Practical Implications
PCR analysis of brain tissue may be helpful in the diagnosis of cerebral toxoplasmosis.
Toxoplasmosis is a widespread infection caused by Toxoplasma gondii, an obligate intracellular protozoan.1 Seroprevalence rates vary greatly, from ∼9% in the United States (with wide regional variations) to more than 85% in Europe.2 The acquired infection is generally asymptomatic in healthy people, causing a self-limited lymphadenopathy or mononucleosis-like illness in about 10% of them.1 CNS toxoplasmosis usually affects immunocompromised hosts, representing 60% of the focal intracerebral mass in patients with AIDS. On the contrary, CNS toxoplasmosis is extremely rare in immunocompetent hosts and its diagnosis is commonly supported by detection of T gondii antibodies.3–7
We describe an immunocompetent patient presenting life-threatening cerebral toxoplasmosis in whom both CSF analysis and serology were uninformative and the diagnosis was achieved by means of molecular amplification of T gondii DNA on brain tissue samples.
CASE REPORT
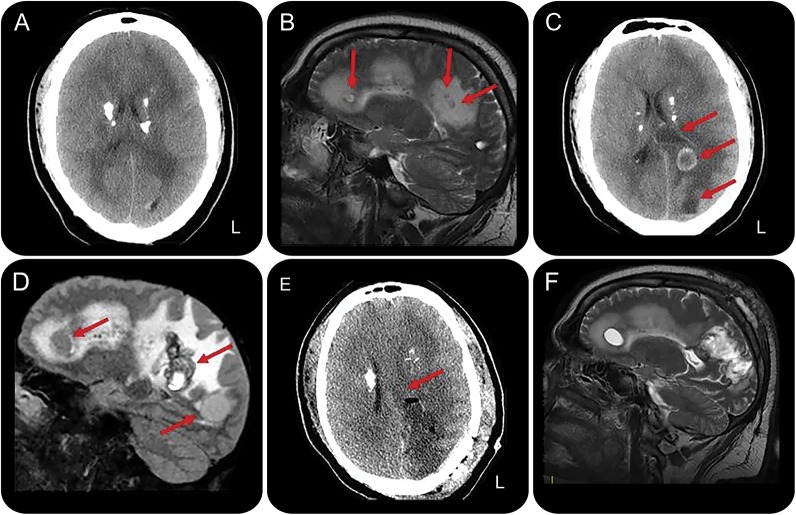
A 30-year-old man was admitted to our neurologic unit for a 5-year history of monthly frontal headache that worsened in frequency and intensity over the last month. Short-lasting episodes of sudden fall without loss of consciousness, followed by unjustified crying, also occurred over the last 5 years. The patient reported the ingestion of poorly cooked pork meat on multiple occasions. Physical examination showed severe obesity without fever or lymphadenopathy. Neurologic examination including fundus oculi was normal. Brain CT scan showed multiple calcifications involving basal ganglia and semioval centers (figure 1A). MRI showed multiple cystic lesions surrounded by edema, not enhancing after contrast (figure 1B). EEG showed mild slowing over the left temporal region. Erythrocyte sedimentation rate was 27 mm/h; C-reactive protein levels were normal. CSF physical–chemical analysis was normal (1 cell/μL, 32 mg/dL proteins). A wide serologic and CSF screening for different infectious diseases, including HIV-I and HIV-II, was negative. Toxoplasmosis serology on blood indicated a chronic infection (immunoglobulin M 3 IU/mL, immunoglobulin G 194 IU/mL). T-lymphocyte count (CD4+ 1,589 cells/μL, range 600–1,564; CD8+ 1,549 cells/μL, range 336–952; CD4+/CD8+ 1.03, range 1.2–2.5) and normal serum immunoglobulin levels excluded immunodeficiency. DNA extraction from CSF (QIAamp DNA Mini Kit; Qiagen, Venlo, Netherlands) and PCR amplification of the B1 region from T gondii (Nested Mix Toxoplasma gondii; Clonit, Milan, Italy) was normal. Total body CT showed mild splenomegaly and lymphadenopathy in the left groin. The patient was discharged with topiramate up to 200 mg/d, but episodes of falling persisted. The patient did not come to our observation over the following 20 months. Thereafter, due to daily severe headache and occurrence of secondary generalized seizures, he was admitted again to our unit. Brain CT and MRI showed a marked size increase of cystic lesions with ring enhancement; diffuse perilesional edema with midline shift was evident (figure 1, C and D). Emergency brain surgery with removal of 2 large cysts was undertaken (figure 1E), with no relevant sequelae. Histopathology showed spherical cysts with several bradyzoites (figure e-1 at Neurology.org). Then, molecular amplification of brain tissue using protocols described for CSF revealed the presence of T gondii DNA. Antiparasitic treatment with pyrimethamine, sulfadiazine, and folinic acid, along with dexamethasone, was started. After 8 days, sulfadiazine was replaced with clindamycin due to skin rash. At 20-month follow-up, the patient referred headache resolution with persistence of sporadic partial motor seizures. Control MRI showed mild size decrease of cystic lesions with reduction of perilesional edema and contrast enhancement (figure 1F).
Figure 1. Neuroimaging findings in an immunocompetent patient at clinical onset, before and after surgical therapy.
(A) CT scan displays multiple calcifications involving basal ganglia and semioval centers. (B) Sagittal T2-weighted image shows multiple cystic lesions (arrows) surrounded by edema. (C) CT scan shows 2 cystic lesions in the left hemisphere (arrows). Midline shift is also evident. (D) Sagittal 3D fluid-attenuated inversion recovery image shows a marked size increase of the cystic lesions with diffuse perilesional edema (arrows). Note the absence of intracystic scolex. (E) Postsurgical CT image shows removal of the cystic lesions with mass effect reduction. (F) Control (at 2-month follow-up) sagittal T2-weighted image displays mild size decrease of cystic lesions.
DISCUSSION
In this immunocompetent patient, the diagnosis of neurotoxoplasmosis was difficult due to absence of fever, slowly progressive clinical course, negativity of serologic screening for recent infectious diseases, and normality of CSF findings including PCR for T gondii. To our knowledge, 12 immunocompetent patients with isolated CNS toxoplasmosis have been reported in the literature (table e-1).3–7,e1–e6 Among these patients, 6 had fever and 10 presented an acute onset including altered sensorium, headache, vomiting, seizures, focal deficits, or meningeal signs. Diagnosis was achieved by serology in 5 out of 12 patients, by PCR on blood in 1 patient, while in 2 patients it relied on ex juvantibus criteria; in 4 patients, histopathology had a diagnostic role. CSF analysis revealed proteinorrachia in 6 patients and pleocytosis in 2 patients only, while PCR was negative in the only tested patient. In our patient, only PCR on brain tissue samples allowed a definite diagnosis, despite the negative results of this procedure on CSF.
Cerebral toxoplasmosis should be suspected in immunocompetent hosts when neuroradiologic findings suggests such diagnosis, even in the absence of classical clinical and laboratory findings. In case of negativity of serologic and CSF analysis, PCR for T gondii on brain tissue sample should be performed promptly in order to start adequate treatment.
Supplementary Material
Footnotes
Supplemental data at Neurology.org/cp
AUTHOR CONTRIBUTIONS
G. Pustorino: acquisition of data, analysis and interpretation of data, writing of manuscript. E. Ferlazzo: acquisition of data, analysis and interpretation of data, study supervision, critical revision of manuscript for intellectual content. M.S. Carpentieri: acquisition of data, analysis and interpretation of data. V. Cianci: acquisition of data, analysis and interpretation of data. S. Gasparini: acquisition of data, analysis and interpretation of data. M. Campello: acquisition of data, analysis and interpretation of data. G.L. Milardi: molecular analyses and interpretation of data. A. Gangemi: acquisition of data, analysis and interpretation of data. U. Aguglia: acquisition of data, analysis and interpretation of data, study supervision, critical revision of manuscript for intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
G. Pustorino reports no disclosures. E. Ferlazzo has received speaker honoraria from Eisai. M.S. Carpentieri, V. Cianci, S. Gasparini, M. Campello, G.L. Milardi, and A. Gangemi report no disclosures. U. Aguglia serves on the editorial board of Neurological Sciences. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004;363:1965–1976. [DOI] [PubMed] [Google Scholar]
- 2.Heller HM, Weller PF, McGovern BH. Toxoplasmosis in immunocompetent hosts. In: UpToDate [online]. Available at: UpToDateInc.com. Accessed October 14, 2016. [Google Scholar]
- 3.Habek M, Ozretić D, Žarković K, et al. Unusual cause of dementia in an immunocompetent host: toxoplasmic encephalitis. Neurol Sci 2009;30:45–49. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Raja A, Mahadevan A, Shankar SK. Toxoplasma granuloma of brainstem: a rare case. Neurol India 2008;56:189–191. [PubMed] [Google Scholar]
- 5.Galli-Tsinopoulou A, Kyrgios I, Giannopoulou EZ, et al. Acquired toxoplasmosis accompanied by facial nerve palsy in an immunocompetent 5-year-old child. J Child Neurol 2010;25:1525–1528. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran R, Radhan P, Anand R, Subramanian I, Santosham R, Sai V. CNS toxoplasmosis in an immunocompetent individual. Radiol Case Rep 2015;9:e00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltrame A, Venturini S, Crichiutti G, Meroni V, Buonfrate D, Bassetti M. Recurrent seizures during acute acquired toxoplasmosis in an immunocompetent traveller returning from Africa. Infection 2016;44:259–262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.