Abstract
Previous studies have suggested that CD47, an essential cell-surface protein, plays an important role in polymorphonuclear neutrophil (PMN) transmigration across tissue cells and extracellular matrix. In the current study, the role of CD47 in PMN transmigration and infiltration into tissues was further evaluated by investigating the function of CD47−/− PMN and inflammatory conditions induced in CD47−/− mice. Using in vitro time-course assays, we found that CD47−/− PMN exhibited no impediment, but slightly enhanced response to and transmigration toward, the chemoattractant fMLF. In vivo analysis in CD47−/− mice by inducing acute peritonitis and aggressive colitis observed consistent results, indicating that both PMN and monocytes effectively infiltrated inflammatory sites despite the absence of CD47 on these leukocytes or the surrounding tissue cells. Although PMN transmigration was not delayed in CD47−/− mice, fewer PMN were found in the intestine at the postacute/chronic stage of chronic colitis induced with sustained low-dose dextran sulfate sodium. Further analysis suggested that the paucity of PMN accumulation was attributable to attenuated granulopoiesis secondary to assessed lower levels of IL-17. Administration of exogenous IL-17A markedly increased PMN availability and rapidly rendered severe colitis in CD47−/− mice under dextran sulfate sodium treatment.
Polymorphonuclear neutrophil (PMN) transmigration across the vasculature into tissues plays a crucial role in innate immunity but is also associated with severe tissue injury, as observed in many inflammatory diseases. CD47, an Ig superfamily transmembrane protein broadly expressed in leukocytes and most tissue cells, has been suggested to play an important role in regulating PMN transmigration. In particular, in vitro leukocyte transmigration assays showed that CD47-specific mAbs, such as C5D5 and B6H12.2, potently inhibit PMN transmigration across endothelial monolayers, collagen-coated filters, and epithelial monolayers in response to chemoattractants fMLF and IL-8 (1–3). Using a time-course transmigration setup, our previous study further demonstrated that the inhibition by anti-CD47 mAbs is through delaying the PMN transmigration rate via a mechanism involving protein tyrosine phosphorylation–mediated signaling events (2, 4). Our study also suggested that CD47 expressed on both PMN and tissue cells contributes to PMN transmigration but via different mechanisms. CD47 on tissue cells such as endothelial and epithelial cells may facilitate PMN transmigration through interactions with SIRPα, a specific CD47 cellular ligand, on PMN (5, 6). CD47 on PMN, in contrast, appears to mediate the transmigration process through PMN-specific protein interaction and signaling events independent of tissue cells (2, 4).
The role of CD47 in PMN transmigration has also been evaluated by inducing inflammatory conditions in CD47-deficient (CD47−/−) mice. The initial study by Lindberg et al. (7), who also established the mouse strain, showed that CD47 deficiency rendered mice vulnerable to bacterial infection, which was possibly attributable to a certain defect of PMN transmigration, as attenuated PMN accumulation at the infectious site was detected at the late phase. In other studies, CD47−/− mice displayed reduced severities in bacteria and LPS-induced lung inflammation (8) and trinitrobenzene sulfgonic acid–induced colitis (9). Decreases in PMN infiltration may be involved in these cases, as PMN-mediated tissue damage is associated with the severity of these conditions.
In the current study, we further determined how CD47 deficiency affects PMN infiltration during inflammation. We employed the same in vitro PMN transmigration system that previously demonstrated inhibitory effects of anti-CD47 mAbs and also examined PMN infiltration in vivo under different inflammatory conditions. To our surprise, CD47−/− PMN failed to display defect in chemotactic response and transmigration in response to inflammatory challenge in vitro and in vivo. When inducing chronic colitis with low doses of dextran sulfate sodium (DSS), we found that PMN accumulation in intestines was reduced, but not until the postacute/chronic stage and likely due to largely attenuated granulopoiesis. Administration of exogenous IL-17A stimulated granulopoiesis, reinstated PMN recruitment, and intensified colitis in CD47−/− mice under DSS treatment.
Materials and Methods
Mice and inflammation models
CD47−/− mice obtained from The Jackson Laboratory were housed with free access to autoclaved water and food in a pathogen-free animal facility. To induce colitis, mice (female, 6–8 wk or 20–22 g) were treated with either 4% DSS (MP Biomedicals) for 7 d or 2% DSS for 15 d through drinking water (10). Mice were evaluated daily for change in body weight and overall body condition including distress and colitic symptoms (wet stool, diarrhea, and bloody diarrhea). Zymosan-induced peritonitis was instigated through i.p. injection of 0.5 mg zymosan A (Sigma-Aldrich) in 0.5 ml PBS followed by evaluation of macrophages, PMN, and monocytes in the peritoneum at varying time points (10). For adoptive transfer of leukocytes (10), total leukocytes obtained from femur bones of wild-type (WT) and CD47−/− donor mice were labeled with CFSE and DiIC16 (3) (Invitrogen), respectively. The cells were then mixed at 1:1 ratio of PMN, as determined by myeloperoxidase (MPO) assays and anti–Ly-6G staining, followed by i.v. injecting into WT or CD47−/− recipient mice. Protocols of using animals and procedures of animal care and handling were approved by the Institutional Animal Care and Use Committee of Georgia State University.
Measurement of cytokines
Cytokines in the peripheral blood (PB) serum were assayed by standard sandwich ELISA. In brief, anti-cytokine Ab–coated ELISA plates were incubated with serum of different dilutions (2 h, 25°C). After washing, the wells were incubated with biotin-conjugated detecting Abs and HRP-conjugated streptavidin followed by detection with HRP substrates. Recombinant murine IL-1β, IL-6, IL-17A, and G-CSF used as standards were obtained from PeproTech. To assess macrophage production of IL-6, macrophages obtained from the peritoneal lavage were cultured in DMEM with 10% FBS followed by stimulation with 0.5 mg zymosan A. Cell supernatants were then collected at various time points, and levels of IL-6 were detected by ELISA.
In vitro PMN chemotaxis assay
This assay was performed using collagen-coated transwell devices with transfilters (0.33 cm2, 5-μm pore size) as described previously (2, 3, 11). Briefly, bone marrow (BM) leukocytes obtained from mouse femur bones were subjected to further separation to isolate PMN according to a protocol of Dong and Wu (12). PMN (~2 × 106) in 150 μl HBSS were then added into the upper chambers of the transwell devices followed by inducing chemotaxis by adding the chemoattractant fMLF (10 μM) into the lower chamber and incubation at 37°C. PMN that transmigrated into the lower chambers were quantified by MPO assays.
Tissue sectioning and staining
Colons removed from mice were immediately frozen and sectioned to 5–10 μm thickness using a Cryostat-microtome. For histological staining, the thin sections were stained with H&E. To visualize PMN infiltration into tissues, the sections were blocked with 5% BSA followed by staining with fluorescence-conjugated anti-mouse Ly-6G Ab followed by fluorescence microscopy.
Results
CD47 deficiency does not restrain PMN transmigration
To further understand how CD47 regulates PMN transmigration, CD47−/− PMN obtained from CD47−/− mice were tested in vitro by chemotactic transmigration assays (2). As shown in Fig. 1A, CD47−/− PMN displayed no inhibition in chemotactic response or transmigration, but potently migrated across collagen-coated filters toward the chemoattractant fMLF in a manner similar to PMN isolated from WT mice. Variation of the fMLF concentration found that CD47−/− PMN generally had the same optimal response as that of WT PMN, but responded slightly better at the low range of fMLF concentrations (Fig. 1B).
FIGURE 1.
Assaying PMN chemotactic transmigration in vitro. BM PMN (2 × 106) obtained from femur bones of WT and CD47−/− mice were applied into the collagen-coated transwells and induced to transmigrate by fMLF (37°C). (A) PMN chemotaxis induced by 10 μM fMLF for 1 and 2 h. (B) PMN responses to fMLF of 0.1–100 μM. PMN transmigration was measured at 1 h. Data are expressed as means ± SEM and represent one of three independent experiments with PMN samples from three mice/group and each sample assayed in triplicates. *p < 0.01.
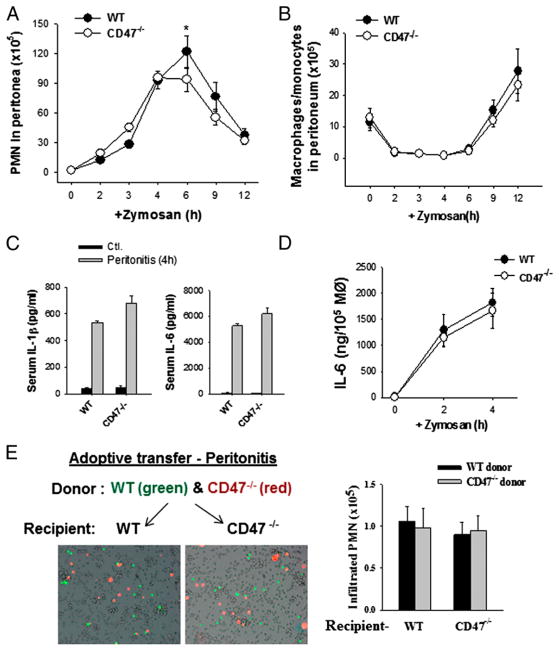
PMN transmigration in vivo was tested by zymosan-induced peritonitis. As shown in Fig. 2A, zymosan administration induced rapid PMN infiltration into the peritoneum starting at 2 h and reaching a peak at 4–6 h, followed by a fast clearance. Compared to WT mice, CD47−/− mice exhibited consistently no delay, but a slight increase (10–30%) of PMN infiltration at early time periods (2 to 3 h). However, PMN accumulation in the peritoneum at 6 h tended to be moderately attenuated (~20–30%) in CD47−/− mice. No differences were observed between CD47−/− and WT mice on the number of inflammatory monocytes (Gr-1+ F4/80+) that infiltrated into the peritoneum after PMN (>6 h) and the key cytokines, IL-6 and IL-1β, associated with zymosan-induced peritonitis (10) (Fig. 2B, 2C). Isolation of peritoneal macrophages and in vitro testing their response to zymosan confirmed that CD47−/− macrophages responded and produced IL-6 similarly as WT macrophages (Fig. 2D).
FIGURE 2.
Analysis of PMN infiltration in vivo by zymosan-induced peritonitis. Zymosan (0.5 mg) was administered (i.p.) into mice (female, 20–22 g) followed by measuring PMN infiltration and other changes at different time points. (A) PMN infiltration into the peritoneum. The data are expressed as means ± SEM. (B) Dynamic changes of F4/80+ macrophages and monocytes in the peritoneum. (C) Serum inflammatory cytokines associated with peritonitis at 4 h post–zymosan injection. (D) In vitro assay of macrophage response. Peritoneal macrophages obtained from WT and CD47−/− mice were challenged with 0.5 mg zymosan in vitro followed by detection of IL-6 released. (E) Adoptive transfer of leukocytes followed by inducing peritonitis confirmed that CD47 deficiency does not impede PMN infiltration. BM leukocytes derived from WT and CD47−/− mice were labeled with CFSE (green) and DiIC16 (3) (red), respectively, and mixed at 1:1 ratio followed by transferring into recipient WT or CD47−/− mice. Zymosan-induced peritonitis was then induced in recipient mice, and peritoneal in-filtration of donor PMN at 4 h was analyzed. The data are expressed as means ± SEM. Original magnification ×100. *p < 0.01. MØ, Macrophages.
Because CD47 is broadly expressed on both leukocytes and tissue cells, we designed adoptive-transfer experiments to separately address the role of CD47 on PMN or tissue cells. In these experiments, PMN were isolated from WT and CD47−/− mice. After labeling with different dyes (red and green), these cells were mixed and cotransferred into other WT or CD47−/− recipient mice. As shown in Fig. 2E, inducing peritonitis in recipient mice by zymosan found that WT and CD47−/− PMN were equally recruited into the peritoneum in either WT or CD47−/− recipient mice. Thus, these results suggest that CD47 expression on both PMN and tissue cells were dispensable for PMN infiltration in vivo.
Resistance of CD47−/− mice to chronic but not highly aggressive acute colitis
It was perplexing in the current study to observe that CD47 depletion caused no inhibition in PMN transmigration, whereas previous studies suggested that CD47−/− mice were associated with decreased levels of inflammation presumably due to decreases in PMN infiltration (7–9). To investigate the discrepancies between our results and previous findings, we induced two sets of colitis in CD47−/− mice and studied PMN infiltration under these conditions. In the first set, mice were given 4% DSS in the drinking water to induce highly progressive acute colitis. As shown in Fig. 3A, all tested CD47−/− and WT mice rapidly developed intensive colitis and succumbed to the treatment in 5–7 d. Examination of the intestines on day 6 revealed significantly shortened large intestines in both strains (Fig. 3B). Tissue sectioning and staining demonstrated severe structural damage of mucosal epithelial layers (Fig. 3C) and aggressive PMN infiltration (Fig. 3D). Thus, CD47−/− offered no protection in acute colitis.
FIGURE 3.
CD47−/− mice do not resist 4% DSS-induced colitis. Mice (female, 20–22 g) were given 4% DSS in drinking water continuously. (A) Development of colitis monitored by body weight loss, diarrhea, and other body conditions (clinical scores evaluated on day 6 are shown in right panel). (B) Shortening of the large intestines on day 6. (C) Histology of colon tissues (H&E staining). (D) PMN infiltration into intestines detected by anti–Ly-6G staining and measuring of tissue-associated MPO (per 200 μg of protein in tissue lysates) on day 6. Original magnification ×100 (C, D).
In the second set of experiments, mice were treated with 2% DSS via drinking water to induce a relatively chronic form of colitis. We observed that CD47−/− mice were protected against this form of inflammatory condition. As shown in Fig. 4, WT mice manifested body weight loss and colitis symptoms (diarrhea) starting from day 6 of the treatment. The colitic condition was exacerbated at around day 9, and the mice were eventually exhausted by days 12–15. In contrast, CD47−/− mice showed no significant body weight loss and only mild signs of distress throughout the treatment (Fig. 4A). A direct comparison on day 12 (Fig. 4B) showed that WT lost >50% of large intestines, whereas CD47−/− mice had only moderate (10–25%) shortening of the intestine. Colon tissue analysis found that CD47−/− mice had significantly reduced damage in mucosal and much less PMN infiltration compared with that seen in WT mice, which suffered massive mucosal damage and excessive PMN infiltration (Fig. 4C). In the end, CD47−/− mice survived the entire course (15 d) of 2% DSS treatment and exhibited only low-level colitis.
FIGURE 4.
CD47−/− mice resist 2% DSS-induced chronic colitis. Mice (female, 20–22 g) were given 2% DSS in drinking water for 15 d. (A) Body weight loss, death, and other symptoms (clinical score on day 12). (B) Large intestines of WT and CD47−/− mice on day 12. (C) Histology of colon tissues and PMN infiltration (tissue MPO) on day 12. (D) Time-course detection of PMN infiltration in intestines as assayed by anti–Ly-6G staining and tissue-associated MPO activity. Original magnification ×100 (C, D).2**p < 0.005, ***p < 0.001 versus respective control groups. Ctl., Control.
The fact that CD47−/− mice resisted to 2%, but not by 4%, DSS-induced colitis was intriguing, given that PMN transmigration and tissue infiltration, per se, were not impaired, as shown in the earlier study (Figs. 1, 2). We then examined the colitis development along with PMN infiltration into the intestines during 2% DSS treatment. As shown in Fig. 4D, PMN infiltrated into intestines in both CD47−/− and WT mice soon after 2% DSS treatment had begun (days 2–5). However, in WT mice, there was an abrupt, exponential increase of PMN infiltration after day 6, resulting in large numbers of PMN in intestines at the late stage. In contrast, no extensive increase of PMN infiltration was observed in CD47−/− mice after day 6. Instead, CD47−/− mice displayed only low levels of PMN infiltration throughout the entire DSS treatment. These results suggest that a mechanism that significantly enhances PMN infiltration at the late, postacute phase was absent in CD47−/− mice. As a result, CD47−/− mice experienced an overall mild form of colitis.
Reduced PMN infiltration is associated with deficit of IL-17A at postacute stage of colitis in CD47−/− mice
Because our previous study (10) demonstrated that elevation of IL-17A in the serum is critical for enhancing PMN infiltration at the postacute stage of chronic colitis, we thereby compared IL-17A levels in WT and CD47−/− mice during 2% DSS-induced colitis. As shown in Fig. 5A, in WT mice, the IL-17A level started to increase after day 6 and continued rising to >1.5 ng/ml on day 12. A transient wave of IL-6, a key cytokine that triggers IL-17A production, was also detected between days 6 and 9 in WT mice. In contrast, CD47−/− mice were completely deficient in IL-17A and IL-6 elevation in the serum even at the late phase of DSS treatment (Fig. 5A). Analysis of PMN transmigration in response to zymosan-induced peritonitis in mice that had been treated with 2% DSS for 10 d observed a significantly increased PMN recruitment at 2 h in WT mice (Fig. 5B), consistent with our previous finding indicating that PMN infiltration is enhanced under chronic colitis (10). However, testing DSS-treated CD47−/− mice by the same zymosan-induced peritonitis observed not only no enhanced PMN recruitment at 2 h, but also a general deficit of PMN infiltration. As shown in Fig. 5B, insignificant PMN were detected in the peritoneum even at 4 h. Because CD47−/− does not hinder PMN infiltration, these results of general shortage of PMN infiltration during inflammation suggest that CD47−/− mice with extended DSS treatment might be associated with depletion of the PMN supply.
FIGURE 5.
CD47−/− mice were associated with inadequate granulopoiesis at the postacute stage of colitis. (A) CD47−/− mice failed to elevate IL-17A and IL-6 in the serum at the postacute stage. (B) Zymosan-induced peritonitis performed in mice treated with 2% DSS for 10 d. Analysis of PMN infiltration into the peritoneum at 2 and 4 h. (C) Analysis of PMN pools in BM and PB in WT and CD47−/− mice. Peripheral WBC and BM leukocytes were labeled with Abs against CD11b and Gr-1 followed by FACS. Populations of PMN (Gr-1highCD11b+) and monocytes (Gr-1intCD11b+, only in BM) were labeled in the figure. (D) Serum levels of G-CSF measured by ELISA. **p < 0.005, ***p < 0.001 versus respective control groups.
Inadequate granulopoiesis in CD47−/− mice during chronic colitis and reversal by IL-17A
To determine whether CD47−/− mice under colitis suffered neutropenia, we examined PMN pools prior to and after DSS treatment. We observed that CD47−/− mice maintained ample PMN pools (Gr-1highCD11b+) in both BM and PB prior to DSS treatment (Fig. 5C). However, CD47−/− mice displayed depletion of PMN pools after extended DSS treatment. As shown in Fig. 5C, largely depleted PMN pools (circled) were observed in BM of CD47−/− mice on day 10 of 2% DSS treatment, whereas the PMN pools were sustained, or even slightly increased, in WT mice. These results suggest that, unlike WI mice, CD47−/− mice were retarded in granulopoieisis and incapable of replenishment of PMN that had been consumed. Interestingly, CD47 deficiency caused no depletion of monocytes as determined by Gr-1int CD11b+ labeling.
Because IL-17A was reported to stimulate granulopoiesis through inducing G-CSF (13–16), we suspected that lacking IL-17A was the reason for inadequate granulopoiesis in CD47−/− mice during colitis. As shown in Fig. 5D, examination of G-CSF in the serum found that CD47−/− mice were subjected to very inadequate elevation of this cytokine during 2% DSS-induced colitis. To further determine the role of IL-17A, we administrated exogenous, functional IL-17A into CD47−/− mice on days 6 and 9 during 2% DSS treatment. As shown in Fig. 6, administration of exogenous IL-17A significantly increased G-CSF levels and largely replenished PMN pools in CD47−/− mice. IL-17A administration also instantly eliminated CD47−/−-mediated resistance to colitis in mice and markedly increased PMN infiltration into intestines. Separate assays by performing zymosan-induced peritonitis in DSS-treated CD47−/− mice observed significantly enhanced PMN recruitment into the peritoneum at 2 h after IL-17A administration. IL-17A administration also increased CD34+ granulocyte precursors (CD11b+CD34+) in BM, suggestive of active granulopoiesis. Altogether, these results suggest that a defect of IL-17A elevation in CD47−/− mice served as a prominent mechanism for inadequate granulopoiesis, resulting in decreases in PMN infiltration and a weakened inflammatory reaction at the late stage of colitis.
FIGURE 6.
Administration of IL-17A eliminated CD47−/−-mediated resistance to colitis. CD47−/− mice under 2% DSS treatment were given IL-17A (20 μg) (i.v.) or vehicle (PBS) on days 6 and 9. (A) Loss of body weight during 2% DSS treatment. (B) Analysis of granulopoiesis in CD47−/− mice on day 12. (C) Serum levels of G-CSF measured by ELISA. (D) Tissue analysis of large intestines on day 12. PMN infiltration into large intestines was determined by anti–Ly-6G staining of colon sections and measuring of tissue-associated MPO activity. Original magnification ×100. (E) IL-17A administration restores PMN infiltration at the postacute stage of colitis as assayed by zymosan-induced peritonitis on day 10. (F) IL-17A increased CD34+ precursors in BM. ***p < 0.001 versus respective controls.
Discussion
Previous studies conducted in vitro demonstrated that functional Abs against cell-surface CD47 significantly delay or inhibit PMN chemotactic transmigration across epithelial and endothelial monolayers and acellular filters (1–3). Several studies carried out in CD47−/− mice also showed that the mice are at increased vulnerability to bacterial infection (7), but resistant to nonpathogen inflammatory conditions (8, 9). The hypothesis that CD47, expressed on both leukocytes and tissue cells (epithelial and endothelial cells), plays a crucial role in facilitating PMN transmigration has been suggested to account for these findings. However, the mechanism through which CD47 enables PMN transmigration into a provoked inflammatory region remains obscure.
In this study, we further investigated the role of CD47 in PMN transmigration by examining the behavior of CD47−/− PMN and CD47−/− mice under various inflammatory conditions. We found that, instead of displaying an impediment of transmigration, PMN deficient in CD47 from CD47−/− mice exhibited no obstacle in migrating toward chemoattractant fMLF. Inducing acute peritonitis by zymosan, and aggressive colitis by 4% DSS, in CD47−/− mice also demonstrated prompt PMN response and infiltration into inflammatory sites. Adoptive transfer of CD47−/− PMN into WT mice, or the other way around, WT PMN into CD47−/− mice, confirmed that depletion of CD47 in either PMN or tissue cells offered little hindrance to PMN transmigration in vivo. Not only was there an absence of inhibition to transmigration, but also in all of the in vitro and in vivo transmigration assays, CD47−/− PMN migrated even slightly faster than PMN of WT mice. In summary, our results conclude that CD47 is dispensable in mounting inflammatory reactions and driving PMN transmigration. These results suggest that CD47 mAb-mediated delay of PMN transmigration is possibly through Ab ligation–triggered inhibitory signaling in PMN or Ab interference of CD47 dynamic interactions on the cell surface resulting in hindrance of PMN transmigration. It is also possible that CD47−/− mice develop a mechanism that compensates the loss of CD47 and supports PMN transmigration. In addition, both in vitro and in vivo assays also showed that CD47 deficiency had no effect on macrophage response and production of proinflammatory cytokines, including IL-1β and IL-6, upon zymosan challenge. Infiltration of monocytes during peritonitis was also unaffected in CD47−/− mice.
However, although PMN response and transmigration were not delayed by CD47 deficiency, decreases in PMN infiltration in CD47−/− mice have been reported (7–9) and were observed in our experiments when inducing relatively chronic colitis using low doses (2%) of DSS. As shown by our results, the initial responses to 2% DSS treatment, including immediate PMN infiltration into intestines, were similar in both CD47−/− and WT mice. After the acute stage (first 6 d) of low-level inflammation, drastic changes occurred in WT mice, and these changes were accompanied by significantly enhanced PMN infiltration. As shown in our results, PMN infiltration into intestines was exponentially increased in WT mice at the postacute phase of DSS treatment, resulting in exaggerated tissue damage and rapidly deteriorated colitic conditions (10). However, CD47−/− mice experienced no such changes, nor enhanced PMN infiltration. In addition, the prominent increase of IL-17A associated with enhanced PMN infiltration observed in WT mice (10) was also missing in CD47−/− mice.
The role of IL-17 in promoting inflammation has been shown previously in various models of inflammation as well as in mice deficient of IL-17. In particular, IL-17 (IL-17A/F) is suggested to induce G-CSF, which in turn promotes granulopoiesis in BM (13–16) and also promotes PMN release into the circulation (17–19). During 2% DSS–induced colitis, high levels of IL-17A produced at the postacute stage largely promote granulopoiesis. CD47−/− mice, however, lacked IL-17 and thus were unable to induce G-CSF and granulopoiesis during colitis. As a result, CD47−/− mice depleted PMN stores during chronic inflammation and displayed attenuation of PMN infiltration at the late phase. The mechanism that accounts for IL-17 preclusion in CD47−/− mice is likely associated with defective recruitment of SIRPα+CD103− DC to lymph nodes, leading to insufficient polarization of Th17 cells (9). Administration of exogenous IL-17A into CD47−/− mice, as shown in our study, induced G-CSF and remarkably replenished the missing PMN pools, leading to instant aggravation of the inflammatory condition. Our studies also suggest that the BM machinery of granulopoiesis is not defective in CD47−/− mice.
Interestingly, although CD47−/− mice displayed only low-level inflammation throughout 2% DSS treatment, these mice suffered yet another phenotypically distinctive colitis after the DSS treatment was terminated and eventually died (data not shown). Thus, additional studies are required to further investigate CD47 deficiency-mediated chronic inflammation and to ask whether CD47−/− mice are defective in producing critical mediators and/or triggering macrophage phagocytosis (20–24) that are required for inflammation resolution and tissue recovery.
Acknowledgments
We thank Dr. Jill Leslie Littrell and Daniel Jordan for critical reading and revision of the manuscript. We also thank Dr. Ke Zen for the constructive discussion.
Abbreviations used in this article
- BM
bone marrow
- DSS
dextran sulfate sodium
- MPO
myeloperoxidase
- PB
peripheral blood
- PMN
polymorphonuclear neutrophil
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Cooper D, Lindberg FP, Gamble JR, Brown EJ, Vadas MA. Transendothelial migration of neutrophils involves integrin-associated protein (CD47) Proc Natl Acad Sci USA. 1995;92:3978–3982. doi: 10.1073/pnas.92.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J Biol Chem. 2001;276:40156–40166. doi: 10.1074/jbc.M104138200. [DOI] [PubMed] [Google Scholar]
- 3.Parkos CA, Colgan SP, Liang TW, Nusrat A, Bacarra AE, Carnes DK, Madara JL. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J Cell Biol. 1996;132:437–450. doi: 10.1083/jcb.132.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zen K, Liu Y. Role of different protein tyrosine kinases in fMLP-induced neutrophil transmigration. Immunobiology. 2008;213:13–23. doi: 10.1016/j.imbio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 5.de Vries HE, Hendriks JJ, Honing H, De Lavalette CR, van der Pol SM, Hooijberg E, Dijkstra CD, van den Berg TK. Signal-regulatory protein alpha-CD47 interactions are required for the transmigration of monocytes across cerebral endothelium. J Immunol. 2002;168:5832–5839. doi: 10.4049/jimmunol.168.11.5832. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Bühring HJ, Zen K, Burst SL, Schnell FJ, Williams IR, Parkos CA. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem. 2002;277:10028–10036. doi: 10.1074/jbc.M109720200. [DOI] [PubMed] [Google Scholar]
- 7.Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 8.Su X, Johansen M, Looney MR, Brown EJ, Matthay MA. CD47 deficiency protects mice from lipopolysaccharide-induced acute lung injury and Escherichia coli pneumonia. J Immunol. 2008;180:6947–6953. doi: 10.4049/jimmunol.180.10.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortin G, Raymond M, Van VQ, Rubio M, Gautier P, Sarfati M, Franchimont D. A role for CD47 in the development of experimental colitis mediated by SIRPalpha+CD103- dendritic cells. J Exp Med. 2009;206:1995–2011. doi: 10.1084/jem.20082805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bian Z, Guo Y, Ha B, Zen K, Liu Y. Regulation of the inflammatory response: enhancing neutrophil infiltration under chronic inflammatory conditions. J Immunol. 2012;188:844–853. doi: 10.4049/jimmunol.1101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zen K, Reaves TA, Soto I, Liu Y. Response to genistein: assaying the activation status and chemotaxis efficacy of isolated neutrophils. J Immunol Methods. 2006;309:86–98. doi: 10.1016/j.jim.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Dong X, Wu D. Methods for studying neutrophil chemotaxis. Methods Enzymol. 2006;406:605–613. doi: 10.1016/S0076-6879(06)06047-2. [DOI] [PubMed] [Google Scholar]
- 13.Jovcić G, Bugarski D, Krstić A, Vlaski M, Petakov M, Mojsilović S, Stojanović N, Milenković P. The effect of interleukin-17 on hematopoietic cells and cytokine release in mouse spleen. Physiol Res. 2007;56:331–339. doi: 10.33549/physiolres.930944. [DOI] [PubMed] [Google Scholar]
- 14.Krstic A, Mojsilovic S, Jovcic G, Bugarski D. The potential of interleukin-17 to mediate hematopoietic response. Immunol Res. 2012;52:34–41. doi: 10.1007/s12026-012-8276-8. [DOI] [PubMed] [Google Scholar]
- 15.Schwarzenberger P, Huang W, Ye P, Oliver P, Manuel M, Zhang Z, Bagby G, Nelson S, Kolls JK. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J Immunol. 2000;164:4783–4789. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- 16.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, et al. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 17.Richards MK, Liu F, Iwasaki H, Akashi K, Link DC. Pivotal role of granulocyte colony-stimulating factor in the development of progenitors in the common myeloid pathway. Blood. 2003;102:3562–3568. doi: 10.1182/blood-2003-02-0593. [DOI] [PubMed] [Google Scholar]
- 18.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 19.Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108:812–820. doi: 10.1182/blood-2005-10-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25:1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, Zhu M, Winkler JW, Petasis NA. Novel proresolving aspirin-triggered DHA pathway. Chem Biol. 2011;18:976–987. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory—pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]