Abstract
Platelet-rich plasma (PRP) contains a variety of cytokines, some of which ameliorate oX-LDL (oxidized low-density lipoprotein)-induced endothelial cell (EC) injury. Therefore, we hypothesized that PRP might alleviate oX-LDL-induced injury.
Methodology
Human umbilical vein endothelial cells (HUVECs) were divided into four groups: a PPP (platelet-poor plasma) group, an oX-LDL group, an oX-LDL+PRP group and a PRP group. CCK-8 (Cell Counting Kit) assay, Annexin V-FITC/7-AAD and Hochest 33342 staining were performed to assess cell proliferation and apoptosis. Tube formation and cell migration assays were performed to evaluate HUVEC-mediated vasculogenesis and migration. Expression levels of Bcl-2, Bax, caspase-3, cleaved caspase-3, PI3K, Akt, eNOS p-Akt, p-eNOS, IL-6 and IL-1 were detected by western blotting or immunofluorescence.
Principal findings
PRP promoted HUVEC proliferation in a non-linear pattern, protected HUVECs against oX-LDL-induced apoptosis and attenuated oX-LDL-mediated inhibition of HUVEC migration and vasculogenesis. Additionally, compared to the PPP group, PRP downregulated pro-apoptotic proteins (ratio of Bax/Bcl-2, caspase-3 and cleaved caspase-3) as well as IL-6 and IL-1. Moreover, the PI3K/Akt/eNOS pathway was activated by PRP and inactivated by oX-LDL.
Conclusions
It was demonstrated that PRP protected HUVECs against oX-LDL-induced injury and that the PI3K/Akt/eNOS pathway was activated in this process.
Keywords: Platelet-rich plasma, oX-LDL, HUVECs, PI3K/Akt/eNOS
1. Introduction
Atherosclerosis is a primary inducer of peripheral artery disease (PAD), cerebrovascular disease and coronary artery disease, important causes of morbidity and mortality worldwide [1, 2]. The pathogenesis of atherosclerosis is associated with foam cell formation and endothelial cell (EC) dysfunction; the latter plays a crucial role in accelerating disruption of blood vessel integrity, which is considered an initial sign of atherosclerosis. ECs constitute the innermost portion of the barrier that protects the three histological layers of the arterial wall from damage [3, 4]. Oxidized low-density lipoprotein (oX-LDL), which induces high levels of oxidative stress, causes EC dysfunction and has important roles in enhancing lipid accumulation in the subendothelium and in inducing smooth muscle cell proliferation, changes that lead to atherosclerosis [5, 6]. Progressive atherosclerosis causes strictures in the aorta and other large vessels, leading to poor outcomes such as limb amputation and death. Thus, compounds that can protect ECs from oX-LDL-induced damage may be useful as early therapies for atherosclerosis.
Multiple drugs (e.g., 7,8-dihydroxy-3-methyl-isochromanone-4 and tetramethylpyrazine), cell factors [e.g., vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF)] and even Chinese herbal extracts [propyl gallate and tribulus terrestris] reportedly protect against oX-LDL-induced EC dysfunction [7, 8, 9, 10, 11, 12]. Activated platelet-rich plasma (PRP), which is not FDA (Food and Drug Administration) labeled, contains a multitude of biological factors, including an abundance of pro-angiogenic cytokines such as platelet-derived epidermal growth factor (PD-EGF), platelet-derived growth factor (PDGF), VEGF, basic fibroblast growth factor (bFGF), angiopoietin (Ang), and transforming growth factor beta (TGF-β) [13, 14, 15]. TGF-β1 and VEGF concentrations as high as 23.738±6848 (mean ± SD in pg/ml) and 642±208, respectively, in PRP have been reported [16]. PRP has been used as a treatment for osteoarthritis (OA), wrinkles, hair loss and tendon strains for decades. PRP has also been used to induce bone regeneration [17, 18, 19, 20, 21], and PRP may exert pro-angiogenic effects in vitro and in vivo, possibly by mediating phosphorylation in the Erk1/2, Akt and angiopoietin 1-Tie 2 pathways [22, 23]. Nonetheless, the protective effects of PRP on oX-LDL-induced injury have seldom been investigated. We hypothesized that the multiple cell growth factors found in PRP exert their functions through a variety of pro-angiogenic receptors.
To determine whether EC apoptosis and inhibition of EC migration and EC-mediated vasculogenesis are enhanced by oX-LDL and attenuated by PRP, we performed several experiments involving human umbilical vein endothelial cells (HUVECs). Our results show that PRP can prevent oX-LDL from inhibiting HUVEC-mediated vasculogenesis and can activate the PI3K/Akt/eNOS pathway.
2. Materials and methods
2.1. Cell culture
The HUVECs used were generously donated by Zhongshan Medical College, Sun Yat-sen University (Guangzhou, Guangdong, China) and cultured in endothelial growth medium 2 (EGM-2, LONZA, California, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, California, USA) and antibiotics (penicillin 100 IU/ml and streptomycin 100 μg/ml, Invitrogen, California, USA) at 37°C in a humidified atmosphere with 5% CO2 (Thermo Fisher Scientific, Massachusetts, USA). Highly concentrated oX-LDL (90.0±9.9 nmol MDA/mg protein) was purchased from Yiyuan Biotechnologies (Guangzhou, China). HUVECs were assigned to separate groups and incubated with oX-LDL, PRP, oX-LD L+PRP or no additional agents. After treatment, the cells were seeded in appropriate chambers or used for subsequent experiments.
2.2. Preparation of PRP extract
We used a BD Vacutainer® (BD, New Jersey, USA) containing acid-citrate-dextrose solution formula A (ACD-A) anticoagulant to collect 54 ml of human blood from 6 volunteers (Supplementary Table 1) who were in good health and had provided informed content to participate in the study. The blood samples were first centrifuged (Eppendorf, Hamburg, Germany) at 450×g for 7 minutes, after which the plasma and the thin layer above the precipitated hemoglobin were transferred to a flow tube (Corning, New York, USA). This material was centrifuged for 5 minutes at 1600×g, and approximately 3/4 of the resulting mixture was collected as PPP (platelet-poor plasma). The remaining portion (PRP), containing a large amount of inactivated platelets, was activated with 0.5 M CaCl2:1000 U of bovine thrombin = 1:1 (V/V) (mixed reagent) [22] at a ratio of 10:1 (V/V) for 10 minutes at room temperature before being centrifuged at 2015×g for 15 minutes. The supernatant was then collected and stored at −20°C for use. This study conformed to ethical guidelines and was approved by the Ethics Committee of Sun Yat-sen University.
2.3. Cell proliferation and toxicity assay
Cell Counting Kit-8 (CCK-8, DOJINDO, Shanghai, China) was utilized to evaluate the toxicity induced by oX-LDL and to confirm that PRP stimulates dose-dependent increases in HUVEC proliferation. A total of 1×104 cells were grown in 96-well plates and treated with the appropriate agent for 24 h. The medium was then replaced, and the cells were treated with 10 μl CCK-8 reagent. The optical density (OD) value was recorded. We assessed the proliferation of HUVECs treated with PRP at increasing concentrations, namely, 1%, 3%, 5%, and 10% (V/V). To evaluate oX-LDL-induced toxicity, we incubated the HUVECs with 5, 10, 15 and 20 μg/ml oX-LDL; the cells in the oX-LDL+PRP group were treated with 3% (V/V) PRP for 10 h before being treated with oX-LDL. Absorbance was measured at a dual wavelength of 450 nm and 655 nm using an iMark Microplate Reader (Bio-Rad, California, USA), and cell viability was calculated as follows: cell viability = (|treated cells-blank|)/(untreated cells-blank).
2.4. Apoptosis assay
A total of 5×104 cells were seeded in a 24-well plate and treated with oX-LDL for 24 h; the cells in the oX-LDL+PRP group were incubated with 3% (V/V) PRP for 10 h before being treated with oX-LDL. The following steps were according to the Annexin V-FITC/7-AAD (Abnova, Wuhan, China) and Hochest 33342 staining kit (Beyotime, Shanghai, China) instructions. A fluorescence microscope (Olympus, Tokyo, Japan) and flow cytometer (Beckman coulter, California, USA) were utilized to observe changes in the morphology of the stained cells and the ratio of apoptotic cells.
2.5. Cell migration assay
Corning transwell chambers (pore size: 8.0 μm, Corning, New York, USA) were used to evaluate HUVEC migration ability. After 24 h of treatment, HUVECs were washed, digested, re-suspended, counted and then seeded in 24-well plates containing chamber inserts. The HUVECs in the oX-LDL+PRP group were pre-treated with 3% (V/V) PRP before being treated with oX-LDL. Each chamber contained 2×104 cells and 200 μl serum-free Dulbecco’s modified Eagle medium (DMEM, Gibco, California, USA). After 24 h, the cells that had migrated through the polycarbonate membrane were washed, fixed (pure formaldehyde for 15 minutes) and stained (0.1% crystal violet dye for 30 minutes). Four photographs taken at 100× magnification were analyzed using ImageJ (National Institute of Health, Maryland, USA).
2.6. Tube formation assay
The cells were grown on a Matrigel basement membrane matrix (Corning, New York, USA) to assess HUVEC tube formation. The Matrigel basement membrane matrix was melted in a freezer overnight at 4°C and then added to a 48-well plate in 10-μl aliquots. The matrix was then allowed to solidify for 30 minutes in an incubator, and 5×104 cells were seeded on its surface to form tubes; the cells were not seeded on areas of the matrix in which vesicles or scratches were present. The cells were allowed to grow for an appropriate period of time, during which the plates were observed under a microscope every other hour. Photographs were taken at 200× magnification after 6 h, and the total lengths of the tubes and the numbers of junctions that had formed were calculated using ImageJ.
2.7. Immunofluorescence assay
Intracellular IL-6 (1:50, Santa Cruz, California, USA) and IL-1 (1:50, Santa Cruz, California, USA) induced by oX-LDL were stained as previously described [24]. Cells were seeded into 6-well plates and allowed to grow for 24 h. The next day, the cells were washed with phosphate-buffered saline (PBS) and incubated with 3% (V/V) PRP. After removing the PBS, the cells were washed twice and exposed to PRP or oX-LDL for 48 h. Fluorescence was observed and photographed (magnification 200×) using an inverted fluorescence microscope (Olympus Corporation, Tokyo, Japan).
2.8. Western blot analysis
A total of 2.5×105 cells were seeded in 6-well plates. As in the above experiments, the cells in the oX-LDL+PRP group were pre-incubated with 3% (V/V) PRP to assess the protective effects of PRP against oX-LDL-induced injury. After 48 h of treatment, the cells were washed three times in cold PBS and lysed with 100 μl lysis buffer [RIPA buffer (10×):protease inhibitor cocktail set I:phosphatase inhibitor cocktail set II:ddH2O = 10:1:1:88]; 80 μl of this mixture was subsequently placed in a 1.5-ml EP tube and stored at −80°C until use. The sample was centrifuged at 12,000×g for 20 minutes at 4°C, and the protein concentration was determined with a BCA Protein Assay Kit (Thermo Fisher Scientific, Massachusetts, USA). The proteins were mixed with loading buffer (5×, Thermo Fisher Scientific, Massachusetts, USA) and boiled for 5 minutes at 95°C, and 30 μg total protein was separated on an 8% polyacrylamide gel. The phosphorylation of specific proteins was detected within 24 h after the above experiments to ensure that the proteins had not been degraded. The proteins were then electrotransferred to a polyvinylidene fluoride (PDVF, Millipore, Massachusetts, USA) membrane with transfer buffer containing 20% (V) methylalcohol. The membrane was subsequently incubated with 5% bovine serum albumin (BSA) diluted with TBST (20 mmol/l Tris-HCl (pH 7.6), 1 ImageJ mmol/l NaCl and 0.1% (V/V) Tween-20) to prevent nonspecific binding before being incubated with primary antibodies, which had been diluted 1:1000 (β-actin, Bcl-2, Bax, caspase-3, and cleaved caspase-3; CST, Massachusetts, USA), 1:1500 (PI3K, Akt, p-Akt, eNOS, and p-eNOS; CST, Massachusetts, USA) or 1:500 (IL-6 and IL-1, Santa Cruz, California, USA ), overnight at 4°C. The membrane was then incubated with a secondary anti-rabbit antibody (1:1000, CST, Massachusetts, USA) diluted with TBST containing 5% BSA for 50 minutes at room temperature. The blots were subsequently exposed to Kodak X-ray films in the dark, and gray values were calculated using ImageJ software.
2.9. Statistical analysis
All experiments were repeated three times and performed independently. Continuous values are presented as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to assess differences among the groups, and all tests were two-sided; p<0.05 was considered statistically significant. All calculations were performed with SPSS 13.0 (IBM Corp., New York, USA), and all histograms were exported with GraphPad Prism 6.0 (GraphPad, California, USA).
3. Results
3.1. Protective effects of PRP against oX-LDL-induced toxicity
To investigate the separate and combined effects of oX-LDL and PRP on HUVECs, we performed the CCK-8 assay, the results of which indicated that treatment with oX-LDL for 24 h concentration-dependently reduced HUVEC viability from 100% to 2.70%±3.65% (Figure 1A). As shown in Figure 1B, HUVEC proliferation peaked at 181%±9.96% after treatment with 3% (V/V) PRP, indicating that 5% and 10% (V/V) PRP did not induce further increases in proliferation. Cell viability was similar between the groups of HUVECs pretreated with 3% and 5% (V/V) PRP for 10 h, with viable cell percentages of 62.52%±5.82% and 63.10%±6.25%, respectively (Figure 1C). Treatment with 1% (V/V) PRP yielded a cell viability percentage of 61.39%±4.23%; however, the difference in cell viability between the oX-LDL (10 μg/μl) and oX-LDL (10 μg/μl) + PRP (1%, V/V) groups was not significant (p>0.05). The percentage of viable cells increased significantly to 83.48%±17.03% in cells treated with 10% (V/V) PRP, likely because of increases in the levels of the multiple factors constituting PRP; however, treatment with 10% (V/V) PRP had no additional effect on HUVEC proliferation. Therefore, we hypothesized that PRP induced a non-linear pattern of changes in HUVEC viability and proliferation because PRP at a concentration greater than 3% (V/V) did not result in higher cell viability compared to 3% PRP. Based on the results of the above experiments, we determined that 10 μg/μl oX-LDL, which decreased HUVEC viability by approximately 50%, and 3% (V/V) PRP were the most appropriate concentrations for assessing the protective effects of PRP against oX-LDL-induced EC injury in subsequent experiments.
Figure 1.
The protective effects of PRP against oX-LDL-induced toxicity (mean ± SD). A: HUVECs were treated with increasing oX-LDL concentrations. B: HUVECs were treated with PRP in gradient concentrations. C: HUVECs were pre-treated with 3% (V/V) PRP before treatment with oX-LDL.
3.2. PRP attenuates the effects of oX-LDL on EC migration and tube formation
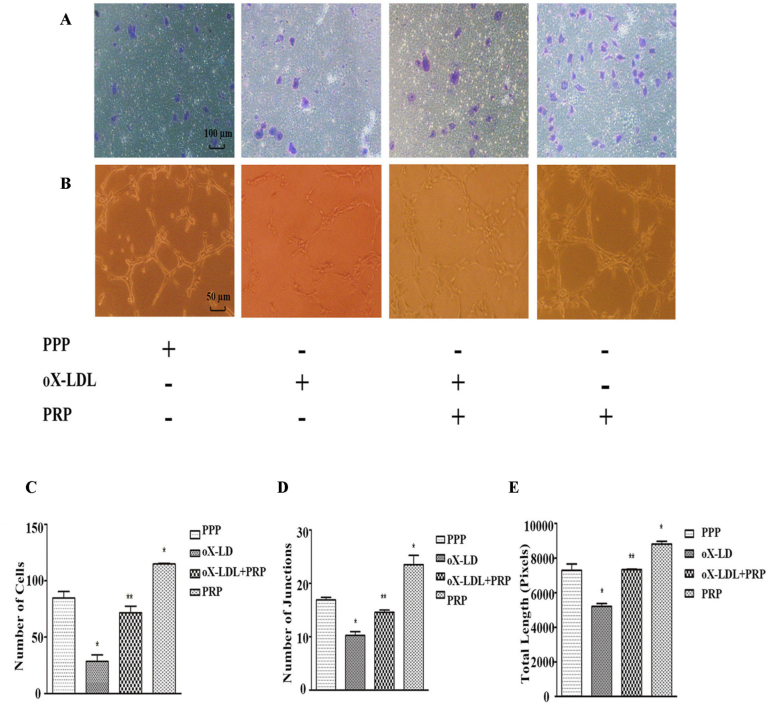
To evaluate the effects of PRP on migration of oX-LDL-treated ECs, we counted the numbers of cells that successfully penetrated the polycarbonate membrane of the transwell chamber in the four groups mentioned above (Figure 2A). Treatment with oX-LDL significantly reduced the average number of migrating cells compared with the PPP group. Specifically, we noted that 28.33±10.26 cells had migrated in the oX-LDL group. The oX-LDL+PRP was pre-treated with 3% PRP (V/V) over 10 h before being exposed to 10 μg/μl oX-LDL, and an average of 71.67±10.07 cells had migrated in this group, higher than the corresponding number in the oX-LDL group (Figure 2C). As expected, HUVECs treated with PRP alone displayed increased migration ability compared with HUVECs treated with PPP. A tube formation assay was performed to determine whether HUVECs formed more junctions and longer branches in response to treatment with PRP (Figure 2B). As shown in Figure 2D, the number of junctions formed by ECs was significantly decreased in the oX-LDL group (10.25±1.25) but significantly increased in the PRP group (23.50±3.03) compared with the PPP group (16.92±0.80). Similarly, the number of junctions formed by ECs in the oX-LDL+PRP group (14.58±0.76) increased significantly compared with that in the oX-LDL group. The results of this assay also showed that oX-LDL + PRP group ECs (7330±45 pixels) formed longer and more continuous tube branches than oX-LDL group ECs (5206±283 pixels) (Figure 2E). Treatment with PRP alone (8802±273 pixels) also increased EC tube formation compared with PPP treatment (7290±627 pixels).
Figure 2.
Capacity of migration and tube formation (mean ± SD). A: Cells that had migrated through the membrane were stained with crystal violet. B: Junctions and length of loops formed by HUVECs were calculated and analyzed. C: Number of migrated cells. D: Number of joints. E: Total length. *p<0.05 versus control; **p<0.05 versus oX-LDL.
3.3. PRP protects HUVECs from oX-LDL-induced apoptosis
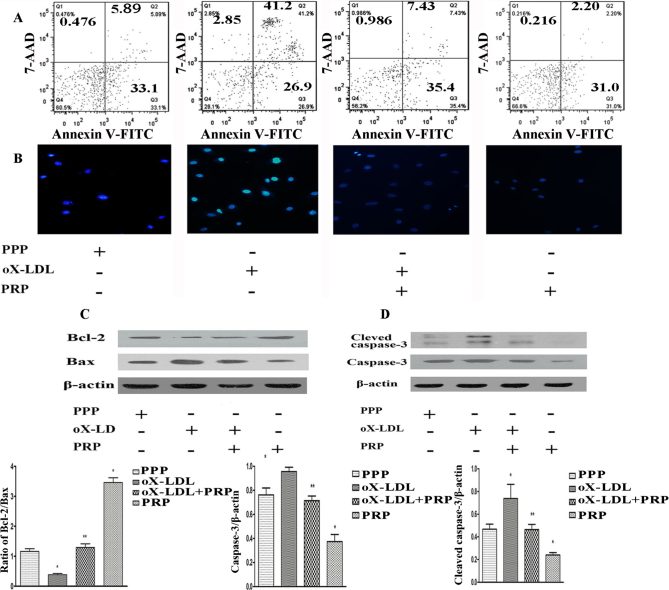
oX-LDL-induced UVEC apoptosis was assessed by Annexin V-FITC/7-AAD staining followed by flow cytometry detection; Hoechst 33342/PI staining was used for observation of changes in nuclear morphometry. As shown in Figure 3A, more apoptotic cells (41.2%) were observed in the 10 μg/μl oX-LDL group than in the PPP group (5.89%). Pre-treatment with 3% (V/V) PRP reduced the number of apoptotic cells to 7.43%, and an average of apoptotic cells was noted in the PRP-only group. The presence of bright and irregular chromatin (Figure 3B) is indicative of the occurrence of apoptosis, and the outcomes of Hochest 33342 staining were in accordance with the results of flow cytometry.
Figure 3.
Apoptotic cells and changes in death-related proteins were detected. A: Apoptotic cells were detected by flow cytometry. B: Cells emitting blue fluorescence were stained with Hoechst 33342. C: Bcl-2 and Bax were detected by western blotting. D: Cleaved caspase-3 and caspase-3 were detected by western blotting. *p<0.05 versus control; **p<0.05 versus oX-LDL.
3.4. PRP up-regulates the Bcl-2/Bax ratio and down-regulates caspase activity
The presence of the anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax may indicate that cells are undergoing apoptosis, especially in cases in which the Bcl-2/Bax ratio is significantly decreased. Treatment with 10 μg/μl oX-LDL significantly decreased Bcl-2 expression and increased Bax expression, thereby decreasing the Bcl-2/Bax ratio (Figure 3C), whereas pre-treatment with PRP significantly increased the Bcl-2/Bax ratio. Treatment with PRP alone promoted Bcl-2 expression and suppressed Bax expression compared with the PPP group. As caspase-3 and cleaved caspase-3, which are downstream molecules in the mitochondrial death pathway, are the primary executors of apoptosis and participate in oX-LDL-induced apoptosis, we examined whether treatment with PRP significantly modulated caspase-3 and cleaved caspase-3 expression by western blotting. As shown in Figure 3D, treatment with oX-LDL increased both caspase-3 and cleaved caspase-3 levels compared with untreated cells and PRP-pre-treated cells, whereas treatment with PRP alone resulted in a lack of caspase-3 and cleaved caspase-3 activation.
3.5. PRP attenuates oX-LDL-induced PI3K/Akt/eNOS signaling pathway inhibition
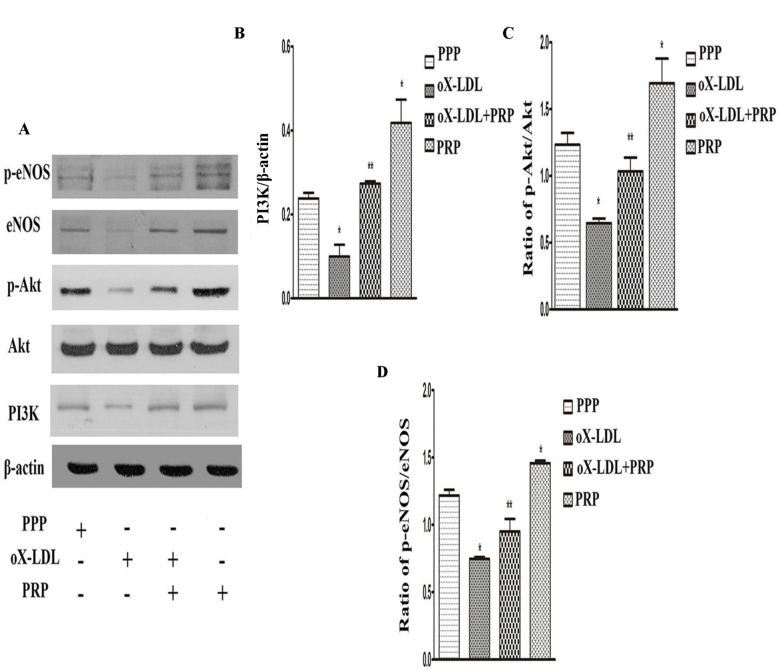
The PI3K/Akt/eNOS signaling pathway is believed to play a pivotal role in protecting cells from apoptosis and in stimulating EC proliferation and migration [3]. To determine whether this signaling pathway is associated with the protective effects of PRP, we assessed the expression levels of proteins associated with the pathway via western blotting. As shown in Figure 4A, 4B and 4C, oX-LDL suppressed PI3K expression and inactivated Akt; however, these effects were apparently inversed by PRP. Neither oX-LDL nor PRP had an impact on the level of total Akt protein (Figure 4A). We also examined endothelial nitric oxide synthase (eNOS), an important downstream molecule of Akt, and its phosphorylated form and found that treatment with oX-LDL decreased total and phosphorylated eNOS as well as the p-eNOS/eNOS ratio (Figure 4A; 4D). Additionally, treatment with PRP alone enhanced PI3K expression and eNOS production and increased Akt and eNOS phosphorylation. All of these results indicate that PRP can protect against oX-LDL-induced HUVEC injury and that the PI3K/Akt/eNOS signaling pathway plays a vital role.
Figure 4.
Effects of PRP on modulation of the PI3K/Akt/eNOS pathway (mean ± SD). A: PI3K, Akt, p-Akt, p-eNOS and eNOS were detected by western blotting. B: PI3K. C: p-Akt/Akt. D: p-eNOS/eNOS. *p<0.05 versus control; **p<0.05 versus oX-LDL.
3.6. PRP suppresses IL-6 and IL-1 expression
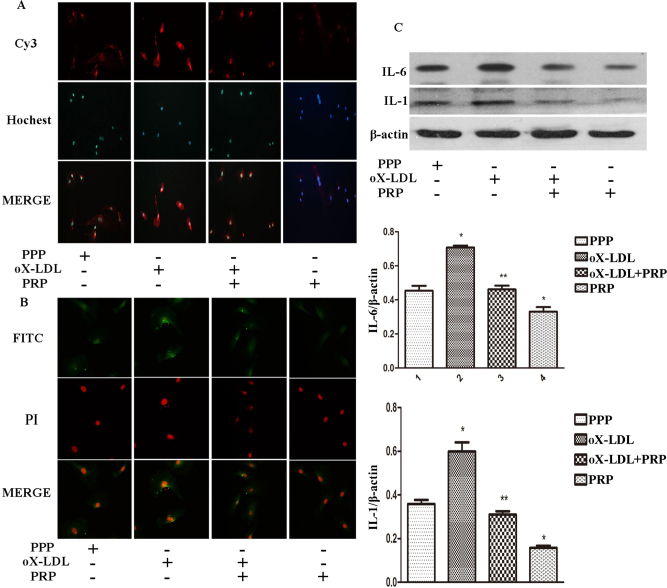
As shown in Figure 5A and 5B, the fluorescence intensity of cells exposed to oX-LDL was much higher than control cells, with many foci where IL-6 and IL-1 accumulated. In contrast, cells pretreated with PRP exhibited a signal that was close to that of the control cells. Moreover, IL-6 and IL-1 levels in cells treated with PRP alone were much lower than in the control cells. Quantification of western blot analysis showed higher levels of IL-6 and IL-1 in the ox-LDL group than in the control group (Figure 5C); in contrast, pretreatment with PRP caused a remarkable reduction in IL-6 and IL-1 compared to the oX-LDL group. Furthermore, the levels of IL-6 and IL-1 in cells treated with PRP alone was lower than those in control cells.
Figure 5.
IL-6 and IL-1 expression in four different groups. Secondary antibodies conjugated with Cy3 or FITC were used to detect IL-6 and IL-1, respectively. Cell nuclei were stained by Hochest 33342 and PI. A: IL-6 expression via immunofluorescence. B: IL-1 expression via immunofluorescence. C: IL-6 and IL-1 expression detected by western blotting (mean ± SD). *p<0.05 versus control; **p<0.05 versus oX-LDL.
4. Discussion
PRP is a platelet concentrate that originates in autologous blood and can store 4-to-9 fold more platelets than blood at baseline [25]. Platelets play a critical role in the initial phase of thrombosis, a process in which they first adhere to and are then activated by the conversion of fibrinogen to fibrin in the presence of coagulation factors.
The above pathological process enhances platelet activation. When platelet production is stimulated, thousands of biomolecules, including growth factors, messengers, enzymes, enzyme inhibitors and other compounds, are quickly released into the circulation [26]. Thus, concentrated volumes of plasma should have higher concentrations of particles than non-concentrated volumes of plasma. Michael O. Schar [16] reported that the concentration of VEGF, the most potent stimulator of vasculogenesis, increased to 642±208 pg/ml in cells after PRP treatment. PRP is an emerging biomedicine that is currently widely used for the treatment of osteoarthritis (OA), muscle (tendon) injuries, wrinkles and hair loss [17, 18, 21]. PRP has also been found to promote angiogenesis in vitro and in vivo by increasing Erk1/2 and Akt phosphorylation [22]. Given that PRP contains an abundance of cellular growth factors, the pro-angiogenic processes in which it participates is expected to be mediated by more pathways than only Erk1/2 and Akt signaling. However, researchers have seldom investigated the mechanism underlying the protective effects of PRP against oX-LDL-induced EC cell injury or the mechanism underlying its pro-angiogenic effects.
It is universally known that oX-LDL-induced EC injury is vital for the occurrence and development of atherosclerosis [27, 28, 29]. oX-LDL usually interacts with ECs via low-density lipoprotein receptor-1 (LOX-1), which can be up-regulated by tumor necrosis factor alpha (TNF α), interleukin-1 (IL-1), interferon gamma (IFNγ), angiotensin II, endothelin-1 and oX-LDL, subsequently impacting expression of downstream molecules, such as apoptosis-related proteins (Bcl-2 and Bax), PI3K, Akt and eNOS and leading to EC dysfunction [30, 31, 32]. IL-8/CXCL8, IL-6/CXCL6, and MCP-1/CCL2 are downstream proteins of IL-1β that have been shown to be down-regulated by PRP, indicating that downstream signal transduction pathways may play important roles in protecting cells and in preserving their functions [33]. Our results indicated that even treatment with 10% (V/V) PRP could not increase cell viability to 100% (Figure 1C). It has been reported that PRP exerts stimulatory effects on human dermal fibroblasts and human adipose-derived stem cells, leading to a pattern of increases in cell proliferation resembling a bell-shaped curve [34]; however, these results are somewhat consistent with those of our experiments. We presumed that the pattern of the stimulatory effects of PRP on PI3K/Akt pathway activity and EC proliferation possibly occurs in a non-linear pattern, most likely because of the presence of oX-LDL [35, 36]. Notably, our experiments also showed that PRP ameliorates the impairments in HUVEC migration and HUVEC-mediated angiogenesis induced by oX-LDL and that PI3K/Akt is the potential pathway through which this occurs. PI3K and Akt proteins are both under the control of oX-LDL and PRP, and the changes in their expression induced by treatment with the above drugs induced corresponding alterations in the expression of eNOS, which plays essential roles in EC relaxation, migration and vasculogenesis and reportedly also plays a pivotal role in protecting cells from apoptosis [37]. Therefore, drugs that directly modulate eNOS activation may have potential as treatment for ox-LDL-induced cell injury.
Similar to previous studies, our findings showed that highly concentrated oX-LDL can cause HUVEC apoptosis [38]. Caspase-3 protein, an effector of apoptosis, acts at the step at which the two-major programmed cell death-related pathways, i.e., the death receptor and cell stress pathways, converge [39]. Cleaved caspase-3 is the truncated and active form of pro-caspase-3, and overexpression of both pro-caspase-3 and cleaved caspase-3, which is activated by oX-LDL, is suggestive of the occurrence of apoptosis. Our results demonstrated that oX-LDL and PRP had contrasting effects on the expression levels of apoptosis-related proteins Bax and Bcl-2, which are located in the mitochondrial membrane [40]. Bax and Bcl-2 exist as a heterodimer, and decreases in the Bcl-2/Bax ratio are also indicative of apoptosis. Moreover, major reductions in IL-1 and IL-6 (Figure 5A; 5B; 5C) suggest a weakened inflammatory response of ECs by PRP. However, extracellular data for IL-1, IL-6 and NO, which can directly demonstrate the protective effect of PRP, were not available. Despite this minor deficiency, final conclusion may not optimally but sufficiently made. Influx of monocytes and smooth muscle cells and their conversion to foam cells is also influenced by EC functionality and is important for initiation of atherosclerosis. Although we found that PRP can reverse the changes induced by oX-LDL and enhance HUVEC survival rates, it remains to be determined whether atherosclerosis progression can be blocked.
It is speculated that the protective effects of PRP against oX-LDL-induced injury are not mediated by the PI3K/Akt/eNOS pathway alone. TGF-β, PDGF-AB, VEGF and IGF, all of which have been shown to be abundant in PRP, likely influence the activity of downstream transduction pathways by increasing the expression of their respective receptors. However, further investigation is warranted to determine whether other pathways mediate the effects of oX-LDL and PRP on EC injury.
Although our study utilized only HUVECs, we successfully demonstrated that PRP can exert protective effects against oX-LDL-induced injury by modulating caspase-3 expression and up-regulating PI3K/Akt/eNOS phosphorylation and the Bcl-2/Bax ratio. Our data suggest that PRP also enhances the self-repair capacity of HUVECs, and our findings provide novel insight regarding the mechanisms through which PRP exerts protective effects against oX-LDL-induced injury. results in less immune rejection than other treatments and can be easily accessible.
Acknowledgments
This investigation was financed by the National Natural Science Fund (NO. 81070257) and the Science and Technology Planning Project of Guangdong Province (NO. 2014A020212481).
Footnotes
Conflicts of interest: Authors state no conflict of interest.
References
- [1].Vemulapalli S., Patel M.R., Jones W.S.. Limb ischemia. cardiovascular diagnosis and management from head to toe. Curr Cardiol Rep. 2015;17:611. doi: 10.1007/s11886-015-0611-y. [DOI] [PubMed] [Google Scholar]
- [2].Ma X., Qiu R., Dang J., Li J., Hu Q., Shan S.. et al. ORMDL3 contributes to the risk of atherosclerosis in Chinese Han population and mediates oxidized low-density lipoprotein-induced autophagy in endothelial cells. Sci Rep. 2015:5, 17194. doi: 10.1038/srep17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lu J., Xiang G.D., Liu M., Mei W., Xiang L., Dong J.. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis. 2015;243:438–448. doi: 10.1016/j.atherosclerosis.2015.10.020. [DOI] [PubMed] [Google Scholar]
- [4].Gimbrone M.A. Jr, García-Cardeña G.. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huang Y.Q., Cai A.P., Chen J.Y., Huang C., Li J., Feng Y.Q.. The relationship of plasma miR-29a and oxidized low density lipoprotein with atherosclerosis. Cell Physiol Biochem. 2016;40:1521–1528. doi: 10.1159/000453202. [DOI] [PubMed] [Google Scholar]
- [6].Ahmad N., Thomas G.N., Chan C., Gill P.. Ethnic differences in lower limb revascularisation and amputation rates. Implications for the aetiopathology of Atherosclerosis. 2014;233:503–507. doi: 10.1016/j.atherosclerosis. [DOI] [PubMed] [Google Scholar]
- [7].Ma L., Zhu X.F., Wu Y.Y., Chen K.J., Shi D.Z., Yin H.J.. Protective effect of propyl gallate against oxidized low-density lipoprotein-induced injury of endothelial cells. Chin J Integr Me. 2015;21:299–306. doi: 10.1007/s11655-014-1980-6. [DOI] [PubMed] [Google Scholar]
- [8].Wang G.F., Shi C.G., Sun M.Z., Wang L., Wu S.X., Wang H.F.. et al. aTetramethylpyrazine attenuates atherosclerosis development and protects endothelial cells from ox-LDL. Cardiovasc Drugs Ther. 2013;27:199–210. doi: 10.1007/s10557-013-6440-6. [DOI] [PubMed] [Google Scholar]
- [9].Liu J.H., Lü Y., Zhang L.K., Du J., Zeng X.J., Hao G.. et al. [Oxidized low density lipoprotein and peroxisome proliferator-activated receptor α induced endogenous fibroblast growth factor 21 upregulation is protective against apoptosis in cardiac endothelial cells] Zhonghua xin xue guan bing zhi. 2010;38:1113–1117. [PubMed] [Google Scholar]
- [10].Kuzuya M., Ramos M.A., Kanda S., Koike T., Asai T., Maeda K.. et al. VEGF Protects against oxidized LDL toxicity to endothelial cells by an intracellular glutathione-dependent mechanism through the KDR receptor. Arterioscler Thromb Vasc Biol. 2001;21:765–770. doi: 10.1161/01.atv.21.5.765. [DOI] [PubMed] [Google Scholar]
- [11].Fu R., Wang Q., Guo Q., Xu J., Wu X.. XJP-1 protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by inhibiting NADPH oxidase subunit expression and modulating the PI3K/Akt/eNOS pathway. Vascul Pharmacol. 2013;58:78–86. doi: 10.1016/j.vph.2012.08.004. [DOI] [PubMed] [Google Scholar]
- [12].Jiang Y.H., Yang C.H., Li W., Wu S., Meng X.Q., Li D.N.. Aqueous extracts of tribulusterrestris protects against oxidized low-density lipoprotein-induced endothelial dysfunction. Chin J Integr Med. 2016;22:193–200. doi: 10.1007/s11655-015-2321-0. [DOI] [PubMed] [Google Scholar]
- [13].Italiano J.E. Jr, Richardson J.L., Patel-Hett S., Battinelli E., Zaslavsky A., Short S.. et al. Angiogenesis is regulated by a novel mechanism: pro-and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Klement G.L., Yip T.T., Cassiola F., Kikuchi L., Cervi D., Podust V.. et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113:2835–2842. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pintucci G., Froum S., Pinnell J., Mignatti P., Rafii S., Green D.. Trophic effects of platelets on cultured endothelial cells aremediated by platelet-associated fibroblast growth factor-2 (FGF-2) and vascularendothelial growth factor (VEGF) Thromb Haemost. 2002;88:834–842. [PubMed] [Google Scholar]
- [16].Schär M.O., Diaz-Romero J., Kohl S., Zumstein M.A., Nesic D.. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res. 2015;473:1635–1643. doi: 10.1007/s11999-015-4192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Assirelli E., Filardo G., Mariani E., Kon E., Roffi A., Vaccaro F.. et al. Effect of two different preparations of platelet-rich plasma on synoviocytes. Knee Surg Sports Traumatol Arthrosc. 2015;23:2690–2703. doi: 10.1007/s00167-014-3113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kamakura T., Kataoka J., Maeda K., Teramachi H., Mihara H., Miyata K.. et al. Platelet-rich plasma with basic fibroblast growth factor for treatment of wrinkles and depressed areas of the skin. Plast Reconstr Surg. 2015;136:931–939. doi: 10.1097/PRS.0000000000001705. [DOI] [PubMed] [Google Scholar]
- [19].Gentile P., Cole J.P., Cole M.A., Garcovich S., Bielli A., Scioli M.G.. et al. Evaluation of not-activated and activated PRP in hair loss treatment: role of growth factor andcytokine concentrations obtained by different collection systems. Int J Mol Sci. 2017;18:408. doi: 10.3390/ijms18020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schneppendahl J., Jungbluth P., Sager M., Benga L., Herten M., Scholz A.. et al. Synergistic effects of HBO and PRP improve bone regeneration with autologous bone grafting. Injury. 2016;47:2718–2725. doi: 10.1016/j.injury.2016.09.039. [DOI] [PubMed] [Google Scholar]
- [21].Guillodo Y., Madouas G., Simon T., Le Dauphin H., Saraux A.. Platelet-rich plasma (PRP) treatmentof sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2015;5:284–288. doi: 10.11138/mltj/2015.5.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kakudo N., Morimoto N., Kushida S., Ogawa T., Kusumoto K.. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol. 2014;47:83–89. doi: 10.1007/s00795-013-0045-9. [DOI] [PubMed] [Google Scholar]
- [23].Mammoto T., Jiang A., Jiang E., Mammoto A.. Platelet rich plasma extract promotes angiogenesis through the angio poietin1-tie2 pathway. Microvasc Res. 2013;89:15–24. doi: 10.1016/j.mvr.2013.04.008. [DOI] [PubMed] [Google Scholar]
- [24].Rival C., Theas M.S., Guazzone V.A., Lustig L.. Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J Reprod Immunol. 2006;70:43–58. doi: 10.1016/j.jri.2005.10.006. [DOI] [PubMed] [Google Scholar]
- [25].Marx R.E.. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- [26].Huber S.C., Cunha J.L., Montalvão S.A.L., da Silva L.Q., Paffaro A.U., da Silva F.A.R.. et al. In vitro study of the role of thrombin in platelet rich plasma (PRP) preparation: utility for gel formation and impact in growth factors release. J Stem Cells Regen Me. 2016;2:12. doi: 10.46582/jsrm.1201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Qin M., Luo Y., Meng X.B., Wang M., Wang H.W., Song S.Y.. et al. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: an insight into PI3K/Akt activation and STAT3 signaling pathways. Vascul Pharmacol. 2015;70:23–34. doi: 10.1016/j.vph.2015.03.002. [DOI] [PubMed] [Google Scholar]
- [28].Mitra S., Deshmukh A., Sachdeva R., Lu J., Mehta J.L.. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am J Med Sci. 2011;342:135–142. doi: 10.1097/MAJ.0b013e318224a147. [DOI] [PubMed] [Google Scholar]
- [29].Pirillo A., Norata G.D., Catapano A.L.. LOX-1, OxLDL, and atherosclerosis. Mediators Inflam. 2013:152786. doi: 10.1155/2013/152786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yao Y., Wang Y., Zhang Y., Liu C.. Klotho ameliorates oxidized low density lipo protein (ox-LDL)-induced oxidativestress via regulating LOX-1 and PI3K/Akt/eNOS pathways. Lipids Health Dis. 2017;16:77. doi: 10.1186/s12944-017-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang H.H., Chen Y., Gao C.Y., Cui Z.T., Yao J.M.. Protective effects of MicroRNA-126 on human cardiac microvascular endothelial cells against hypoxia/reoxygenation-induced injury and inflammatory response by activating PI3K/Akt/eNOS signaling pathway. Cell Physiol Biochem. 2017;42:506–518. doi: 10.1159/000477597. [DOI] [PubMed] [Google Scholar]
- [32].Ahsan A., Han G., Pan J., Liu S., Padhiar A.A., Chu P.. et al. Phosphocreatine protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Apoptosis. 2015;20:1563–1576. doi: 10.1007/s10495-015-1175-4. [DOI] [PubMed] [Google Scholar]
- [33].Andia I., Rubio-Azpeitia E., Maffulli N.. Platelet-rich plasma modulates the secretion of inflammatory/angiogenic proteins by inflamed tenocytes. Clin Orthop Relat Res. 2015;473:1624–1634. doi: 10.1007/s11999-015-4179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kakudo N., Minakata T., Mitsui T., Kushida S., Notodihardjo F.Z., Kusumoto K.. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122:1352–1360. doi: 10.1097/PRS.0b013e3181882046. [DOI] [PubMed] [Google Scholar]
- [35].Ou H.C., Lee W.J., Lee S.D., Huang C.Y., Chiu T.H., Tsai K.L.. et al. Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-inducedapoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol Appl Pharmacol. 2010;248:134–143. doi: 10.1016/j.taap.2010.07.025. [DOI] [PubMed] [Google Scholar]
- [36].Zhou J., Abid M.D.N., Xiong Y., Chen Q., Chen J.. Ox-LDL downregulates eNOS activity via LOX-1-mediated endoplasmic reticulum stress. Int J Mol Med. 2013;32:1442–1450. doi: 10.3892/ijmm.2013.1513. [DOI] [PubMed] [Google Scholar]
- [37].Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A.M.. Activation of nitric oxide synthase inendothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- [38].Veas C., Jara C., Willis N.D., Pérez-Contreras K., Gutierrez N., Toledo J.. et al. Overexpression of LOXIN protects endothelial progenitor cells from apoptosis induced by oxidized low density lipoprotein. J Cardiovasc Pharmacol. 2016;67:326–335. doi: 10.1097/FJC.0000000000000358. [DOI] [PubMed] [Google Scholar]
- [39].Sugawara T., Fujimura M., Noshita N., Kim G.W., Saito A., Hayashi T.. et al. Neuronal death/survival signaling pathways in cerebral ischemia. Neurorx. 2004;1:17–25. doi: 10.1602/neurorx.1.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hockenbery D., Nuñez G., Milliman C., Schreiber R.D., Korsmeyer S.J.. Bcl-2 is an inner mitochondrialmembrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]