ABSTRACT
Pseudomonas aeruginosa causes chronic pulmonary infections in patients with cystic fibrosis (CF). P. aeruginosa mucoid conversion, defined by overproduction of the exopolysaccharide alginate, correlates with accelerated decline in CF patient lung function. Recalcitrance of the mucoid phenotype to clearance by antibiotics and the immune response is well documented. However, despite advantages conferred by mucoidy, mucoid variants often revert to a nonmucoid phenotype both in vitro and in vivo. Mixed populations of mucoid isolates and nonmucoid revertants are recovered from CF lungs, suggesting a selective benefit for coexistence of these variants. In this study, cocultures of mucoid and nonmucoid variants exhibited enhanced resistance to two host antimicrobials: LL-37, a cationic antimicrobial peptide, and hydrogen peroxide (H2O2). Alginate production by mucoid isolates protected nonmucoid variants in consortia from LL-37, as addition of alginate exogenously to nonmucoid variants abrogated LL-37 killing. Conversely, nonmucoid revertants shielded mucoid variants from H2O2 stress via catalase (KatA) production, which was transcriptionally repressed by AlgT and AlgR, central regulators of alginate biosynthesis. Furthermore, extracellular release of KatA by nonmucoid revertants was dependent on lys, encoding an endolysin implicated in autolysis and extracellular DNA (eDNA) release. Overall, these data provide a rationale to study interactions of P. aeruginosa mucoid and nonmucoid variants as contributors to evasion of innate immunity and persistence within the CF lung.
KEYWORDS: Pseudomonas aeruginosa, alginate, antimicrobial peptides, catalase, exopolysaccharide, polymicrobial
IMPORTANCE
P. aeruginosa mucoid conversion within lungs of cystic fibrosis (CF) patients is a hallmark of chronic infection and predictive of poor prognosis. The selective benefit of mixed populations of mucoid and nonmucoid variants, often isolated from chronically infected CF patients, has not been explored. Here, we show that mixed-variant communities of P. aeruginosa demonstrate advantages in evasion of innate antimicrobials via production of shared goods: alginate and catalase. These data argue for therapeutically targeting multiple constituents (both mucoid and nonmucoid variants) within diversified P. aeruginosa communities in vivo, as these variants can differentially shield one another from components of the host response.
INTRODUCTION
Cystic fibrosis (CF) is one of the most common lethal genetic diseases (1, 2). CF patients exhibit impaired mucociliary clearance, leading to recurrent pulmonary infections (3). During later stages of disease, the Gram-negative bacterium Pseudomonas aeruginosa predominates in the CF lung, exacerbating pathology and hastening patient mortality (4). P. aeruginosa infection promotes excessive influx of neutrophils into the lung, driving tissue damage, fibrosis, and organ dysfunction (5, 6). CF neutrophils overproduce antimicrobials such as reactive oxygen species (ROS) (e.g., hydrogen peroxide [H2O2] and hypochlorite [HOCl]) that damage host tissues and bacteria (7). Neutrophils and CF lung epithelium also secrete cationic antimicrobial peptides (AMPs) in excess (8, 9). One such AMP, LL-37, is a multifunctional cathelicidin that is bactericidal and immunomodulatory (10).
Importantly, exposure to sublethal concentrations of ROS and AMPs promotes bacterial mutagenesis and mucoid conversion, a critical P. aeruginosa pathoadaptation (11–13). The mucoid phenotype in P. aeruginosa is defined by overproduction of the polyanionic exopolysaccharide alginate (14). Nonmucoid, environmental isolates of P. aeruginosa initially colonize CF patients (15). However, exposure to host-derived mutagens (e.g., H2O2 and LL-37) promotes mutation of mucA (see Fig. S1 in the supplemental material), which encodes a transmembrane anti-sigma factor that sequesters its cognate sigma factor, AlgT (also known as AlgU or σ22) (16, 17). mucA mutation liberates AlgT, which promotes enhanced transcription of the alginate biosynthetic operon [algD(PA3540)-algA(PA3551)], and genes encoding ancillary transcription factors essential for alginate biosynthesis: algB, algR, and amrZ (18–20). Though AlgT is critical for mucoid conversion, the AlgT regulon is predicted to consist of 293 open reading frames, indicating a broad role in P. aeruginosa gene regulation (21, 22).
Paradigm for P. aeruginosa mucoid conversion and reversion to nonmucoid phenotype during chronic CF infection. The CF lung is first colonized with nonmucoid P. aeruginosa progenitors (mucA+ algT+). Via exposure to host-derived mutagens in vivo (e.g., neutrophils and neutrophil effectors, H2O2 and LL-37) P. aeruginosa acquires a mutation in mucA and converts to a mucoid phenotype (mucA mutant [“mucA-”] algT+), which is defined by overproduction of the exopolysaccharide alginate. Nonmucoid revertants (mucA mutant and algT mutant [“algT-”]) of P. aeruginosa can arise spontaneously from a subset of mucoid variants via second-site, suppressor mutations in algT, which encodes the sigma factor essential for upregulation of the alginate biosynthetic operon. Both mucoid variants and nonmucoid revertants are often coisolated from CF patients chronically infected with P. aeruginosa. Download FIG S1, TIF file, 0.2 MB (238.2KB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mucoid conversion is correlated with decline in CF patient lung function and marks a transition to the progressively debilitating stages of disease (23). Compared to nonmucoid isolates, mucoid P. aeruginosa exhibits enhanced resistance to multiple antibiotics (24–27) and to host immune effectors (12, 28, 29). Apart from the alginate polysaccharide, P. aeruginosa expresses multiple factors that enable evasion of the host response, including proteases, rhamnolipids, and lipases (30). Additionally, P. aeruginosa catalases, encoded by katA and katB, are critical virulence factors that neutralize H2O2 stress (31, 32).
Despite recalcitrance of mucoid P. aeruginosa, both mucoid and nonmucoid variants are often isolated together from CF lung specimens (33–37). The majority of nonmucoid P. aeruginosa variants present within the CF lung in late disease have reverted from mucoid strains (Fig. S1) (33). These revertants predominantly arise via spontaneous suppressor mutations in algT in vitro (mucA and algT mutants) (38, 39). The propensity of mucoid P. aeruginosa to revert to the nonmucoid phenotype has been attributed to energetic costs of alginate production, which may be disadvantageous under certain conditions (39–41).
However, host factors selecting for nonmucoid revertants in vivo are not known. In light of benefits conferred by mucoidy, the copresence of nonmucoid revertants within hyperinflammatory CF airways suggests both variants contribute to P. aeruginosa persistence. As such, in this study, we hypothesized that mixed populations of mucoid and nonmucoid variants have an advantage in evading innate antimicrobials, wherein both P. aeruginosa morphotypes exhibit differential mechanisms to combat host factors. Indeed, we show when grown in consortia, mucoid and nonmucoid variants demonstrate enhanced resistance to LL-37 and H2O2. Each P. aeruginosa phenotype contributes a portion of immune protection, benefiting the community as a whole: mucoid variants protect both themselves and nonmucoid variants from LL-37 stress via alginate production. Conversely, nonmucoid revertants protect themselves and mucoid variants from H2O2 via catalase (KatA) production. We demonstrate katA is transcriptionally repressed when AlgT is active, via downstream transcription factor, AlgR. Additionally, extracellular release of catalase depends on lys, which mediates autolysis and extracellular DNA (eDNA) release. In total, these data provide important insights regarding mixed-variant P. aeruginosa interactions that enable evasion of critical components of host immunity.
RESULTS
Cocultures of mucoid and nonmucoid isolates exhibit enhanced resistance to host antimicrobials.
To determine whether there is a selective advantage of mixed mucoid and nonmucoid P. aeruginosa populations in evading host effectors, we focused on two innate antimicrobials found within the CF lung: LL-37 and H2O2 (7–9). We exposed monocultures or cocultures of mucoid and nonmucoid variants to either LL-37 or H2O2 for 1 h, followed by plating for colony-forming units (CFU). The clinical mucoid isolate FRD1 (mucA mutant) and isogenic nonmucoid strains (FRD1 algD or FRD1 algT) were differentially drug marked by streptomycin and rifampin (RIF), respectively, to independently track their survival in coculture. algD, the first gene in the alginate biosynthetic operon, encodes a GDP-mannose dehydrogenase essential for alginate production (18); FRD1 algD is an algD insertional mutant. FRD1 algT, which harbors both a mucA mutation and point mutation in a sigma-factor-encoding gene, algT, was first isolated as a spontaneous nonmucoid revertant of FRD1 (42) (see Table S1 in the supplemental material).
All strains, plasmids, and primers used in this study. Download TABLE S1, PDF file, 0.3 MB (320.3KB, pdf) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
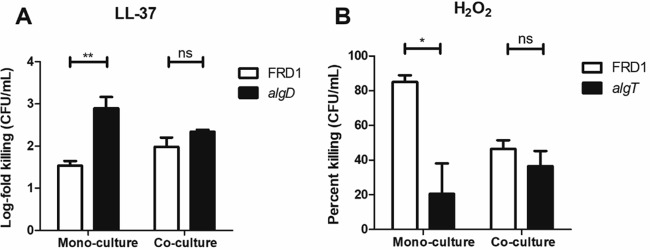
In monoculture, the mucoid strain, FRD1, was significantly more resistant to LL-37 than the nonmucoid strain, FRD1 algD, as previously reported (12); however, in coculture, the susceptibilities of both strains to LL-37 were similar, suggesting that FRD1 algD was partially rescued from LL-37 by FRD1 (Fig. 1A). Conversely, in monoculture, FRD1 was significantly more susceptible to H2O2 than FRD1 algT; however, in coculture, the susceptibility of FRD1 to H2O2 was almost identical to that of FRD1 algT and significantly reduced compared to the monoculture condition (Fig. 1B). This suggested that the copresence of nonmucoid FRD1 algT protected FRD1 from H2O2 stress. The rationale for using the algT revertant (not FRD1 algD) in these H2O2 sensitivity experiments is clarified further below: there was no difference in H2O2 susceptibilities of FRD1 and FRD1 algD in monoculture (see Fig. 3). These results indicated an advantage for mixed-variant, mucoid/nonmucoid populations of P. aeruginosa in evading two critical innate immune effectors.
FIG 1 .
Mucoid and nonmucoid P. aeruginosa variants in coculture exhibit enhanced resistance to LL-37 and H2O2. (A) Monocultures and cocultures of FRD1 (mucA) and FRD1 algD exposed to 50 µg/ml LL-37 for 1 h. (B) Monocultures and cocultures of FRD1 and FRD1 algT exposed to 25 mM H2O2 for 1 h. Data are represented as log fold (A) or percentage of killing (B) compared to the no-treatment control for each strain/condition. Experiments were performed in duplicate on three independent occasions. Data are presented as mean ± standard error of the mean (SEM). Statistical significance was determined by an unpaired, two-tailed Student’s t test. *, P < 0.05; **, P < 0.01; ns, not significant.
Alginate is sufficient to protect bacteria from LL-37 killing.
We next sought to define differential mechanisms of immune protection employed by mucoid and nonmucoid variants against LL-37 and H2O2, respectively. Additionally, we endeavored to understand how these immune evasion strategies might be transferrable between variants in consortia, resulting in advantages observed under coculture conditions (Fig. 1A and B).
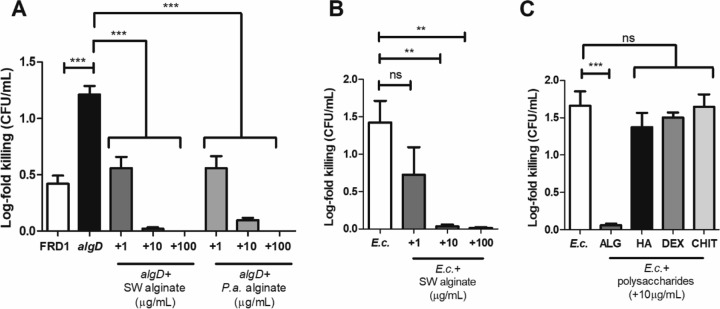
Our previous study identified that LL-37 contributes to mucoid conversion and that mucoid isolates are more resistant to LL-37-mediated killing than algD mutants (12). Here, we hypothesized that if the alginate polysaccharide protects against LL-37, then purified alginate added exogenously to nonmucoid P. aeruginosa should abrogate LL-37 killing. For these experiments, we used both commercially available, seaweed (SW)- and P. aeruginosa (FRD1)-derived alginate. The principal difference between seaweed alginate and P. aeruginosa alginate is that only bacterial alginate is O-acetylated (43). Alginate was added exogenously to FRD1 algD prior to LL-37 (12.5 µg/ml) exposure. Subsequently, bacteria were plated for colony-forming units (CFU), and log fold killing was calculated (Fig. 2A). Concentrations of LL-37 within CF airway secretions have been measured up to 30 µg/ml (8, 9). Therefore, the concentration of LL-37 used here is within the physiologically relevant range.
FIG 2 .
Alginate is sufficient to protect both P. aeruginosa and E. coli from LL-37-mediated killing. (A) FRD1 (mucA), an isogenic FRD1 algD strain, or the FRD1 algD strain with exogenously added Pseudomonas aeruginosa (P.a.) or seaweed (SW) alginate at three different concentrations (1, 10, and 100 µg/ml) was treated for 1 h with LL-37 (12.5 µg/ml). Bacteria were plated for CFU per milliliter. Data are presented as log fold killing compared to the no-treatment control. (B) Log fold killing of E. coli HB101 by 12.5 µg/ml LL-37 with or without the exogenous addition of SW alginate. (C) Log fold killing of E. coli HB101 by 12.5 µg/ml LL-37 with or without the exogenous addition of 10 µg/ml of differentially charged polysaccharides: ALG, SW alginate (anionic); HA, hyaluronic acid (anionic); DEX, dextran (neutral); and CHIT, chitosan (cationic). Experiments were performed in duplicate on three independent occasions. Statistical significance was determined by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple-comparison test. Each condition was compared to either FRD1 algD (A) or E. coli (B and C) alone. Data are presented as mean ± SEM. **, P < 0.01; ***, P < 0.001; ns, not significant.
As previously reported (12), the nonmucoid algD mutant alone was 10-fold more sensitive to LL-37 than the mucoid isolate. However, exogenous addition of both P. aeruginosa- and seaweed-derived alginates rescued algD from LL-37 killing (Fig. 2A). Alginate in CF sputum is quantitated within the range of 10 to 100 µg/ml (44). Thus, we used 1, 10, and 100 µg/ml of alginate. We observed a dose-dependent reduction in LL-37 killing when adding increasing concentrations of alginate (Fig. 2A). Seaweed alginate was also sufficient to protect Escherichia coli (HB101) from LL-37 dose dependently (Fig. 2B). These results suggested that mucoid P. aeruginosa resistance to LL-37 is alginate dependent and that alginate as a released product can protect nonmucoid variants and nonpseudomonad species from LL-37.
We further sought to determine whether alginate, an anionic polysaccharide, serves as an electrostatic sink for cationic LL-37. We reasoned if alginate, due to its negative charge, protects bacteria from LL-37 killing, then a different negatively charged polysaccharide may be similarly protective. Three polysaccharides were added to E. coli culture prior to LL-37 exposure: hyaluronic acid (anionic), chitosan (cationic), and dextran (neutral). Only alginate protected E. coli from LL-37 killing (Fig. 2C). Addition of constituent monosaccharides of alginate, d-mannuronic acid and l-guluronic, acid, alone or in combination, also did not prevent LL-37 killing (see Fig. S2A in the supplemental material). These data suggest alginate’s specific capacity to protect from LL-37 killing is unique as other charged polysaccharides and uronic acid monomers did not confer resistance.
Uronic acid monomers of alginate do not shield bacteria from LL-37 killing, and addition of calcium does not affect the alginate polymer’s capacity to protect from LL-37. (A) Log fold killing of E. coli HB101 (E.c.) by 12.5 µg/ml LL-37 with or without the exogenous addition of 10 µg/ml alginate (ALG) or uronic acids (GA, l-guluronic acid; MA, d-mannuronic acid). (B) Log fold killing of E. coli HB101 by 12.5 µg/ml LL-37 with or without the exogenous addition of 10 µg/ml of alginate, which was preincubated with indicated millimolar concentrations of calcium (Ca2+). A 2.5 mM Ca2+ concentration represents the approximate physiological concentration within the CF airway. Experiments were performed in duplicate on three independent occasions. Statistical significance was measured using one-way ANOVA followed by Dunnett’s multiple-comparison test. Each condition was compared to E. coli alone. Data are presented as mean ± SEM. ***, P < 0.001; ns, not significant. Download FIG S2, TIF file, 0.8 MB (854.9KB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Divalent cations, specifically calcium (Ca2+), change properties of alginate by cross-linking the polysaccharide (45). The Ca2+ concentration is also elevated within CF airway secretions (1.9 to 3.0 mM) (46). To investigate whether the presence of Ca2+ alters alginate’s capacity to protect against LL-37, we preincubated alginate with Ca2+ prior to exogenous addition to bacteria and treatment with LL-37. Physiologically relevant Ca2+ concentrations did not affect alginate’s capacity to prevent LL-37 killing (Fig. S2B). In total, in mixed communities, mucoid variants of P. aeruginosa protect nonmucoid variants from LL-37 via alginate, independent of Ca2+ concentration.
algT mutation confers resistance to H2O2.
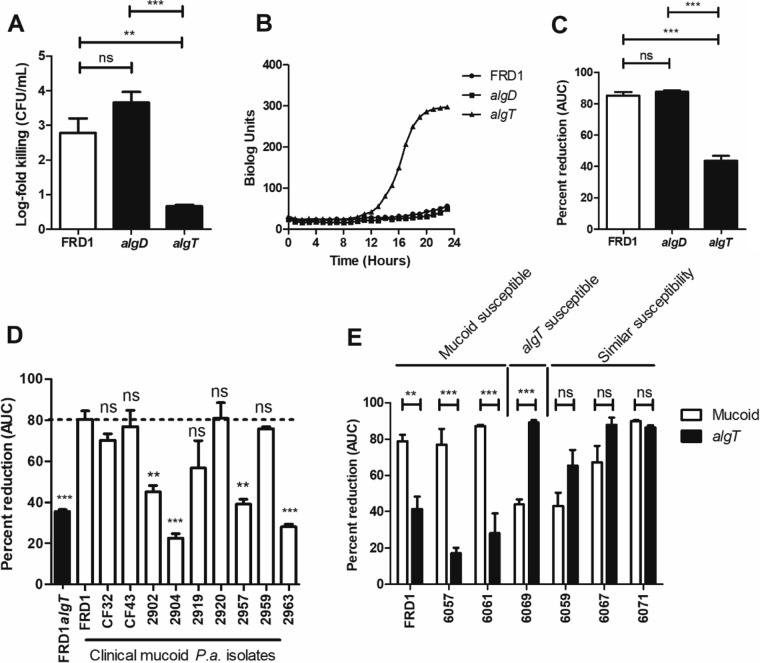
Given that alginate provided significant protection from LL-37, we anticipated it may also protect against other neutrophil-derived antimicrobials, such as ROS. Previous publications suggested that mucoid P. aeruginosa is more resistant to oxidative stress because alginate is a sink for free radicals (47, 48). Therefore, we were surprised at finding no difference in FRD1 and FRD1 algD susceptibilities to H2O2 (Fig. 3A). Paradoxically, FRD1 algT, a nonmucoid revertant, was significantly more resistant to H2O2 killing than the parent strain (Fig. 1B and 3A). Furthermore, in a mixed population, the presence of FRD1 algT was sufficient to protect FRD1 from H2O2 killing (Fig. 1B). The H2O2 concentrations used here are within a physiologically relevant range (12 to 100 mM) (49).
FIG 3 .
The nonmucoid algT revertant is significantly more resistant to hydrogen peroxide than the clinical mucoid isolate, FRD1. (A) One-hour treatment of P. aeruginosa strains (FRD1 [mucA] and the isogenic algT and algD mutants) with 50 mM H2O2, followed by plating for CFU per milliliter. Data are presented as log fold killing relative to the no-treatment condition. (B) Strains grown for 24 h at 37°C in the presence of 25 mM H2O2 via the OmniLog (Biolog, Inc.) system (C) Kinetic growth curve data from panel B were expressed as a percentage of reduction in AUC relative to the no-treatment condition (Fig. S2A). (D) Clinical mucoid isolates grown for 24 h at 37°C in the presence of 25 mM H2O2 via the Biolog system. The dotted line represents H2O2 sensitivity of FRD1. Strains marked as “ns” were as susceptible as FRD1 to H2O2 (not significant statistically compared to FRD1). (E) Paired mucoid and nonmucoid revertants (algT mutants) grown for 24 h at 37°C in the presence of 25 mM H2O2 via the Biolog system. Experiments were performed in triplicate on three independent occasions. Data are presented as mean ± SEM. Statistical significance was measured by one-way ANOVA followed by Tukey’s multiple-comparison test (A, C, and E) or Dunnett’s multiple-comparison test (D). White bars represent mucoid strains. Black bars represent nonmucoid strains. **, P < 0.01; ***, P < 0.001.
We used the high-throughput Biolog system (OmniLog; Biolog, Inc.) to investigate susceptibility of mucoid and nonmucoid variants to H2O2. The Biolog system measures bacterial metabolic activity via a tetrazolium-based dye (50, 51). Untreated FRD1 and nonmucoid (algT or algD) variants demonstrated similar growth (see Fig. S3A in the supplemental material). However, the algT revertant was more resistant to H2O2 than both FRD1 and FRD1 algD (Fig. 3B). Data are represented as the area under the curve (AUC) for each strain treated with H2O2 as a percentage of the no-treatment condition (Fig. 3C). FRD1 algT was also more resistant to hypochlorite (HOCl) than FRD1 (Fig. S3B and C). In total, these findings suggested that H2O2 susceptibility of mucoid P. aeruginosa is relieved by reversion (i.e., algT mutation).
The nonmucoid algT revertant is more resistant to hypochlorite (HOCl) than the clinical mucoid isolate, FRD1 (mucA). (A) Twenty-four-hour Biolog growth curves of FRD1 (mucA), isogenic algT and algD mutants grown in the presence of medium alone. (B) Twenty-four-hour Biolog growth curves of strains grown in the presence of 11.25 mM HOCl. (C) Kinetic growth curve data from panel B expressed as percentage of reduction in AUC relative to the no-treatment condition. Experiments were performed in triplicate on at least three independent occasions. Statistical significance was measured using one-way ANOVA followed by Dunnett’s multiple-comparison test, wherein each strain was compared to FRD1 (C). Data are presented as mean ± SEM. *, P < 0.05; ns, not significant. Download FIG S3, TIF file, 0.4 MB (409KB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Clinical mucoid P. aeruginosa isolates demonstrate susceptibility to H2O2.
Given that mucoidy did not protect against H2O2 killing, we sought to investigate whether this phenotype was specific to FRD1 or generalizable across multiple clinical mucoid strains. We first tested the H2O2 susceptibilities of nine additional mucoid CF isolates. We found that 5/9 strains tested exhibited H2O2 sensitivity similar to that of FRD1, while 4/9 did not (Fig. 3D). Given that each isolate tested overproduced alginate, the sensitivity differences observed between these two groups is unlikely to be attributable to alginate production and reveals that the H2O2 sensitivity of mucoid strains may not be specific to FRD1.
We subsequently tested H2O2 susceptibility of multiple isogenic mucoid and algT revertant pairs of P. aeruginosa strains (Fig. 3E). Mucoid strains in this screen represent clinical isolates from CF patients (33); spontaneous algT revertants were isolated either from patients or via growth of the mucoid variant in vitro (33). Although not all strains tested behaved as FRD1 or FRD1 algT, two pairs did recapitulate the previously observed phenotype, wherein the mucoid isolate was more susceptible to H2O2. While differences in H2O2 sensitivities among clinical isolates were intriguing, these were not attributable to mucoidy alone. All mucoid isolates tested overproduced alginate (see Fig. S4A in the supplemental material). There was no correlation between H2O2 susceptibilities and alginate production (Fig. S4B). These results supported our previous findings suggesting that mucoidy alone was insufficient to protect P. aeruginosa from H2O2 stress.
Alginate production by paired mucoid and algT isolates does not correlate with H2O2 susceptibility phenotype. (A) Quantification of alginate production by paired mucoid and algT revertants via carbazole assay. The limit of detection was set equal to zero. (B) H2O2 susceptibility of each paired mucoid and algT strain plotted versus alginate production by each strain. Lines of best fit for both mucoid and algT strains are shown with r2 values of 0.0008 and 0.002, respectively. Data are presented as mean ± SEM. Alginate quantitation of all strains was performed in duplicate on three independent occasions. Download FIG S4, TIF file, 0.8 MB (832KB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
algT mutant supernatants protect FRD1 from H2O2 stress via KatA.
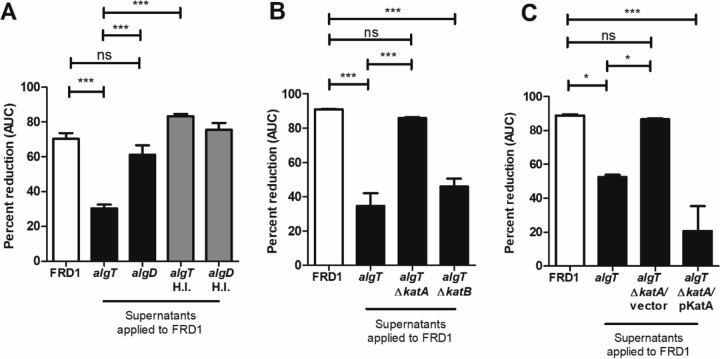
Given that three pairs of mucoid and algT revertants tested (including FRD1) showed algT mutants were more resistant to H2O2, we sought to elucidate the mechanism underlying this phenotype. In coculture, FRD1 algT protected FRD1 from H2O2 killing (Fig. 1B), suggesting secretion of a soluble antioxidant. To test this experimentally, we filter sterilized supernatants from stationary-phase cultures of FRD1 and algT and algD mutant strains. FRD1 was resuspended in these supernatants, and H2O2 susceptibility was tested. Only supernatants from FRD1 algT significantly protected FRD1 from H2O2 killing (Fig. 4A). Supernatants derived from FRD1 algD or FRD1 itself did not protect from H2O2. Heat inactivation of algT supernatants abrogated protection, suggesting a heat-labile protein was responsible for H2O2 resistance (Fig. 4A).
FIG 4 .
Supernatants derived from the algT revertant are sufficient to protect mucoid P. aeruginosa from H2O2 killing via KatA. (A) FRD1 (mucA) was resuspended in cell-free supernatants derived from the algT or algD strain prior to growth for 24 h in the presence of 25 mM H2O2 via the Biolog system. Supernatants were collected and filter sterilized from strains after overnight growth. Heat-inactivated supernatants, incubated at 80°C for 30 min, are designated “HI.” Growth curve data are shown as percentage of reduction in AUC relative to the no-treatment condition. (B and C) FRD1 resuspended in supernatants derived from algT, algT ΔkatA, and algT ΔkatB mutants (B) or algT ΔkatA/vector and algT ΔkatA/pKatA (C) prior to 24 h of growth in the presence of 25 mM H2O2 via the Biolog system. Experiments were performed in triplicate on three independent occasions. Data are presented as mean ± SEM. Statistical significance was measured by one-way ANOVA followed by Tukey’s multiple-comparison test (A to C). *, P < 0.05; ***, P < 0.001; ns, not significant.
Two catalases produced by P. aeruginosa, KatA and KatB, play a vital role in protection against H2O2 stress (31, 52–54). Supernatants derived from an algT ΔkatA mutant abrogated protection of FRD1 against H2O2 compared to the parent algT revertant. In contrast, supernatants from the algT ΔkatB mutant still protected FRD1 from H2O2 (Fig. 4B). Protection from H2O2 was restored in the algT ΔkatA mutant by expression of katA in trans (Fig. 4C). These data suggested the revertant protected mucoid P. aeruginosa from H2O2 via KatA, which was released into the extracellular milieu.
AlgT indirectly represses katA transcription via AlgR.
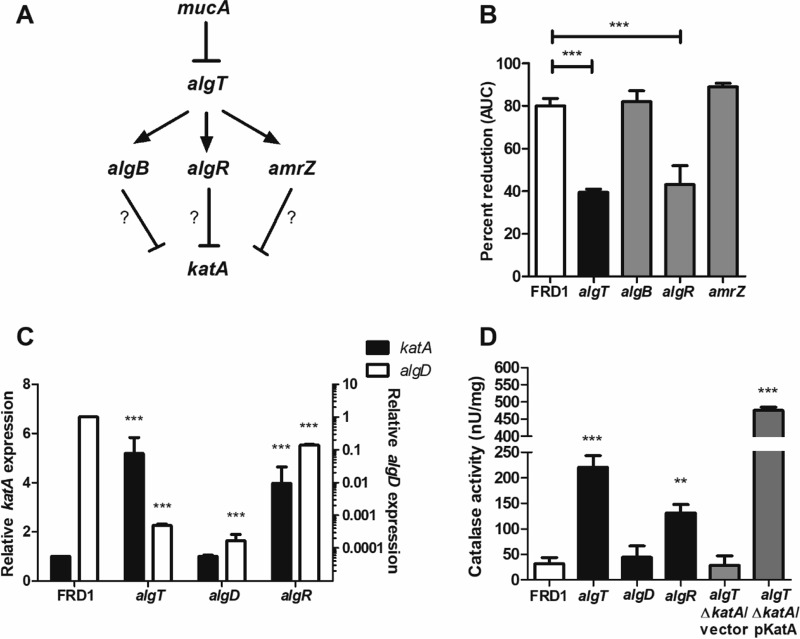
Given that mutation of algT provided enhanced resistance to H2O2 through KatA, we proposed algT may act as a repressor of katA transcription. Alternative sigma factors typically suppress gene transcription indirectly, via downstream transcription factors (55–57). In the alginate system, three main transcription factors lie downstream of AlgT: AlgB, AlgR, and AmrZ (Fig. 5A) (19, 20). Each of these factors (including AlgT) is necessary for expression of the alginate biosynthetic operon. We reasoned that if these factors directly repressed katA transcription, then mutation of genes that encode these factors in a mucA background should result in enhanced resistance to H2O2.
FIG 5 .
katA transcription is negatively regulated by AlgT, via AlgR. (A) Potential pathway for regulation of katA transcription by AlgT through one of three downstream transcription factors: AlgB, AlgR, and AmrZ. (B) FRD1 (mucA) and the isogenic algT, algB, algR, and amrZ mutants were grown for 24 h in the presence of 25 mM H2O2 via the Biolog system. Data are plotted as the percentage of AUC relative to the no-treatment condition. (C) katA and algD mRNA levels quantitated by qRT-PCR, relative to FRD1. (D) Quantitation of catalase protein activity within cell-free supernatants of P. aeruginosa strains using the BioVision catalase activity colorimetric assay. Experiments were performed in triplicate (B and C) or duplicate (D) on at least three independent occasions. Statistical significance was measured using one-way ANOVA followed by Tukey’s multiple-comparison test (B and C) or Dunnett’s multiple-comparison test (D), wherein each strain was compared to FRD1. Data are presented as mean ± SEM. **, P < 0.01; ***, P < 0.001; ns, not significant.
Indeed, algR mutation resulted in enhanced resistance to H2O2 compared to FRD1, while algB and amrZ mutants remained H2O2 sensitive (Fig. 5B). Consistent with this finding, katA transcription is elevated in both algT and algR mutants relative to FRD1 (Fig. 5C). katA transcription was unchanged in the algD mutant compared to FRD1. We also measured algD transcript as an additional control in this experiment. Consistent with previously published work (19, 57), mutation of algT, algR, or algD results in significant reduction of algD transcription relative to the mucA isolate.
Catalase protein activity was quantitated by a commercially available catalase enzyme activity kit. Catalase activity was significantly higher in supernatants of algT and algR mutants relative to FRD1 (Fig. 5D), corresponding with the elevated katA transcription (Fig. 5C). Supernatants from the algT ΔkatA strain demonstrated significant loss of catalase activity; katA complementation restored activity (Fig. 5D). In total, these data suggested AlgT is an indirect repressor of katA transcription via AlgR.
Extracellular release of KatA is dependent on lys-mediated cell lysis.
Previous publications had shown KatA within the periplasmic space and predicted KatA is released via cell lysis (53). However, a clear mechanism linking autolysis in P. aeruginosa and KatA release was not elucidated.
Recently, a bacteriophage endolysin encoded by lys (PA0629), found within the R- and F-pyocin gene cluster, was shown to mediate explosive cell lysis and extracellular DNA (eDNA) release in P. aeruginosa (58). Here, we sought to determine whether lys also has a role in KatA release. Two previously published studies supported this investigation. First, the expression of lys was elevated in response to H2O2 exposure in P. aeruginosa, suggesting that cell lysis may be an adaptive response against H2O2 stress (59). Furthermore, in our previous work comparing the transcriptomes of FRD1 and an isogenic algT mutant, lys expression was upregulated in the algT mutant, suggesting that cell lysis in FRD1 algT might contribute to H2O2 resistance (60).
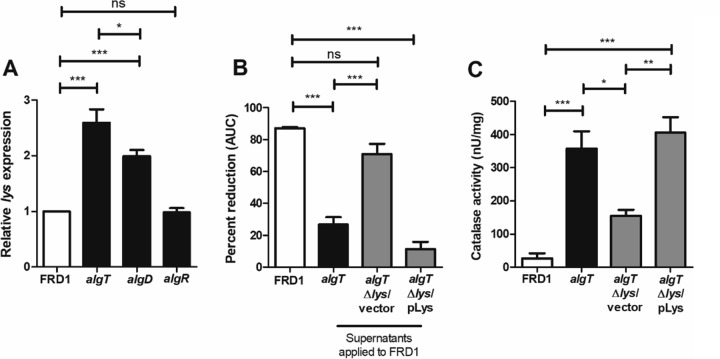
Consistent with previous findings, lys mRNA was elevated in FRD1 algT compared to FRD1 (Fig. 6A). Surprisingly, lys transcription was also elevated in the algD mutant but not in the algR mutant. These results suggested that algT mutation likely derepresses lys transcription via an algR-independent pathway. Subsequently, we generated both a lys mutant and its complement in the FRD1 algT background. To validate that the Δlys mutant exhibited reduced cell lysis, we measured eDNA present within the supernatants of our strains as a surrogate for cell lysis. We derived supernatants from FRD1 wild-type and algT, algT Δlys, and algTΔ lys/plys strains, and similar to a previously published approach (61), these supernatants were analyzed by agarose gel electrophoresis. A high-molecular-weight band was observed for each strain (see Fig. S5A in the supplemental material), suggestive of eDNA. Quantification of band intensity revealed that the algT revertant underwent more cell lysis (i.e., showed greater eDNA release) than FRD1 (Fig. S5B). Furthermore, the algT Δlys strain showed reduced cell lysis, which was restored by complementation (Fig. S5B).
FIG 6 .
Deletion of lys abrogates catalase release in algT revertants. (A) lys mRNA levels quantitated by qRT-PCR relative to FRD1. (B) Percentage of reduction in AUC for FRD1 resuspended in supernatants from the algT, algT Δlys, and algT Δlys/pLys strains prior to 24 h of growth in the presence of H2O2 by the Biolog system. (C) Quantitation of catalase protein activity within cell-free supernatants of P. aeruginosa strains using the BioVision catalase activity colorimetric assay. Experiments were performed in triplicate (A and B) or duplicate (C) on at least three independent occasions. Statistical significance was measured using one-way ANOVA followed by Tukey’s multiple-comparison test (A to C). Data are presented as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
Deletion of lys reduces eDNA release by algT revertants. (A) eDNA visualized via electrophoresis of cell-free supernatants from FRD1 (mucA) or algT, algT Δlys, and algT Δlys/pLys mutants. eDNA was observed as a high-molecular-weight band (>3,000 bp). MWM, molecular weight marker. One representative gel image is shown. (B) Quantification of eDNA band intensity using the densitometry plugin in ImageJ. Densitometry results represent the average from three independent experiments. Statistical significance was measured using one-way ANOVA followed by Tukey’s multiple-comparison test. Data are presented as mean ± SEM. ***, P < 0.001; ns, not significant. Download FIG S5, TIF file, 1.9 MB (2MB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Next, we wanted to ascertain whether the algT Δlys strain also released less catalase than its parent strain. We hypothesized that if deletion of lys resulted in reduced catalase release, then supernatants from the algT Δlys strain would be less effective in protecting FRD1 from H2O2 stress. Indeed, FRD1 resuspended in supernatants derived from the algT Δlys strain was significantly more susceptible to H2O2 than when resuspended in supernatants from the algT or complemented Δlys mutant (Fig. 6B). Correspondingly, there was reduced catalase activity in cell-free supernatants of the algT Δlys mutant as well, which was restored by complementation (Fig. 6C). To our knowledge, these results for the first time link a specific mechanism for cell lysis and extracellular release of P. aeruginosa catalase.
DISCUSSION
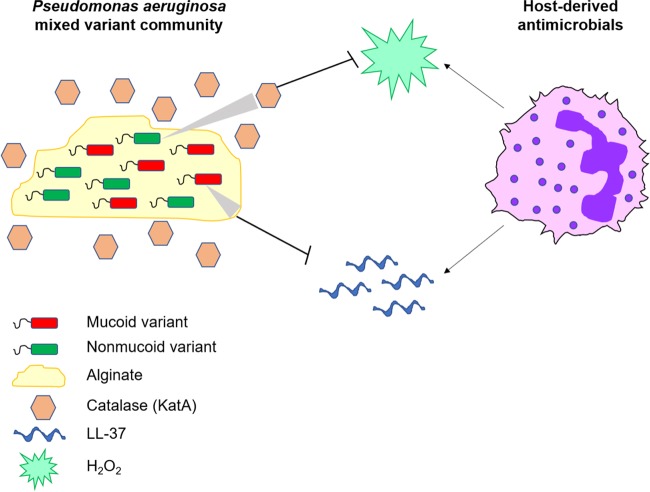
Mixed communities of mucoid and nonmucoid P. aeruginosa strains are frequently isolated from chronically infected CF patients (33–37). Given the well-understood recalcitrance of the mucoid phenotype to clearance by antibiotics and immune cells, the selective benefit of nonmucoid variants within these mixed communities has not been elucidated. Here, we have shown that mixed-variant P. aeruginosa populations have an advantage in evading two critical immune effectors: LL-37 and H2O2. This benefit of coexistence is mediated by production and sharing of two public goods: mucoid variants overproduce alginate to protect against LL-37, and nonmucoid algT revertants overproduce catalase (KatA) to neutralize H2O2 (Fig. 7).
FIG 7 .
Model of mixed communities of mucoid variants with nonmucoid revertants evading host-derived antimicrobials within the CF lung. Mucoid P. aeruginosa variants protect mixed-variant populations from LL-37 via alginate production. Nonmucoid revertants protect the mixed population by releasing catalase (KatA), which neutralizes H2O2.
We had previously shown that LL-37 contributes to mucoid conversion in P. aeruginosa and that mucoid isolates were significantly more resistant than isogenic nonmucoid variants to this peptide (12). Here, we demonstrated that the addition of alginate exogenously to a nonmucoid algD mutant was sufficient to rescue this strain from LL-37 killing. Furthermore, alginate was sufficient to protect E. coli from LL-37-killing. This substantiates previous work showing alginate added exogenously to other Gram-negative pathogens (e.g., Klebsiella pneumoniae) can provide resistance to AMPs such as polymyxin B and α-defensin-1 (HNP-1) (62). The presence of free alginate in CF sputum and in association with lung mucosa is well documented (34, 35, 44). Our results, taken together with past findings, suggest that within the extracellular milieu of the CF airway, alginate protects bacteria from AMPs, independent of genera/species.
In investigating whether the polyanionic charge of alginate plays a role in LL-37 evasion, we found an uncharged polysaccharide (dextran) and a cationic polysaccharide (chitosan) did not protect from LL-37. Hyaluronic acid, a negatively charged polysaccharide, also did not shield from LL-37 killing. It is tempting to conclude based on these data that a unique property of alginate, independent of its anionic charge, is responsible for LL-37 resistance. Nonetheless, previous studies demonstrate that the primary mode of interaction between alginate and AMPs is likely electrostatic in nature: alginate binds AMPs via ionic interactions, which induce peptide α-helix formation and aggregation, inhibiting AMP bactericidal function (63, 64). In one study, the amount of negative charge per chemical repeating unit within polyanionic polysaccharides correlated with protection against LL-37 killing (65). Hyaluronic acid has half the negative charge density of alginate (66). Thus, the greater negative charge of alginate may explain how it binds and protects from LL-37 more effectively than does hyaluronic acid.
Although P. aeruginosa susceptibility to LL-37 was alginate dependent, H2O2 sensitivity was significantly reduced in a nonmucoid algT revertant compared to the mucoid parent. These results were surprising for two reasons: First, both LL-37 and H2O2 are known to induce mucoid conversion, and as is true for LL-37, we expected mucoid variants to be resistant to their own pathoadaptive triggers, including ROS (11, 12). Second, two often-cited publications illustrate how alginate acts as a sink for ROS (47, 48). Both publications demonstrate that addition of alginate to stimulated phagocytes reduces detection of ROS without affecting viability of the immune cells. However, another publication directly contradicted these results, showing that addition of alginate to neutrophils enhances oxidative burst (44). In support of our findings, Brown et al. found that catalase protein activity was lower in FRD1 than that in an algT mutant, suggesting H2O2 susceptibility of mucoid isolates may be attributable to catalase, not to alginate (31).
Nevertheless, to investigate whether mucoidy is sufficient to protect against ROS and to rule out that FRD1 is unique in its susceptibility to H2O2, we screened the H2O2 susceptibilities of a panel of clinical mucoid P. aeruginosa isolates as well as isogenic pairs of mucoid algT revertants. These data reassured us that the FRD1 or FRD1 algT phenotype was represented in multiple, but not all P. aeruginosa isolates from CF patients. However, we did observe some variability among clinical isolates: in the second screen, some mucoid algT pairs exhibited no difference in H2O2 susceptibility, and in one case, the mucoid isolate was more resistant to H2O2 than the algT revertant. Differences in alginate production among these strains did not account for the different H2O2 susceptibility phenotypes. One possible explanation for these differences could be the algT revertants have distinct algT mutations, which perturb sigma factor function differently, resulting in variable H2O2 sensitivity phenotypes. Future work will seek to test this hypothesis through sequencing of the algT locus across multiple nonmucoid revertants to determine whether specific algT mutations cluster with H2O2 susceptibility phenotypes.
In focusing our mechanistic studies here on FRD1, we found that supernatants from FRD1 algT protected the mucoid strain from H2O2 stress in a katA-dependent manner. In P. aeruginosa, katA encodes a constitutively expressed catalase, whereas katB expression is induced upon exposure to H2O2 (31). Both catalases are localized in different cellular compartments: while KatB is restricted to the cytosol, KatA is found in both the cytosol and periplasm, suggesting KatA may be secreted or released (31). This may explain why only supernatants derived from the algT ΔkatA strain, but not those from the algT ΔkatB strain, showed complete loss of protection from H2O2. These data corroborate previous findings showing KatA (but not KatB) in the extracellular milieu of P. aeruginosa (53, 54).
We further demonstrated that katA transcription is negatively regulated by AlgT, via AlgR. Although an aforementioned study had shown that catalase protein activity is higher in FRD1 algT than in FRD1, the H2O2 susceptibility of these strains and a pathway for algT-dependent transcriptional repression of katA were not investigated (31). Furthermore, Lizewski et al. published that an algR mutant in a non-mucA strain background (PAO1) exhibits greater resistance to H2O2 than the wild-type strain (67). In later work, via microarray, they also showed that katA transcription is elevated (1.8-fold) in the algR mutant compared to PAO1, without attributing this to possible AlgT-dependent effects (68). Our findings here connect the prior work by Brown et al. and Lizewski et al. by providing evidence for AlgT repression of katA transcription via AlgR, thus elucidating a specific mechanism for enhanced H2O2 tolerance of algT revertants.
We also linked lys-mediated autolysis to the release of catalase and evasion of H2O2 killing in P. aeruginosa. While lys expression was elevated in FRD1 algT, it was not increased in the algR mutant, despite katA expression, catalase protein activity, and H2O2 resistance being elevated in both strains. This finding suggests two possibilities: either lys transcriptional regulation is algT dependent and algR independent (i.e., lys is directly repressed by a different transcription factor downstream of AlgT), or algR mutants exhibit autolysis in a lys-independent manner, explaining the detection of catalase in cell-free supernatants of the algR mutant, albeit less than in the algT mutant. Examining the validity of these hypotheses will be the subject of future work.
Given the long-term persistence of P. aeruginosa mucoid variants within the CF lung, it seems logical that H2O2-susceptible mucoid variants may be shielded and sustained by the presence of coinfecting nonmucoid variants. Moreover, ROS such as H2O2 (and HOCl) may represent important host factors that select for revertants within the CF lung. However, these data also begged the question of whether nonmucoid progenitor strains (mucA+ and algT+) of P. aeruginosa, wherein wild-type MucA would be predicted to antagonize AlgT activity, are equally as resistant to H2O2 as nonmucoid revertants (mucA and algT mutants) (Fig. S1). Although the progenitor of FRD1 has never been isolated, we generated a “pseudoprogenitor” via complementation of mucA in FRD1 (FRD1/pMucA). Indeed, both the nonmucoid revertant and the progenitor were more resistant to H2O2 than the mucoid variant (see Fig. S6A in the supplemental material). Furthermore, supernatants derived from both the progenitor and revertant protected FRD1 from H2O2 stress (Fig. S6B). These data suggest that H2O2 resistance depends on inactivation of AlgT, and both nonmucoid progenitors and algT revertants could play a role in evasion of H2O2 within mixed-variant communities.
Complementation of mucA restores H2O2 resistance of FRD1. (A) FRD1 (mucA) and the isogenic algT and mucA/pMucA strains were grown for 24 h in the presence of 25 mM H2O2 via the Biolog system. Data are plotted as a percentage of the AUC relative to the no-treatment condition. (B) mucA cells resuspended in supernatants derived from algT and mucA/pMucA cells prior to 24 h of growth in the presence of 25 mM H2O2 by the Biolog system. Experiments were performed in triplicate on at least three independent occasions. Statistical significance was measured using one-way ANOVA followed by Tukey’s multiple-comparison test. Data are presented as mean ± SEM. ***, P < 0.001; ns, not significant. Download FIG S6, TIF file, 0.5 MB (501.9KB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
All experiments in this study were performed under in vitro conditions with planktonic cultures of bacteria, wherein mucoid and nonmucoid variants were mixed in a 1:1 ratio. As such, we acknowledge that the advantages of mucoid and nonmucoid communities demonstrated here in evading an antimicrobial peptide and ROS may only capture part of the total benefits realized in these populations in vivo. Nevertheless, the remarkable capacity of P. aeruginosa to adapt to stress via the acquisition of stable mutations is well established (69–71). Multiple variants of P. aeruginosa with different colony morphotypes have been found to coexist within the CF lung, including mucoid variants, nonmucoid revertants, and small-colony variants (SCVs), among others (72). The selective advantage of these mixed P. aeruginosa populations in evading the host response illustrates the insurance hypothesis: an ecologic principle postulating, the fitness of a community to withstand stress is enhanced by genotypic/phenotypic diversity (73, 74). The CF lung represents an environment that changes over time, through the age of the patient, stage of disease, coinfecting microbes, and treatment with various therapeutics (75–79). The genotypic and functional diversification of P. aeruginosa likely contributes to adaptation under these stressful conditions, enabling long-term colonization of the CF airway. This study argues for continued examination of mixed-variant P. aeruginosa communities as significant contributors to disease pathology.
MATERIALS AND METHODS
Strains and growth conditions.
All P. aeruginosa strains were maintained on Pseudomonas isolation agar (PIA), followed by growth in Luria broth with no salt (LBNS). E. coli strains were maintained on Luria agar (LA), followed by growth in Luria broth (LB). All gene mutations were made as previously described by overlap extension PCR (80). For plasmid maintenance, 100 µg/ml (E. coli) or 300 µg/ml (P. aeruginosa) ampicillin was added to the media. In coculture experiments, parental and derivative strains were selected with 150 µg/ml streptomycin or 100 µg/ml rifampin (RIF), respectively. Arabinose (0.2%) was used to induce expression of genes from the pHERD20T arabinose-inducible vector. All primers, plasmids, and strains used are delineated in Text S1 and Table S1 in the supplemental material).
Supplemental materials and methods. Download TEXT S1, PDF file, 0.4 MB (377.3KB, pdf) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
One-hour bacterial killing.
Bacterial strains were grown to mid-exponential phase (approximately an optical density at 600 nm [OD600] of ~0.5 or 2 × 108 CFU/ml). For H2O2 killing assays, bacteria were mixed 1:1 with H2O2 diluted in LBNS and incubated for 1 h at 37°C, followed by plating for CFU per milliliter on PIA. Data were expressed as log fold killing relative to the no-treatment condition. For the LL-37 (Sigma) killing assays, bacteria at the exponential phase were pelleted and resuspended in sodium phosphate buffer (SPB) at pH 6.4. Bacteria were mixed 1:1 with LL-37 diluted in SPB and incubated 1 h at 37°C, followed by plating for CFU per milliliter on either PIA or LA. For experiments in which mono- or polysaccharides were added exogenously, seaweed alginate (Sigma), hyaluronic acid (Sigma), dextran (Sigma), chitosan (MP Biomedicals), mannuronic acid (Sigma), and guluronic acid (Carbosynth, Compton, United Kingdom) were obtained commercially. P. aeruginosa alginate was purified as described below.
Monoculture versus coculture bacterial killing.
Monoculture versus coculture killing assays were performed identically to the 1-h killing assay protocol described above. Under the coculture conditions, strains were mixed 1:1 prior to exposure to either LL-37 or H2O2. Cultures were plated on selective media to determine CFU.
Alginate purification and quantitation.
P. aeruginosa alginate was purified and quantitated as previously described (57). Additional details are provided in Text S1.
Biolog growth inhibition and supernatant protection assays.
Overnight bacterial cultures were diluted to an OD600 of 0.24. To generate a master mix for each bacterial strain, 150 µl of bacterial culture was added to 850 µl LBNS with 12 µl of Biolog dye A. Fifty microliters was transferred to a Biolog 96-well plate in triplicate. Then, 50 µl of H2O2 (diluted in LBNS at the desired concentration) or LBNS alone was added to each well containing bacteria. Plates were placed in the OmniLog incubator at 37°C for 24 h. The output of the system is growth curves, which can be plotted (as Biolog units versus time in hours) using Biolog’s kinetic software (OL_FM_12) package. Data are also presented as the area under the curve (AUC), which was generated from Biolog’s parametric (OL_PR_12) software. The percentage of reduction (AUC) was calculated by taking the AUC in the presence of H2O2 as a percentage of the no-treatment condition.
To assess if bacterial supernatants from various strains were sufficient to protect FRD1 from H2O2 stress, overnight bacterial cultures were pelleted. Supernatants were collected and filter sterilized. Overnight FRD1 culture was diluted in fresh medium to an OD600 of 0.24. Five hundred microliters was pelleted and resuspended with 500 μl of supernatant from desired strains. H2O2 susceptibility was then assayed by Biolog as detailed above.
qRT-PCR.
Quantitative reverse transcriptase PCR (qRT-PCR) was performed to measure mRNA levels of desired genes in bacterial strains of interest as described previously (81). Additional details are provided in Text S1.
Catalase activity assays.
To measure catalase protein activity in the cell-free supernatants of P. aeruginosa strains, a commercially available kit (BioVision catalase activity colorimetric/fluorometric assay) was used per the manufacturer’s instructions. Additional details are provided in Text S1.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism v.5 (GraphPad Software, Inc.). Statistical significance was determined using a P value of <0.05. Three biological replicates were performed in triplicate for all experiments unless otherwise specified.
ACKNOWLEDGMENTS
We acknowledge Nationwide Children’s Hospital (NCH) Laboratory Service for isolation of mucoid P. aeruginosa strains from CF patient sputum samples. We additionally thank Oana Ciofu (Department of Immunology and Microbiology, University of Copenhagen, Denmark) for providing paired isogenic mucoid algT revertant isolates. DNA sequences were obtained from the Pseudomonas Genome Database (82). We thank Christopher Jones, Erin Gloag, Anirudh Singh, and Lucia Rosas for comments on the manuscript.
This study was supported by a TL1 predoctoral fellowship awarded to S.M. by the Center for Clinical and Translational Science (CCTS), The Ohio State University College of Medicine (TL1TR001069) and by NIH grants awarded to D.J.W. (R01AI34895 and R01AI097511). The funders did not have any role in study design, data collection and interpretation, decision to publish, or preparation of the manuscript. The authors declare no conflicts of interest.
Footnotes
Citation Malhotra S, Limoli DH, English AE, Parsek MR, Wozniak DJ. 2018. Mixed communities of mucoid and nonmucoid Pseudomonas aeruginosa exhibit enhanced resistance to host antimicrobials. mBio 9:e00275-18. https://doi.org/10.1128/mBio.00275-18.
REFERENCES
- 1.Spoonhower KA, Davis PB. 2016. Epidemiology of cystic fibrosis. Clin Chest Med 37:1–8. doi: 10.1016/j.ccm.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Scotet V, Duguépéroux I, Saliou P, Rault G, Roussey M, Audrézet MP, Férec C. 2012. Evidence for decline in the incidence of cystic fibrosis: a 35-year observational study in Brittany, France. Orphanet J Rare Dis 7:14. doi: 10.1186/1750-1172-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, Mall MA. 2017. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res 367:537–550. doi: 10.1007/s00441-016-2562-z. [DOI] [PubMed] [Google Scholar]
- 4.Murray TS, Egan M, Kazmierczak BI. 2007. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr 19:83–88. doi: 10.1097/MOP.0b013e3280123a5d. [DOI] [PubMed] [Google Scholar]
- 5.Cantin AM, Hartl D, Konstan MW, Chmiel JF. 2015. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros 14:419–430. doi: 10.1016/j.jcf.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, Greene C, McElvaney G, Mall M, Döring G. 2012. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros 11:363–382. doi: 10.1016/j.jcf.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Galli F, Battistoni A, Gambari R, Pompella A, Bragonzi A, Pilolli F, Iuliano L, Piroddi M, Dechecchi MC, Cabrini G, Working Group on Inflammation in Cystic Fibrosis . 2012. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim Biophys Acta 1822:690–713. doi: 10.1016/j.bbadis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Chen CI-U, Schaller-Bals S, Paul KP, Wahn U, Bals R. 2004. Beta-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J Cyst Fibros 3:45–50. doi: 10.1016/j.jcf.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Bergsson G, Reeves EP, McNally P, Chotirmall SH, Greene CM, Greally P, Murphy P, O’Neill SJ, McElvaney NG. 2009. LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J Immunol 183:543–551. doi: 10.4049/jimmunol.0803959. [DOI] [PubMed] [Google Scholar]
- 10.Hancock REW, Haney EF, Gill EE. 2016. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol 16:321–334. doi: 10.1038/nri.2016.29. [DOI] [PubMed] [Google Scholar]
- 11.Mathee K, Ciofu O, Sternberg C, Lindum PW, Campbell JIA, Jensen P, Johnsen AH, Givskov M, Ohman DE, Molin S, Høiby N, Kharazmi A. 1999. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 145:1349–1357. doi: 10.1099/13500872-145-6-1349. [DOI] [PubMed] [Google Scholar]
- 12.Limoli DH, Rockel AB, Host KM, Jha A, Kopp BT, Hollis T, Wozniak DJ. 2014. Cationic antimicrobial peptides promote microbial mutagenesis and pathoadaptation in chronic infections. PLoS Pathog 10:e1004083. doi: 10.1371/journal.ppat.1004083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-Rojas A, Makarova O, Müller U, Rolff J. 2015. Cationic peptides facilitate iron-induced mutagenesis in bacteria. PLoS Genet 11:e1005546. doi: 10.1371/journal.pgen.1005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govan JR, Deretic V. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60:539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May TB, Shinabarger D, Maharaj R, Kato J, Chu L, DeVault JD, Roychoudhury S, Zielinski NA, Berry A, Rothmel RK. 1991. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev 4:191–206. doi: 10.1128/CMR.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin DW, Schurr MJ, Mudd MH, Govan JR, Holloway BW, Deretic V. 1993. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc Natl Acad Sci U S A 90:8377–8381. doi: 10.1073/pnas.90.18.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucher JC, Yu H, Mudd MH, Deretic V. 1997. Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect Immun 65:3838–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramsey DM, Wozniak DJ. 2005. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol Microbiol 56:309–322. doi: 10.1111/j.1365-2958.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 19.Wozniak DJ, Ohman DE. 1994. Transcriptional analysis of the Pseudomonas aeruginosa genes algR, algB, and algD reveals a hierarchy of alginate gene expression which is modulated by algT. J Bacteriol 176:6007–6014. doi: 10.1128/jb.176.19.6007-6014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baynham PJ, Wozniak DJ. 1996. Identification and characterization of AlgZ, an AlgT-dependent DNA-binding protein required for Pseudomonas aeruginosa algD transcription. Mol Microbiol 22:97–108. doi: 10.1111/j.1365-2958.1996.tb02659.x. [DOI] [PubMed] [Google Scholar]
- 21.Wood LF, Ohman DE. 2009. Use of cell wall stress to characterize σ22 (AlgT/U) activation by regulated proteolysis and its regulon in Pseudomonas aeruginosa. Mol Microbiol 72:183–201. doi: 10.1111/j.1365-2958.2009.06635.x. [DOI] [PubMed] [Google Scholar]
- 22.Wood LF, Ohman DE. 2012. Identification of genes in the σ22 regulon of Pseudomonas aeruginosa required for cell envelope homeostasis in either the planktonic or the sessile mode of growth. mBio 3:e00094-12. doi: 10.1128/mBio.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Kosorok MR, Farrell PM, Laxova A, West SEH, Green CG, Rock MJ, Splaingard ML. 2005. Infection and lung disease progression in children with cystic fibrosis. JAMA 293:581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 24.Hodges NA, Gordon CA. 1991. Protection of Pseudomonas aeruginosa against ciprofloxacin and 1-lactams by homologous alginate. Antimicrob Agents Chemother 35:2450–2452. doi: 10.1128/AAC.35.11.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, Parsek MR. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol 183:5395–5401. doi: 10.1128/JB.183.18.5395-5401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goltermann L, Tolker-Nielsen T. 2017. Importance of the exopolysaccharide matrix in antimicrobial tolerance of Pseudomonas aeruginosa aggregates. Antimicrob Agents Chemother 61:e02696-16. doi: 10.1128/AAC.02696-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hengzhuang W, Wu H, Ciofu O, Song Z, Høiby N. 2011. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 55:4469–4474. doi: 10.1128/AAC.00126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pier GB, Coleman F, Grout M, Franklin M, Ohman DE. 2001. Role of alginate O acetylation in resistance of mucoid Pseudomonas aeruginosa to opsonic phagocytosis. Infect Immun 69:1895–1901. doi: 10.1128/IAI.69.3.1895-1901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK. 2005. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J Immunol 175:7512–7518. doi: 10.4049/jimmunol.175.11.7512. [DOI] [PubMed] [Google Scholar]
- 30.Gonçalves-de-Albuquerque CF, Silva AR, Burth P, Rocco PRM, Castro-Faria MV, Castro-Faria-Neto HC. 2016. Possible mechanisms of Pseudomonas aeruginosa-associated lung disease. Int J Med Microbiol 306:20–28. doi: 10.1016/j.ijmm.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Brown SM, Howell ML, Vasil ML, Anderson AJ, Hassett DJ. 1995. Cloning and characterization of the katB gene of Pseudomonas aeruginosa encoding a hydrogen peroxide-inducible catalase: purification of KatB, cellular localization, and demonstration that it is essential for optimal resistance to hydrogen peroxide. J Bacteriol 177:6536–6544. doi: 10.1128/jb.177.22.6536-6544.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JS, Heo YJ, Lee JK, Cho YH. 2005. KatA, the major catalase, is critical for osmoprotection and virulence in Pseudomonas aeruginosa PA14. Infect Immun 73:4399–4403. doi: 10.1128/IAI.73.7.4399-4403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciofu O, Lee B, Johannesson M, Hermansen NO, Meyer P, Høiby N, Scandinavian Cystic Fibrosis Study Consortium . 2008. Investigation of the algT operon sequence in mucoid and non-mucoid Pseudomonas aeruginosa isolates from 115 Scandinavian patients with cystic fibrosis and in 88 in vitro non-mucoid revertants. Microbiology 154:103–113. doi: 10.1099/mic.0.2007/010421-0. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Haagensen JAJ, Jelsbak L, Johansen HK, Sternberg C, Høiby N, Molin S. 2008. In situ growth rates and biofilm development of Pseudomonas aeruginosa populations in chronic lung infections. J Bacteriol 190:2767–2776. doi: 10.1128/JB.01581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjarnsholt T, Jensen PØ, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Høiby N. 2009. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol 44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 36.Høiby N, Ciofu O, Bjarnsholt T. 2010. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol 5:1663–1674. doi: 10.2217/fmb.10.125. [DOI] [PubMed] [Google Scholar]
- 37.Bragonzi A, Wiehlmann L, Klockgether J, Cramer N, Worlitzsch D, Döring G, Tümmler B. 2006. Sequence diversity of the mucABD locus in Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 152:3261–3269. doi: 10.1099/mic.0.29175-0. [DOI] [PubMed] [Google Scholar]
- 38.Schurr MJ, Martin DW, Mudd MH, Deretic V. 1994. Gene cluster controlling conversion to alginate-overproducing phenotype in Pseudomonas aeruginosa: functional analysis in a heterologous host and role in the instability of mucoidy. J Bacteriol 176:3375–3382. doi: 10.1128/jb.176.11.3375-3382.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeVries CA, Ohman DE. 1994. Mucoid-to-nonmucoid conversion in alginate-producing Pseudomonas aeruginosa often results from spontaneous mutations in algT, encoding a putative alternate sigma factor, and shows evidence for autoregulation. J Bacteriol 176:6677–6687. doi: 10.1128/jb.176.21.6677-6687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Govan JRW, Fyfe JAM, McMillan C. 1979. The instability of mucoid Pseudomonas aeruginosa: fluctuation test and improved stability of the mucoid form in shaken culture. J Gen Microbiol 110:229–232. doi: 10.1099/00221287-110-1-229. [DOI] [PubMed] [Google Scholar]
- 41.Wyckoff TJO, Thomas B, Hassett DJ, Wozniak DJ. 2002. Static growth of mucoid Pseudomonas aeruginosa selects for non-mucoid variants that have acquired flagellum-dependent motility. Microbiology 148:3423–3430. doi: 10.1099/00221287-148-11-3423. [DOI] [PubMed] [Google Scholar]
- 42.Ohman DE, Chakrabarty AM. 1981. Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect Immun 33:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hay ID, Ur Rehman ZU, Moradali MF, Wang Y, Rehm BHA. 2013. Microbial alginate production, modification and its applications. Microb Biotechnol 6:637–650. doi: 10.1111/1751-7915.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen SS, Kharazmi A, Espersen F, Høiby N. 1990. Pseudomonas aeruginosa alginate in cystic fibrosis sputum and the inflammatory response. Infect Immun 58:3363–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russell NJ, Gacesa P. 1988. Chemistry and biology of the alginate of mucoid strains of Pseudomonas aeruginosa in cystic fibrosis. Mol Aspects Med 10:1–91. doi: 10.1016/0098-2997(88)90002-7. [DOI] [PubMed] [Google Scholar]
- 46.Smith DJ, Anderson GJ, Bell SC, Reid DW. 2014. Elevated metal concentrations in the CF airway correlate with cellular injury and disease severity. J Cyst Fibros 13:289–295. doi: 10.1016/j.jcf.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Simpson JA, Smith SE, Dean RT. 1989. Scavenging by alginate of free radicals released by macrophages. Free Radic Biol Med 6:347–353. doi: 10.1016/0891-5849(89)90078-6. [DOI] [PubMed] [Google Scholar]
- 48.Learn DB, Brestel EP, Seetharama S. 1987. Hypochlorite scavenging by Pseudomonas aeruginosa alginate. Infect Immun 55:1813–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panmanee W, Hassett DJ. 2009. Differential roles of OxyR-controlled antioxidant enzymes alkyl hydroperoxide reductase (AhpCF) and catalase (KatB) in the protection of Pseudomonas aeruginosa against hydrogen peroxide in biofilm vs. planktonic culture. FEMS Microbiol Lett 295:238–244. doi: 10.1111/j.1574-6968.2009.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bochner BR, Savageau MA. 1977. Generalized indicator plate for genetic, metabolic and taxonomic studies with microorganisms. Appl Environ Microbiol 33:434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bochner BR. 2009. Global phenotypic characterization of bacteria. FEMS Microbiol Rev 33:191–205. doi: 10.1111/j.1574-6976.2008.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Britigan BE, Miller RA, Hassett DJ, Pfaller MA, McCormick ML, Rasmussen GT. 2001. Antioxidant enzyme expression in clinical isolates of Pseudomonas aeruginosa: identification of an atypical form of manganese superoxide dismutase. Infect Immun 69:7396–7401. doi: 10.1128/IAI.69.12.7396-7401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hassett DJ, Alsabbagh E, Parvatiyar K, Howell ML, Wilmott RW, Ochsner UA. 2000. A protease-resistant catalase, KatA, released upon cell lysis during stationary phase is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities. J Bacteriol 182:4557–4563. doi: 10.1128/JB.182.16.4557-4563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart PS, Roe F, Rayner J, Elkins JG, Lewandowski Z, Ochsner UA, Hassett DJ. 2000. Effect of catalase on hydrogen peroxide penetration into Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 66:836–838. doi: 10.1128/AEM.66.2.836-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu W, Badrane H, Arora S, Baker HV, Jin S. 2004. MucA-mediated coordination of type III secretion and alginate synthesis in Pseudomonas aeruginosa. J Bacteriol 186:7575–7585. doi: 10.1128/JB.186.22.7575-7585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones AK, Fulcher NB, Balzer GJ, Urbanowski ML, Pritchett CL, Schurr MJ, Yahr TL, Wolfgang MC. 2010. Activation of the Pseudomonas aeruginosa AlgU regulon through mucA mutation inhibits cyclic AMP/Vfr signaling. J Bacteriol 192:5709–5717. doi: 10.1128/JB.00526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones CJ, Ryder CR, Mann EE, Wozniak DJ. 2013. AmrZ modulates Pseudomonas aeruginosa biofilm architecture by directly repressing transcription of the psl operon. J Bacteriol 195:1637–1644. doi: 10.1128/JB.02190-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turnbull L, Toyofuku M, Hynen AL, Kurosawa M, Pessi G, Petty NK, Osvath SR, Cárcamo-Oyarce G, Gloag ES, Shimoni R, Omasits U, Ito S, Yap X, Monahan LG, Cavaliere R, Ahrens CH, Charles IG, Nomura N, Eberl L, Whitchurch CB. 2016. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun 7:11220. doi: 10.1038/ncomms11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang W, Small DA, Toghrol F, Bentley WE. 2005. Microarray analysis of Pseudomonas aeruginosa reveals induction of pyocin genes in response to hydrogen peroxide. BMC Genomics 6:115. doi: 10.1186/1471-2164-6-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tart AH, Wolfgang MC, Wozniak DJ. 2005. The alternative sigma factor AlgT represses Pseudomonas aeruginosa flagellum biosynthesis by inhibiting expression of fleQ. J Bacteriol 187:7955–7962. doi: 10.1128/JB.187.23.7955-7962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McFarland KA, Dolben EL, LeRoux M, Kambara TK, Ramsey KM, Kirkpatrick RL, Mougous JD, Hogan DA, Dove SL. 2015. A self-lysis pathway that enhances the virulence of a pathogenic bacterium. Proc Natl Acad Sci U S A 112:8433–8438. doi: 10.1073/pnas.1506299112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Llobet E, Tomás JM, Bengoechea JA. 2008. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology 154:3877–3886. doi: 10.1099/mic.0.2008/022301-0. [DOI] [PubMed] [Google Scholar]
- 63.Chan C, Burrows LL, Deber CM. 2004. Helix induction in antimicrobial peptides by alginate in biofilms. J Biol Chem 279:38749–38754. doi: 10.1074/jbc.M406044200. [DOI] [PubMed] [Google Scholar]
- 64.Chan C, Burrows LL, Deber CM. 2005. Alginate as an auxiliary bacterial membrane: Binding of membrane-active peptides by polysaccharides. J Pept Res 65:343–351. doi: 10.1111/j.1399-3011.2005.00217.x. [DOI] [PubMed] [Google Scholar]
- 65.Foschiatti M, Cescutti P, Tossi A, Rizzo R. 2009. Inhibition of cathelicidin activity by bacterial exopolysaccharides. Mol Microbiol 72:1137–1146. doi: 10.1111/j.1365-2958.2009.06707.x. [DOI] [PubMed] [Google Scholar]
- 66.Toppazzini M, Coslovi A, Boschelle M, Marsich E, Benincasa M, Gennaro R, Paoletti S. 2011. Can the interaction between the antimicrobial peptide LL-37 and alginate be exploited for the formulation of new biomaterials with antimicrobial properties? Carbohydr Polym 83:578–585. doi: 10.1016/j.carbpol.2010.08.020. [DOI] [Google Scholar]
- 67.Lizewski SE, Lundberg DS, Schurr MJ. 2002. The transcriptional regulator AlgR is essential for Pseudomonas aeruginosa pathogenesis. Infect Immun 70:6083–6093. doi: 10.1128/IAI.70.11.6083-6093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lizewski SE, Schurr JR, Jackson DW, Frisk A, Carterson AJ, Schurr MJ. 2004. Identification of AlgR-regulated genes in Pseudomonas aeruginosa by use of microarray analysis. J Bacteriol 186:5672–5684. doi: 10.1128/JB.186.17.5672-5684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winstanley C, O’Brien S, Brockhurst MA. 2016. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol 24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Damkiær S, Yang L, Molin S, Jelsbak L. 2013. Evolutionary remodeling of global regulatory networks during long-term bacterial adaptation to human hosts. Proc Natl Acad Sci U S A 110:7766–7771. doi: 10.1073/pnas.1221466110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clark ST, Diaz Caballero J, Cheang M, Coburn B, Wang PW, Donaldson SL, Zhang Y, Liu M, Keshavjee S, Yau YCW, Waters VJ, Elizabeth Tullis D, Guttman DS, Hwang DM. 2015. Phenotypic diversity within a Pseudomonas aeruginosa population infecting an adult with cystic fibrosis. Sci Rep 5:10932. doi: 10.1038/srep10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boles BR, Thoendel M, Singh PK. 2004. Self-generated diversity produces “insurance effects” in biofilm communities. Proc Natl Acad Sci U S A 101:16630–16635. doi: 10.1073/pnas.0407460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boles BR, Thoendel M, Singh PK. 2005. Genetic variation in biofilms and the insurance effects of diversity. Microbiology 151:2816–2818. doi: 10.1099/mic.0.28224-0. [DOI] [PubMed] [Google Scholar]
- 75.Frayman KB, Armstrong DS, Grimwood K, Ranganathan SC. 2017. The airway microbiota in early cystic fibrosis lung disease. Pediatr Pulmonol 52:1384–1404. doi: 10.1002/ppul.23782. [DOI] [PubMed] [Google Scholar]
- 76.LiPuma JJ. 2010. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev 23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S, Zhang Y, Surendra A, Gong Y, Elizabeth Tullis D, Yau YCW, Waters VJ, Hwang DM, Guttman DS. 2015. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep 5:10241. doi: 10.1038/srep10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pittman JE, Wylie KM, Akers K, Storch GA, Hatch J, Quante J, Frayman KB, Clarke N, Davis M, Stick SM, Hall GL, Montgomery G, Ranganathan S, Davis SD, Ferkol TW, Australian Respiratory Early Surveillance Team for Cystic Fibrosis . 2017. Association of antibiotics, airway microbiome and inflammation in infants with cystic fibrosis. Ann Am Thorac Soc 14:1548–1555. doi: 10.1513/AnnalsATS.201702-121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, Radey M, Accurso FJ, Wolter DJ, Cooke G, Adam RJ, Carter S, Grogan B, Launspach JL, Donnelly SC, Gallagher CG, Bruce JE, Stoltz DA, Welsh MJ, Hoffman LR, McKone EF, Singh PK. 2017. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med 195:1617–1628. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heckman KL, Pease LR. 2007. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc 2:924–932. doi: 10.1038/nprot.2007.132. [DOI] [PubMed] [Google Scholar]
- 81.Xu B, Soukup RJ, Jones CJ, Fishel R, Wozniak DJ. 2016. Pseudomonas aeruginosa AmrZ binds to four sites in the algD promoter, inducing DNA-AmrZ complex formation and transcriptional activation. J Bacteriol 198:2673–2681. doi: 10.1128/JB.00259-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, Brinkman FSL. 2016. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res 44:D646–D653. doi: 10.1093/nar/gkv1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Paradigm for P. aeruginosa mucoid conversion and reversion to nonmucoid phenotype during chronic CF infection. The CF lung is first colonized with nonmucoid P. aeruginosa progenitors (mucA+ algT+). Via exposure to host-derived mutagens in vivo (e.g., neutrophils and neutrophil effectors, H2O2 and LL-37) P. aeruginosa acquires a mutation in mucA and converts to a mucoid phenotype (mucA mutant [“mucA-”] algT+), which is defined by overproduction of the exopolysaccharide alginate. Nonmucoid revertants (mucA mutant and algT mutant [“algT-”]) of P. aeruginosa can arise spontaneously from a subset of mucoid variants via second-site, suppressor mutations in algT, which encodes the sigma factor essential for upregulation of the alginate biosynthetic operon. Both mucoid variants and nonmucoid revertants are often coisolated from CF patients chronically infected with P. aeruginosa. Download FIG S1, TIF file, 0.2 MB (238.2KB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
All strains, plasmids, and primers used in this study. Download TABLE S1, PDF file, 0.3 MB (320.3KB, pdf) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Uronic acid monomers of alginate do not shield bacteria from LL-37 killing, and addition of calcium does not affect the alginate polymer’s capacity to protect from LL-37. (A) Log fold killing of E. coli HB101 (E.c.) by 12.5 µg/ml LL-37 with or without the exogenous addition of 10 µg/ml alginate (ALG) or uronic acids (GA, l-guluronic acid; MA, d-mannuronic acid). (B) Log fold killing of E. coli HB101 by 12.5 µg/ml LL-37 with or without the exogenous addition of 10 µg/ml of alginate, which was preincubated with indicated millimolar concentrations of calcium (Ca2+). A 2.5 mM Ca2+ concentration represents the approximate physiological concentration within the CF airway. Experiments were performed in duplicate on three independent occasions. Statistical significance was measured using one-way ANOVA followed by Dunnett’s multiple-comparison test. Each condition was compared to E. coli alone. Data are presented as mean ± SEM. ***, P < 0.001; ns, not significant. Download FIG S2, TIF file, 0.8 MB (854.9KB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The nonmucoid algT revertant is more resistant to hypochlorite (HOCl) than the clinical mucoid isolate, FRD1 (mucA). (A) Twenty-four-hour Biolog growth curves of FRD1 (mucA), isogenic algT and algD mutants grown in the presence of medium alone. (B) Twenty-four-hour Biolog growth curves of strains grown in the presence of 11.25 mM HOCl. (C) Kinetic growth curve data from panel B expressed as percentage of reduction in AUC relative to the no-treatment condition. Experiments were performed in triplicate on at least three independent occasions. Statistical significance was measured using one-way ANOVA followed by Dunnett’s multiple-comparison test, wherein each strain was compared to FRD1 (C). Data are presented as mean ± SEM. *, P < 0.05; ns, not significant. Download FIG S3, TIF file, 0.4 MB (409KB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Alginate production by paired mucoid and algT isolates does not correlate with H2O2 susceptibility phenotype. (A) Quantification of alginate production by paired mucoid and algT revertants via carbazole assay. The limit of detection was set equal to zero. (B) H2O2 susceptibility of each paired mucoid and algT strain plotted versus alginate production by each strain. Lines of best fit for both mucoid and algT strains are shown with r2 values of 0.0008 and 0.002, respectively. Data are presented as mean ± SEM. Alginate quantitation of all strains was performed in duplicate on three independent occasions. Download FIG S4, TIF file, 0.8 MB (832KB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Deletion of lys reduces eDNA release by algT revertants. (A) eDNA visualized via electrophoresis of cell-free supernatants from FRD1 (mucA) or algT, algT Δlys, and algT Δlys/pLys mutants. eDNA was observed as a high-molecular-weight band (>3,000 bp). MWM, molecular weight marker. One representative gel image is shown. (B) Quantification of eDNA band intensity using the densitometry plugin in ImageJ. Densitometry results represent the average from three independent experiments. Statistical significance was measured using one-way ANOVA followed by Tukey’s multiple-comparison test. Data are presented as mean ± SEM. ***, P < 0.001; ns, not significant. Download FIG S5, TIF file, 1.9 MB (2MB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Complementation of mucA restores H2O2 resistance of FRD1. (A) FRD1 (mucA) and the isogenic algT and mucA/pMucA strains were grown for 24 h in the presence of 25 mM H2O2 via the Biolog system. Data are plotted as a percentage of the AUC relative to the no-treatment condition. (B) mucA cells resuspended in supernatants derived from algT and mucA/pMucA cells prior to 24 h of growth in the presence of 25 mM H2O2 by the Biolog system. Experiments were performed in triplicate on at least three independent occasions. Statistical significance was measured using one-way ANOVA followed by Tukey’s multiple-comparison test. Data are presented as mean ± SEM. ***, P < 0.001; ns, not significant. Download FIG S6, TIF file, 0.5 MB (501.9KB, tif) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplemental materials and methods. Download TEXT S1, PDF file, 0.4 MB (377.3KB, pdf) .
Copyright © 2018 Malhotra et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.