Abstract
The phytochemical analysis of the polar extracts of Cedrus brevifolia needles yielded 20 compounds, namely from the methanol extract we isolated three flavonoids (1–3), one hydrolysable tannin (4), eleven phenolic derivatives (5–15) and one apocarotenoid (16), while from the methanol: water (5:1) extract we isolated four flavonoids (17–20). Chemical structures of all isolated compounds were determined by 1D, 2D-NMR (1 Dimension, 2 Dimensions Nuclear Magnetic Resonance) and UV-Vis (Ultraviolet-Visible) spectroscopy. Furthermore, the antioxidant potentials and the anti-inflammatory activities of both crude extracts and isolates were evaluated through DPPH radical scavenging capability, linoleic acid lipid peroxidation inhibition, and soybean LOX inhibition assays. This is the first report on the chemical profile of C. brevifolia needles. Catechin was the main compound derived from the methanol extract. According to our results, 4-O-β-d-glucopyranyl trans-p-coumaric acid and taxifolin were the most active ingredients.
Keywords: C. brevifolia; flavonoids; catechin; simple phenols; apocarotenoids; bioactivity, antioxidant; reducing power; total antioxidant capacity; reactive oxygen species
1. Introduction
Cedrus brevifolia (Pinaceae) is an important endemic tree of Cyprus flora with narrow distribution. It is well-differentiated from other species of the genus based on morphological and eco-physiological traits, such as short needles and slow growth, resistance to aphids, and the highest tolerance to drought in all cedar species [1]. In ancient times, Theophrastus (371–287 B.C.) was the first to mention the existence of Cedrus in Cyprus, Phoenicia and Syria as an important forest tree of that period [2]. Cedar wood has been highly appreciated since ancient times for building temples, palaces, and ships [2,3]. The Roman author and architect Marcus Vitruvius Pollio wrote that the material used for the roof of the Greek temple of Artemis in Ephesus was from Cedrus wood [4]. In ancient Egypt, it was known that cedar was very resistant to insects and pathogenic microorganisms, so they used its essential oil to mummify corpses [3].
In South-West Turkey the tar extract from C. libani, under the common name katran, is used internally and externally to heal wounds, fight parasites, and cure various diseases [3]. It is noteworthy that the tar extract has been proposed to be recognized for its therapeutic value by the French pharmacopoeia [5]. C. brevifolia bark is a source of compounds with antioxidant capacity and 15-lipoxygenase inhibitory activity [6]; C. deodara needles water extract exhibits antibacterial activity [7].
Taking in consideration the importance and uses of Cedrus species, this study was designed to investigate the chemical composition of the methanol and the aqueous methanol [MeOH:H2O (5:1)] extracts prepared from needles of C. brevifolia and to evaluate their total antioxidant capacity and anti-inflammatory activity, as well as of the isolates.
2. Results
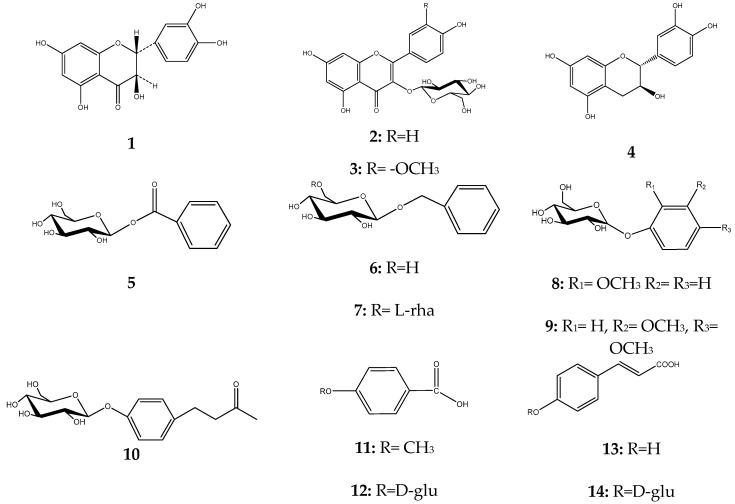
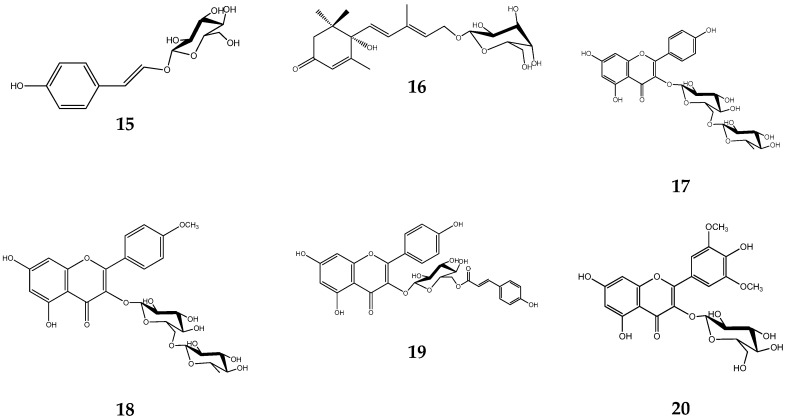
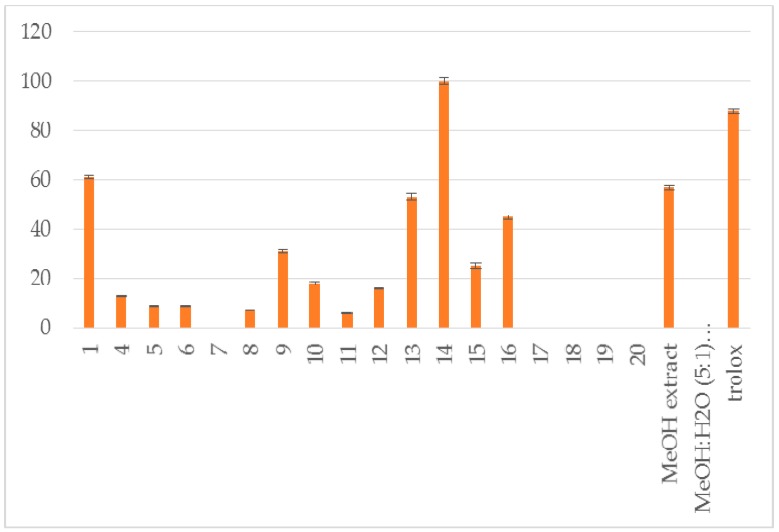
The methanol extract (6.5 g) yielded taxifolin (1) [8], astragalin (2) [9], isorhamnetin 3-O-β-d-glucoside (3) [10], (−)-catechin (4) [11], benzoate glucoside (5) [12], benzyl-β-d-glucoside (6) [13], benzyl-β-d-rutinoside (7) [14], 2-methoxy-phenyl-β-d-glucoside (8) [15], 3,4-dimethoxyphenyl-β-d-glucoside (9) [16], raspberry ketone (10) [17], p-anisic acid (11) [18], 4-hydroxybenzoic acid 4-O-β-d-glucoside (12) [19], p-coumaric acid (13, 6.0 mg) and its glucoside (14) [20,21], trans-vaginoside (15) [22] and abscisic alcohol glucoside (16) [23]. The methanol:water (5:1) extract afforded kaempferol-3-O-β-rutinoside (17) [24], kaempferide-3-O-β-rutinoside (18) [25], tiliroside (19) [26], and syringetin 3-O-β-d-glucoside (20) [27] (Figure 1). Furthermore, both crude extracts and isolated compounds were examined for their inhibitory potency on lipoxygenase and lipid peroxidation, as well as for their antioxidant activity, in comparison to known antioxidants, e.g., caffeic acid, nor-dihydroguaretic acid (NDGA) and trolox. AAPH (2,2′-azobis (2-amidino-propane) dihydrochloride), DPPH (2,2-diphenyl-1-picrylhydrazyl) and soybean lipoxygenase (LOX) assays were used for the tests. This is the first report on the chemical profile of C. brevifolia needles. Catechin was the main compound derived from the methanol extract (See Supplementary Data, Table S1. According to our results of the in vitro tests, both extracts were found to possess potential antioxidant activity due to their high phenolic contents. Moreover, 4-O-β-d-glucopyranyl trans-p-coumaric acid and taxifolin were the most active ingredients (Figure 2, Figure 3 and Figure 4, Table 1).
Figure 1.
Structures of isolated compounds from C. brevifolia needles.
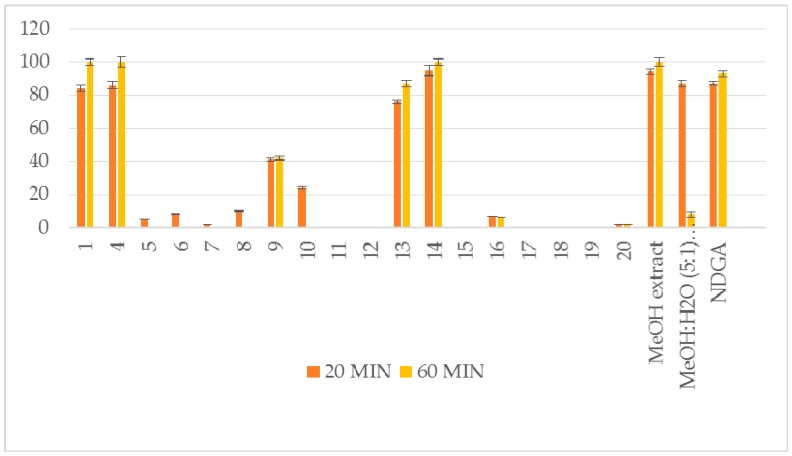
Figure 2.
Reducing ability (RA %) at 0.1 mM. Interaction with DPPH.
Figure 3.
% Inhibition of soybean lipoxygenase (LOX) at 0.1 mM.
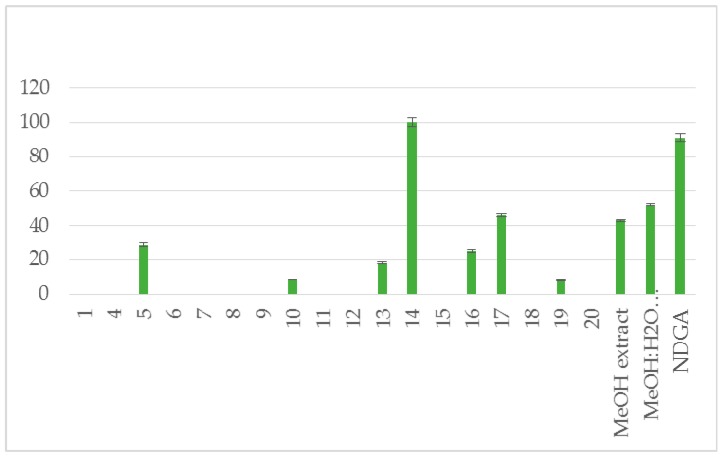
Figure 4.
Percent inhibition of lipid peroxidation induced by AAPH at 0.1 mM.
Table 1.
In vitro reducing ability (RA %) in DPPH assay, soybean lipoxygenase inhibition (% LOX inhbt) and anti-lipid peroxidation activity (A-LP %).
| Compound | RA # % ± SD, DPPH, (20 min) | RA # % ± SD, DPPH, (60 min) | % LOX ± SD Inhbt @ (0.1 mM) | A-LP % ± SD @ (0.1 mM) |
|---|---|---|---|---|
| 1 | 84 ± 1.8 * | 100 ± 2.1 ** | no | 61 ± 0.6 ** |
| 4 | 86 ± 2.2 ** | 100 ± 3.1 ** | no | 13 ± 0.3 * |
| 5 | 5 ± 0.1 * | no | 29 ± 1.1 ** | 9 ± 0.1 * |
| 6 | 8 ± 0.3 ** | no | no | 9 ± 0.1 * |
| 7 | 2 ± 0.0 * | no | no | no |
| 8 | 9.8 ± 0.4 * | no | no | 7 ± 0.1 * |
| 9 | 41 ± 1.0 ** | 42 ± 1.3 ** | no | 31 ± 0.7 * |
| 10 | 24 ± 0.8 ** | no | 8.5 ± 0.1 ** | 18 ± 0.6 ** |
| 11 | no | no | no | 6 ± 0.1 * |
| 12 | no | no | no | 16 ± 0.1 * |
| 13 | 76 ± 1.1 ** | 87 ± 1.9 ** | 18 ± 0.6 ** | 53 ± 1.2 ** |
| 14 | 95 ± 3.2 ** | 100 ± 2.1 ** | 100 ± 2.5 ** | 100 ± 1.4 ** |
| 15 | no | no | no | 25 ± 1.0 * |
| 16 | 7 ± 0.1 * | 6 ± 0.0 * | 25±1.2 ** | 45 ± 0.9 ** |
| 17 | nt # | nt # | 46 ± 1.0 ** | nt # |
| 18 | nt # | nt # | no | no |
| 19 | no | no | 8 ± 0.3 * | no |
| 20 | 2 ± 0.0 * | 2 ± 0.0 * | no | no |
| MeOH extract | 94 ± 1.9 ** | 100 ± 2.5 ** | 43 ± 0.4 * | 57 ± 1.0 ** |
| MeOH:H2O (5:1) extract | 87 ± 2.1 ** | 8 ± 1.8 ** | 52 ± 0.7 ** | no |
| NDGA | 87 ± 1.1** | 93 ± 1.8 ** | 91 ± 2.3 ** | |
| trolox | 88 ± 0.9 ** |
# Final concentration 0.1mM; no: no activity under the experimental conditions; * p < 0.05; ** p < 0.01; nt #: not tested (The amount of the compounds was very small for the experiments to be performed. Thus, we decided for these compounds to test only their enzyme inhibitory activity for the sake of comparison); significant differences are relative to the solvent control.
3. Discussion
Overall, 20 compounds were isolated from C. brevifolia needles. The isolates were categorized as simple phenols, polyphenolic derivatives, and one apocarotenoid. Taking into account the phenolic nature of the isolates, we decided to evaluate their in vitro antioxidant activity using two different antioxidant assays: (a) interaction with the stable free radical DPPH, as this method can be used for polar and nonpolar constituents [28], (b) interaction with the water-soluble azo compound AAPH in order to measure the radical-scavenging activity in vitro [29]. The antioxidant ability of the isolates was measured in comparison to positive controls, such as NDGA and trolox. The results are shown in Table 1 and Figure 2, Figure 3 and Figure 4. The interaction, which indicates their radical scavenging ability in an iron-free system, was measured at 100μM after 20 and 60 min. In the DPPH assay, particularly effective antioxidants are the phenoxide anions from phenolic compounds like compounds 1, 4, 13 and 14, as well as nor-dihydroguaretic acid (NDGA), which was used as a reference. For these compounds it was observed an increase in their antioxidant activity after 60 min. Methanol and methanol: water extracts presented high reducing activity. This could be correlated to the presence of phenolic derivatives. The rest isolates did not present any interesting result. Due to low amounts, compounds 2 and 3 were not tested.
The % inhibition of lipid peroxidation given in Table 1indicates three potent isolates, 1, 13 and 16. The methanol extract also exhibits anti lipid peroxidation activity.
We also decided to further evaluate the presented isolates and extracts for their ability to inhibit soybean LOX since most of the LOX inhibitors are antioxidants or free radical scavengers. Perusal of the % inhibition values (Table 1) shows that the most potent inhibitor is isolate 14 followed by 17 which seem to be less potent. It should to be noticed that both methanol extracts are almost equipotent. This inhibition is related to their antioxidant ability.
The investigation revealed that the polar extracts of C. brevifolia needles are abundant in phenolic compounds, which could explain its strong antioxidant activity.
4. Materials and Methods
4.1. Plant Material
C. brevifolia needles were collected on April 2013 from Cedar valley near Paphos (Cyprus) and authenticated by Mr. Konstantinos Nikolaou. A voucher specimen is kept at the Herbarium of Department of Forests, Cyprus, under the number: CYP 1467.
4.2. Equipment and Reagents
Optical rotation was recorded on a Perkin Elmer 341 polarimeter. The [α]D values were obtained in methanol at 20 °C. UV spectra were recorded on a Shimadzu UV-160A spectrophotometer, according to [30] (1970). IR spectra were carried out by Perkin-Elmer Paragon 500 FT-IR spectrophotometer (PerkinElmer, Inc., Waltham, MA, USA). 1H, 13C and 2D-NMR spectra were recorded on a Bruker DRX 400 (Bruker BioSpin GmbH, Silberstetten, Germany), and on a Bruker AC 200 (50.3 MHz for 13C-NMR) spectrometers at 295 K. Chemical shift are reported in ppm (δ) using the residual solvent signal (δH 3.31 in 1H and δC 49.0 in 13C, CD3OD) as reference. Correlation spectroscopyΥ (COSY); Heteronuclear Single Quantum Correlation (HSQC); Heteronuclear Multiple Bond Correlation (HMBC); Nuclear Overhauser Effect Spectroscopy (NOESY); Rotating-frame Overhauser Effect Spectroscopy (ROESY) experiments were performed using standard Bruker microprograms. Vacuum liquid chromatography (VLC) was performed on a silica gel (Merck: 43–63 μm) (Merck KGaA, Darmstadt, Germany) [31], column chromatography (CC) on silica gel 60H (SDS: 40–63 μm), Cellulose (Merck, Art. 2330) (Merck KGaA, Darmstadt, Germany) and Sephadex LH 20 (Pharmacia, Sweden). Gradient elution with the solvents mixtures indicated in each case. Semi-preparative RP18-HPLC (Reversed Phase18-High Liquid Performance Chromatography) was performed on a HPLC system: PU-2080 pump Plus (JASCO, Tokyo, Japan); refractive index detector RID-10A (Shimazdu, Kyoto Japan); software: Clarity (JASCO, Tokyo, Japan), with Kromasil RP-18 columns (i.d.:10 mm, length: 250 mm, 10 µm); flow rate 1.5 mL/min. Prep. Thin Layer Chromatography plates pre coated with silica gel 60 (Merck, Art. 5721). Fractions monitoring to follow separation was performed by thin layer chromatography (TLC) on silica gel 60 F254 (Merck, Art. 5554) and cellulose (Merck Art. 5552). Compounds were detected using UV absorbance (λ 254 and λ 365 nm). Vanillin/sulphuric acid reagent (vanillin 5% in H2SO4/MeOH 1:1) and Neu’s reagent [32] were used for detection at TLC chromatography. Analytical solvents were obtained from Panreac Quimica SA (Barcelone, Spain, Italy), while deuterated solvents were purchased from Merck, KGaA (Darmstadt, Germany). Medium-pressure liquid chromatographic (MPLC) separations were carried out using Büchi C-615 system & pump Büchi 688 with reverse phase column packed with SiO2. Desiccators were activated by anhydrous di-phosphorus pentoxide analytical reagent (a.r.) grade (Art. CL 00. 0631; Chem-Lab, N.V., Belgium).
4.3. Equipment and Reagents for In Vitro Experiments
Soybean lipoxygenase, sodium linoleate, 2,2-azobis (2-amidinopropane) dihydrochloride (AAPH), 1,1-diphenyl-2-picrylhydrazyl (DPPH) were obtained from Sigma Chemical, Co. (St. Louis, MO, USA). For the in vitro tests, UV-Vis spectra were obtained on a 554 double beam spectrophotometer Perkin-Elmer (Perkin-Elmer Corporation Ltd., Lane Beaconsfield, Bucks, UK).
4.4. Extraction and Chromatography
C. brevifolia needles (0.143 kg) were extracted successively with dichloromethane, methanol, and methanol: water 5:1, and concentrated to dryness to yield residues of 23.0 g and 18.3 g, respectively.
10 g of the MeOH extract was submitted to RP18-MPLC (41.0 × 4.0 cm) using a H2O:MeOH gradient system (100% H2O→100% MeOH; steps of 10% MeOH) to yield 11 fractions (A-K) of 500 mL each. Based on TLC results, combined fractions D and E (149.6 mg) were further applied to CC over silica gel eluted with mixtures of DM: MeOH: H2O of increasing polarity (95:5:0.5 to 50:50:5) and gave 41 sub-fractions (D′A-D′L); subfraction D′L (6.0 mg) was identified as compound 13; subfraction D′I (7.6 mg) was subjected to prep. TLC on silica gel with DM:MeOH:H2O 7:3:0.3 and afforded compound 8 (4.0 mg). Fraction H (236.7mg) was subjected to repeated CC over Sephadex LH-20 (MeOH 100%) and silica gel (DM: MeOH: H2O 95:5:0.5 to 50:50:5) and yielded compounds 11 (3.0 mg), 15 (2.8 mg). Fraction I (1.5 g) was submitted to CC over silica gel using mixtures of DM: MeOH:H2O (100:0:0 to 0:0:100) and 15 fractions were obtained. Subfraction IF (9.1 mg; eluted with DM: MeOH:H2O 80:20:2) was identified as compound 1 (9.1 mg); subfraction II (75.0 mg; eluted with DM: MeOH: H2O 70:30:3) was subjected to prep. RP18-HPLC (MeOH:AcOH 5% 4:6) and yielded compounds 2 (0.4 mg; Rt 40.4), 3 (0.5 mg; Rt 39.4), 5 (12.6 mg; Rt 14.8), 6 (3.8 mg; Rt 15.7), 9 (2.7 mg; Rt 9.39), 10 (8.4 mg; Rt 11.4), 12 (1.9 mg; Rt 43.6), 16 (3.4 mg; Rt 28.2); subfraction IJ (358.7 mg; eluted with DM: MeOH:H2O 70:30:3) was subjected to CC over silica gel (EtOAc:MeOH:H2O 100:0:0 to 70:30:3) and afforded compound 4 (19.0 mg); fraction IJK derived from the latter subfraction IJ (17.5 mg; eluted with EtOAc:MeOH:H2O 90:10:1) was further fractionated by RP18-HPLC (MeOH:AcOH 5% 4:6) and yielded compound 7 (2.8 mg; Rt 25.0); subfraction IL (259.6 mg) was submitted to CC over Cellulose (isocratic elution with AcOH:H2O15:85) and afforded compounds 4 (14.5 mg) and 14 (3.5 mg). Sub-fraction IK (142.0 mg) was subjected to CC over silica gel eluted with mixtures of increasing polarity of EtOAc/MeOH and yielded 4 (7.5 mg).
5.0 g of the methanol:water (5:1) extract also was submitted to RP18-MPLC (41.0 × 4.0 cm) using mixtures of decreasing polarity of H2O:MeOH (100:0 to 0:100; steps of 10%) and gave us 11 fractions (A-K). Fraction I (157.0 mg; eluted with H2O:MeOH 20:80) was subjected to CC over Sephadex LH-20 (MeOH 100%), and subfraction IF (70.5 mg) was further analyzed by RP18-HPLC (MeOH:AcOH 5% 4:6) and yielded compound 20 (0.8 mg; Rt 14.0). Subfraction IE (44.0 mg) submitted to CC over silica gel (DM:MeOH 95:5 to 0:100) and yielded compound 17 (1.0 mg; eluted with DM: MeOH 80:20). Fraction J (256.0 mg; eluted with H2O:MeOH 10:90) was applied to CC over silica gel (DM:MeOH: H2O 98:2:0.2 to 0:50:50); combined subfractions JK, JN (6.9 mg; eluted with DM:MeOH: H2O 97:3:0.2 to 95:5:0.3) and subfraction JR (8.2 mg; eluted DM:MeOH: H2O 85:15:0.6), were further fractionated by prep. TLC on silica gel (DM:MeOH:H2O 8:2:0.2) and afforded compounds 19 (5.7 mg) and 18 (2.8 mg), respectively. All obtained extracts, fractions and isolated compounds were evaporated to dryness in vacuum under low temperature, and then were put in activated desiccators with P2O5 until their weights had stabilized.
4.5. DPPH Radical Scavenging Activity
The reducing ability of C. brevifolia extracts and of the isolated compounds was determined using the method described by Pontiki et al. [33]. To an ethanolic solution of DPPH 1mL from an 100µM stock solution (freshly prepared), an equal volume of the extracts (stock solutions 5 mg/mL) and pure compounds (stock solutions 10mM) dissolved in EtOH, were added separately. The mixture was shaken vigorously and incubated at room temperature for 20 and 60 min. Absorbance was measured spectrophotometrically at 517 nm. NDGA was used as reference substance. All tests were performed in triplicate and the averages of the results were calculated.
4.6. AAPH Induced Linoleic Acid Lipid Peroxidation Assay
The anti-lipid peroxidation activity of C. brevifolia extracts and isolated compounds were determined, as reported previously [33], i.e. 10 μL of the 16 mM sodium linoleate was added to the UV cuvette containing 0.93 mL of 0.05 M phosphate buffer, pH 7.4, which was previously pre-thermostated at 37 °C. The oxidation reaction was initiated under air by the addition of 50 μL of 40 mM AAPH solution. Oxidation was carried out in the presence of samples (10 μL) without an antioxidant, and lipid peroxidation was calculated in the presence of same level of EtOH at 234 nm.
4.7. Soybean LOX Inhibition
LOX inhibition of the extracts and isolates were determined by using the method described by Pontiki et al. [33]. The samples, dissolved in EtOH, were incubated at room temperature with sodium linoleate (100 μL) and 200 μL enzyme solution (1/9 × 10−4 w/v in saline). The transformation of sodium linoleate to 13-hydroperoxylinoleate sodium was measured spectrophotometrically at 234 nm and compared with the appropriate reference NDGA (nor-dihydroguaiaretic acid).
4.8. Statistics
Experiments were performed in triplicate. The results were expressed as mean ± standard deviation (SD). When needed statistical comparisons were made using the Kruskal Wallis test. Statistically significant difference was defined as p < 0.05.
The reducing abilities are given only as % inhibition since the majority of the tested compounds presented lower than 50% antioxidant ability at 100 μM. Considering the LOX inhibition as well as the anti-lipid peroxidation activity, only one and two compounds respectively exhibited high activities at 100 μM. Thus for the sake of comparison we did not determine the IC50 values for them. The same concept was followed for the DPPH interaction results.
4.9. NMR Data of Compounds 1–20
The 1H- and 13C-NMR data of these compounds (1–20) are listed as follows (see also Supplementary Material):
Compound 1: 1H-NMR (400 MHz, CD4O) δH: 6.95 d (1H, H-2′, J = 1.8 Hz), 6.85 dd (1H, H-6′, J = 8.0, 1.8 Hz), 6.80 d (1H, H-5′, J = 8.0 Hz), 5.92 d (1H, H-8, J = 1.9 Hz), 5.88 d (1H, H-6, J = 1.9 Hz), 4.89 d (1H, H-2, J = 12.2 Hz), 4.50 d (1H, H-3, J = 12.2 Hz).
NOESY: nOe signals between H-2/H-3; H-3/H-6′.
Compound 2: 1H-NMR (400 MHz, CD4O) δH: 8.06 d (2H, H-2′, H-6′, J = 8.7 Hz) 6.90 d (2H, H-3′, H-5′, J = 8.7 Hz) 6.40 s (1H, H-8) 6.21 s (1H, H-6) 5.23 d (1H, H-1′′, J = 7.8 Hz) 3.42–3.20 m (3H, H-2′′,3′′,4′′,5′′), 3.69 dd (1H, H-6a′′, J = 12.0, 5.8 Hz) 3.53 dd (1H, H-6b′′ J = 12.0, 2.1 Hz).
13C-NMR (50.3 MHz CD4O) δC: 161.0 (C-2) 100.0 (C-6) 94.4 (C-8) 158.4 (C-9) 104.0 (C-10) 123.0 (C-1′) 131.9 (C-2′,6′) 159.2 (C-4′) 115.1 (C-3′,5′) 103.7 (C-1′′) 76.2 (C-2′′) 78.1a (C-3′′) 71.3 (C-4′′) 78.4a (C-5′′) 62.3 (C-6′′).
a: interchangeable signals.
Compound 3: 1H-NMR (400 MHz, CD4O) δH: 7.93 d (1H, H-2′, J = 2.0 Hz) 7.59 dd (1H, H-6′, J = 8.6, 2.0 Hz) 6.89 d (1H, H-5′, J = 2.0 Hz) 6.19 d (1H, H-6, J = 2.0 Hz) 6.38 d (1H, H-8, J = 2.0 Hz) 3.95 s (3H, OCH3) 5.40 d (1H, H-1′′, J = 7.8 Hz) 3.40–3.20 m (3H, H-2′′,3′′,4′′,5′′) 3.22 dd (1H, H-5′′, J = 4.8, 3.0) 3.73 dd (1H, H-6a′′, J = 12.0, 5.3 Hz) 3.52 dd (1H, H-6b′′, J = 12.0, 1.8 Hz).
13C-NMR (50.3 MHz CD4O) δC: 159.0 (C-2) 100.4 (C-6) 95.2 (C-8) 157.3 (C-9) 104.0 (C-10) 121.0 (C-1′) 114.7 (C-2′) 151.3 (3′) 149.7 (C-4′) 116.4 (C-5′) 103.7 (C-1′′) 123.8 (C-6′) 103.7 (C-1′′) 75.5 (C-2′′) 78.0 (C-3′′) 71.6 (C-4′′) 78.2 (C-5′′) 62.8 (C-6′′).
Compound 4: 1H-NMR (400 MHz, CD4O) δH: 6.82 d (1H, H-2′, J = 1.9 Hz) 6.74 d (1H, H-5′, J = 8.0 Hz) 6.71 dd (1H, H-6′ J = 8.0, 1.9 Hz) 5.82 d (1H, H-6, J = 2.2 Hz) 5.94 d (1H, H-8, J = 2.2 Hz) 4.56 (1H, H-2, J = 7.7 Hz) 3.97 ddq (1H, H-3, J = 8.1, 7.7, 5.4 Hz) 2.84 dd (1H, H-4ax, J = 16.1, 5.4 Hz) 2.50 dd (1H, H-4eq, J = 16.1, 8.1 Hz).
13C-NMR (50.3 MHz CD4O) δC: 82.8 (C-2) 68.8(C-3) 28.4 (C-4) 157.6 (C-5) 95.5 (C-6) 157.8 (C-7) 96.3 (C-8) 156.9 (C-9) 100.8 (C-10) 132.4 (C-1′) 115.3 (C-2′) 146.4 (3′,4′) 116.0 (C-5′) 119.9 (C-6′).
Compound 5: 1H-NMR (400 MHz, CD4O) δH: 8.10 br d (2H, H-2,6, J = 7.7 Hz) 7.50 br t (1H, H-3,5, J = 7.7 Hz) 7.63 (1H, H-4, J = 7.7 Hz). 5.73 d (1H, H-1′, J = 7.6 Hz) 3.52 dd (1H, H-2′, J = 7.6, *Hz) 3.49–3.43 m (3H, H-3′,4′,5′), 3.86 dd (1H, H-6a,′ J = 12.1, 2.1 Hz) 3.71 dd (1H, H-6b′, J = 12.1, 5.1 Hz).
13C-NMR (50.3 MHz CD4O) δC: 133.2 (C-1) 130.7 (C-2,6) 128.4(C-3,5) 132.4 (C-4) 166.2 (C-7) 94.1 (C-1′) 73.4 (C-2′) 77.8a (C-3′) 71.3 (C-4′) 78.4a (C-5′) 62.1 (C-6′).
*: partially overlapped signal; a: interchangeable signals.
Compound 6: 1H-NMR (400 MHz, CD4O) δH: 7.42 br d (2H, H-2,6, J = 7.3 Hz) 7.33 br dd (2H, H-3,5, J = 7.7, 7.3 Hz) 7.28 br d (1H, H-4, J = 7.3 Hz) 4.95 d (1H, H-7a, J = 11.8) 4.69 d (1H, H-7b, J = 11.8 Hz). 4.38 d (1H,H-1′, J = 7.4 Hz) 3.43-3.31 m (3H, H-2′,3′,4′,5′) 3.91 dd (1H, H-6a′, J = 2.1, 1.7 Hz) 3.70 dd (1H, H-6b′, J = 11.7, 5.8 Hz).
13C-NMR (50.3 MHz CD4O) δC: 138.9 (C-1) 128.9 (C-2,6) 128.9 (C-3,5) 128.4 (C-4) 71.4 (C-7) 103.0 (C-1′) 74.9 (C-2′) 78.0a (C-3′) 71.6 (C-4′) 77.9a (C-5′) 62.4 (C-6′).
a: interchangeable signals.
Compound 7: 1H-NMR (400 MHz, CD4O) δH: 7.42 br d (2H, H-2,6, J = 7.3 Hz) 7.33 br dd (2H, H-3,5, J = 7.7, 7.3 Hz) 7.28 br d (1H, H-4, J = 7.3 Hz) 4.87 d (1H, H-7a, J = 11.8) 4.64 d (1H, H-7b, J = 11.8 Hz) 4.32 d (1H,H-1′, J = 7.9 Hz) 3.24 dd (1H, H-2′, J = 8.5, 7.9) 3.70-3.20 m* (3H, H-3′,4′,5′), 3.99 dd (1H, H-6a′, J = 11.7, 1.7 Hz) 3.64 dd (1H, H-6b′, J = 11.3, 5.9 Hz) 4.81 d (1H,H-1′′, J = 1.7 Hz) 3.86 dd (1H, H-2′′ J = 3.1, 1.7 Hz) 3.68 m (3H, H-3′′, J = 9.5 3.1 Hz), 3.34 * (1H H-4′′) 3.67 (1H H-5′′) 1.27 d (3H H-6′′, J = 6.2Hz).
13C-NMR (50.3 MHz CD4O) δC: 128.8 (C-2,6) 128.8 (C-3,5) 128.3 (C-4) 71.4 (C-7) 103.0 (C-1′) 75.0 (C-2′) 77.9a (C-3′) 71.3 (C-4′) 77.4a (C-5′) 67.6 (C-6′) 102.1 (C-1′′) 72.2(C-2′′) 71.1 (C-3′′) 73.7 (C-4′′) 71.1 (C-5′′) 17.9 (C-6′′).
*: partially overlapped signals; a: interchangeable signals.
Compound 8: 1H-NMR (400 MHz, CD4O) δH: 7.14 br d (1H, H-3, J = 8.2 Hz) 7.09-7.07 m (2H, H-4,5) 6.92 dd (1H, H-6, J = 8.2, 1.8 Hz). 4.89* (1H,H-1′) 3.50 (1H, H-2′) 3.82 (-OCH3) 3.48 m (1H, H-3′) 3.40 (1H, H-4′) 3.91 dd (1H, H-6a′, J = 11.7, 1.7 Hz) 3.70 dd (1H, H-6b′, J = 11.7, 5.8 Hz).
13C-NMR (50.3 MHz CD4O) δC: 117.0a (C-3) 112.0 (C-4) 116.8 a (C-5) 120.2 (C-6) –OCH3 (56.1) 102.2 (C-1′) 74.5 (C-2′) 77.5 (C-3′) 71.0 (C-4′) 78.0 (C-5′) 62.0 (C-6′).
*: partially overlapped by methanol-d4 moisture; a: interchangeable signals.
Compound 9: 1H-NMR (400 MHz, CD4O) δH: 6.83 d (1H, H-2, J = 2.8 Hz) 6.86 d (2H, H-5, J = 8.8 Hz) 6.67 dd (1H, H-6, J = 8.8, 2.9 Hz) 3.82 (-OCH3-3), 3.79 (-OCH3-4). 4.79 d (1H, H-1′, J = 7.5 Hz) 3.46–3.35 m (4H, H-2′,3′,4′,5′) 3.91 dd (1H, H-6a′, J = 11.7, 1.7 Hz) 3.70 (1H, H-6b′, J = 11.7, 5.8 Hz).
13C-NMR (50.3 MHz CD4O) δC: 153.9 (C-1) 103.7 (C-2) 145.7 (C-3) 150.6 (C-4) 113.5 (C-5) 109.0 (C-6) –OCH3 (56.1, 57.5) 103.1 (C-1′) 74.9 (C-2′) 77.4a (C-3′) 71.3 (C-4′) 77.9a (C-5′) 62.2 (C-6′).
a: interchangeable signals.
Compound 10: 1H-NMR (400 MHz, CD4O) δH: 7.11 d (2H, H-3,5, J = 8.5 Hz) 7.00 d (2H, H-2,6, J = 8.5 Hz) 2.80–2.76 m (2H, H-7,8) 2.10 s (–CH3). 4.85 d (1H,H-1′, J = 7.8 Hz) 3.43 (1H,H-2′) 3.40–3.30 (2H, H-3′,4′,5′) 3.88 dd (1H, H-6a′, J = 12.0, 1.8 Hz) 3.69 (1H, H-6b′, J = 12.0, 5.0 Hz).
13C-NMR (50.3 MHz CD4O) δC: 157.3 (C-1) 117.5 (C-2,6) 129.0 (C-3,5) 135.3 (C-4) 29.68 (C-7) 45.6 (C-8) 29.63(C-9 –CH3) 210.3 (>C = 0) 102.0 (C-1′) 74.6 (C-2′) 77.8 (C-3′) 71.0 (C-4′) 77.7 (C-5′) 62.0 (C-6′).
Compound 11: 1H-NMR (400 MHz, CD4O) δH: 7.96 d (2H, H-2,6, J = 7.89 Hz).
7.01 d (2H, H-3,5, J = 7.04 Hz) 3.88 s (-OCH3).
13C-NMR (50.3 MHz CD4O) δC: 131.7 (C-2,6) 116.0 (C-3,5) 56.0 (–OCH3).
Compound 12: 1H-NMR (400 MHz, CD4O) δH: 8.06 d (2H, H-2,6, J = 8.1 Hz) 6.90 d (2H, H-3,5, J = 8.1 Hz). 4.96 d (1H,H-1′, J = 7.4 Hz) 3.50–3.30 m (4H, H-2′, 3′, 4′, 5′) 3.91 dd (1H, H-6a′, J = 11.7, 1.7 Hz) 3.70 (1H, H-6b′, J = 11.7, 5.8 Hz).
13C-NMR (50.3 MHz CD4O) δC: 132.0 (C-2,6) 116.2 (C-3,5) 101.0 (C-1′) 75.0 (C-2′) 78.0 (C-3′) 71.8 (C-4′) 78.0 (C-5′) 62.3 (C-6′).
Compound 13: 1H-NMR (400 MHz, CD4O) δH: 7.44 d (2H, H-2,6, J = 8.3 Hz) 6.79 d (2H, H-3,5, J = 8.3 Hz) 7.56 d (1H, H-7, J = 16.0 Hz) 6.29 d (1H, H-8, J = 16.0 Hz).
13C-NMR (200 MHz CD4O) δC: 131.4 (C-2,6) 124.3 (C-3,5) 146.7 (C-7) 115.4 (C-8).
Compound 14: 1H-NMR (400 MHz, CD4O) δH: 7.54 d (2H, H-2,6, J = 7.9 Hz) 7.12 dd (2H, H-3,5, J = 7.9 Hz) 7.60 d (1H, H-7, J = 16.2 Hz) 6.37 d (1H, H-8, J = 16.2 Hz). 4.96 d (1H, H-1′, J = 7.2 Hz) 3.70–3.41 m (4H, H-2′, 3′, 4′, 5′) 3.88 dd (1H, H-6a′, J = 12.1, 2.1) 3.70 (1H, H-6b′, J = 12.1, 8.1 Hz).
13C-NMR (50.3 MHz CD4O) δC: 129.2 (C-1) 132.0 (C-2,6) 117.4 (C-3,5) 159.5 (C-4) 144.7 (C-7) 117.8 (C-8) 170.3 (C-9 –COOH) 101.2 (C-1′) 74.4 (C-2′) 77.5 (C-3′) 70.7 (C-4′) 78.0 (C-5′) 62.2 (C-6′).
Compound 15: 1H-NMR (400 MHz, CD4O) δH: 7.48 d (2H, H-2,6, J = 8.2 Hz) 7.08 d (2H, H-3,5, J = 8.2 Hz) 7.37 d (1H, H-7, J = 16.0 Hz) 6.40 d (1H, H-8, J = 16.0 Hz). 4.94 d (1H, H-1′) 3.55–3.28 m (4H, H-2′,3′,4′,5′) 3.89 dd (1H, H-6a′, J = 11.8, 2.1) 3.70 (1H, H-6b′, J = 11.8, 4.4 Hz).
13C-NMR (50.3 MHz CD4O) δC: 129.4 (C-2, 6) 117.6 (C-3, 5) 139.9 (C-4) 129.9 (C-7) 102.2 (C-1′) 74.3 (C-2′) 77.6 (C-3′) 70.8 (C-4′) 76.5 (C-5′) 62.1 (C-6′).
Compound 16: 1H-NMR (400 MHz, CD4O) δH: 2.45 d (1H, H-2a, J = 17.0 Hz) 2.22 d (1H, H-2b, J = 17.0 Hz) 5.90 tt (1H, H-4, J = 6.9 1.2 Hz) 5.91 d (1H, H-7, J = 15.9 Hz) 6.82 d (1H, H-8, J = 15.9 Hz) 1.89 brs (3H, H-10 CH3) 1.07 s (3H, H-11 CH3) 1.03 s (3H, H-12 CH3) 1.90 d (3H, H-13 CH3 1.2 Hz) 5.63 t (1H, H-14, J = 7.0 Hz) 4.51 dd (1H, H-15a, J = 12.3 7.6 Hz) 4.38 dd (1H, H-15b, J = 12.3 5.9 Hz) 4.28 d (1H, H-1′) 3.17 t (1H, H-2′, J = 8.0 Hz) 3.27–3.35 m (4H, H-3′, 4′, 5′) 3.88 dd (1H, H-6a′, J = 12.0, 1.7) 3.68 (1H, H-6b′, J = 12.0, 5.7 Hz).
13C-NMR (50.3 MHz CD4O) δC: 42.7 (C-1) 49.6 (C-2) 200.0 (C-3 –C=O) 127.4 (C-4) 166.9 (C-5) 80.0 (C-6) 132.0 (C-7) 127.7 (C-8) 136.0 (C-9) 19.4 (C-10) 23.4 (C-11) 24.5 (C-12) 20.4 (C-13) 127.0 (C-14) 65.0 (C-15) 103.0 (C-1′) 74.6 (C-2′) 77.7 (C-3′) 71.3 (C-4′) 77.8 (C-5′) 62.7 (C-6′).
Compound 17: 1H-NMR (400 MHz, CD4O) δH: 8.07 d (2H, H-2′,6′, J = 8.7 Hz) 6.90 d (2H, H-3′,5′, J = 8.7 Hz) 6.34 d (1H, H-8, J = 2.1 Hz) 6.16 d (1H, H-6, J = 2.1 Hz) 5.06 d (1H, H-1′′, J = 7.8 Hz) 3.44 dd (1H, H-2′, J = 7.9, 7.5) 3.43–3.32 m* (3H, H-3′,4′,5′), 3.88 brd (1H, H-6a′, J = 11.0, 1.7 Hz) 3.64 dd (1H, H-6b′, J = 11.0, 5.9 Hz) 4.52 d (1H, H-1′′′ 1.7 Hz) 3.31* (1H, H-2′′′) 3.68 m (2H, H-3′′′, H-5′′′), 3.34* (1H H-4′′′)* 1.27 d (3H H-6′′′, J = 6.2 Hz).
13C-NMR (50.3 MHz CD4O)δC: 159.5 (C-2) 136.1(C-3) 180.2 (C-4) 164.0 (C-5) 100.8 (C-6) 167.1 (C-7) 94.9 (C-8) 159.2 (C-9) 107.5 (C-10) 123.6 (C-1′) 132.2 (C-2′, 6′) 117.0 (C-3′, 5′) 162.2 (C-4′) 101.7 (C-1′′) 76.7 (C-2′′) 78.9 (C-3′′) 72.0a (C-4′′) 78.0 (C-5′′) 69.3 (C-6′′) 102.9 (C-1′′′) 72.9a (C-2′′′) 73.1 (C-3′′′) 74.3 (C-4′′′) 70.5 (C-5′′′) 18.6 (C-6′′′).
*: overlapped signals; a: interchangeable signals.
Compound 18: 1H-NMR (400 MHz, CD4O) δH: 8.04 d (2H, H-2′,6′, J = 8.7 Hz) 6.82 d (2H, H-3′,5′, J = 8.7 Hz) 6.33 d (1H, H-8, J = 2.1 Hz) 6.22 d (1H, H-6, J = 2.1 Hz) 3.94 (3H, –OCH3) 5.84 d (1H, H-1′′, J = 7.8 Hz) 3.24 dd (1H, H-2′′, J = 8.5, 7.9) 3.43–3.32 ma (3H, H-3′′,4′′,5′′), 3.99 dd (1H, H-6a′′, J = 11.7, 1.7 Hz) 3.64 dd (1H, H-6b′′, J = 11.3, 5.9 Hz). 4.51 d (1H, H-1′′′, J = 1.7 Hz) 3.86 dd (1H, H-2′′′, J = 3.1, 1.7 Hz) 3.68 m (3H, H-3′′′, J = 9.5 3.1 Hz), 3.34a (1H H-4′′′) 3.67 (1H H-5′′′) 1.27 d (3H H-6′′′, J = 6.2 Hz).
13C-NMR (50.3 MHz CD4O) δC: 128.8* (C-2′,6′) 128.8 (C-3′,5′) 128.3* (C-4′) 71.4 (C-7)103.0 (C-1′′) 75.0 (C-2′′) 77.9b (C-3′′) 71.3 (C-4′′) 77.4b (C-5′′) 67.6 (C-6′′) 102.1 (C-1′′′) 72.2(C-2′′′) 71.1α (C-3′′′) 73.7 (C-4′′′) 71.1α (C-5′′′) 17.9 (C-6′′′).
a,b: overlapped signals; *: interchanged signals.
Compound 19: δ(ppm) 7.98 d (2H, H-2′, H-6′, J = 8.8 Hz), 7.44 d (1H, H-7′′′, J = 15.6 Hz), 7.36 d (2H, H-2′′′& H-6′′′, J = 9.2 Hz), 6.77 d (2H, d, H-3′ & H-5′, J = 8.8 Hz), 6.77 d (2H, H-3′′′ & H-5′′′, J = 9.2 Hz), 6.37 d (1H, H-8, J = 2.1 Hz), 6.14 d (1H, H-6 J = 2.1 Hz), 6.07 d (1H, H-8′′′, J = 15.6 Hz), 5.20 d (1H, H-1′′, J = 7.6 Hz), 4.35 dd (1H, H-6′′a, J = 12.6, 2.0 Hz), 4.21 dd (1H, H-6′′b, J = 12.8, 6.8 Hz), 3.35–3.51 m (4H, H-2″,3″,4′′,5′′) 159.5 (C-2) 137.0 (C-3) 179.4 (C-4) 162.7 (C-5) 99.7 (C-6) 165.8 (C-7) 94.9 (C-8) 158.3 (C-9) 105.7 (C-10) 123.4 (C-1′) 132.2 (C-2′,6′) 118.0 (C-3′,5′) 161.4 (C-4′) 104.1 (C-1′′) 74.9 (C-2′′) 77.9 (C-3′′) 71.8 (C-4′′) 75.2 (C-5′′) 64.5 (C-6′′) 123.2 (C-1′′′) 131.5 (C-2′′′ ,6′′′) 117.2 (C-3′′′ ,5′′′) 157.5 (C-4′′′) 147.5 (C-7′′′) 115.9 (C-8′′′) 168.7 (C-9′′′).
Compound 20: 7.53 s (2H, H-2,6); 6.42 d (1H, H-8, J = 2 Hz), 6.21 d (1H, H-6, J = 2 Hz), 5.47 d (1H, H-1′′, J = 8 Hz), 3.94 s (6H, OCH3 × 2 at C-3′ and at C-5′) 5.47 d (1H, H-1′′, 7.8 Hz) 3.42–3.20 m (3H, H-2′′,3′′,4′′,5′′), 3.74 dd (1H, H-6a′′ 12.0, 5.8 Hz) 3.58 dd (1H, H-6b′′ 12.0, 2.1 Hz).
13C-NMR (50.3 MHz CD4O) δC: 107.4 (C-2′,6′) 99.6 (C-6) 94.4 (C-8) 56.5 (–OCH3) 102.8 (C-1′′) 76.2 (C-2′′) 78.1a (C-3′′) 71.0 (C-4′′) 77.3a (C-5′′) 62.2 (C-6′′).
a: overlapped signals.
Supplementary Materials
The following are available online at www.mdpi.com/2223-7747/7/1/1/s1.
Author Contributions
K.N. collected and identified the plant material; H.S. conceived and designed the experiments, contributed in the writing, and also supervised the chemical analyses; A.D. contributed in the writing and carried out all chemical analyses; D.H.-L. contributed to writing and biological evaluations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dagher-Kharrat M.B., Mariette S., Lefèvre F., Fady B., March G.G., Plomion C., Savoure A. Geographical diversity and genetic relationship among Cedrus species estimated by AFLP. Tree Genet. Genomes. 2006;3:275–285. doi: 10.1007/s11295-006-0065-x. [DOI] [Google Scholar]
- 2.Theophrasti E. In: Opera. Wimmer F., editor. Didot; Paris, France: 1866. (In Greek) [Google Scholar]
- 3.Kurt Y., Kacar M.S., Isik K. Traditional Tar Production from Cedrus libani A. Rich on the Taurus Mountains in Southern Turkey. Econ. Bot. 2008;62:615–620. doi: 10.1007/s12231-008-9023-x. [DOI] [Google Scholar]
- 4.Pollio V. Vitruvius: The Ten Books on Architecture. Harvard University Press; New York, NY, USA: 1914. pp. 154–196. [Google Scholar]
- 5.Creţu E., Trifan A., Aprotosoaie A.C., Miron A. 15-lipoxygenase inhibition, superoxide and hydroxyl radicals scavenging activities of Cedrus brevifolia bark extracts. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2013;117:250–256. [PubMed] [Google Scholar]
- 6.Hafizoglu H., Barne H. Studies on the Chemistry of Cedrus libani A. Rich.-III. Oleoresin Composition of Cones and Bark from Cedrus libani. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood. 1987;41:141–145. doi: 10.1515/hfsg.1987.41.3.141. [DOI] [Google Scholar]
- 7.Zeng W.C., He Q., Sun Q., Zhong K., Gao H. Antibacterial activity of water-soluble extract from pine needles of Cedrus deodara. Int. J. Food Microbiol. 2012;153:78–84. doi: 10.1016/j.ijfoodmicro.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Usmana A., Vera Thossb V., Nur-e-Alamc M. Isolation of Taxifolin from Trichilia emetica Whole Seeds. Am. Sci. Res. J. Eng. Technol. Sci. 2016;21:77–82. [Google Scholar]
- 9.Saito S., Silva G., Santos R., Gosmann G., Pungartnik C., Brendel M. Astragalin from Cassiaalata induces DNA Adducts in Vitro and Repairable DNA damage in the yeast Saccharomyces cerevisiae. Int. J. Mol. Sci. 2012;13:2846–2862. doi: 10.3390/ijms13032846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Hawiet A.M., Toaima S.M., Asaad A.M., Radwan M.M., El-Sebakhy N.A. Chemical constituents from Astragalus annularis Forssk. and A. trimestris L., Fabaceae. Rev. Bras. Pharmacogn. 2009;20:860–865. doi: 10.1590/S0102-695X2010005000047. [DOI] [Google Scholar]
- 11.Lee S.I., Yang J.H., Kim D.K. Antioxidant Flavonoids from the Twigs of Stewartia koreana. Biomol. Ther. 2010;18:191–196. doi: 10.4062/biomolther.2010.18.2.191. [DOI] [Google Scholar]
- 12.Chen Y.-H., Chang F.-R., Lu M.-C., Hsieh P.-W., Wu M.-J., Du Y.-C., Wu Y.-C. New Benzoyl Glucosides and Cytotoxic Pterosin Sesquiterpenes from Pteris ensiformis Burm. Molecules. 2008;13:255–266. doi: 10.3390/molecules13020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwab W., Schreier P. Aryl β-d-glucosides from Carica papaya fruit. Phytochemistry. 1987;27:1813–1816. doi: 10.1016/0031-9422(88)80450-3. [DOI] [Google Scholar]
- 14.Hamerski L., Bomm M.D., Silva D.H.S., Claudia M., Young M., Furlan M., Eberlin M.N., Gamboa I.C., Cavalheiro A.J., Bolzani S. Phenylpropanoid glucosides from leaves of Coussarea Hydrangeifolia (Rubiaceae) Phytochemistry. 2005;66:1927–1932. doi: 10.1016/j.phytochem.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Fujimatu E., Ishikawa T., Kitajima J. Aromatic compound glucosides, alkyl glucoside and glucide from the fruit of Anisei. Phytochemistry. 2003;63:609–616. doi: 10.1016/S0031-9422(03)00179-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Xu J., Xiao L., Zeng G.Z., Sun Z.H., Tan N.H. A New Phenolic Glycoside from Chamaecyparis obtuse var. Breviramea f. crippsii. Molecules. 2013;18:1255–1261. doi: 10.3390/molecules18011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pabst A., Barron D., Adda J., Schreier P. Phenylbutan-2-one β-d-glucosides from raspberry fruit. Phytochemistry. 1990;29:3853–3858. doi: 10.1016/0031-9422(90)85346-H. [DOI] [Google Scholar]
- 18.Marquesa D.D., Machado M.I.L., de Carvalhob M.G., da C. Meleirab L.A., Braz-Filhoc R. Isoflavonoids and Triterpenoids Isolated from Pterodon Polygalaeflorus. J. Braz. Chem. Soc. 1998;9:295–301. doi: 10.1590/S0103-50531998000300014. [DOI] [Google Scholar]
- 19.Fiorentino A., Abrosca B., Pacifico S., Mastellone C., Piscopo V., Caputo R., Monaco P. Isolation and Structure Elucidation of Antioxidant Polyphenols from Quince (Cydonia vulgaris) Peels. J. Agric. Food Chem. 2008;56:2660–2667. doi: 10.1021/jf800059r. [DOI] [PubMed] [Google Scholar]
- 20.Hussain M., Rahman M., Jabbar A., Rashid M. Phytochemical and biological investigations of Albizzia lebbeck Benth. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 2008;7:273–278. [Google Scholar]
- 21.Johnssona P., Peerlkampa N., Kamal-Eldina A., Andersson R., Anderssona R., Lundgren L., Aman P. Polymeric fractions containing phenol glucosides in flaxseed. Food Chem. 2002;76:207–212. doi: 10.1016/S0308-8146(01)00269-2. [DOI] [Google Scholar]
- 22.Abdel-Kade M.S. Two New Nor-phenylpropanoid Glucosides and Hemipholin from the Flowers of Ononis vaginalis. J. Braz. Chem. Soc. 1997;8:637–639. doi: 10.1590/S0103-50531997000600012. [DOI] [Google Scholar]
- 23.Lutz A., Winterhalter P. Isolation of Additional Carotenoid Metabolites from Quince Fruit (Cydonia oblonga Mill.) J. Agric. Food Chem. 1992;40:1116–1120. doi: 10.1021/jf00019a005. [DOI] [Google Scholar]
- 24.Akkola E.K., Süntara I., Keleşb H., Sezika E., Gürlerc G. Bioassay-guided isolation and characterization of wound healer compounds from Morus nigra L. (Moraceae) Rec. Nat. Prod. 2015;9:484–495. [Google Scholar]
- 25.Moustafa A.M.Y., Khodair A.I., Saleh M.A. Isolation, structural elucidation of flavonoid constituents from Leptadenia pyrotechnica and evaluation of their toxicity and antitumor activity. Pharm. Biol. 2009;47:539–552. doi: 10.1080/13880200902875065. [DOI] [Google Scholar]
- 26.Mekhelfi T., Kerbab K., Guella G., Zaiter L., Benayache S., Benayache F. Phytochemical constituents of Thymelaea microphylla Coss. et Dur. from Algeria. Pharm. Lett. 2014;6:152–156. [Google Scholar]
- 27.Gutzeit D., Wray V., Winterhalter P., Jerz G. Preparative Isolation and Purification of Flavonoids and Protocatechuic Acid from Sea Buckthorn Juice Concentrate (Hippophaë rhamnoides L. ssp. rhamnoides) by High-Speed Counter-Current Chromatography. Chromatographia. 2007;65:1–7. doi: 10.1365/s10337-006-0105-6. [DOI] [Google Scholar]
- 28.Koleva I.I., van Beek T.A., Linssen J.P.H., de Groot A., Evstatieva L.N. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochem. Anal. 2001;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- 29.Liegois C., Lermusieau G., Colin S.J. Measuring Antioxidant Efficiency of Wort, Malt, and Hops against the 2,2′-Azobis(2-amidinopropane) Dihydrochloride-Induced Oxidation of an Aqueous Dispersion of Linoleic Acid. Agric. Food Chem. 2000;48:1129–1134. doi: 10.1021/jf9911242. [DOI] [PubMed] [Google Scholar]
- 30.Mabry T.G., Markham K.R., Thomas M.B. The Systematic Identification of Flavonoids. Springer; New York, NY, USA: 1970. [Google Scholar]
- 31.Coll J.C., Bowden B.F. The application of vacuum liquid chromatography to the separation of terpene mixtures. J. Nat. Prod. 1986;49:934–936. doi: 10.1021/np50047a033. [DOI] [Google Scholar]
- 32.Neu R. Chelate von Diarylborsäuren mit aliphatischen Oxyalkylaminen als Reagenzien für den Nachweis von Oxyphenyl-benzo-γ-pyronen. Naturwissenschaften. 1957;44:181–183. doi: 10.1007/BF00599857. [DOI] [Google Scholar]
- 33.Pontiki E., Hadjipavlou-Litina D., Litinas K., Nicolotti O., Carotti A. Design, synthesis and pharmacological evaluation of novel acrylic acid derivatives acting as lipoxygenase and cyclooxygenase-1 inhibitors with antioxidant and anti-inflammatory activities. Eur. J. Med. Chem. 2011;46:191–200. doi: 10.1016/j.ejmech.2010.10.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.