Abstract
Background
Inflammation, coagulation, and cell stress contribute to atherosclerosis and its adverse events. A biomarker risk score (BRS) based on the circulating levels of biomarkers C-reactive protein, fibrin degradation products, and heat shock protein-70 representing these 3 pathways was a strong predictor of future outcomes. We investigated whether soluble urokinase plasminogen activator receptor (suPAR), a marker of immune activation, is predictive of outcomes independent of the aforementioned markers and whether its addition to a 3-BRS improves risk reclassification.
Methods and Results
C-reactive protein, fibrin degradation product, heat shock protein-70, and suPAR were measured in 3278 patients undergoing coronary angiography. The BRS was calculated by counting the number of biomarkers above a cutoff determined using the Youden’s index. Survival analyses were performed using models adjusted for traditional risk factors. A high suPAR level ≥3.5 ng/mL was associated with all-cause death and myocardial infarction (hazard ratio, 1.83; 95% confidence interval, 1.43–2.35) after adjustment for risk factors, C-reactive protein, fibrin degradation product, and heat shock protein-70. Addition of suPAR to the 3-BRS significantly improved the C statistic, integrated discrimination improvement, and net reclassification index for the primary outcome. A BRS of 1, 2, 3, or 4 was associated with a 1.81-, 2.59-, 6.17-, and 8.80-fold increase, respectively, in the risk of death and myocardial infarction. The 4-BRS was also associated with severity of coronary artery disease and composite end points.
Conclusions
SuPAR is independently predictive of adverse outcomes, and its addition to a 3-BRS comprising C-reactive protein, fibrin degradation product, and heat shock protein-70 improved risk reclassification. The clinical utility of using a 4-BRS for risk prediction and management of patients with coronary artery disease warrants further study.
Keywords: biomarker, cardiovascular outcomes, coronary artery disease, prognosis, risk score
Coronary artery disease (CAD) continues to be the leading cause of mortality worldwide.1 The mechanisms involved in the development of atherosclerosis and subsequent plaque rupture that precipitate acute coronary events and death are complex and involve several pathways, including key contributions of inflammation, immune dysregulation, stress, and thrombosis.2 Clinical tools, such as the Framingham risk score, predict long-term risk of outcomes in the healthy population,3,4 but fail to reliably prognosticate risk of adverse outcomes in patients with established CAD.5
We recently identified a simple noninvasive risk score based on the concept that the greater the number of pathways related to plaque rupture that are activated in a given patient, the greater the risk of actual plaque rupture.6 This aggregate biomarker strategy for risk prediction was tested using the biomarkers C-reactive protein (CRP), representing inflammation, fibrin degradation products (FDP), representing the coagulation pathway, and heat shock protein-70 (HSP-70), representing cell stress. The biomarker risk score (BRS) strategy was highly successful in predicting risk of near-term myocardial infarction (MI) and death in patients with suspected or established CAD.6
Soluble urokinase plasminogen activator receptor (suPAR) is a marker of immune activation and inflammation that seems to orchestrate cellular adhesion, migration, and proliferation during development of the atherosclerotic plaque.7 Higher circulating levels of suPAR are associated with incident cardiovascular disease (CVD) in the healthy population,8,9 and we and others have found that it also predicts incident CVD events and chronic kidney disease in subjects with CAD.10–12
The purpose of the present investigation is to determine (1) whether suPAR levels are associated with outcomes independent of the aforementioned markers of inflammation (CRP), hypercoagulable state (FDP), and cellular stress (HSP-70), and (2) whether addition of suPAR to the 3-BRS improves prediction of future adverse events.
Methods
Study Population
Subjects were recruited as a part of the Emory Cardiovascular Biobank and consisted of 3278 patients undergoing left heart catheterization for diagnosis of suspected CAD in Emory Healthcare hospitals between 2003 and 2009. Subjects with heart transplantation, severe valvular heart disease, congenital heart disease, severe anemia, recent blood transfusion, myocarditis, active inflammatory diseases, and cancer were excluded. Demographics, medical, smoking status, and risk factor prevalence were documented as previously described.6 Smoking was classified as nonsmoker or current smoker. Subjects were noted to have hypertension or dyslipidemia if they had a documented history or were on treatment. Acute MI at enrollment was defined using universal criteria.13 Briefly, MI was diagnosed with detection of a rise of cardiac troponin with either ischemic symptoms, dynamic EKG changes, or identification of an intracoronary thrombus by angiography. The study was approved by the Emory University Institutional Review Board. All subjects provided written informed consent.
Outcomes and Follow-Up
To minimize physician-imposed bias, we selected a composite end point consisting of hard outcomes of ischemic heart disease, including all-cause death and nonfatal MI. Follow-up was conducted between 1 and 5 years for determination of the primary composite end point of all-cause death and nonfatal MI and the secondary end points of cardiovascular death, all-cause death/MI/revascularization, and all-cause death/MI/stroke. Follow-up data were collected by personnel blinded to the biomarker data through telephone interview, chart review, and query of the Social Security Death Index and State records. Two independent cardiologists, both blinded to the clinical and biomarker data, adjudicated the cause of death. Cardiovascular death was defined as death attributable to an ischemic cardiovascular cause (ie, fatal MI, stroke, or peripheral arterial disease) or sudden death because of an unknown but presumed cardiovascular cause in high-risk patients. Medical records were accessed to validate all self-reported events, including MI.13
Identification of CAD and Severity Scoring
Luminal narrowing of coronary arteries were quantified using a modified American Heart Association/American College of Cardiology classification of the coronaries.14 Patients were classified as having nonsignificant CAD (visible plaque resulting in <50% luminal stenosis) or significant CAD (at least 1 major epicardial vessel with ≥50% stenosis). Normal coronaries were defined as those with no visual stenosis of major epicardial arteries and smooth appearance during angiography and without history of prior coronary artery bypass graft surgery or angioplasty. Quantitative angiographic scoring was performed using the Gensini score that quantifies CAD severity by a nonlinear points system for degree of luminal narrowing and has been shown to have prognostic significance.15,16
Sample Collection
Fasting arterial blood samples for serum and plasma were drawn before angiography and stored at −80°C (mean, 4.9 years). Details of the biomarker assays have been previously described.6,11 Briefly, serum CRP and FDP measurements were determined using a sandwich immunoassay by FirstMark, Inc (San Diego, CA). Serum HSP-70 was measured with a sandwich enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) and optimized by FirstMark. Plasma suPAR levels were measured using commercially available kits (suPARnostic kit; Virogates, Copenhagen, Denmark). Minimum detectable CRP, FDP, HSP-70, and suPAR were 0.1 mg/L, 0.06 μg/mL, 0.313 ng/mL, and 0.1 ng/mL, respectively.
Statistical Analyses
Continuous variables are presented as mean (±SD) or median (interquartile range) and categorical variables as proportions (%). The student’s t test and χ2 tests were used when appropriate. Mann–Whitney U or Kruskal–Wallis nonparametric tests were performed on non-normally distributed variables. The relationship between biomarkers and outcomes was determined using the Cox proportional hazards regression in unadjusted models and in models adjusted for established risk factors that include clinically relevant covariates for CVD outcomes (age, sex, race, history of hypertension, diabetes mellitus, dyslipidemia, previous MI, acute MI at presentation, estimated glomerular filtration rate, Gensini score, body mass index, left ventricular ejection fraction, history of coronary artery bypass graft, smoking status, and the use of aspirin, clopidogrel, and statins). Fine and Gray’s subdistribution hazard model was used to analyze cardiovascular death outcome, considering noncardiovascular death as the competing risk event.
The best discriminatory cutoff for each biomarker in association with the death/MI outcome was determined using the Youden’s index (sensitivity–[1–specificity]) from the receiver operating characteristic analysis to identify high versus low levels. The Youden index is a global measure of a biomarker’s effectiveness that calculates to the maximum difference between sensitivity and 1 specificity.17 Cut points for CRP, FDP, HSP-70, and suPAR were 3 mg/L, 1.0 μg/mL, 0.313 ng/mL, and 3.5 ng/mL, respectively, as described previously.6,11 A 3-BRS was derived by counting the number of biomarkers above respective cut points.
Discrimination analysis for the prediction of each end point was calculated as the difference in C statistic comparing the baseline model incorporating traditional risk factors (model 1), a second model including the 3 biomarkers (model 2), and finally a model containing the 3 biomarkers in addition to suPAR levels (model 3). C statistics, continuous net reclassification index, and integrated discrimination improvement metrics comparing model 3 to models 1 and 2 were calculated using the R package survC1 and survIDINRI.18–21
Average annual event rates for each outcome measure were calculated by dividing the observed number of events by the observed event-specific number of person-years of follow-up. Interaction terms of each covariate on the association of the aggregate BRS and the primary end point were evaluated and demonstrated using a forest plot.22 The independent association of the BRS with the presence of ≥50% stenosis in any major coronary artery was evaluated with a binary logistic regression model adjusting for known cardiovascular risk factors, including age, sex, diabetes mellitus, hypertension, dyslipidemia, and smoking status. P values <0.05 from 2-sided tests were considered statistically significant. Statistical analyses were performed with SAS (Version 9.3; SAS Institute, NC).
Results
Relationship Between Biomarker Risk Score and Clinical Risk Factors
The relationships between the categories of the BRS and the clinical and demographic factors are listed in Table 1. Although the absolute differences in most of the parameters were small, subjects with a higher BRS were more likely to be older, female, black, diabetic, hypertensive, and active smoker and to have lower left ventricular ejection fraction and lower estimated glomerular filtration rate. There was no significant difference in management strategy (ie, medical versus revascularization) between patients in different categories of the BRS.
Table 1.
Baseline Characteristics
| Baseline Characteristics | Total (N=3278) | Number of Elevated Biomarkers | |||||
|---|---|---|---|---|---|---|---|
| 0 (N=923) | 1 (N=1141) | 2 (N=796) | 3 (N=332) | 4 (N=86) | P Value for Trend | ||
| Demographics | |||||||
| Age, y | 63±12 | 62±11 | 62±13 | 64±12 | 66±11 | 66±11 | <0.001 |
| Male sex, N (%) | 2105 (64%) | 686 (74%) | 740 (65%) | 437 (55%) | 189 (57%) | 53 (62%) | <0.001 |
| White, N (%) | 2709 (83%) | 793 (86%) | 939 (82%) | 647 (81%) | 264 (79%) | 66 (78%) | 0.015 |
| Systolic BP, mm Hg | 137±23 | 136±20 | 137±23 | 138±23 | 138±26 | 132±24 | 0.062 |
| Diastolic BP, mm Hg | 76±12 | 76±11 | 76±12 | 75±12 | 75±13 | 74±11 | 0.045 |
| BMI, kg/m2 | 30±6 | 29±5 | 30±6 | 30±7 | 30±7 | 28±6 | <0.001 |
| Comorbidities | |||||||
| Acute MI on presentation, N (%) | 389 (11%) | 57 (6.3%) | 141 (12.5%) | 110 (14%) | 60 (18%) | 1 (1.2%) | <0.001 |
| History of prior MI, N (%) | 981 (31%) | 251 (28%) | 319 (29%) | 259 (33%) | 124 (38%) | 28 (34%) | 0.001 |
| Diabetes mellitus, N (%) | 1033 (31%) | 199 (22%) | 329 (29%) | 316 (40%) | 146 (44%) | 43 (50%) | <0.001 |
| Hypertension, N (%) | 2362 (72%) | 632 (68%) | 818 (72%) | 597 (75%) | 255 (77%) | 60 (70%) | 0.010 |
| Dyslipidemia, N (%) | 2291 (70%) | 660 (71%) | 805 (71%) | 550 (69%) | 229 (69%) | 47 (55%) | 0.024 |
| Current smoking, N (%) | 484 (15%) | 90 (10%) | 191 (17%) | 143 (18%) | 53 (17%) | 7 (8.4%) | <0.001 |
| History of prior CABG, N (%) | 721 (22%) | 192 (21%) | 233 (20%) | 169 (21%) | 96 (29%) | 31 (36%) | <0.001 |
| History of prior angioplasty, N (%) | 1342 (42%) | 382 (42%) | 462 (41%) | 328 (42%) | 137 (42%) | 33 (39%) | 0.963 |
| LVEF, % | 53±12 | 55±11 | 54±12 | 52±14 | 50±14 | 44±18 | <0.001 |
| Medications | |||||||
| Statin use, N (%) | 2378 (72%) | 696 (75%) | 831 (73%) | 568 (71%) | 231 (70%) | 52 (60%) | 0.016 |
| Aspirin use, N (%) | 2652 (81%) | 749 (81%) | 931 (82%) | 637 (80%) | 272 (82%) | 63 (73%) | 0.371 |
| Clopidogrel use, N (%) | 1516 (46%) | 412 (45%) | 532 (47%) | 353 (44%) | 183 (55%) | 36 (42%) | 0.010 |
| Beta-blocker use, N (%) | 2075 (63%) | 523 (57%) | 720 (63%) | 538 (68%) | 235 (71%) | 59 (69%) | <0.001 |
| ACE-inh/ARB use, N (%) | 2042 (62%) | 536 (58%) | 748 (67%) | 504 (63%) | 208 (63%) | 46 (53%) | 0.004 |
| Angiographic findings | |||||||
| Gensini angiographic score, median (IQR) | 12 (0-50) | 11 (0-50) | 11 (0-48) | 12 (0-48) | 20 (2.1-61) | 20 (1.4-85) | 0.001 |
| ≥50% epicardial vessel stenosis, N (%) | 2026 (66%) | 547 (63%) | 706 (66%) | 494 (68%) | 225 (73%) | 54 (68%) | 0.024 |
| Visually normal coronaries, N (%) | 656 (20%) | 208 (22.5%) | 240 (21%) | 155 (19%) | 44 (13%) | 9 (11%) | 0.001 |
| Laboratory values | |||||||
| GFR, mL/min | 76±47 | 81±44 | 82±49 | 71±49 | 60±44 | 54±43 | <0.001 |
| LDL, mg/dL | 99±37 | 98±35 | 102±39 | 100±38 | 96±39 | 97±35 | 0.070 |
| HDL, mg/dL | 42±13 | 43±12 | 42±13 | 41±12 | 40±13 | 42±15 | 0.002 |
| Biomarkers | |||||||
| CRP, mg/L, median (IQR) | 3.0 (1.2-7.6) | 1.1 (0.6-1.9) | 3.4 (1.45-6.9) | 6.0 (3.2-10) | 9.6 (4.7-18) | 8.9 (4.9-22) | <0.001 |
| HSP-70, ng/mL, median (IQR) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-8.0) | 4.0 (0.0-189.2) | 264 (106-635) | <0.001 |
| FDP, μg/mL, median (IQR) | 0.5 (0.3-0.8) | 0.4 (0.3-0.5) | 0.5 (0.3-0.7) | 0.6 (0.4-1.0) | 1.2 (0.7-2.1) | 1.7 (1.2-4.6) | <0.001 |
| suPAR, ng/mL, median (IQR) | 3.0 (2.3-3.9) | 2.4 (2.0-2.9) | 2.8 (2.2-3.4) | 3.9 (3.0-4.9) | 4.5 (3.7-6.0) | 5.3 (4.1-7.1) | <0.001 |
| Management strategy | 0.539 | ||||||
| Medical management, N (%) | 1863 (57%) | 551 (60%) | 632 (55%) | 443 (56%) | 188 (57%) | 49 (57%) | … |
| Revascularization, N (%) | 1260 (38%) | 337 (36%) | 449 (39.4%) | 316 (40%) | 127 (38.3%) | 31 (36%) | … |
| Other, N (%) | 155 (4.7%) | 35 (3.8%) | 60 (5.3%) | 37 (4.6%) | 17 (5.1%) | 6 (7%) | … |
| Follow-up events | |||||||
| MI, N (%) | 116 (3%) | 16 (2%) | 40 (3%) | 25 (3%) | 29 (9%) | 6 (7%) | <0.001 |
| All-cause death, N (%) | 269 (8%) | 21 (2%) | 46 (4%) | 79 (10%) | 86 (26%) | 37 (43%) | <0.001 |
| Cardiovascular death, N (%) | 153 (5%) | 14 (1%) | 27 (2%) | 43 (5%) | 48 (14%) | 21 (24%) | <0.001 |
| All-cause death/MI, N (%) | 363 (11%) | 34 (4%) | 82 (7%) | 98 (12%) | 109 (33%) | 40 (46%) | <0.001 |
| All-cause death/MI/revascularization, N (%) | 628 (19%) | 92 (10%) | 187 (16%) | 167 (21%) | 138 (42%) | 44 (51%) | <0.001 |
| All-cause death/MI/stroke, N (%) | 392 (12%) | 39 (4%) | 91 (8%) | 108 (14%) | 113 (34%) | 41 (48%) | <0.001 |
ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass graft; CRP, C-reactive protein; FDP, fibrin degradation products; GFR, glomerular filtration rate; HDL, high-density lipoprotein; HSP, heat shock protein; IQR, interquartile range; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; MI, myocardial infarction; and suPAR, soluble urokinase plasminogen activator receptor.
Relationship of Biomarker Risk Score With Angiographic CAD
Patients with higher BRS were more likely to have at least one epicardial vessel with ≥50% stenosis and higher burden of CAD as quantified by higher Gensini score (Table 1). Conversely, visually normal coronary arteries were more likely to be present in those with a lower compared with a higher BRS. Compared with those with a BRS of 0 or 1, those with BRS ≥2 had 25% increased odds of having significant CAD (>50% stenosis; odds ratio, 1.25; P=0.013), independent of age, sex, diabetes mellitus, hypertension, dyslipidemia, and smoking status. Similarly, the BRS was an independent correlate of severity of CAD assessed by the Gensini score after adjustment for variables listed above.
Clinical and Demographic Predictors of Incident Adverse Outcomes
Over a median follow-up of 2.3 years and a total of 7539 patient-years of follow-up, 269 subjects died (8.2%), 116 had an MI (3.5%), 153 (4.7%) had cardiovascular death, 35 had stroke (1.1%), and 353 underwent revascularization (10.8%; Table 1). In a Cox proportional hazard model for the composite end point of all-cause death/MI adjusting for all the aforementioned demographic and clinical covariates, significant predictors were age (years; hazard ratio [HR], 1.02; 95% confidence interval [CI], 1.01–1.03), estimated glomerular filtration rate (mL/min; HR, 0.99; 95% CI, 0.993–0.999), acute MI (HR, 1.97; 95% CI, 1.49–2.59), diabetes mellitus (HR, 1.65; 95% CI, 1.32–2.08), active smoking (HR, 1.42; 95% CI, 1.05–1.93), left ventricular ejection fraction (%; HR, 0.98; 95% CI, 0.97–0.98), Gensini score (HR, 1.003; 95% CI, 1.001–1.005), aspirin use (HR, 0.69; 95% CI, 0.46-0.85), and clopidogrel use (HR, 1.43; 95% CI, 1.11–1.84); P value for all <0.05. We think that the elevated hazard ratio associated with clopidogrel use is likely related to a high-risk inherent to individuals taking this antiplatelet medication.
Relationship Between Biomarker Risk Score and Outcomes
Correlation analyses between the 4 biomarkers revealed weak but significant correlations (Table 2). An increased (above cutoff) level of each biomarker was independently associated with future risk of all-cause death, cardiovascular death, composite of death and MI, combined death, MI, and revascularization, and composite of death, MI, and stroke after adjustment for the noted clinical covariates. Only elevated CRP was not significantly associated with future cardiovascular death (Table 3). Importantly, a high suPAR level was an independent predictor of all incident outcome measures in models adjusted for all aforementioned variables, as well as a 3-BRS comprising CRP, FDP, and HSP-70. For instance, a high suPAR level was independently associated with incident all-cause death/MI (HR, 1.83; P<0.001; Table 3).
Table 2.
Associations Between Biomarkers
| HSP-70 | FDP | CRP | |
|---|---|---|---|
| suPAR | |||
| r* | 0.16 | 0.27 | 0.27 |
| p | <0.001 | <0.001 | <0.001 |
| CRP | |||
| r | 0.072 | 0.22 | |
| p | <0.001 | <0.001 | |
| FDP | |||
| r | 0.18 | ||
| p | <0.001 | ||
CRP indicates C-reactive protein; FDP, fibrin degradation products; HSP, heat shock protein; and suPAR, soluble urokinase plasminogen activator receptor.
r value represents correlation coefficient of the Spearman bivariate correlation test. Statistically significant direct 2-by-2 correlations were observed between biomarkers.
Table 3.
Association Between Major Cardiovascular Events and the Biomarkers Individually and as the BRS
| HR (95% CI); P Value | |||||
|---|---|---|---|---|---|
| All-Cause Death | Cardiovascular Death* | All-Cause Death and MI | All-Cause Death, MI, Revascularization | All-Cause Death, MI, Stroke | |
| All biomarkers in same model | |||||
| CRP ≥3.0 mg/L | 1.69 (1.26-2.27), P<0.001 |
1.57 (0.39-6.38), P=0.53 |
1.58 (1.24-2.02), P<0.001 |
1.31 (1.09-1.56), P=0.003 |
1.58 (1.25-2.00), P<0.001 |
| HSP-70 >0.313 ng/mL | 1.98 (1.46-2.67), P<0.001 |
1.79 (1.17-2.73), P=0.01 |
2.22 (1.71-2.87), P<0.001 |
1.94 (1.60-2.35), P<0.001 |
1.99 (1.56-2.55), P<0.001 |
| FDP ≥1.0 μg/mL | 1.87 (1.42-2.46), P<0.001 |
1.79 (1.25-2.58), P=0.002 |
1.57 (1.23-1.99), P<0.001 |
1.41 (1.16-1.70), P<0.001 |
1.60 (1.27-2.01), P<0.001 |
| suPAR ≥3.5 ng/mL | 2.31 (1.71-3.13), P<0.001 |
2.12 (1.37-3.26), P<0.001 |
1.83 (1.43-2.35), P<0.001 |
1.29 (1.07-1.55), P=0.006 |
1.78 (1.40-2.25), P<0.001 |
| Categorical | |||||
| 1 vs 0 markers | 1.68 (0.96-2.93), P=0.065 |
1.59 (0.79-3.19), P=0.20 |
1.81 (1.19-2.76), P=0.005 |
1.63 (1.25-2.11), P<0.001 |
1.74 (1.18-2.59), P=0.005 |
| 2 vs 0 markers* | 3.40 (2.0-5.76), P<0.001 |
2.52 (1.27-5.01), P=0.01 |
2.59 (1.70-3.94), P<0.001 |
1.93 (1.47-2.54), P<0.001 |
2.49 (1.68-3.69), P<0.001 |
| 3 vs 0 markers* | 7.59 (4.46-12.91), P<0.001 |
5.60 (2.72-11.56), P<0.001 |
6.17 (4.05-9.40), P<0.001 |
3.68 (2.75-4.91), P<0.001 |
5.60 (3.76-8.34), P<0.001 |
| 4 vs 0 markers* | 11.87 (6.5-21.65), P<0.001 |
6.76 (2.81-16.26), P<0.001 |
8.8 (5.32-14.57), P<0.001 |
4.17 (2.82-6.17), P<0.001 |
7.99 (4.93-12.93), P<0.001 |
Analyses were adjusted for age, sex, race, body mass index, glomerular filtration rate, acute myocardial infarction, history of previous myocardial infarction, hypertension, dyslipidemia, diabetes mellitus, left ventricular ejection fraction, history of coronary bypass graft surgery, history of coronary angioplasty, active smoking, Gensini angiographic severity score, aspirin use, statin use, and clopidogrel use. BRS indicates biomarker risk score; CI, confidence interval; CRP, C-reactive protein; FDP, fibrin degradation products; HR, hazard ratio; HSP, heat shock protein; MI, myocardial infarction; and suPAR, soluble urokinase plasminogen activator receptor.
Fine and Gray’s subdistribution hazard model was used to analyze cardiovascular death outcome, considering noncardiovascular death as the competing risk event. Patients with 0 elevated marker was treated as the reference category for the analysis of cardiovascular death.
The 4-BRS was associated with a graded increase in risk of each of the end points noted. When analyzed as a numeric scale in Cox proportional hazard models adjusting for all previously described variables, each 1 U increase in the BRS was associated with 1.94-fold increased risk of all-cause death, 1.71-fold increased risk of cardiovascular death, 1.77-fold increased risk of death/MI, 1.45-fold increase in the risk of death/MI/revascularization, and 1.72-fold increased risk of death/MI/stroke (P<0.001 for all). Comparison of hazard ratios for those with 1, 2, 3, and 4 elevated biomarkers compared with those with no elevated biomarker are presented in Table 3, and cumulative incidence plots are shown in Figure 1. Thus, compared with those with a BRS of 0, those with a BRS of 4 had an 8.8-fold (P<0.001) increased odds of having death or MI or a 6.76-fold (P<0.001) increased risk of cardiovascular death. Average annual event rates increased in a graded fashion across the categories of the BRS, with the 2.6% of subjects with a BRS of 4 having on average 21% per year risk of death/MI compared with a 1.1% event rate in the 28% of subjects with a BRS of 0 (Figure 2). Average annual event rates for each end point across categories of the BRS are presented in Figure 2.
Figure 1.
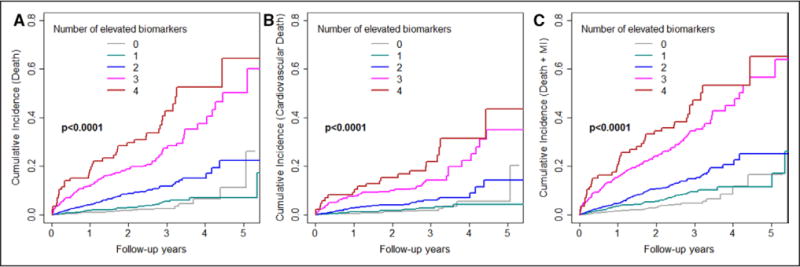
Cumulative incidence plots for all-cause death (A), cardiovascular death (B), and all-cause death/MI (C) per category of the biomarker risk score. MI indicates myocardial infarction.
Figure 2.
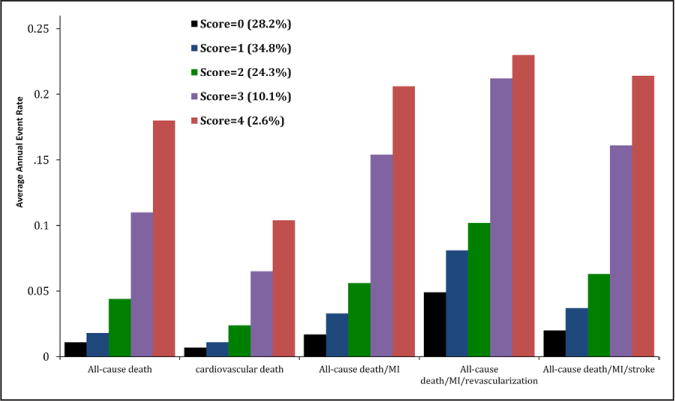
Annual event rates of major cardiovascular events in each category of the biomarker risk score. Percent of patients within each biomarker risk category is listed beside each score. MI indicates myocardial infarction.
Discrimination Testing
The addition of suPAR to a model consisting of clinical covariates and the 3-BRS was associated with significant improvement in risk reclassification metrics and C statistic with respect to the primary end point of all-cause death and MI, as well as the outcomes of all-cause death, cardiovascular death, and the combined outcomes (Table 4).
Table 4.
Discrimination Analysis of the Biomarker Risk Score With Major Cardiovascular Events
| Model | C Statistic (95% CI) | ΔC Statistic (95% CI) | Continuous NRI (95% CI) | Relative IDI (95% CI) |
|---|---|---|---|---|
| All-cause death/MI | ||||
| 3-biomarker risk score | 0.71 (0.67-0.75) | … | … | … |
| 3-biomarker risk score+suPAR | 0.74 (0.70-0.78) | 0.03 (0.01-0.05) | 0.60 (0.28-0.96) | 0.10 (0.03-0.17) |
| All-cause death | ||||
| 3-biomarker risk score | 0.71 (0.67-0.75) | … | … | … |
| 3-biomarker risk score+suPAR | 0.74 (0.70-0.78) | 0.03 (0.01-0.06) | 0.61 (0.27-0.99) | 0.11 (0.03-0.19) |
| Cardiovascular death | ||||
| 3-biomarker risk score | 0.71 (0.66-0.76) | … | … | … |
| 3-biomarker risk score+suPAR | 0.73 (0.68-0.79) | 0.02 (0.00-0.05) | 0.60 (0.13-0.86) | 0.08 (0.01-0.16) |
| All-cause death/MI/revascularization | ||||
| 3-biomarker risk score | 0.66 (0.63-0.69) | … | … | … |
| 3-biomarker risk score+suPAR | 0.67 (0.64-0.70) | 0.01 (0.00-0.02) | 0.57 (0.26-0.83) | 0.05 (0.01-0.09) |
| All-cause death/MI/stroke | ||||
| 3-biomarker risk score | 0.70 (0.66-0.75) | … | … | … |
| 3-biomarker risk score+suPAR | 0.73 (0.70-0.77) | 0.03 (0.01-0.05) | 0.60 (0.10-0.99) | 0.10 (0.02-0.16) |
Baseline model comprised age, sex, race, body mass index, a history of smoking, glomerular filtration rate, history of myocardial infarction, history of revascularization (coronary bypass graft or percutaneous coronary intervention), hypertension, dyslipidemia, diabetes mellitus, left ventricular ejection fraction, presence of obstructive coronary artery disease, aspirin use, statin use, and clopidogrel use. CI indicates confidence interval; IDI, integrated discrimination improvement; MI, myocardial infarction; NRI, net reclassification index; and suPAR, soluble urokinase plasminogen activator receptor.
Discussion
Several pathways are involved and act synergistically in the development of atherosclerotic plaque and its progression to the stage of plaque instability and rupture. We had previously identified a BRS comprising CRP, FDP, and HSP-70 that predicted risk of incident MI and death but did not correlate with the presence or severity of CAD.6 The present study demonstrates that the circulating level of suPAR is an independent predictor of adverse CVD outcomes after adjustment for all traditional cardiovascular risk factors and levels of CRP, FDP, and HSP-70. Moreover, the addition of suPAR to a 3-BRS based on the aforementioned biomarkers improves risk reclassification metrics of the C statistic, net reclassification index, and integrated discrimination improvement. When comparing this 4-BRS strategy to our prior study involving 3 biomarkers, we found that only the 4-BRS and not the 3-BRS was independently associated with both presence and severity of CAD.
The urokinase plasminogen activator is a serine protease produced by smooth muscle cells, vascular endothelial cells, macrophages, monocytes, and fibroblasts, and when bound to its receptor (urokinase plasminogen activator receptor), it leads to the generation of plasmin.7,23 Urokinase plasminogen activator receptor is involved in several functions, including migration, adhesion, fibrinolysis, and cell proliferation.24–26 Plasma suPAR reflects cellular shedding of urokinase plasminogen activator receptor, which is induced during inflammation; shedding seems to be free of circadian changes and is relatively stable during periods of acute stress.9,27 suPAR has been reported to predict incident CVD independent of the Framingham risk score in healthy populations free of CVD28,29 and in those with CVD.10,11
CRP has been widely studied in populations free of CVD,30,31 as well as in cohorts with established CAD; it predicts incident CVD and major adverse cardiovascular events independent of traditional cardiovascular risk factors.32,33 HSPs are abundant intracellular proteins that aid in a cell’s response to acute stress and are involved in protein folding and transport.34 HSP-70 is one of the more extensively studied HSPs; yet, its relationship with CAD has been controversial.35 It seems that while lower levels are associated with long-term development of atherosclerotic plaque,35 higher levels predispose to higher risk of plaque rupture and incident future outcomes.6 FDPs are measures of ongoing fibrin/fibrinogen degradation. Increased plasma FDP level predicts incident cardiovascular events in patients with peripheral vascular disease.36 Plasma D-dimer, which is one of the products included in the FDP analysis, is a marker of turnover of cross-linked fibrin; it was higher in those with CAD compared with that in healthy controls37 and predicted adverse cardiovascular events in healthy individuals independent of cardiovascular risk factors.38 Similarly, in the BARI 2D study (Bypass Angioplasty Revascularization Investigation 2 Diabetes), people with diabetes mellitus with CAD had higher D-dimer levels that were associated with an increased risk of cardiac events.39 FDP ELISA assay used in this study detects the full complement of FDP components, including D-dimer as well as fragments D and E and any additional intermediate products from fibrin degradation.
Previous studies investigating the role of multiple biomarkers in populations without CVD have demonstrated only slight improvements in predictive potential (using C statistic) when added to standard clinical models.40–42 In contrast, our study establishes the value of a multimarker aggregate score in a high-risk population with suspected or established CAD, a group in which traditional risk scores such as Framingham have failed to identify risk of recurrent CVD events. We have shown that despite a statistically significant 2-by-2 correlation between the 4 biomarkers, the observed correlation coefficients were rather modest. This finding is compatible with the concept that these biomarkers act independently and do so with their predominant activities involving separate biological pathways. It is well known that the immune system, partly because of stimulation of inflammatory pathways, plays an important role in the pathogenesis of atherosclerosis and in the activation of pathways leading to plaque rupture.43 Here we have shown that a BRS comprising suPAR (a biomarker of immune system activation and of inflammation) in addition to CRP, FDP, and HSP-70 significantly predicts all major outcomes, as well as the severity of CAD. Thus, although the HR for increase in risk of death and MI was 5-fold greater in those who had 3 positive biomarkers compared with those with a BRS of 0 using the risk score described previously, the HR was 8.8-fold greater in those with 4 positive biomarkers using the 4 aggregate BRS.6 This corresponds to an average annual event rate of 21% in these patients. Use of this BRS in conjunction with other readily available clinical factors could potentially guide clinicians in appropriate identification of patients at highest risk with use of more aggressive treatment options, risk factor control, and behavioral modification counseling. Conversely, identification of patients at low risk could prevent unnecessary testing in this population. Whether this improvement in the ability to predict risk impacts clinical management, however, remains to be determined and requires the establishment and study of therapeutic algorithms based on these biomarkers.
Strengths and Limitations
Our study has several strengths. We enrolled individuals of both genders and races, those with acute MI, and patients with a range of left ventricular ejection fraction, reflecting a population that is at high risk yet typical of those undergoing cardiac catheterization. Biomarker evaluation was performed at one time point by the same laboratory personnel, which minimized variability. C statistic, net reclassification index, and integrated discrimination improvement were calculated using survival models, which allows for better model discrimination and overall predictive ability. Limitations of our study include a one-time measurement of biomarkers that may not reflect fluctuations in their levels over time. Despite rigorous attempts in controlling for confounding variables, our inability to adjust for a well-validated comorbidity index in this study further adds to limitations. Our results need to be further validated and should not be generalized to a population without suspected or known CAD. Furthermore, future studies are needed to assess cost-effectiveness of using this multimarker BRS in routine clinical practice.
In conclusion, a 4-BRS representing inflammation, coagulation, cell stress, and immune pathways significantly predicts risk of MI and death and significantly improves risk reclassification beyond a 3-BRS. Whether more aggressive medical management in individuals with high BRS would lead to a decrease in the BRS and whether such a decrease, if it occurs, modifies subsequent risk of outcomes remain to be studied.
WHAT IS KNOWN
A 3-biomarker risk score incorporating levels of C-reactive protein, fibrin degradation products, and heat shock protein-70 representing pathways of inflammation, hypercoagulable state, and cellular stress was previously shown to significantly improve risk prediction in patients with or at high risk of cardiovascular disease.
Soluble urokinase plasminogen activator receptor, thought to be a marker of immune activation, is strongly predictive of outcomes in various populations
WHAT THE STUDY ADDS
Soluble urokinase plasminogen activator receptor is associated with worse outcomes independently of traditional risk factors, as well as C-reactive protein, fibrin degradation products, and heat shock protein-70.
Addition of soluble urokinase plasminogen activator receptor to a biomarker risk score incorporating the aforementioned biomarkers significantly improved risk prediction.
The clinical utility of a multimarker approach to risk prediction warrants investigation.
Acknowledgments
We thank the personnel at GenWay for analyzing the original 3 biomarkers (fibrin degradation product [FDP], heat shock protein [HSP- 70], and C-reactive protein [CRP]) and the members of the Emory Cardiovascular Biobank team, Emory Clinical Cardiovascular Research Institute (ECCRI), and Atlanta Clinical and Translational Science Institute for recruitment of participants, compilation of data, and preparation of samples. Drs Ghasemzadeh, Hayek, and Quyyumi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
Dr Quyyumi is supported by 5P01HL101398-02, 1P20HL113451-01, 1R56HL126558-01, 1RF1AG051633-01, R01 NS064162-01, R01 HL89650-01, HL095479-01, 1U10HL110302-01, 1DP3DK094346-01, and 2P01HL086773-06A1. Funding for collection and management of samples was received from the Robert W. Woodruff Health Sciences Center Fund (Atlanta, GA), Emory Heart and Vascular Center (Atlanta, GA), Katz Family Foundation Preventive Cardiology Grant (Atlanta, GA), and National Institutes of Health (NIH) Grants UL1 RR025008 from the Clinical and Translational Science Award program. Soluble urokinase plasminogen activator receptor (suPAR) sample kits were provided by ViroGates (Denmark). High-sensitivity C-reactive protein measurements were conducted by FirstMark, Division of GenWay Biotech Inc (San Diego, CA). High-sensitivity troponin-I was measured by Abbott Laboratories (Abbott Park, IL).
Footnotes
Disclosures
Drs Epstein and Quyyumi are equity holders in GenWay Biotech and receive consulting fees. T Pielak and C.W. Thorball are employed by Virogates (Denmark). The other authors report no conflicts.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430.. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 4.Pencina MJ, D’Agostino RB, Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shlipak MG, Ix JH, Bibbins-Domingo K, Lin F, Whooley MA. Biomarkers to predict recurrent cardiovascular disease: the Heart and Soul Study. Am J Med. 2008;121:50–57. doi: 10.1016/j.amjmed.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, Nanjundappa RA, Sikora S, Malayter D, Wilson PW, Sperling L, Quyyumi AA, Epstein SE. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. 2013;62:329–337. doi: 10.1016/j.jacc.2013.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuhrman B. The urokinase system in the pathogenesis of atherosclerosis. Atherosclerosis. 2012;222:8–14. doi: 10.1016/j.atherosclerosis.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 8.Eugen-Olsen J, Andersen O, Linneberg A, Ladelund S, Hansen TW, Langkilde A, Petersen J, Pielak T, Møller LN, Jeppesen J, Lyngbaek S, Fenger M, Olsen MH, Hildebrandt PR, Borch-Johnsen K, Jørgensen T, Haugaard SB. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296–308. doi: 10.1111/j.1365-2796.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 9.Lyngbæk S, Marott JL, Sehestedt T, Hansen TW, Olsen MH, Andersen O, Linneberg A, Haugaard SB, Eugen-Olsen J, Hansen PR, Jeppesen J. Cardiovascular risk prediction in the general population with use of su-PAR, CRP, and Framingham Risk Score. Int J Cardiol. 2013;167:2904–2911. doi: 10.1016/j.ijcard.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Lyngbæk S, Marott JL, Møller DV, Christiansen M, Iversen KK, Clemmensen PM, Eugen-Olsen J, Jeppesen JL, Hansen PR. Usefulness of soluble urokinase plasminogen activator receptor to predict repeat myocardial infarction and mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous intervention. Am J Cardiol. 2012;110:1756–1763. doi: 10.1016/j.amjcard.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Eapen DJ, Manocha P, Ghasemzadeh N, Patel RS, Al Kassem H, Hammadah M, Veledar E, Le NA, Pielak T, Thorball CW, Velegraki A, Kremastinos DT, Lerakis S, Sperling L, Quyyumi AA. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc. 2014;3:e001118. doi: 10.1161/JAHA.114.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, Wei C, Hotton AL, French AL, Sperling LS, Lerakis S, Quyyumi AA, Reiser J. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 14.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(4 suppl):5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 15.Gensini G. Coronary Arteriography. New York, NY: Futura Publishing Co; 1975. [Google Scholar]
- 16.Sinning C, Lillpopp L, Appelbaum S, Ojeda F, Zeller T, Schnabel R, Lubos E, Jagodzinski A, Keller T, Munzel T, Bickel C, Blankenberg S. Angiographic score assessment improves cardiovascular risk prediction: the clinical value of SYNTAX and Gensini application. Clin Res Cardiol. 2013;102:495–503. doi: 10.1007/s00392-013-0555-4. [DOI] [PubMed] [Google Scholar]
- 17.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32:2430–2442. doi: 10.1002/sim.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207. [DOI] [PubMed] [Google Scholar]
- 22.Clark O, Djulbegovic B. Forest plots in excel software(Data sheet) 2001 [Google Scholar]
- 23.Waltz DA, Fujita RM, Yang X, Natkin L, Zhuo S, Gerard CJ, Rosenberg S, Chapman HA. Nonproteolytic role for the urokinase receptor in cellular migration in vivo. Am J Respir Cell Mol Biol. 2000;22:316–322. doi: 10.1165/ajrcmb.22.3.3713. [DOI] [PubMed] [Google Scholar]
- 24.Madsen CD, Sidenius N. The interaction between urokinase receptor and vitronectin in cell adhesion and signalling. Eur J Cell Biol. 2008;87:617–629. doi: 10.1016/j.ejcb.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 26.Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol. 2007;177:927–939. doi: 10.1083/jcb.200612058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157–172. doi: 10.3233/DMA-2009-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kjellman A, Akre O, Gustafsson O, Høyer-Hansen G, Lilja H, Norming U, Piironen T, Törnblom M. Soluble urokinase plasminogen activator receptor as a prognostic marker in men participating in prostate cancer screening. J Intern Med. 2011;269:299–305. doi: 10.1111/j.1365-2796.2010.02284.x. [DOI] [PubMed] [Google Scholar]
- 29.Persson M, Engström G, Björkbacka H, Hedblad B. Soluble urokinase plasminogen activator receptor in plasma is associated with incidence of CVD. Results from the Malmö Diet and Cancer Study. Atherosclerosis. 2012;220:502–505. doi: 10.1016/j.atherosclerosis.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 30.Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett-Connor E, Benjamin EJ, Björkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D’Agostino RB, Sr, Dankner R, Davey-Smith G, Deeg D, Dekker JM, Engström G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, J0rgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil- Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J, Emerging Risk Factors Collaboration C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–2011. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 32.Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, Hsia J, Gersh BJ, Rifai N, Ridker PM, Pfeffer MA, Braunwald E, PEACE Investigators Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 33.Hemingway H, Philipson P, Chen R, Fitzpatrick NK, Damant J, Shipley M, Abrams KR, Moreno S, McAllister KS, Palmer S, Kaski JC, Timmis AD, Hingorani AD. Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med. 2010;7:e1000286. doi: 10.1371/journal.pmed.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–1559. doi: 10.1161/01.atv.0000029720.59649.50. [DOI] [PubMed] [Google Scholar]
- 35.Zhu J, Quyyumi AA, Wu H, Csako G, Rott D, Zalles-Ganley A, Ogunmakinwa J, Halcox J, Epstein SE. Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:1055–1059. doi: 10.1161/01.ATV.0000074899.60898.FD. [DOI] [PubMed] [Google Scholar]
- 36.Fowkes FG, Lowe GD, Housley E, Rattray A, Rumley A, Elton RA, MacGregor IR, Dawes J. Cross-linked fibrin degradation products, progression of peripheral arterial disease, and risk of coronary heart disease. Lancet. 1993;342:84–86. doi: 10.1016/0140-6736(93)91288-w. [DOI] [PubMed] [Google Scholar]
- 37.Koenig W, Rothenbacher D, Hoffmeister A, Griesshammer M, Brenner H. Plasma fibrin D-dimer levels and risk of stable coronary artery disease: results of a large case-control study. Arterioscler Thromb Vasc Biol. 2001;21:1701–1705. doi: 10.1161/hq1001.097020. [DOI] [PubMed] [Google Scholar]
- 38.Empana JP, Canoui-Poitrine F, Luc G, Juhan-Vague I, Morange P, Arveiler D, Ferrieres J, Amouyel P, Bingham A, Montaye M, Ruidavets JB, Haas B, Evans A, Ducimetiere P, PRIME Study Group Contribution of novel biomarkers to incident stable angina and acute coronary syndrome: the PRIME Study. Eur Heart J. 2008;29:1966–1974. doi: 10.1093/eurheartj/ehn331. [DOI] [PubMed] [Google Scholar]
- 39.Sobel BE, Hardison RM, Genuth S, Brooks MM, McBane RD, 3rd, Schneider DJ, Pratley RE, Huber K, Wolk R, Krishnaswami A, Frye RL, BARI 2D Investigators Profibrinolytic, antithrombotic, and antiinflammatory effects of an insulin-sensitizing strategy in patients in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2011;124:695–703. doi: 10.1161/CIRCULATIONAHA.110.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St-Pierre AC, Cantin B, Bergeron J, Pirro M, Dagenais GR, Després JP, Lamarche B. Inflammatory markers and long-term risk of ischemic heart disease in men A 13-year follow-up of the Quebec Cardiovascular Study. Atherosclerosis. 2005;182:315–321. doi: 10.1016/j.atherosclerosis.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D’Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 42.Kim HC, Greenland P, Rossouw JE, Manson JE, Cochrane BB, Lasser NL, Limacher MC, Lloyd-Jones DM, Margolis KL, Robinson JG. Multimarker prediction of coronary heart disease risk: the Women’s Health Initiative. J Am Coll Cardiol. 2010;55:2080–2091. doi: 10.1016/j.jacc.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 43.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]


