Abstract
To evaluate the pharmacoeconomics of three therapeutic schemes in treating anti-tuberluosis therapy -induced liver injury (anti-TB DILI).
Methods
In the construction of a decision tree model, the efficacy and safety parameters came from the results of the randomized, controlled trial conducted here, the effect parameters were derived from expert advice, and the cost parameters, such as usage specification, number, and unit price, came from literature, expert advice, and so on.
Results
The cost-effectiveness analysis (CEA) based on the effect degrees showed that bicyclol had the best effect (4.63562). The incremental cost-effectiveness ratio (ICER) (206.03270) of bicyclol was the lowest. The cost-effectiveness ratio of silibinin was the lowest (68.59987). The CEA based on the complete normalization rate showed that bicyclol had the highest complete normalization rate (83.562%), the lowest cost-effectiveness ratio (4.63627), and the smallest ICER (4.63504). Sensitivity analyses proved the robustness of the results.
Conclusions
Bicyclol is the most cost-effective therapy and the preferred choice for treating anti-TB DILI.
Keywords: Cost-effectiveness analysis, Decision trees analysis, Anti-TB DILI, Pharmacoeconomics, Prospective
1. Introduction
In 2014, about 1.5 million people died of tuberculosis, which has become the number one cause of death from infectious diseases in the world [1]. Tuberculosis is a major infectious disease in China, with a prevalence of 459 per 100,000. China has the second-highest incidence of tuberculosis in the world, with up to 1,300,000 new cases each year, accounting for 14.3% of new cases in the world. Tuberculosis has become a major public health and social problem in China. China is one of 22 countries in the world with a high burden of tuberculosis [2, 3].
A combination of anti-tuberculosis drugs is needed to treat tuberculosis. Adverse reactions may occur during anti-tuberculosis treatment, which has become a serious problem for tuberculosis treatment staff, especially basic-level medical workers. Anti-tuberluosis drug-induced liver injury (anti-TB DILI) is the most common and the most harmful adverse reaction [3, 4].
The prevalence of anti-TB DILI among Chinese patients is 8%-30%. The clinical manifestations are loss of appetite, nausea, vomiting, fatigue, discomfort or pain in the liver area, and increased level of aminotransferase. In severe cases, liver failure may occur, which may be life-threatening. If not handled properly, the liver injury may not only cause great pain but also cause some patients to terminate anti-tuberculosis treatment or to change therapeutic schemes, thus reducing the efficacy of the treatment and leading to drug resistance, which may affect the evolution of the disease and the prognosis of the patient [5]. Medical consultations, treatment, and hospitalization of such patients due to anti-TB DILI increase the financial burden on their families and the economic burden on society.
Clinicians attach great importance to this problem. The identification and timely treatment of anti-TB DILI, as well as the reasonable application of anti-liver injury drugs in anti-tuberculosis treatment, can not only improve patient compliance, but also ensure the smooth progress of the anti-tuberculosis treatment and increase the recovery rate [3,6], thus reducing the financial burden on patients and obtaining good cost-effectiveness. The diagnosis and treatment guidelines for and the expert advice on anti-TB DILI point out that for mild-to-moderate liver cell damage and mixed-type anti-TB DILI, based on the degree of abnormality of alanine aminotransferase (ALT), different treatment schemes can be adopted with clinical symptoms and signs as a reference. Treatment schemes include liver-protecting therapy with bicyclol, glycyrrhizin or silibinin [6, 7].
Starting in the United States in the late 1970s [8], pharmacoeconomics has been studied and applied widely in more and more countries with the sharp increase in global medical expenses. Pharmacoeconomics is the evaluation of drug resources through the evaluation of the utilization level of drug resources, and provides the theoretical basis for scientific decision-making. At present, the prospective observational study is considered the ideal standard for pharmacoeconomic research [9]. The decision analysis approach is an effective tool to choose the optimal scheme from a series of therapeutic schemes.
As the anti-TB DILI-related pharmacoeconomic research indicates, bicyclol has the advantage of cost-effectiveness [10, 11] for treating anti-TB DILI compared with diammonium glycyrrhizinate enteric-coated capsules, silibinin capsules, polyene phosphatidylcholine capsules, and tiopronin enteric-coated tablets. Most of the clinical studies are based on meta-analyses conducted on searches of the literature [12, 13]. There are fewer studies on the prospective economics or evaluation of the prospective pharmacoeconomics aimed at the treatment of anti-TB DILI.
We performed a prospective pharmacoeconomic study of different treatments for anti-TB DILI. The cost-effectiveness of three commonly used and efficient anti-liver injury drugs were evaluated: diammonium glycyrrhizinate enteric-coated capsules, bicyclol tablets, and silibinin capsules. The aim of this study was as follows: (1) Identify an economic, reasonable, safe, efficient, and optimal clinical pathway. (2) Reduce the burden of anti-TB DILI patients. (3) Provide a scientific and rational medical decision-making basis for clinicians. (4) Optimize the use of health resources and medical expenses.
2. Patients and methods
2.1. Ethics statement and research design
The study was carried out at the Sixth People’s Hospital of Zhengzhou, the Zhoukou Infectious Disease Hospital, and the Hebi Infectious Disease Hospital between November 2014 and January 2016. With the use of SPSS 22.0 statistical software, 225 patients were randomly assigned to 3 treatment groups at a 1:1:1 ratio.
The study was in full compliance with the Declaration of Helsinki and good clinical research practice. The study was approved by the ethics committees of each hospital, and all patients provided written consents after they had been informed of the potential benefits and risks of the study.
2.2. Objective of the study
The inclusion criteria were as follows: (1) Men and women aged 18-65 years. (2) Primary treated pulmonary tuberculosis defined according to the diagnostic criteria of pulmonary tuberculosis (WS288-2008) [14]. (3) Anti-TB DILI defined as ≥6 points according to the Roussel Uclaf Causality Assessment Method scoring system [15]. (4) ALT and/or aspartate aminotransferase (AST) ≥2 times the upper limit of the normal value (ULN), and total bilirubin (TBIL) and alkaline phosphatase (ALP) <3 times ULN before treatment. The ULN of liver function indexes in three research centers were identical as follows: ALT: 40 U/L, AST: 40 U/L, TBIL: 17.1 mmol/L, ALP: 135 U/L.
2.3. Treatment protocols
2.3.1. Grouping and medication
There were 225 patients being treated in 3 research centers at the same time, with 75 patients at each center. The patients were randomly divided into three groups. Patients in group A took 75 mg of bicyclol (Beijing Union Pharmaceutical Factory) orally each day, 25 mg once 3 times a day. Patients in group B took 450 mg of diammonium glycyrrhizinate (Jiangsu Zhengda Tianqing Pharmaceutical Co.) orally each day, 150 mg once 3 times a day. Patients in group C took 210 mg of silibinin (Tianjin Tasly Pharmaceutical Co.) orally each day, 70 mg once 3 times a day. All patients took the medication for 4 weeks and received liver function examinations once a week.
It was planned to give patients active treatment or remove them from the study if they developed severe liver injury or their original disease became serious. However, no such events occurred during the study.
2.3.2. Principles of management of anti-TB DILI
2.3.2.1 Patients whose ALT is <3 times ULN, without dramatic symptoms or jaundice, who are under close observation, can receive anti-liver injury treatment, and conditionally stop the use of anti-tuberculosis drugs that frequently cause liver injury.
2.3.2.2 Patients whose ALT is not <3 times ULN or whose TBIL is not <2 times ULN, who are under close observation, can receive anti-liver injury treatment, and stop the use of anti-tuberculosis drugs that frequently cause liver injury.
2.3.2.3 Patients whose ALT is not <5 times ULN or whose ALT is not <3 times ULN, who have jaundice, nausea, vomiting, fatigue, or other symptoms, or whose TBIL is not <3 times ULN, and should stop the use of all anti-tuberculosis drugs immediately and receive active anti-liver injury treatment.[6]
2.4. Criteria for therapeutic efficacy
The therapeutic efficacy can be evaluated according to the following two criteria.
2.4.1. For patients with only one abnormal liver function index (ALT, AST, TBIL, or ALP), the therapeutic efficacy can be evaluated according to the following parameters
Complete normalization: The abnormal liver function index reverts to normal after treatment.
Partial improvement: The abnormal liver function index decreases by not less than 50% compared with baseline but does not revert to normal after treatment.
No improvement: The abnormal liver function index does not show complete normalization or partial improvement after treatment.
2.4.2. For patients with no less than two abnormal liver function indexes (ALT, AST, TBIL, or ALP), the therapeutic efficacy can be evaluated according to the following parameters
Complete normalization: All abnormal liver function indexes return to normal after treatment.
Partial improvement: Although not all abnormal liver function indexes before treatment revert to normal, at least two liver function indexes decrease by not less than 50% compared with baseline.
No improvement: The abnormal liver function indexes do not show complete normalization or partial improvement after treatment.
2.5. Pharmacoeconomics evaluation
2.5.1. Construction of the decision tree model
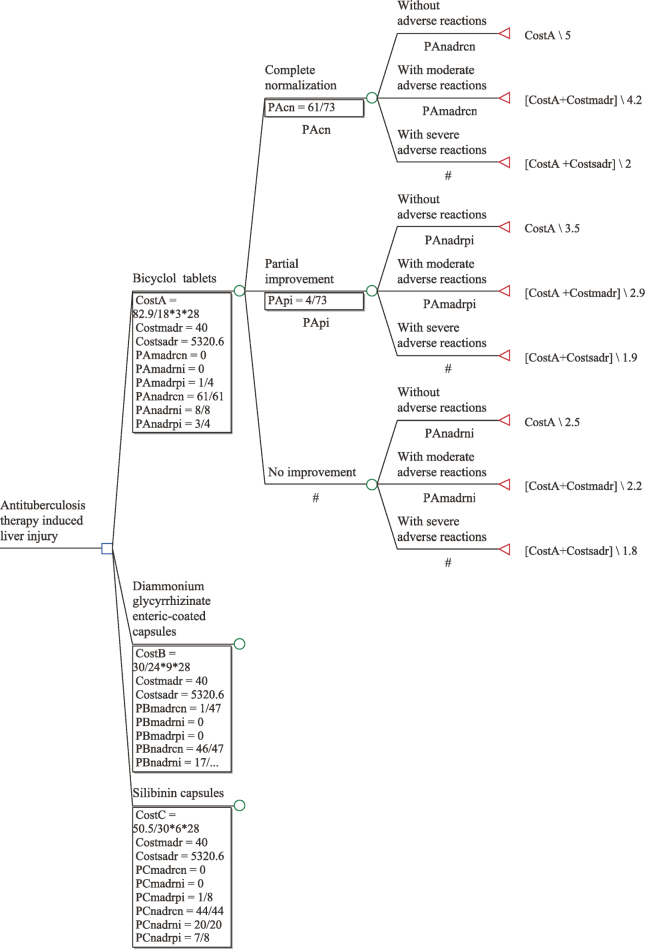
The decision tree model is a mature, dynamic decision analytic model that predicts the value of a target variable based on several input variables. The decision tree consists of possible outcomes produced by the decision point, that is, the branch of the decision tree. The decision node refers to the pharmacologic treatment protocol, and the branch of the decision tree refers to the therapeutic response and its probability. The present study used anti-TB DILI as research object, and the time span was 4 weeks. The TreeAge pro2011 software was used to construct the model. The structure of the decision tree model in this study is shown in Figure 1.
Figure 1.
Decision tree model structure
The decision node is represented with “□” and it is the starting point of the decision problem. The state point is represented with “O,” indicating the status that the scheme has met with. The lines drawn from the state point indicate a variety of possible states that may occur; next to them, all status contents are marked, and the degree of possibility for development is expressed by probability. The terminal point of decision is expressed by “Δ,” next to which the assigned value of each effect degree is marked.
2.5.2. Model parameters
2.5.2.1 Efficacy and safety parameters: The parameters are derived from the rates of complete normalization, partial improvement, no improvement, moderate adverse reactions, and other numerical data from randomized, controlled trials (Table 1).
Table 1.
Comparison of the general conditions of the three groups at baseline
| General condition | A group (n = 73) | B group (n = 71) | C group (n = 72) | Statistic | P |
|---|---|---|---|---|---|
| Age (year) | |||||
| Mean ± SD | 35.9 ± 15.1 | 38.7 ± 13.3 | 41.2 ± 14.9 | 2.391 | 0.094 |
| 45–65 | 24 | 25 | 30 | 1.292 | 0.524 |
| 18–44 | 49 | 46 | 42 | ||
| Sex | |||||
| Male | 60 | 52 | 55 | 1.697 | 0.428 |
| Female | 13 | 19 | 17 | ||
| Serum biochemistry | |||||
| ALT (U/L) | 141.0 ± 41.5 | 139.2 ± 40.4 | 139.4 ± 36.6 | 0.044 | 0.957 |
| AST (U/L) | 131.2 ± 39.9 | 128.2 ± 40.5 | 130.8 ± 37.7 | 0.119 | 0.888 |
| TBIL (mmol/L) | 13.0 ± 5.4 | 12.2 ± 5.9 | 13.2 ± 5.8 | 0.537 | 0.585 |
| ALP (U/L) | 153.1 ± 50.5 | 152.7 ± 53.1 | 154.9 ± 49.1 | 0.037 | 0.693 |
A group received bicyclol tablets, B group received diammonium glycyrrhizinate enteric–coated capsules, and C group received silibinin capsules. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin.
2.5.2.2 Effect parameters: In the analysis of pharmacoeconomics, the effect mainly means the state that the patient has recovered or has been in a stable condition after receiving treatment. Values were assigned for different degrees of effect to conduct a quantitative analysis. The Chinese basic pharmacoeconomics evaluation recommends a medical subjective scale be used to assign and grade the main treatment outcomes [16].
The evaluation criteria of the effect degrees in this study referred to a five-point scale mutually agreed upon by experts. The impact of therapeutic efficacy and safety of patients were taken into consideration. Assuming that the patients were in the same condition before treatment, the state of patients’ therapeutic efficacy after treatment could be divided into nine levels. Complete normalization without moderate or severe adverse reactions was considered the best outcome, and no improvement with severe adverse reactions was considered the worst outcome. Lower marks indicated worse effects (Table 3).
Table 3.
Parameters used in the decision tree model
| Parameters | A group (n = 73) | B group (n = 71) | C group (n = 72) | Sensitive |
|---|---|---|---|---|
| Efficacy and safety parameters | ||||
| Complete normalization rate | 61/73 | 47/71 | 44/72 | |
| Partial improvement rate | 4/73 | 7/71 | 8/72 | |
| No improvement rate | 8/73 | 17/71 | 20/72 | |
| moderate adverse reactions rate | 1/73 | 1/71 | 1/72 | |
| Effect parameters | ||||
| Complete normalization without moderate or severe adverse reactions | 5 | |||
| Complete normalization with moderate adverse reactions | 4.2 | |||
| Complete normalization with severe adverse reactions | 2 | |||
| Partial improvement without moderate or severe adverse reactions | 3.5 | |||
| Partial improvement y with moderate adverse reactions | 2.9 | |||
| Partial improvement with severe adverse reactions | 1.9 | |||
| No improvement without moderate or severe adverse reactions | 2.5 | |||
| No improvement with moderate adverse reactions | 2.2 | |||
| No improvement with severe adverse reactions | 1.8 | |||
| Cost parameters | ||||
| Drug expense (CNY/28 days) | 386.87 | 315 | 282.80 | -10% |
| Processing costs of moderate adverse reactions (CNY) | 40 | |||
| Processing costs of severe adverse reactions (CNY/average hospitalization expense) | 5320.6 |
A group received bicyclol tablets, B group received diammonium glycyrrhizinate enteric–coated capsules, and C group received silibinin capsules.
2.5.2.3 Cost parameters: According to the pharmacoeconomics principle, the cost of a particular treatment protocol includes the direct, indirect, and implicit costs, and it is quantified by monetary unit. The direct cost is the cost of medical services, including the direct medical costs and the direct nonmedical costs. At present, there is no unified approach to convert and calculate the implicit cost (the expenses incurred by the pain, sadness, and mental trauma caused by disease). The indirect costs (the loss incurred by the failure to work due to disease), the implicit costs, and the direct nonmedical costs could be negligible in the study, and only the costs of the anti-liver injury drugs and the processing costs of adverse reactions were calculated to avoid the data deviation. So we established the calculation formula as Ccost = Cdrug+ Cadverse reaction. The costs of the drugs used were calculated based on their prices in local hospitals in 2015. Mild adverse reactions did not need to be treated and thus did not incur any costs. Other adverse reactions were divided into two levels. Patients with moderate adverse reactions required treatment with simple drugs. Based on expert opinion, the cost of treatment of moderate adverse reactions was set at 40 CNY per person. Patients with severe adverse reactions required hospitalization. Hospitalization costs were based on the cost of hospitalization in national secondary-level public hospitals between January and May 2015, according to the National Health and Family Planning Commission of the People’s Republic of China. The cost was set at 5320.5 CNY per person. Because of the short length of the study, there was no discount for extended treatment (Table 3).
2.6. Analytic techniques
2.6.1. Statistical analysis of therapeutic efficacy
The sample size was calculated according to the results of previous studies. The complete normalization rate for the liver function index of diammonium glycyrrhizinate enteric–coated capsules was 51.11%, that of silibinin capsules was 41.50%, and that of bicyclol tablets was 73.10%. The sample size of the test was calculated with PASS 11.0 software with a proportion of 1:1:1, α = 0.05, and test power of 90%. The calculated required sample size was 180 cases. To allow for factors such as loss, 20% was added to the sample size. Thus, the number of selected cases was 225, with 75 cases in each group. The measured data obeyed the normal distribution and were expressed as mean values ± standard deviation. Numerical data were presented as number of cases (percentage). Measured data were compared among three groups by single-factor analysis of variance. Numerical data were analyzed by the chi-square test. Ranked data were compared by the Kruskal-Wallis H test. If any statistically significant differences were found between groups, further pairwise comparisons were conducted. All statistical tests were two-sided. In the comparisons among three groups, a difference was considered statistically significant when P was less than 0.05; for pairwise comparisons, a difference was considered statistically significant when P was less than 0.05/3 (0.017). All statistical analyses were performed with SPSS 22.0 statistical software.
2.6.2. Pharmacoeconomic analysis
TreeAge pro2011 was used to construct and analyze the decision tree model. The study adopted a cost-effectiveness analysis (CEA). CEA is one of the main analytic methods of pharmacoeconomic evaluation. The incremental cost-effectiveness ratio (ICER) is the most frequently used analysis index in CEA; it represents the average incremental cost associated with one additional unit of the measure of effect.
3. Results
3.1. Analysis of efficacy and safety
3.1.1. Comparison of the baseline information of three groups of patients
A total of 225 patients satisfying the inclusion criteria entered the study, 9 patients dropped out, and 216 patients completed the study according to the plan. Of the patients who completed the study, 73 received bicyclol, 71 received diammonium glycyrrhizinate, and 72 received silibinin. There were 35, 38 and 34 patients in each of these groups stopped taking anti-tuberculosis drugs No statistically significant differences were observed between the groups in age, sex ratio, state of illness, or other characteristics (Table 1).
3.1.2. Comparison of clinical efficacy and safety for three groups of patients
After 4 weeks of treatment, the rate of complete normalization in the bicyclol, diammonium glycyrrhizinate, and silibinin groups was 83.6%, 66.2%, and 61.1%, respectively. The rate of complete normalization in the bicyclol group was significantly higher than that in the diammonium glycyrrhizinate group (P = 0.016) and also much higher than that in the silibinin group (P = 0.003). The rate of complete normalization in the diammonium glycyrrhizinate group was not significantly different from that in the silibinin group (P = 0.531). The adverse reactions were as follows: mild reactions included rash and dizziness in five patients in the bicyclol group, edema, itchy skin, and dry mouth in five patients in the diammonium glycyrrhizinate group, and anorexia and nausea in five patients in the silibinin group. The differences between the groups were not statistically significant (P = 0.919). Moderate reactions included skin rash in one patient in the bicyclol group, abdominal distension in one patient in the diammonium glycyrrhizinate group, and “feeling sick” in one patient in the silibinin group. The differences between the groups were not statistically significant (P = 1.000). No patient in the study had severe adverse reactions (Table 2).
Table 2.
Comparison of the efficacy and safety of treatment in the three groups
| Variable | A group (n = 73) | B group (n = 71) | C group (n = 72) | X2 | P |
|---|---|---|---|---|---|
| Therapeutic efficacy n (%) abc | |||||
| Complete normalization individual quantity | 61(83.6%) | 47(66.2%) | 44(61.1%) | ||
| Partial improvement individual quantity | 4(5.5%) | 7(9.9%) | 8(11.1%) | 9.612 | 0.008 |
| No improvement individual quantity | 8(10.9%) | 17(23.9%) | 20(27.8%) | ||
| Safety n (%) with adverse reactions | |||||
| Mild | 5(6.85%) | 6(8.45%) | 5(6.94%) | 0.168 | 0.919 |
| Moderate | 1(1.4%) | 1(1.4%) | 1(1.4%) | 0.000 | 1.000 |
| Severe | 0(0%) | 0(0%) | 0(0%) | ||
A group received bicyclol tablets, B group received diammonium glycyrrhizinate enteric–coated capsules, and C group received silibinin capsules.
Statistical significance of differences between groups in therapeutic efficacy:
a : A vs B, significant (P = 0.016).
b: A vs C, very significant (P = 0.003).
c: B vs C, not statistically significant (P = 0.531).
3.2. Pharmacoeconomic analysis
3.2.1. Table of model parameters (Table 3)
3.2.2. CEA based on the effect degrees
The analysis showed that the three therapeutic regimens were safe and efficient against anti-TB DILI. From the point of view of economics, bicyclol and silibinin were the superior treatment strategies and diammonium glycyrrhizinate was the inferior treatment strategy (Figure 2). Among the superior strategies, the cost-effectiveness ratio of silibinin was the smallest (68.59987), and the calculated effect of bicyclol was 4.63562, which was better than that of silibinin, with a minimum ICER of 206.03270. No significant differences were observed among the drugs in safety, and therefore bicyclol was the preferred choice to treat anti-TB DILI. Silibinin had the lowest effect and the lowest cost. Thus, silibinin was an economical and efficient therapeutic drug for patients in poor economic conditions (Table 4).
Figure 2.
Cost-effectiveness frontier
Table 4.
Cost-effectiveness analysis of three groups based on effect degrees
| Therapeutic regimen | Cost (C, CNY) | DC | Effect(E) | DE | C/E | ICER (DC/DE) |
|---|---|---|---|---|---|---|
| Silibinin capsules | 283.35556 | 0 | 4.13056 | 0 | 68.59987 | 0 |
| Bicyclol tablets | 387.41461 | 104.05906 | 4.63562 | 0.50506 | 83.57348 | 206.03270 |
| Diammonium glycyrrhizinate enteric–coated capsules | 315.56338 | 32.20782 | 4.24225 | 0.11170 | 74.38579 | 288.34746 |
ICER, incremental cost-effectiveness ratio.
3.2.3. CEA based on the rate of complete normalization
Anti-TB DILI is caused by toxic damage or anaphylactic reactions of patients treated with therapeutic doses of anti-tuberculosis drugs. Treating anti-TB DILI can enhance patients’ compliance with anti-tuberculosis therapy and ensure the continuation of anti-tuberculosis treatment. Therefore, for treating anti-TB DILI, complete normalization is more meaningful. As Table 5 indicated, bicyclol had the highest rate of complete normalization (83.562%), the lowest cost-effectiveness ratio (4.63627), and the best ICER (4.63504). Therefore, for patients seeking complete normalization, bicyclol was the best solution (Table 5).
Table 5.
Cost-effectiveness analysis of three groups based on the rate of complete normalization
| Therapeutic scheme | Cost (C, CNY) | DC | Effect(E, %) | DE | C/E | ICER (DC/DE) |
|---|---|---|---|---|---|---|
| Silibinin capsules | 283.35556 | 0 | 61.111 | 0 | 4.63673 | 0 |
| Bicyclol tablets | 387.41461 | 104.05906 | 83.562 | 22.451 | 4.63627 | 4.63504 |
| Diammonium glycyrrhizinate enteric–coated capsules | 315.56338 | 32.20782 | 66.197 | 5.086 | 4.76702 | 6.33255 |
ICER, incremental cost-effectiveness ratio.
3.2.4. Sensitivity analysis
The variables used in pharmacoeconomic research are often difficult to measure accurately, and the uncertainty of the data may bias the analytic result. The object of sensitivity analysis is to determine the influence that the data involved in the analysis has on the conclusion [17]. Drug prices’ reduction is an inevitable trend because of the increased reform of the Chinese medical system. In this study, the sensitivity analysis was conducted on the condition that the drug price decreases by 10%. The result indicated that the reduction in drug prices had no impact on the CEA of all the therapeutic regimens.
4. Discussion
Anti-tuberculosis therapy-induced liver injury (anti-TB DILI) is a pathological process that is caused by toxic damage to liver cells by medications or their metabolites, or by an allergic reaction of the liver to medications or their metabolites, which can occur in any population [6].
The first-line drugs for anti-tuberculosis combined therapy include isoniazid, rifampicin, and pyrazinamide. Isoniazid (INH) is associated with one of the highest incidences of liver failure; it can lead to immune-mediated liver toxicity or autoimmunity [18, 19]. When only isoniazid is used for 1 month, aminotransferase levels increase significantly in 28% of patients. When only rifampicin is used, the incidence of liver injury is about 2%. When these two drugs are used together, the incidence of liver injury is higher than when only isoniazid is used, because rifampicin can promote the generation of toxic metabolites of isoniazid. The hepatotoxicity of pyrazinamide is dose-dependent and can inhibit dehydrogenation from producing free radicals and lead to liver injury by inducing lipid peroxidation [20].
American Thoracic Society(ATS) statement showed that first line anti-TB drugs, especially rifampin, should not be discontinued for mild gastrointestinal complaints. If serum transaminase concentrations are more than five times ULN or more than three times the ULN with jaundice and/or hepatitis symptoms, then potentially hepatotoxic medications should be stopped immediately and the patient evaluated promptly [21]. For patients with severe DILI, the principle of management of anti-TB drug liver injury in China is in line with ATS’ statement, as stop all of antituberculsis drugs. For patients with mild or medium DILI, Chinese experts advise to stop the use of anti-tuberculosis drugs that frequently cause liver injury. In this study, there were 35, 38 and 34 patients in 3 groups stop all of antituberculsis drugs.
This study showed that three therapeutic schemes had favorable efficacy in treating anti-TB DILI, and abnormal liver functions of the patients recovered rapidly. When evaluating a therapeutic scheme from the perspective of pharmacoeconomics, it is necessary to strike a balance between cost and effectiveness by selecting a therapeutic scheme with satisfactory efficacy, good safety, and reasonable cost. Based on the effect degrees, bicyclol had the best effect with the smallest ICER and silibinin had the lowest effect but had the lowest cost and a minimum cost-effectiveness ratio. On the basis of the rate of complete normalization, bicyclol had the best rate of complete normalization, and its ICER was the most advantageous. Therefore, when a better clinical efficacy is needed, bicyclol is the most cost-effective treatment. The sensitivity analysis showed that this result was reliable.
The results of this study were consistent with previous findings [10, 11] on the pharmacoeconomic evaluation of bicyclol. A study by Wang and colleagues [10] suggested that the silibinin capsule was the inferior treatment. Although both the present study and Wang’s study showed that the silibinin capsule had the lowest effect and the lowest complete normalization rate, the dosage of silibinin capsules in Wang’s study was twice that in the present study. The silibinin capsule had the highest cost-effectiveness ratio in Wang’s study because the cost was higher than that of bicyclol and diammonium glycyrrhizinate enteric–coated capsules, while the cost of silibinin in this study was the lowest.
The recovery of liver function in patients with anti-TB DILI ensures the smooth progress of anti-tuberculosis therapy. Silibinin capsules can be used for patients with lower financial resources and lower treatment expectations. But if the patient requires a better recovery of liver function, bicyclol is the preferred therapeutic regimen. A variety of anti-liver injury drugs with different efficacy, safety, and prices are available in China. Anti-TB DILI patients and clinicians should choose appropriate drugs that can not only recover patients’ liver functions and keep their tuberculosis treatment going, but also ensure the economy and safety of the therapy.
According to the Chinese Society of Hepatology guidelines for the diagnosis and treatment of DILI, the principles for DILI treatment included treating DILI with appropriate anti-inflammatory and hepatoprotective agents according to the clinical patterns of DILI [22]. Research confirms that glycyrrhizin has a protective efficacy against liver damage caused by rifampin and isoniazid [23, 24] and has good therapeutic efficacy against anti-TB DILI [25]. Silibinin is a flavonoid derived from the fruit of milk thistle (Silybum marianum), which has many pharmacological actions including resisting lipid peroxidation, eliminating free radicals, maintaining stabilization of the cell membrane, and promoting hepatocellular regeneration [26, 27]. It can effectively prevent anti-TB DILI [28]. Bicyclol has anti-inflammatory and hepatic protective pharmacological action on several experimental liver injuries. Its mechanism of action is closely related to the increase of anti-inflammatory factors, the elimination of free radicals, anti-lipid peroxidation, and the protection of biological membranes and mitochondrial function [29, 30, 31]. Clinical studies have confirmed that bicyclol can prevent and cure anti-TB DILI [32].
5. Conclusions
With the advantage of cost-effectiveness, bicyclol can be used as a safe, efficient, and economical anti-liver injury drug to treat anti-TB DILI. This study explored the prospective economic evaluation of the treatment of anti-TB DILI with anti-liver injury drugs. Because of the limited number of cases included in the study, only the pharmacoeconomic analysis of a 4-week course was conducted, and indirect costs, implicit costs, and direct nonmedical costs were not calculated in this study. It is necessary to carry out a larger-scale and more thorough pharmacoeconomic study to evaluate the economic effect of anti-liver injury drugs in anti-TB DILI treatment.
Footnotes
Conflicts of interest: Authors state no conflict of interest.
References
- [1].Glaziou P., Floyd K., Weil D., Raviglione M.. TB deaths rank alongside HIV deaths as top infectious killer. Int J. Tuberc Lung Dis. 2016;2:143–144. doi: 10.5588/ijtld.15.0985. [DOI] [PubMed] [Google Scholar]
- [2].The technical guidance group of the fifth national tuberculosis epidemiological random sampling survey, the office of the fifth national tuberculosis epidemiological random sampling survey, The report of the fifth national tuberculosis epidemiological random sampling survey in 2010. Chin J. Antituberculosis prevention. 2012;8:485–508. [Google Scholar]
- [3].Donglou X., Yu M., Lizhen Z. Diagnostic manual for adverse reactions of antituberculosis drugs. 1st. People’s Medical Publishing House; Beijing: 2009. [Google Scholar]
- [4].Chengwei C.. Pathogenesis and treatment strategies of drug-induced liver injury. Zhonghua Jie He He Hu Xi Za Zhi. 2013;10:726–728. doi: 10.3760/cma.j.issn.1001-0939.2013.10.004. [DOI] [PubMed] [Google Scholar]
- [5].Heping X., Jin G.. Clinical characteristics of antituberculosis drug-induced liver injury. Chin J. tuberculosis prevention. 2013;7:485–487. [Google Scholar]
- [6].Chinese journal of tuberculosis and respiratory, editorial board, tuberculosis branch of the Chinese Medical Association, Expert advice on diagnosis and treatment of the antituberculosis therapy Induced Liver Injury. Chin J. Tuberculosis and Respiratory Diseases. 2013;10:732–736. doi: 10.3760/cma.j.issn.1001-0939.2013.10.007. [DOI] [Google Scholar]
- [7].Interpretation of guidelines for diagnosis and treatment of drug induced liver injury. 1st. Shanghai Science and Technology Press; 2015. The Drug-Induced Liver Disease Group, Branch Association of Hepatology, Chinese Medical Association; p. Shanghai. [Google Scholar]
- [8].Kermode M.. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004;1:95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- [9].Evaluation guidelines of Chinese pharmacoeconomics by the research group, a guide for China’s pharmaceutical economics evaluation: 2011 edition. Chin J. Pharmaceutical economics. 2011;3:6. doi: 10.3969/j.issn.1673-5846.2011.03.001. [DOI] [Google Scholar]
- [10].Aihua W., Hongbo T., Xinlei L., Ning Z., Xin F.. Evaluation of pharmacoeconomics of oral anti-inflammatory hepatoprotective drugs in the treatment of drug-induced liver injury. Chin J. Gastroenterol Hepatol. 2015;10:1232–1237. doi: 10.3969/j.issn.1006-5709.2015.10.021. [DOI] [Google Scholar]
- [11].Guangyu H., Yuming W.. Pharmacoeconomics profiles of four hepatoprotective drugs used for the treatment of drug-induced liver injury. Chin J. Hepatol. 2014;10:763–768. doi: 10.3760/cma.j.issn.1007-3418.2014.10.010. [DOI] [PubMed] [Google Scholar]
- [12].Martello JL, Pummer TL, Krenzelok EP. Cost minimization analysis comparing enteral N-acetylcysteine to intravenous acetylcysteine in the management of acute acetaminophen toxicity. Clin Toxicol (Phila) 2010;1:79–83. doi: 10.3109/15563650903409799. [DOI] [PubMed] [Google Scholar]
- [13].Senarathna SM, Sri Ranganathan S, Buckley N, Fernandopulle R. A cost effectiveness analysis of the preferred antidotes for acute paracetamol poisoning patients in Sri Lanka. BMC Clin Pharmacol. 2012;12:6. doi: 10.1186/1472-6904-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ministry of Health of the People’s Republic of China, Diagnostic Criteria for Pulmonary Tuberculosis. People’s Republic of China sanitary industry standard: WS 288-2008 [Google Scholar]
- [15].Danan G, Benichou C. Causality assessment of adverse reactions to drugs I, A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- [16].Xiangnan G., Sheng H., Lu X., Luwen S.. The primary exploration of the economic evaluation method for essential drugs in China. Chin Pharmaceutical J. 2011;12:963–966. [Google Scholar]
- [17].Xiaodong K.. Pharmacoeconomics: Concepts, Methods and applications. Foreign medical sciences Pharmaceutical fascicule. 1994;1:18. doi: 10.13220/j.cnki,jipr.1994.01.003. [DOI] [Google Scholar]
- [18].Metushi IG., Uetrecht J.. Isoniazid-induced liver injury and immune response in mice. J Immunotoxicol. 2014;4:383–392. doi: 10.3109/1547691X.2013.860644. [DOI] [PubMed] [Google Scholar]
- [19].Metushi IG., Cai P., Zhu X., Nakagawa T., Uetrecht JP.. A Fresh Look at the Mechanism of Isoniazid-Induced Hepatotoxicity. Clin Pharmacol Ther. 2011;6:911–914. doi: 10.1038/clpt.2010.355. [DOI] [PubMed] [Google Scholar]
- [20].Chengwei C. Drug and toxic liver disease. 2nd. Shanghai Science and Technology Press; Shanghai: 2013. [Google Scholar]
- [21].Saukkonen JJ., Cohn DL., Jasmer RM., Schenker S., Jereb JA., Nolan CM.. et al. An Official ATS Statement: Hepatotoxicity of Antituberculosis Therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- [22].Yuecheng Y., Yimin M., Chengwei C., Jinjun C., Jun C., Wenming C.. et al. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int. 2017;11:221–241. doi: 10.1007/s12072-017-9793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shen C., Cheng X., Li D., Meng Q.. Investigation of rifampicin-induced hepatotoxicity in rat hepatocytes maintained in gel entrapment culture. Cell Biol Toxicol. 2009;3:265–274. doi: 10.1007/s10565-008-9076-8. [DOI] [PubMed] [Google Scholar]
- [24].Shen C, Zhang H, Zhang G, Meng Q. Isoniazid-induced hepatotoxicity in rat hepatocytes of gel entrapment culture. Toxicol Lett. 2006;1:66–74. doi: 10.1016/j.toxlet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- [25].Miyazawa N, Takahashi H, Yoshiike Y, Ogura T, Watanuki Y, Sato M. et al. Effect of glycyrrhizin on anti-tuberculosis drug-induced hepatitis. Kekkaku. 2003;1:15–19. [PubMed] [Google Scholar]
- [26].E-Gazayerly ON, Makhlouf AI, Soelm AM, Mohmoud MA. Antioxidant and hepatoprotective effects of silymarin phytosomes compared to milk thistle extract in CCl4 induced hepatotoxicity in rats. J Microencapsul. 2014;1:23–30. doi: 10.3109/02652048.2013.805836. [DOI] [PubMed] [Google Scholar]
- [27].Parveen R, Baboota S, Ali J, Ahuja A, Vasudev SS, Ahmad S. Effects of silymarin nanoemulsion against carbon tetrachloride-induced hepatic damage. Arch Pharm Res. 2011;5:767–774. doi: 10.1007/s12272-011-0510-8. [DOI] [PubMed] [Google Scholar]
- [28].Li J., Lin WF, Pan YY, Zhu XY. Protective effect of silibinin on liver injury induced by antituberculosis drugs. Zhonghua Gan Zang Bing Za Zhi. 2010;5:385–386. doi: 10.3760/cma.j.issn.1007-3418.2010.05.019. [DOI] [PubMed] [Google Scholar]
- [29].Zhao J., Chen H., Li Y.. Protective effect of bicyclol on acute alcohol-induced liver injury in mice. European Journal of Pharmacology. 2008(1–3):322–331. doi: 10.1016/j.ejphar.2008.02.059. [DOI] [PubMed] [Google Scholar]
- [30].Wang HP., Li Y.. Protective effect of bicyclol on acute hepatic failure induced by lipopolysaccharide and D-galactosamine in mice. European Journal of Pharmacology. 2006(1–3):194–201. doi: 10.1016/j.2005.12.080. [DOI] [PubMed] [Google Scholar]
- [31].Liu G.T., Li Y., Wei HL., Zhang H., Xu JY., Yu LH.. Mechanism of protective action of bicyclol against CCl4-induced liver injury in mice. Liver International. 2005;25:872–879. doi: 10.1111/j.1478-3231.2005.01103.x. [DOI] [PubMed] [Google Scholar]
- [32].Chu N.H., Li L., Zhang X., Gu J., Du YD., Cai C.. et al. Role of bicyclol in preventing drug-induced liver injury in tuberculosis patients with liver disease. Int J. Tuberc Lung Dis. 2015;4:475–480. doi: 10.5588/ijtld.14.0579. [DOI] [PubMed] [Google Scholar]