Practical Implications
Focal β-amyloid angiopathy manifesting as mass lesions should be included in the differential diagnosis of symptomatic, enlarging, variably contrast-enhancing lesions, and can occur independently from coexistent classical or inflammatory cerebral amyloid angiopathy, amyloidoma, Alzheimer disease, or other distributed amyloid pathology.
We report on a 70-year-old right-handed woman who presented with an 11-year history of a gradually progressive motor and cognitive-behavioral syndrome caused by focal cerebral β-amyloid angiopathy manifesting as discrete mass lesions. At age 59, she developed left leg clumsiness and cramping, evolving within a year into leg stiffness. Similar symptoms gradually affected her left arm. At age 65, she noted progressive word-finding difficulties, anxiety, mental rigidity, and compulsive traits. By age 68, she had difficulties with processing speed, planning, and navigation. Her history was notable for mild hypertension, hyperlipidemia, hypothyroidism, sleep apnea, bilateral carpal tunnel syndrome, and uterine fibroids; her mother had late-onset parkinsonism. She never smoked or used illicit drugs.
On examination at age 67, the patient had left sensory drift, graphanesthesia, limb kinetic apraxia, bradykinesia, mild weakness, hypertonia, and hyperreflexia, affecting the leg more than the arm. On gait examination, she had decreased left arm swing with dystonic posturing and left leg circumduction. On cognitive testing, she was slow, made occasional inattentive self-corrected errors, and had mildly low lexical and moderately low semantic fluency, whereas global Mini-Mental State Examination and Montreal Cognitive Assessment cognitive screening tests were normal (30/30).
Brain MRI revealed juxtaventricular frontoparietal and anterior temporal white matter mass-like fluid-attenuated inversion recovery hyperintensities, with concordant restricted diffusion, and variable enhancement on longitudinal T1 postgadolinium sequences (figure 1). Postcontrast cervical spine MRI did not reveal meningeal or cord abnormalities. CT angiography revealed only mild large vessel disease without major stenosis. CSF analysis at age 64 at an outside institution revealed elevated protein (61 mg/dL) and CSF oligoclonal bands (corresponding serum sample not sent) and normal glucose (51 mg/dL). The patient was diagnosed with multiple sclerosis and treated with SQ interferon-β-1a without benefit.
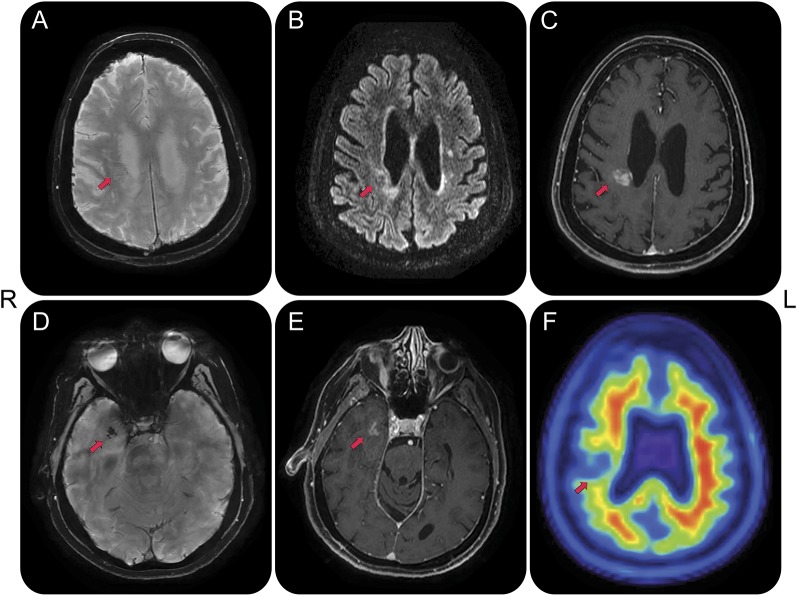
Figure 1. MRI and molecular amyloid PET.
MRI acquisitions obtained at age 68 years reveal 2 focal contrast-enhancing lesions in the right frontoparietal and temporal white matter and amyloid PET acquisition obtained at age 70 reveals decreased tracer uptake in the right frontoparietal lesion. Arrows point to lesions. (A) Susceptibility-weighted angiography sequence indicative of faint linear susceptibility artifact of the frontoparietal lesion, likely reflective of prominent vascularity, without features of microhemorrhages. (B) Fluid-attenuated inversion recovery sequence reveals hyperintense signal around the center of the lesion, possibly reflective of a combination of edema and white matter damage. (C) T1 with contrast sequence indicates blood–brain barrier disruption at the lesion center. (D) Susceptibility-weighted angiography sequence reveals confluent punctate susceptibility artifacts within the right temporal lesion. There were no distributed susceptibility artifacts other than the focal changes noted in the right anterior temporal lesion at age 68, which were absent at age 67. (E) Contrast enhancement of the right temporal lesion on T1 with contrast. (F) Amyloid [18F]florbetapir PET image reveals lack of cortical tracer uptake, making Alzheimer disease unlikely, and decreased tracer uptake at the lesion center, supportive of white matter breakdown, decreased perfusion, or lack of tracer sensitivity to specific amyloid pathology. There was no abnormal tracer uptake in the temporal lobe lesion. The temporal lesion remained stable, whereas the frontoparietal increased from 7 to 19 mm between ages 59 and 63, stabilizing thereafter. There was mild atrophy of the dorsolateral frontoparietal and medial temporal lobes, right worse than left (slices not shown).
At age 67, the patient sought evaluation at our institution. Biopsy of the right frontoparietal mass-like lesion revealed non-inflammatory β-amyloid angiopathy restricted to the vessels (figure 2). Amyloid [18F]florbetapir PET (figure 1) was not supportive of Alzheimer disease (AD) or classical cerebral amyloid angiopathy (CAA),1 and instead revealed decreased tracer retention in the frontoparietal lesion compared to the surrounding white matter, which normally shows nonspecific tracer binding. Repeat CSF testing revealed protein of 67 mg/dL, and normal leukocytes, erythrocytes, glucose, immunoglobulin G index (0.6), and oligoclonal bands that were also present in serum. CSF β-amyloid 42 (786.15 pg/mL), total-tau (208.35 pg/mL), and phospho-tau levels (42.1 pg/mL) (Athena Diagnostics) were normal, making coexistent AD pathology unlikely. Whole-body FDG-PET was normal. Autoimmune, infectious, and malignancy testing was unrevealing, including HIV, venereal disease research laboratory test, serum protein electrophoresis, urine protein electrophoresis, and kappa and lambda light chains. Interferon-β-1a was discontinued and treatment focused on vascular risk factor modification. The patient remained stable until she had a perforating branch right pontine stroke at age 70, which transiently aggravated her left pyramidal symptoms.
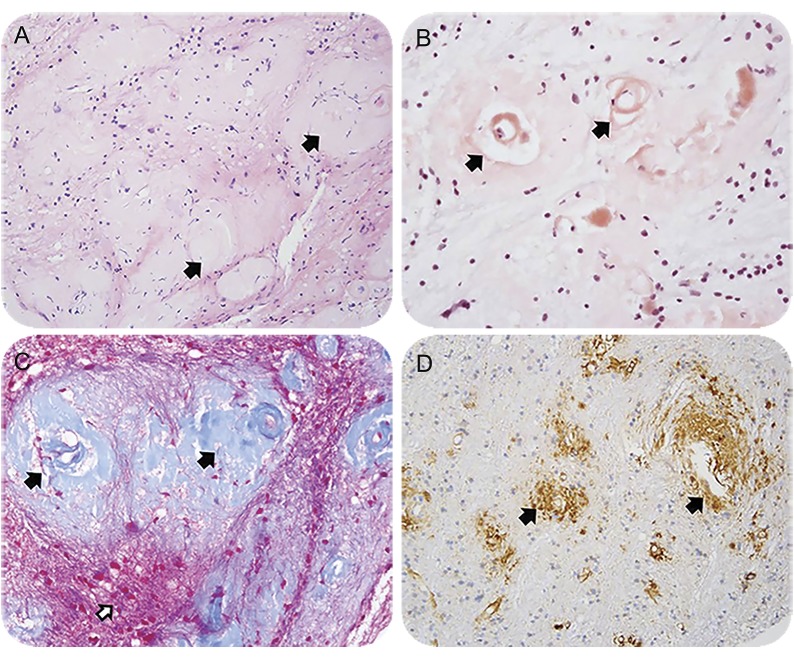
Figure 2. Neuropathology from brain biopsy pathologic staining.
Section staining from needle biopsy of the right frontoparietal lesion. (A) Hematoxylin & eosin stain suggests perivascular damage (arrows) without evidence of a primary inflammatory process. (B) Congo red stain reveals abnormal protein deposition in the vessel wall and presence of a false lumen around the tunica intima (arrows). (C) Trichrome stain further supports vascular and perivascular damaged tissue (black arrows) but without direct damage to the background white matter tissue (white arrow). (D) Immunohistochemical stain for β-amyloid clarifies presence of pathologic protein accumulation (arrows). Our staining β-amyloid antibody (4G8, 1:2,000, Covance, NJ) is not able to distinguish between Αβ-40 and Αβ-42, though the former is suspected to be the culprit, given normal CSF Aβ-42.
The patient exhibited a clinicopathologic variant of cerebral β-amyloid angiopathy manifesting as a mass-like lesion, a presentation we call focal cerebral β-amyloid angiopathy (faβ angiopathy). This phenotype is distinct from classical CAA, which is characterized by distributed small vessel disease, especially of the occipital lobes, and in which nearly half of patients display microhemorrhages and 60% superficial siderosis.2 Faβ angiopathy is also distinct from amyloidomas, which exhibit diffuse parenchymal deposition of amyloid, whereas amyloid in faβ angiopathy is restricted to blood vessels.3 Also, faβ angiopathy lesions tend to affect the deeper white matter, in contrast to classical CAA, which usually affects cortical or juxtacortical structures.4 The neuropathology and imaging in our case was not supportive of inflammatory CAA (CAA-i), a rapidly progressive form of CAA with microhemorrhages in an inflamed and edematous area.5 Normal CSF AD biomarkers and amyloid PET make AD copathology unlikely. While amyloid PET has been reported to have high sensitivity for classical CAA and typically reveals elevated cortical tracer uptake,1 its utility appears to be limited in faβ angiopathy, considering it was negative in our case. Finally, although available testing cannot firmly rule out coexisting tau or TDP-43 pathology, symptoms and atrophy usually progress faster in these conditions, whereas faβ lesions could explain the lateralizing atrophy through involvement of projection fibers and neuroglia.
There are only a handful of case reports of faβ angiopathy, usually misdiagnosed as gliomas or lymphomas prior to biopsy, and occasionally presenting with inflammation and edema that responds to steroids.3,6–8 Some of these reports classify this entity as a variant of CAA, CAA-i, or amyloidoma. These reports include both sexes and ages ranging from the mid-30s to the late 80s. Symptoms vary according to size and location of lesions, and presence or absence of edema and inflammation, reflective of dynamic pathologic features. Characteristic MRI findings are (1) a mass-like lesion, usually unifocal, which is hypointense on T1 and variably hyperintense on T2 sequences; (2) presenting in any lobe; (3) with slow, if any, growth; and (4) with variable contrast enhancement, a finding not observed in classical CAA. The above can make radiologic distinction from glioma difficult. There is no evidence in the literature that such cases progress to classical CAA, although follow-up is limited and the sample size small. It is also unclear if systemic copathologies that occasionally relate to systemic amyloid deposition are more frequent in faβ angiopathy, as in our patient’s carpal tunnel syndrome and uterine tumors. Other than corticosteroid and immunosuppressant treatment in inflammatory cases, treatment follows clinical judgment. In addition to vascular risk factor modification, it may be advisable to avoid antiplatelet therapy, especially in cases with evidence of microhemorrhages.9 The potential benefit and safety of anti–amyloid antibody therapies is unknown, but there is a theoretical concern about risk for blood–brain barrier breakdown.10
Faβ angiopathy should be included in the differential diagnosis of symptomatic, enlarging, variably contrast-enhancing lesions and is phenotypically distinct from classical CAA, CAA-i, amyloidoma, AD, or other distributed amyloid pathology.
AUTHOR CONTRIBUTIONS
E. Karageorgiou: study concept and design, acquisition, analysis, and interpretation of data, manuscript compilation, and revision for intellectual content. G. Naasan: acquisition, analysis, and interpretation of data, manuscript revision for intellectual content. S. Pleasure: acquisition, analysis, and interpretation of data, manuscript revision for intellectual content. S. Alexandrescu: acquisition, analysis, and interpretation of data, manuscript revision for intellectual content. J. Gelfand: interpretation of data, manuscript revision for intellectual content. G. Tammewar: acquisition, analysis, and interpretation of data, manuscript revision for intellectual content. B.L. Miller: interpretation of data, manuscript revision for intellectual content. G.D. Rabinovici: acquisition, analysis, and interpretation of data, manuscript revision for intellectual content. L.T. Grinberg: acquisition, analysis, and interpretation of data, manuscript revision for intellectual content, supervision of study.
STUDY FUNDING
This work was supported by the Robert Katzman, MD, Clinical Research Training Fellowship in Alzheimer's Research by the Alzheimer's Association and the American Brain Foundation, and the Tau Consortium. Amyloid PET in this patient was supported by the Imaging Dementia-Evidence for Amyloid Scanning (IDEAS) study (ClinicalTrials.gov Identifier: NCT02420756).
DISCLOSURES
E. Karageorgiou and G. Naasan report no disclosures. S. Pleasure served as Reviewing Editor for Journal of Neuroscience and serves as Neurological Reviews Editor for JAMA Neurology; receives research support from NIH (NIMH, NIDA); and has served as an expert witness in a medico-legal case. S. Alexandrescu reports no disclosures. J. Gelfand serves on a scientific advisory board for Genentech and Medimmune; has received research support from Quest Diagnostics, Genentech, and MedDay; and has served as an expert witness consultant in a medico-legal case. His spouse serves on a scientific advisory board for Eli Lilly; serves as a consultant for Zosano; and receives research support from eNeura and Allergan. G. Tammewar reports no disclosures. B.L. Miller serves on scientific advisory boards for Tau Consortium, The John Douglas French Foundation, The Larry Hillblom Foundation, National Institute for Health Research in Dementia (UK), Stanford University ADRC, University of Washington ADRC, Arizona Alzheimer's Disease Center, and LEARN Consortium; serves as Editor of Neurocase; receives publishing royalties for Behavioral Neurology of Dementia (Cambridge University Press, 2009), Handbook of Neurology (Elsevier Inc., 2009), The Human Frontal Lobes (Guilford Publications, 2008), and Frontotemporal Dementia (Oxford University Press, 2014); and receives research support from Quest Diagnostics Incorporated, NIH/NIA, and CMS. G.D. Rabinovici serves on scientific advisory boards for Roche, Lundbeck, Piramal, Merck, and Genentech; has received speaker honoraria and/or funding for travel from Eisai, Alzheimer's Association, AAN, and Roche; serves as Associate Editor for JAMA Neurology; serves as a consultant for Putnam; and receives research support from Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, Piramal, NIH (NIA, NINDS), Alzheimer's Association, John Douglas French Alzheimer's Foundation, Tau Consortium, American College of Radiology, Association for Frontotemporal Lobar Degeneration, and Michael J. Fox Foundation. L.T. Grinberg has received funding for travel from ConnectMed; serves as an Associate Editor for Frontiers in Dementia and Cell and Tissue Banking and on the editorial board of Journal of Alzheimer's Disease; and receives research support from AVID Radiopharmaceuticals, NIH/NIA, Alzheimer's Association, and Rainwater Foundation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Gurol ME, Becker JA, Fotiadis P, et al. Florbetapir-PET to diagnose cerebral amyloid angiopathy: a prospective study. Neurology 2016;87:2043–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;74:1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karbowniczek A, Wierzba-Bobrowicz T, Mendel T, Nauman P. Cerebral amyloid angiopathy manifested as a brain tumour: clinical and neuropathological characteristics of two cases. Folia Neuropathol 2012;50:194–200. [PubMed] [Google Scholar]
- 4.Yamada M. Cerebral amyloid angiopathy: emerging concepts. J Stroke 2015;17:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eng JA, Frosch MP, Choi K, Rebeck GW, Greenberg SM. Clinical manifestations of cerebral amyloid angiopathy-related inflammation. Ann Neurol 2004;55:250–256. [DOI] [PubMed] [Google Scholar]
- 6.Ronsin S, Deiana G, Geraldo AF, et al. Pseudotumoral presentation of cerebral amyloid angiopathy-related inflammation. Neurology 2016;86:912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landau D, Avgeropoulos N, Ma J. Cerebral amyloidoma mimicking intracranial tumor: a case report. J Med Case Rep 2010;4:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osumi AK, Tien RD, Felsberg GJ, Rosenbloom M. Cerebral amyloid angiopathy presenting as a brain mass. AJNR Am J Neuroradiol 1995;16:911–915. [PMC free article] [PubMed] [Google Scholar]
- 9.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010;75:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiFrancesco JC, Longoni M, Piazza F. Anti-abeta autoantibodies in amyloid related imaging abnormalities (ARIA): candidate biomarker for immunotherapy in Alzheimer's disease and cerebral amyloid angiopathy. Front Neurol 2015;6:207. [DOI] [PMC free article] [PubMed] [Google Scholar]