cytokine would have a similar effect on α4β7+ TEM (33). Using blocking antibody, however, we found that IFN-γ had no significant effect on CCR7 expression in α4β7+ TEM (Fig. 4B).
FIG 4.
TGF-β induces the differentiation of α4β7+ TCM from α4β7+ TEM. (A) α4β7+ TEM were isolated and cultured in the presence of the indicated cytokines for 7 days. The results show the percentage of CD3+ CD8− α4β7+ TCM on day 7 (donors = 5). (B) α4β7+ TEM were isolated and cultured in the presence of anti-IFN-γ (20 μg/ml) blocking antibody. The results show the percentage of CD3+ CD8− α4β7+ TCM on day 7 (donors = 4). The corresponding isotype antibody was used as a control. (C) A representative histogram shows the expression of TGF-βRII on CD3+ CD8− CD4+ α4β7+ TEM on the day of α4β7+ TEM isolation (donors = 8). (D) Gating strategy used to identify the CD3+ CD4+ CD45RA− α4β7+ TCM and CD3+ CD4+ CD45RA− CCR7+ TCM in the assays whose results are shown in panels E and Fi and ii. A representative flow cytometric analysis shows the expression of CCR7 on α4β7+ TEM stimulated with 1 ng/ml TGF-β for 7 days. The positive cells were defined using the corresponding isotype controls. (E) α4β7+ TEM were isolated and cultured in the presence of 1 ng/ml or 10 ng/ml TGF-β. The effect of TGF-β on the induction of CD3+ CD4+ CD45RA− α4β7+ TCM from α4β7+ TEM was determined on day 0 (before TGF-β stimulation), day 1, and day 7 during TGF-β coculture (donors = 8). (F) CCR7− CD4 T cells directly isolated from peripheral blood were treated with TGF-β either in the presence (donors = 6) (i) or in the absence (donors = 6) (ii) of IL-2 (10 ng/ml) and IL-15 (20 ng/ml). The effect of TGF-β on the induction of CD3+ CD4+ CD45RA− CCR7+ TCM was determined on days 0 (before TGF-β stimulation), 1, 3, and 7. (G) Correlation between the percentage of LAP+ CD3+ α4β7+ TEM immediately after α4β7+ TEM isolation and the percentage of CD3+ CD8− CD4+ α4β7+ TCM after an additional 7 days of culture (donors = 9). (H) α4β7+ TEM were isolated and cultured in the presence of the TGF-βRI inhibitor SB431542 for 7 days. The results show the percentage of CD3+ CD8− CD4+ α4β7+ TCM on day 7 (donors = 9). Dimethyl sulfoxide (DMSO) at the same concentration as the relative inhibitor concentration was used as the control. (I) Percentage of viable cells in the presence of 1 μM SB431542 (donors = 7), determined by a trypan blue exclusion assay. DMSO at the same concentration as the relative inhibitor concentration was used as the control. Cell counting was performed by use of a Countess automated cell counter. Data are expressed as the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant.
We then investigated the role of TGF-β because it increases CCR7 expression on antigen-activated memory CD8 T cells and breast cancer cells undergoing epithelial-mesenchymal transition (25, 26). The susceptibility of α4β7+ TEM toward TGF-β was confirmed by the surface expression of TGF-β receptor II (Fig. 4C). Interestingly, when α4β7+ TEM were cultured with recombinant human TGF-β, CCR7 was significantly upregulated as soon as 1 day after TGF-β stimulation. The increase of CCR7 expression was found to be in a dose- and time-dependent manner (Fig. 4D and E).
To validate that the role of TGF-β in inducing CCR7 expression on TEM was not restricted to our in vitro-induced α4β7+ TEM, CCR7− CD4 T cells directly isolated from peripheral blood were stimulated with TGF-β in the presence of IL-2 and IL-15. Consistently, CCR7 was upregulated on CCR7− CD45RA− CD4 TEM by TGF-β (Fig. 4Fi). Interestingly, in the absence of IL-2 and IL-15, CCR7 was also upregulated on TEM by TGF-β, despite a slower expression kinetics and lower expression level (Fig. 4Fii).
In the experiments whose results are shown in Fig. 3Bi, CCR7 was upregulated on α4β7+ TEM even in the absence of exogenous TGF-β. In order to find out the source of TGF-β in our cell culture system, the presence of TGF-β-positive cells, which were defined as latency-associated peptide (LAP)-positive (LAP+) cells, was measured by flow cytometry (34). Although a small frequency of LAP+ cells was found in α4β7+ TEM freshly isolated from α4β7+ MEMT, no correlation between their presence and the percentage of α4β7+ TCM generated after an additional 7 days of culture was observed (Fig. 4G). To investigate whether CCR7 expression would be affected by signaling through the TGF-β receptor (TGF-βR) in the absence of exogenous TGF-β, the specific inhibitor of TGF-βRI kinase, SB431542, was tested. About 40% (43.4% ± 3.1%) fewer α4β7+ TCM were found in the presence of the inhibitor on day 7 (Fig. 4H). The decreased percentage of α4β7+ TCM was not due to the cytotoxicity of the inhibitor because the inhibitor did not induce a significant increase of cell death, as determined by a trypan blue exclusion assay (Fig. 4I). These results suggest that the source of TGF-β for inducing CCR7 expression in α4β7+ TEM is probably the fetal bovine serum (FBS) in the culture medium (35, 36). In fact, active TGF-β was detectable in the acid-treated full medium using a human TGF-β enzyme-linked immunosorbent assay (ELISA) (concentration of TGF-β in full medium, about 559.3 pg/ml).
Collectively, our results suggest that TGF-β can induce the differentiation of TCM from TEM through interacting with ALK5/TGF-β receptor I. Also, the kinetics of differentiation is modulated by T cell activation stimuli, such as IL-2 and IL-15.
α4β7+ TCM migrate toward CCL19 and downregulate CCR7 upon T cell activation.
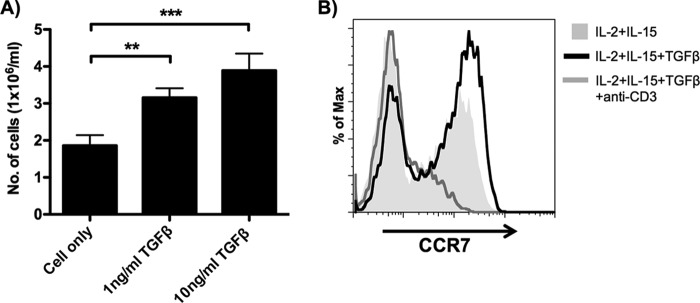
TCM have been demonstrated to have a higher lymph node-homing potential and can differentiate into TEM upon T cell activation (6). To evaluate whether α4β7+ TCM exhibit these two TCM characteristics, α4β7+ TEM were first activated by TGF-β for 7 days and the lymph node-homing potential and differentiation capacity were evaluated by CCL19 chemotaxis as well as TCR activation, respectively. Using an in vitro migration assay, we found that the migratory potential toward the lymph node-homing chemokine CCL19 was significantly higher in TGF-β-stimulated α4β7+ TEM than in the unstimulated control (Fig. 5A). This result suggests that α4β7+ TCM, like the regular TCM, can migrate to lymph nodes through the CCL19/CCR7 axis. To investigate the TEM differentiation capacity of α4β7+ TCM, TGF-β-stimulated α4β7+ TEM were stimulated with anti-CD3 antibody. Interestingly, CCR7 expression on anti-CD3-stimulated TGF-β-stimulated α4β7+ TEM was reduced (Fig. 5B). The downregulation of CCR7 suggests that α4β7+ TCM can differentiate into TEM upon TCR activation. Overall, these results validated the novel role of TGF-β in regulating the differentiation of CD4 TEM to TCM.
FIG 5.
Central memory T cell properties of α4β7+ TCM. (A) α4β7+ TEM either untreated or stimulated with TGF-β for 7 days were tested for CCL19 chemotaxis in vitro, and the number of cells (normalized to 1 × 106/ml) that migrated to the CCL19-containing medium was determined (donors = 6). Data are expressed as the mean ± SEM. **, P < 0.01; ***, P < 0.001. (B) α4β7+ TEM cultured in IL-2, IL-15, and TGF-β for 7 days were stimulated under the indicated conditions. A representative histogram shows the expression of CCR7 on CD3+ CD4+ CD45RA− α4β7+ TEM 3 days after stimulation (donors = 6).
α4β7+ TCM differentiated from TGF-β-stimulated α4β7+ TEM bind to MAdCAM-1.
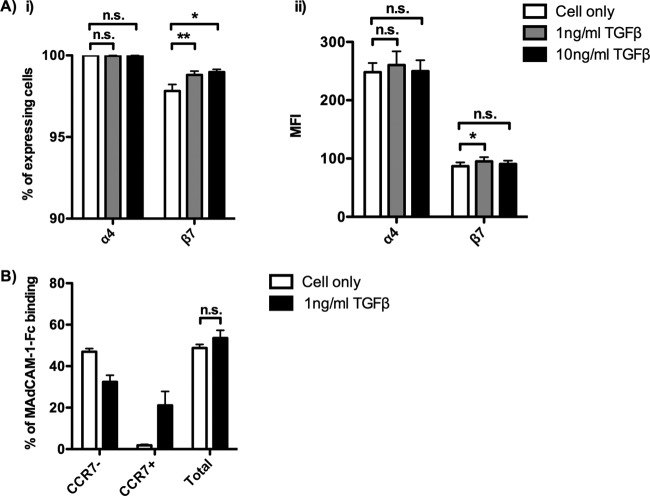
Since the expression of integrin α4β7 promotes the migration of CD4 T cells to the gut, we assessed the effect of TGF-β on α4 and β7 expression on α4β7+ TEM. Neither α4 nor β7 was downregulated by TGF-β after culturing the α4β7+ TEM in the presence of 1 ng/ml or 10 ng/ml TGF-β for 7 days. A small but significant increase in the percentage and MFI of β7 expression was observed in the presence of TGF-β (Fig. 6Ai and ii). Because TGF-β did not downregulate the expression of α4 and β7, we speculated that TGF-β would not affect the binding of MAdCAM-1. As expected, no significant difference in the binding affinity toward MAdCAM-1 was found between the TGF-β-stimulated and unstimulated α4β7+ TEM. Importantly, the α4β7+ TCM induced by TGF-β also interacted with MAdCAM-1, similar to the findings for α4β7+ TEM (Fig. 6B). These results suggest that TGF-β does not affect the function of α4β7 of either α4β7+ TEM or α4β7+ TCM.
FIG 6.
Effect of TGF-β on α4 and β7 expression on α4β7+ TEM and binding toward MAdCAM-1. α4β7+ TEM were isolated and cultured in the presence of 1 ng/ml or 10 ng/ml TGF-β for 7 days. (A) The effect of TGF-β on the percentage (i) and the MFI (ii) of α4 and β7 expression on CD4+ CD45RO+ T cells was determined on day 7 (donors = 5). (B) The effect of TGF-β on the total percentage of MAdCAM-1–Fc binding cells and the binding of MAdCAM-1–Fc on α4β7+ TEM and α4β7+ TCM was determined on day 7 (donors = 3). Data are expressed as the mean ± SEM. *, P < 0.05; **, P < 0.01.
Expression profile of CD62L on α4β7+ TEM and TEM directly isolated from peripheral blood in the presence or absence of TGF-β.
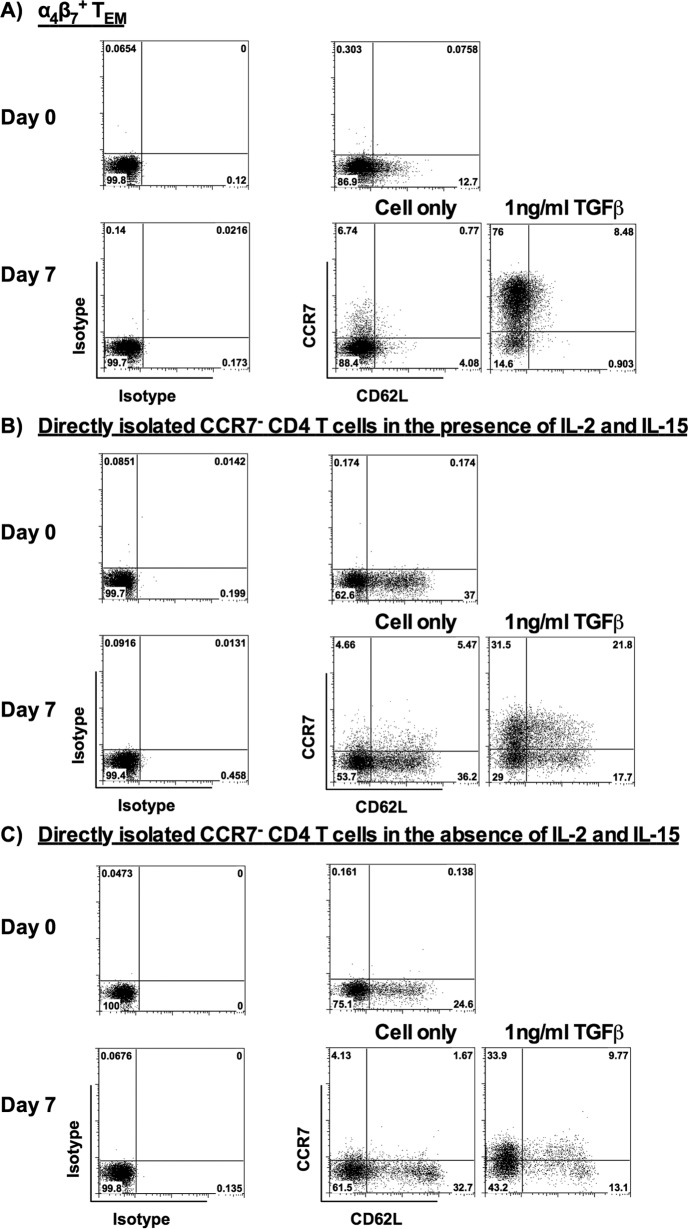
Previous literatures showed that TCM coexpress CCR7 and CD62L (37, 38); therefore, we assessed CD62L expression on α4β7+ TEM generated from our in vitro activation system and CD4 TEM directly isolated from peripheral blood with or without TGF-β stimulation. We found that freshly isolated α4β7+ TEM generated from in vitro activation did not express CD62L or expressed only a low level of CD62L, which is the reported phenotype of TEM (38). Besides, TGF-β did not induce CD62L expression, although CCR7 was upregulated on α4β7+ TEM 7 days after TGF-β stimulation (Fig. 7A). This result demonstrates that α4β7+ TCM do not express CD62L or express only a low level of CD62L. In contrast to α4β7+ TEM, CD62L was expressed on CCR7− CD45RA− CD4 TEM directly isolated from peripheral blood. Interestingly, CCR7 is upregulated on both CD62L− and CD62L+ cells 7 days after TGF-β stimulation either in the presence or in the absence of IL-2 and IL-15 (Fig. 7B and C). Among the tested donors, we found that the percentage of CD3+ CD4+ CD45RA− CCR7− TEM with PD-1 was 13.4% ± 1.9%. This result suggests that only a small percentage of TEM were exhausted cells in our study (39).
FIG 7.
Changes of CD62L expression on α4β7+ TEM and TEM directly isolated from peripheral blood in the presence or absence of TGF-β. (A) Representative flow cytometric analysis shows the expression of CCR7 and CD62L on the isolated α4β7+ TEM (day 0) and the α4β7+ TEM stimulated with 1 ng/ml TGF-β for 7 days (day 7) (donors = 8). (B and C) Representative flow cytometric analysis shows the expression of CCR7 and CD62L on CD3+ CD4+ CD45RA− CCR7− TEM directly isolated from peripheral blood on day 0 and on day 7 after stimulation with 1 ng/ml TGF-β in the presence (donors = 6) (B) or absence (donors = 6) (C) of IL-2 (10 ng/ml) and IL-15 (20 ng/ml). The positive cells were defined using the corresponding isotype controls.
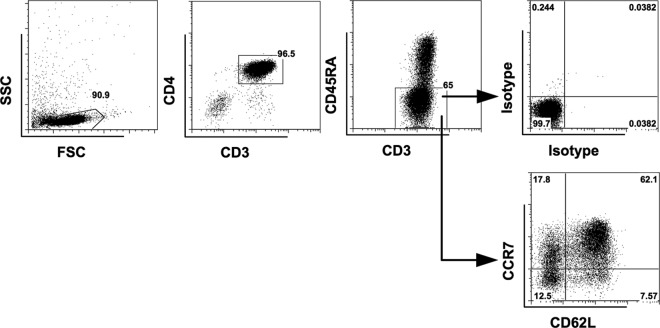
Since α4β7+ TCM do not express CD62L, we sought to understand whether CD62L is expressed on all CCR7+ CD45RA− TCM in peripheral blood-derived CD4 T cells. Although CD45RA− CCR7+ CD4 TCM were mainly CD62L+, there was a minor population of CD62L− TCM. CD62L was also expressed on CD45RA− CCR7− TEM (Fig. 8). The distribution of CD62L+ cells in our findings was similar to that in a previous study (6). These results suggest that TCM include CD62L− cells and TEM include CD62L+ cells if memory CD4 T cells are defined by CD45RA and CCR7, as mentioned in previous studies (6, 40–43). Overall, although they lack CD62L expression, α4β7+ TCM generated from our in vitro activation system still have a TCM phenotype based on CD45RA and CCR7 expression (Fig. 4D). In addition, these cells exert the TCM characteristics, as mentioned above. Importantly, our results reveal that the CD62L+ CCR7+ TCM can be derived from CD62L+ CCR7− CD45RA− CD4 TEM in the presence of TGF-β.
FIG 8.
CD62L expression in different memory T cell subsets of purified CD4 T cells. Representative flow cytometric analysis shows the expression of CD62L on CD3+ CD4+ CD45RA− CCR7+ TCM and CD3+ CD4+ CD45RA− CCR7− TEM in CD4 T cells purified from peripheral blood (donors = 5). The positive cells were defined using the corresponding isotype controls.
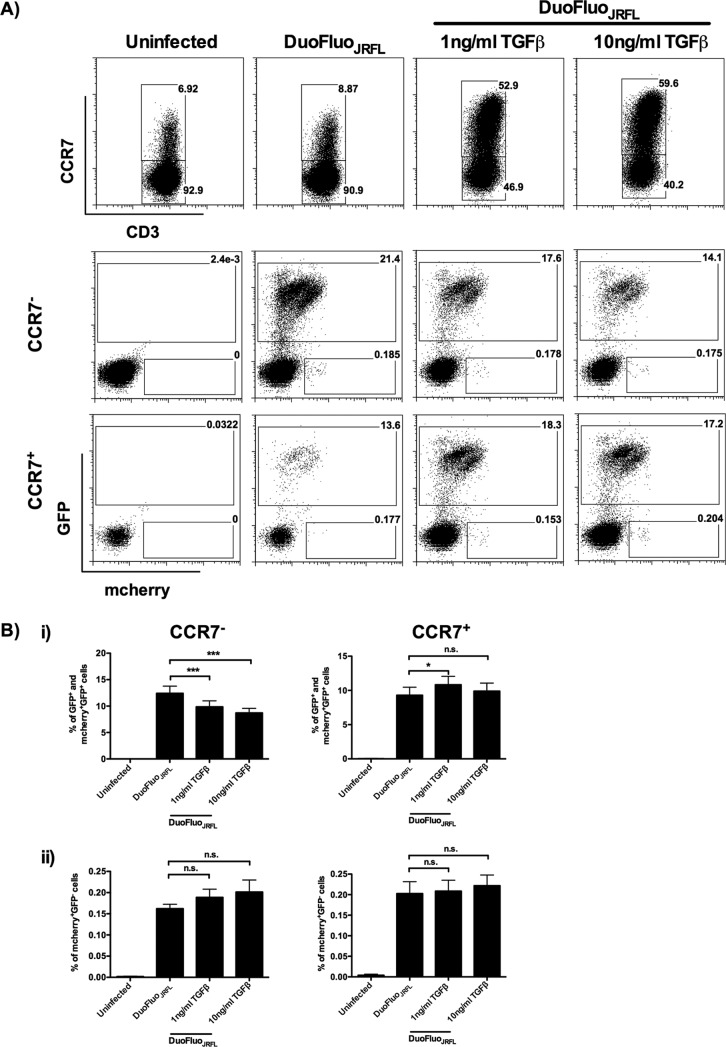
TGF-β induces both productively and latently infected α4β7+ TCM from α4β7+ TEM during HIV-1 infection.
A recent study demonstrated that HIV-1 latency is established directly and early in both resting and activated primary CD4 T cells (17). To investigate whether HIV-1 latency can establish in the TEM-derived TCM, α4β7+ TEM were infected with DuoFluoJRFL in the presence or absence of TGF-β. DuoFluoJRFL is a dual-reporter pseudovirus which can be used to identify productively and latently infected cells early after infection (17). As shown in Fig. 9A, TGF-β facilitated the α4β7+ TEM-to-α4β7+ TCM differentiation by inducing CCR7 expression. In line with our previous results obtained using live replicating HIV-1BJZS7, productively infected cells (green fluorescent protein [GFP] positive [GFP+] and mCherry positive [mCherry+] GFP+) were identified in both α4β7+ TEM and α4β7+ TCM (Fig. 9Bi). The reduction of productively infected cells in TGF-β-stimulated α4β7+ TEM was mainly because of the differentiation of α4β7+ TCM. Interestingly, a small percentage of latently infected cells (mCherry+ GFP negative[GFP−]) was also observed in these two subsets (Fig. 9A and Bii). Critically, although exogenous TGF-β did not affect the percentage of latently infected α4β7+ TEM and α4β7+ TCM (Fig. 9Bii), the percentage of latently infected TCM was expected to be increased in the presence of TGF-β, as there were more CCR7+ cells in the treated group (Fig. 9A). These results suggest that latently infected cells can establish in TEM- and TEM-derived TCM immediately after infection. Importantly, TGF-β increases the amount of latently infected TCM after HIV-1 infection.
FIG 9.
α4β7+ TEM and α4β7+ TCM harbor productively and latently infected HIV-1. α4β7+ TEM were infected with DuoFluoJRFL, and the results were analyzed by flow cytometry at 6 days after infection. (A) A representative flow cytometric analysis shows that CCR7 is upregulated in the presence of TGF-β after infection. The presence of productively infected (GFP+ and mCherry+ GFP+) and latently infected (mCherry+ GFP−) α4β7+ TEM (CCR7−) and α4β7+ TCM (CCR7+) was also demonstrated (donors = 9). (B) Percentage of productively infected (GFP+ and mCherry+ GFP+) (i) and latently infected (mCherry+ GFP−) (ii) α4β7+ TEM (CCR7−) and α4β7+ TCM (CCR7+) (donors = 9). Data are expressed as the mean ± SEM. *, P < 0.05; ***, P < 0.001.
DISCUSSION
HIV-1 preferentially infects α4β7+ CCR5+ gut-homing memory CD4 T cells and selectively targets the TEM (2, 44). Recent studies not only demonstrated the presence of α4β7+ CD4 T cells but also revealed that more than 90% of the CD4 T cells at the portal of HIV-1 entry, such as cervicovaginal tissues, are TEM (1, 3). Although using tissue-derived T cells would be ideal for understanding the earliest events following HIV-1 transmission in vitro, human tissue explants are difficult to obtain. The method for in vitro differentiation of gut-homing T cells from human peripheral blood cells has been well documented (2, 28). Here, we adapted an allogeneic T cell activation method to induce the CCR5-tropic HIV-1-susceptible α4β7+ TEM for studying the roles of TEM after HIV-1 infection.
In contrast to the TCM-to-TEM transition, the mechanisms involved in the differentiation of TEM to TCM remain undetermined. Surprisingly, our results demonstrated that CCR7 upregulation was observed in both α4β7+ TEM and TEM directly isolated from peripheral blood after short-term culture. This upregulation was independent of direct anti-CD3/CD28 stimulation or IL-2 and IL-15, suggesting that some factors in the FBS might play a role in CCR7 expression. TGF-β has been found to increase CCR7 expression in antigen-activated memory CD8 T cells and breast cancer cells undergoing epithelial-mesenchymal transition (25, 26). Although we did not find a correlation between LAP-expressing α4β7+ TEM and CCR7 expression, LAP is abundantly present in FBS (35, 36). LAP is not biologically active under normal pH, but the acidic environment created during cell culture might lead to the release of active TGF-β, which subsequently induces CCR7 expression on TEM. In this study, we demonstrate that CCR7 expression on TEM was significantly inhibited by the TGF-βRI inhibitor (SB431542) in the absence of exogenous TGF-β. These results therefore suggest that TGF-β in FBS, despite being from a different species, probably cross-activates TEM through TGF-βRI and regulates CCR7 expression in TEM. Also, we demonstrate that CCR7 was significantly upregulated on α4β7+ TEM and TEM directly isolated from peripheral blood by recombinant human TGF-β. The role of TGF-β in inducing TEM-to-TCM differentiation was further validated by the increased CCL19 chemotaxis and CCR7 downregulation upon TCR stimulation in the TGF-β-stimulated α4β7+ TEM.
Due to the abundance and the high level of CCR5 expression, TEM at the portal of HIV-1 entry are very likely to be the first cell type infected after mucosal HIV-1 transmission. Previous studies showed that TEM that reside in human gut-associated lymphoid tissues are rapidly eliminated after HIV-1 infection (4, 5). However, apart from being depleted, other possible roles of TEM in HIV/AIDS pathogenesis remain largely unknown. In this study, we found that α4β7+ TCM differentiated from TEM in the presence of TGF-β consisted of both latently and productively infected T cells after DuoFluoJRFL infection. It is of interest to determine whether the infected α4β7+ TCM are raised from infected α4β7+ TEM or from direct infection of α4β7+ TCM. A recent study in rhesus macaques revealed that the genes downstream of the TGF-β signaling pathway are upregulated in tissues containing SIV RNA as early as 1 day after infection (27). Therefore, TGF-β might also be produced immediately at the infection site upon HIV-1 infection, and it might facilitate the establishment of latently infected TCM from TEM early after HIV-1 transmission. This hypothesis is strengthened by our findings, which show that CCR7 expression on TEM was induced as soon as 24 h after TGF-β stimulation. Importantly, the effect of TGF-β on CCR7 expression was enhanced in the presence of proinflammatory cytokines, such as IL-2 and IL-15, as demonstrated using TEM directly isolated from peripheral blood. During acute HIV-1 infection, there is a rapid increase of proinflammatory cytokines in plasma as well as gut-associated and peripheral lymphoid tissues (45, 46). Therefore, it is highly possible that the inflammatory environments after HIV-1 infection amplify the effect of TGF-β on promoting TEM-to-TCM differentiation. Collectively, our results not only reveal that the TGF-β-dependent TEM-to-TCM differentiation is a previously unrecognized mechanism for the formation of the latently infected TCM after HIV-1 infection but also suggest that the rate of latency establishment would be dependent on the degree of inflammation.
In addition, we demonstrated that the TGF-β-induced α4β7+ TCM could bind to MAdCAM-1. MAdCAM-1 is an adhesion molecule selectively expressed on the endothelial cells of intestinal mucosa and gut-associated lymphoid tissues, interacting with integrin α4β7 on T cells for mediating their gut homing (47–49). Therefore, TGF-β signaling might not affect the homing of the latently infected or productively infected α4β7+ gut-homing TEM-derived TCM to the gut-associated lymphoid tissues through the interaction with MAdCAM-1 after HIV-1 infection in vivo (47, 48).
Recently, an HIV-1-susceptible CCR5+ CCR7+ CD4 TCM-like cell subset was identified in lavage fluid from the female genital tract (50). As α4β7+ TCM differentiated from α4β7+ TEM also expressed CCR7 and CCR5 like the cell subset described previously (50), our study therefore provides a possible explanation for the source of this cell subset.
In conclusion, our study reveals a novel role of the immunosuppressive cytokine TGF-β on TEM-to-TCM differentiation and suggests that productively infected and latently infected TCM can be derived from TEM during acute HIV-1 infection. Taken together, our study emphasizes the need to reconsider the importance of TGF-β and TEM in HIV/AIDS pathogenesis.
MATERIALS AND METHODS
Antibodies and reagents.
The following fluorochrome-conjugated anti-human antibodies and their relevant isotype controls were used for flow cytometry and were purchased from BioLegend: CD3-Pacific Blue, CD4-Pacific Blue, CD4-peridinin chlorophyll protein (PerCP)-Cy5.5, CD4-allophycocyanin (APC), CD8-APC, CD8-PerCP-Cy5.5, CCR7-phycoerythrin (PE), CCR7-APC, LAP-PE, CD62L-APC, PD-1–fluorescein isothiocyanate (FITC), mouse IgG1-FITC, mouse IgG1-PE, mouse IgG1-APC, mouse IgG2a-PE, and mouse IgG2a-APC. CD3-FITC, CD45RA-PECy7, CD45RO-FITC, CCR5-PECy7, integrin β7-APC, rat IgG2a-APC, mouse IgG1-PECy7, and mouse IgG2a-PE-Cy7 were purchased from BD Pharmingen. CD49d (integrin α4)-PE was purchased from BD Bioscience. mouse IgG2b-PE, human TGF-βRII-APC, and goat IgG control-APC were purchased from R&D Systems. HIV-1 p24 antigen-FITC was purchased from Beckman Coulter. Alexa Fluor 488 (AF488)-conjugated goat anti-human IgG secondary antibody was purchased from Life Technologies. LEAF-purified anti-human IFN-γ antibody was purchased from BioLegend, and a purified no azide/low endotoxin (NA/LE) mouse IgG1(κ) isotype control, purchased from BD Pharmingen, was used for blocking experiments. The LEAF-purified anti-human CD3 antibody used for T cell activation was purchased from BioLegend. Phytohemagglutinin (PHA), SB431542, and maraviroc were purchased from Sigma. Recombinant human TGF-β1 was purchased from Peprotech.
Preparation and culture of α4β7+ MEMT, α4β7+ TEM, and TEM.
Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coat of blood samples from healthy donors obtained from the Red Cross, Hong Kong SAR, China, by Ficoll-Paque Plus (Amersham Biosciences). The use of buffy coats for this study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. To prepare α4β7+ gut-homing memory CD4 T cells (α4β7+ MEMT), 10 × 106 purified CD4 T cells (purity, >90%; CD4 T cell enrichment cocktails; RosetteSep) were activated by coculturing with 3 × 106 96-Gy gamma-irradiated RPMI8866 cells in full medium (RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin) plus 10 ng/ml IL-2 (Peprotech) and 10 nM retinoic acid (RA; Sigma) for 6 days. IL-15 (10 ng/ml; Peprotech) was added 1 day after the coculture. On day 6, the CD45RO+ cells in the coculture were isolated using CD45RO microbeads (Miltenyi Biotec). α4β7+ MEMT were generated by culturing the purified cells in the presence of 10 nM RA, 10 ng/ml IL-2, and 10 ng/ml IL-15 for an additional 2 days.
α4β7+ TEM were prepared by culturing the day 6 purified CD45RO+ cells in the presence of 10 nM RA, 10 ng/ml IL-2, and 10 ng/ml IL-15 for 5 more days, followed by negative selection using CCR7 microbeads (Miltenyi Biotec). For TEM directly isolated from peripheral blood, CD4 T cells were first enriched from PBMCs as mentioned above. The CCR7− CD4 T cells were then prepared by negative selection using CCR7 microbeads. Purified TEM were cultured in full medium supplemented with 10 ng/ml IL-2 and 20 ng/ml IL-15 for further experiments, unless otherwise indicated.
Preparation of gut-homing CD4 T cells with PHA.
Purified CD4 T cells were stimulated with 5 μg/ml phytohemagglutinin (PHA; Sigma) in the presence of 10 ng/ml IL-2 and 10 nM RA for 6 days in a 24-well plate. Half of the medium was removed, and the culture was replenished with medium containing 10 ng/ml IL-2 and 10 nM RA on day 3. The phenotypes of the cells were analyzed by flow cytometry on day 6.
Studying the role of allogeneic stimulation in the generation of α4β7+ MEMT.
Purified CD4 T cells (10 × 106) were activated by coculturing in the presence or absence of 3 × 106 96-Gy gamma-irradiated RPMI8866 cells in full medium plus 10 ng/ml IL-2 (Peprotech) and 10 nM retinoic acid (RA; Sigma) for 6 days. IL-15 (10 ng/ml; Peprotech) was added 1 day after the coculture. The phenotypes of the cells were analyzed by flow cytometry on day 6 after coculture and before the enrichment of CD45RO+ cells.
Differentiation of α4β7+ TCM to α4β7+ TEM by anti-CD3 stimulation.
Isolated α4β7+ TEM cultured in 10 ng/ml IL-2 and 20 ng/ml IL-15 were stimulated with 1 ng/ml TGF-β for 7 days for the generation of α4β7+ TCM. On day 7, cells were washed 3 times for removing the cytokines. The TGF-β-stimulated α4β7+ TEM were then cultured in 10 ng/ml IL-2 and 20 ng/ml IL-15 alone or together with 1 ng/ml TGF-β or anti-CD3 antibody, as indicated in Fig. 5B. The differentiation of α4β7+ TCM to α4β7+ TEM, as defined by CCR7 downregulation, was measured 3 days after stimulation.
Preparation of DuoFluoJRFL and live replicating HIV-1BJZS7.
DuoFluoJRFL was generated by cotransfecting 293T cells with R7GEmc and a plasmid encoding the monotropic HIV-1 envelope (HIV-1JRFL) using polyethylenimine. Virus-containing cell-free supernatants were collected at 48 h posttransfection.
The live replicating transmitted founder virus HIV-1BJZS7, which was previously isolated from a 20-year-old man in an acute cohort of men who have sex with men with acute HIV-1 infection in Beijing, China (51), was propagated in the AIDS Institute of The University of Hong Kong using α4β7+ MEMT. The use of α4β7+ MEMT was approved by the Institutional Review Board mentioned above. The virus stock was prepared in the MOLT4-CCR5 T cell line in the presence of 1 ng/ml IL-2 for 14 days.
Virus-containing supernatants were concentrated with PEG-it virus precipitation solution (System Bioscience) by following the manufacturer's protocol. The concentration of the virus was determined by an HIV-1/p24 antigen (p24) ELISA (Zeprometrics).
HIV-1 infection.
α4β7+ TEM (0.2 × 106) were infected with 250 ng p24 of DuoFluoJRFL or 40 ng p24 of HIV-1ZS-7 by spinoculation at 1,200 × g for 90 min at 4°C 1 day after isolation. To prevent infection, cells were pretreated with 1 μM maraviroc for 2 h. Following spinoculation, cells were immediately transferred and cultured at 37°C. Virus was removed by washing with full medium 3 times at 2 days after infection. After virus removal, cells were replenished with IL-2- and IL-15-containing full medium. Maraviroc (1 μM) was added back to the cells treated with maraviroc previously to prevent infection of residual virus. The amounts of IL-2 and IL-15 used for α4β7+ TEM were the same as those mentioned above.
Flow cytometry.
Cells were stained with the fluorochrome-conjugated anti-human antibodies and their relevant isotype controls as mentioned above at room temperature for 30 min. After staining, cells were washed and the data were acquired by use of a FACSAria II instrument. Intracellular labeling for p24 was performed on previously labeled cells with Fix/Perm solution (BD Bioscience) according to the manufacturer's introduction. The data acquired were analyzed with FlowJo software (TreeStar).
MAdCAM-1 binding assay.
α4β7+ MEMT and TGF-β-stimulated α4β7+ TEM were washed with phosphate-buffered saline (PBS), followed by incubation with 37°C prewarmed RPMI 1640 medium containing 0.45 M sucrose at room temperature for 15 min. Cells were then stained with 10 μg/ml a recombinant human MAdCAM-1–Fc chimera protein (R&D Systems) for 30 min at 4°C. For α4β7+ MEMT, cells were washed with ice-cold PBS and resuspended in RPMI 1640 medium containing 0.45 M sucrose for staining with AF488-conjugated goat anti-human IgG secondary antibody at 4°C for 30 min. For TGF-β-stimulated α4β7+ TEM, cells were washed as mentioned above and stained with CD3-Pacific Blue, CD4-APC, and CCR7-PE anti-human immunoglobulin antibodies together with AF488-conjugated goat anti-human IgG secondary antibody. Stained cells were washed with ice-cold PBS, and the binding was visualized by flow cytometry.
In vitro migration assay.
Day 7 TGF-β-stimulated α4β7+ TEM were washed twice with PBS, followed by washing once with migration buffer (RPMI 1640 supplemented with 0.5% bovine serum albumin). Cells were then resuspended in migration buffer before migration. Cell viability was determined by a trypan blue exclusion assay. For transwell migration, 0.4 × 106 viable cells were added to the upper chamber and 600 μl migration buffer containing 100 ng/ml CCL19 was added to the lower chamber of the transwell. Transwell inserts with a pore size of 5 μm were from Corning. Migration was determined by quantifying the total number of cells in the lower chamber after 4 h. All cell counting was performed by use of a Countess automated cell counter (Invitrogen).
Measurement of cytokine concentrations.
Active TGF-β in acid-treated full medium was measured by a human TGF-β1 DuoSet ELISA (R&D Systems) according to the manufacturer's protocol. IFN-γ was measured by use of a LEGENDplex human Th17 panel (BioLegend) according to the manufacturer's instructions, and the data were analyzed by use of the FACSAria II instrument.
Statistical analysis.
Data are expressed as the mean ± standard error of the mean (SEM). Statistical significance was determined by a two-tailed paired Student's t test using GraphPad Prism software (GraphPad software). P values smaller than 0.05 were considered statistically significant. For significant results, the strength of significance is designated in terms of P values of <0.05, <0.01, and <0.001.
ACKNOWLEDGMENTS
We thank the Hong Kong Health and Medical Research Fund (12111262), the Hong Kong Research Grant Council (HKU5/CRF/13G), the Hong Kong Council for the AIDS Trust Fund (MSS 183R and 227R), the Hong Kong University Small Project Fund (201109176134 and 201309176059), the University Development Fund of The University of Hong Kong, and the Li Ka Shing Faculty of Medicine Matching Fund to the AIDS Institute for financial support. We also thank the San-Ming Project of Medicine in Shenzhen, China, and the Mega-Projects of National Science Research for the 13th Five-Year Plan (2017ZX10201101-001-007) in China for financial support.
The reagent DuoFluo (R7GEmC) was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH (catalog number 12595; DuoFluo [R7GEmC]), from Vincenzo Calvanez and Eric Verdin.
We have no conflict of interest.
REFERENCES
- 1.Saba E, Grivel JC, Vanpouille C, Brichacek B, Fitzgerald W, Margolis L, Lisco A. 2010. HIV-1 sexual transmission: early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol 3:280–290. doi: 10.1038/mi.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O'Shea A, Patel N, Van Ryk D, Wei D, Pascuccio M, Yi L, McKinnon L, Izulla P, Kimani J, Kaul R, Fauci AS, Arthos J. 2009. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci U S A 106:20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joag VR, McKinnon LR, Liu J, Kidane ST, Yudin MH, Nyanga B, Kimwaki S, Besel KE, Obila JO, Huibner S, Oyugi JO, Arthos J, Anzala O, Kimani J, Ostrowski MA, Toronto HIV Research Group, Kaul R. 2016. Identification of preferential CD4+ T-cell targets for HIV infection in the cervix. Mucosal Immunol 9:1–12. doi: 10.1038/mi.2015.28. [DOI] [PubMed] [Google Scholar]
- 4.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 7.Bromley SK, Thomas SY, Luster AD. 2005. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol 6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 8.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. 2005. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol 6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M Jr, Lifson JD, Maino VC, Axthelm MK, Villinger F. 2006. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest 116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, Qin S, Forster R, Lipp M, Lanzavecchia A. 1999. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol 29:2037–2045. doi:. [DOI] [PubMed] [Google Scholar]
- 11.Schwendemann J, Choi C, Schirrmacher V, Beckhove P. 2005. Dynamic differentiation of activated human peripheral blood CD8+ and CD4+ effector memory T cells. J Immunol 175:1433–1439. doi: 10.4049/jimmunol.175.3.1433. [DOI] [PubMed] [Google Scholar]
- 12.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. 2009. The challenge of finding a cure for HIV infection. Science 323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 13.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. 1995. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 14.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 15.Davey RT Jr, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, Kovacs JA, Polis MA, Walker RE, Falloon J, Masur H, Gee D, Baseler M, Dimitrov DS, Fauci AS, Lane HC. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavez L, Calvanese V, Verdin E. 2015. HIV latency is established directly and early in both resting and activated primary CD4 T cells. PLoS Pathog 11:e1004955. doi: 10.1371/journal.ppat.1004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DI, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH. 2014. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. 2006. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. 2008. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. 2008. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3− effector T cells. Nat Immunol 9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerwenka A, Kovar H, Majdic O, Holter W. 1996. Fas- and activation-induced apoptosis are reduced in human T cells preactivated in the presence of TGF-beta 1. J Immunol 156:459–464. [PubMed] [Google Scholar]
- 24.Kim SV, Xiang WV, Kwak C, Yang Y, Lin XW, Ota M, Sarpel U, Rifkin DB, Xu R, Littman DR. 2013. GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science 340:1456–1459. doi: 10.1126/science.1237013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmadzadeh M, Rosenberg SA. 2005. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol 174:5215–5223. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pang MF, Georgoudaki AM, Lambut L, Johansson J, Tabor V, Hagikura K, Jin Y, Jansson M, Alexander JS, Nelson CM, Jakobsson L, Betsholtz C, Sund M, Karlsson MC, Fuxe J. 2016. TGF-beta1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 35:748–760. doi: 10.1038/onc.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barouch DH, Ghneim K, Bosche WJ, Li Y, Berkemeier B, Hull M, Bhattacharyya S, Cameron M, Liu J, Smith K, Borducchi E, Cabral C, Peter L, Brinkman A, Shetty M, Li H, Gittens C, Baker C, Wagner W, Lewis MG, Colantonio A, Kang HJ, Li W, Lifson JD, Piatak M Jr, Sekaly RP. 2016. Rapid inflammasome activation following mucosal SIV infection of rhesus monkeys. Cell 165:656–667. doi: 10.1016/j.cell.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elahi S, Niki T, Hirashima M, Horton H. 2012. Galectin-9 binding to Tim-3 renders activated human CD4+ T cells less susceptible to HIV-1 infection. Blood 119:4192–4204. doi: 10.1182/blood-2011-11-389585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duarte RF, Chen FE, Lowdell MW, Potter MN, Lamana ML, Prentice HG, Madrigal JA. 2002. Functional impairment of human T-lymphocytes following PHA-induced expansion and retroviral transduction: implications for gene therapy. Gene Ther 9:1359–1368. doi: 10.1038/sj.gt.3301807. [DOI] [PubMed] [Google Scholar]
- 30.Valiante NM, Rengaraju M, Trinchieri G. 1992. Role of the production of natural killer cell stimulatory factor (NKSF/IL-12) in the ability of B cell lines to stimulate T and NK cell proliferation. Cell Immunol 145:187–198. doi: 10.1016/0008-8749(92)90322-G. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez PW, Famiglietti M, Sowrirajan B, DePaula-Silva AB, Rodesch C, Barker E, Bosque A, Planelles V. 2014. Downmodulation of CCR7 by HIV-1 Vpu results in impaired migration and chemotactic signaling within CD4(+) T cells. Cell Rep 7:2019–2030. doi: 10.1016/j.celrep.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. 1998. CCR5 is characteristic of Th1 lymphocytes. Nature 391:344–345. [DOI] [PubMed] [Google Scholar]
- 33.Frasca L, Nasso M, Spensieri F, Fedele G, Palazzo R, Malavasi F, Ausiello CM. 2008. IFN-gamma arms human dendritic cells to perform multiple effector functions. J Immunol 180:1471–1481. doi: 10.4049/jimmunol.180.3.1471. [DOI] [PubMed] [Google Scholar]
- 34.Gandhi R, Farez MF, Wang Y, Kozoriz D, Quintana FJ, Weiner HL. 2010. Cutting edge: human latency-associated peptide+ T cells: a novel regulatory T cell subset. J Immunol 184:4620–4624. doi: 10.4049/jimmunol.0903329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danielpour D, Kim KY, Dart LL, Watanabe S, Roberts AB, Sporn MB. 1989. Sandwich enzyme-linked immunosorbent assays (SELISAs) quantitate and distinguish two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) in complex biological fluids. Growth Factors 2:61–71. doi: 10.3109/08977198909069082. [DOI] [PubMed] [Google Scholar]
- 36.Oida T, Weiner HL. 2010. Depletion of TGF-beta from fetal bovine serum. J Immunol Methods 362:195–198. doi: 10.1016/j.jim.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallusto F, Geginat J, Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 38.Mueller SN, Gebhardt T, Carbone FR, Heath WR. 2013. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 39.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 40.Potter SJ, Lacabaratz C, Lambotte O, Perez-Patrigeon S, Vingert B, Sinet M, Colle JH, Urrutia A, Scott-Algara D, Boufassa F, Delfraissy JF, Theze J, Venet A, Chakrabarti LA. 2007. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J Virol 81:13904–13915. doi: 10.1128/JVI.01401-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL. 2013. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian Y, Babor M, Lane J, Schulten V, Patil VS, Seumois G, Rosales SL, Fu Z, Picarda G, Burel J, Zapardiel-Gonzalo J, Tennekoon RN, De Silva AD, Premawansa S, Premawansa G, Wijewickrama A, Greenbaum JA, Vijayanand P, Weiskopf D, Sette A, Peters B. 2017. Unique phenotypes and clonal expansions of human CD4 effector memory T cells reexpressing CD45RA. Nat Commun 8:1473. doi: 10.1038/s41467-017-01728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, Pereyra F, Zurakowski R, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M. 2014. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 20:139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nie C, Sato K, Misawa N, Kitayama H, Fujino H, Hiramatsu H, Heike T, Nakahata T, Tanaka Y, Ito M, Koyanagi Y. 2009. Selective infection of CD4+ effector memory T lymphocytes leads to preferential depletion of memory T lymphocytes in R5 HIV-1-infected humanized NOD/SCID/IL-2Rgammanull mice. Virology 394:64–72. doi: 10.1016/j.virol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, Self SG, Borrow P. 2009. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilsson J, Kinloch-de-Loes S, Granath A, Sonnerborg A, Goh LE, Andersson J. 2007. Early immune activation in gut-associated and peripheral lymphoid tissue during acute HIV infection. AIDS 21:565–574. doi: 10.1097/QAD.0b013e3280117204. [DOI] [PubMed] [Google Scholar]
- 47.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. 1993. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74:185–195. doi: 10.1016/0092-8674(93)90305-A. [DOI] [PubMed] [Google Scholar]
- 48.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. 1994. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol 152:3282–3293. [PubMed] [Google Scholar]
- 49.Nakache M, Berg EL, Streeter PR, Butcher EC. 1989. The mucosal vascular addressin is a tissue-specific endothelial cell adhesion molecule for circulating lymphocytes. Nature 337:179–181. doi: 10.1038/337179a0. [DOI] [PubMed] [Google Scholar]
- 50.Swaims-Kohlmeier A, Haaland RE, Haddad LB, Sheth AN, Evans-Strickfaden T, Lupo LD, Cordes S, Aguirre AJ, Lupoli KA, Chen CY, Ofotukun I, Hart CE, Kohlmeier JE. 2016. Progesterone levels associate with a novel population of CCR5+CD38+ CD4 T cells resident in the genital mucosa with lymphoid trafficking potential. J Immunol 197:368–376. doi: 10.4049/jimmunol.1502628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y, Li N, Zhang T, Huang X, Cai F, Vandergrift N, Xin R, Meng Z, Zhang X, Jiang C, Xu X, Montefiori DC, Gao F, Wu H. 2015. Comprehensive characterization of the transmitted/founder env genes from a single MSM cohort in China. J Acquir Immune Defic Syndr 69:403–412. doi: 10.1097/QAI.0000000000000649. [DOI] [PMC free article] [PubMed] [Google Scholar]