Abstract
Smartphones are ubiquitous devices that offer a variety of useful applications for human and veterinary medical professionals and the biomedical research community. Smartphones can serve as fomites and potentially transmit pathogens, including bacterial species such as methicillin-resistant Staphylococcus aureus. The goal of this study was to evaluate 6 methods to decrease aerobic bacterial colonies on smartphones, including two 254-nm UVC devices, 70% ethanol spray, quaternary ammonium disinfectant spray, sodium hypochlorite-impregnated wipes, and delicate-task wipes. All methods were individually effective at decreasing aerobic bacterial counts after sanitization. In addition, 254-nm UVC devices providing a dose of 60 mJ/cm2, with UVC bulbs exposing both sides of the smartphone, were an effective nonliquid method for smartphone sanitization.
Portable smart application-based devices have steadily increased in popularity in the United States, with recent surveys suggesting that 72% of adults own a smartphone and 45% own a tablet.17,18 Portable touchscreen devices offer general applications, such as text messaging, calculators, timers, flashlights, and cameras; medical applications including pharmaceutical formularies, medical calculators, and patient communication; and laboratory-animal specific applications, including electronic medical records, animal census tools, and veterinary pharmaceutical formularies.
Mobile phones can harbor bacterial nosocomial pathogens, including methicillin-resistant Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli, Klebsiella spp., Enterobacter spp., enterococci, and streptococci, among others.13-15,20,22-24 The few published reports focusing on touchscreen-based smart devices likewise confirm contamination with bacterial pathogens, with one study finding a higher rate of pathogen contamination for smartphones (34.8%) as compared with nonsmartphones (20.5%).7,11,13
The potential ability of smartphones to serve as fomites has important implications for laboratory animal facilities. Specifically, the use of contaminated smart devices inside vivaria or procedure rooms poses the risk of exposing research animals to potential pathogens. This risk is especially critical when maintaining SPF genetically modified, immunodeficient, and humanized mouse models, because large commercial mouse vendors exclude bacterial pathogens including S. aureus and P. aeruginosa from mice housed in their cleanest health-status barrier facilities.4,9,12,21 Previously, transmission of Corynebacterium bovis to a strain of hirsuite immunologically altered mice was attributed to a mobile tablet shared between 2 housing rooms.5
Despite the number of publications assessing microbiologic contamination of smartphones, limited published reports evaluate the efficacy of sanitization methods, and those that assess decreases in bacterial colonization after smartphone sanitization evaluate alcohol-impregnated lens wipes, quaternary ammonium disinfectant–detergent, and microfiber cloths.7,16,20 Additional methods of smartphone disinfection, including commercially available 254-nm UVC smartphone sanitizing devices and bleach-impregnated wipes, have not previously been evaluated, to our knowledge. The goal of this study was to evaluate the efficacy of UV smartphone sanitizing devices as compared with liquid methods of sanitization. We hypothesized that UV light would be more effective than liquid-based methods for sanitization of smartphones.
Materials and Methods
Smartphone inclusion criteria.
The characteristics of the smartphones sampled are shown in Figure 1. Smartphones were excluded from the study when physical flaws (for example, a cracked screen) were present and when the smartphone case completely enclosed the phone screen. Smartphone owners were queried regarding whether they sanitized their smartphone regularly, and a minimum of 2 wk since the last sanitization was required prior to sampling. Smartphones that underwent repeated sampling over the course of the project had a minimum intersampling interval of 3 wk. All smartphone sampling procedures were considered exempt by the MIT Committee on the Use of Humans as Experimental Subjects. Written informed consent was obtained for all persons volunteering their smartphones.
Figure 1.
Characteristics of smartphones sampled (n = 24).
Smartphone and UV device sampling.
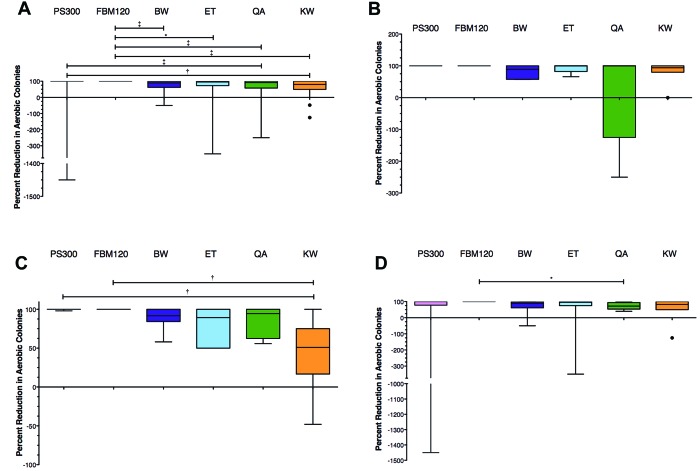
Three separate areas were chosen for sampling and designated as smartphone face, junction, and case. A sterile cotton-tipped applicator (Puritan Medical Products, Guilford, ME) was moistened in tryptic soy broth (Becton Dickinson, Sparks, MD) and swabbed over the smartphone face, smartphone–case junction, and the sides and back of the case (Figure 2 A). Each swab was rolled down and up the center of a plate containing trypticase soy agar with 5% sheep blood (Figure 2 B). A disposable 10-μL inoculating loop (Greiner Bio-One, Monroe, NC) was used to repeatedly spread colonies across the plate, perpendicular to the initial swab (Figure 2 B). Each area was sampled before and after sanitization. The smartphone without a case was sampled by using 2 swabs, encompassing the smartphone face and combined smartphone sides and back. The same persons performed all swab procedures and initial inoculation of agar plates (MTL) and dispersion of the swab inoculum (CMM). As a control, UVC devices were sampled by moistening a cotton-tipped applicator in tryptic soy broth and swabbing the surface where the smartphone would be placed. The swabs were inoculated onto the first quadrant of trypticase soy agar with 5% sheep blood, and a sterile inoculating loop was used to streak for colony isolation. Plates were incubated overnight at 37 °C with 5% CO2.
Figure 2.
Smartphone sampling procedure and contamination measured by using aerobic bacterial counts at each sampling site. (A) A sterile cotton-tipped applicator moistened with tryptic soy broth (TSB) was used to sample the front of the smartphone face, smartphone-case junction, and sides and back of the smartphone case as designated by yellow dashed lines. (B) Swabs from smartphone sampling were plated down the center of a tryptic soy agar plate with 5% sheep blood, and the initial swab inoculum was spread in perpendicular manner by using a 10-µL disposable inoculating loop. (C) The smartphone face was significantly less contaminated than the phone-case junction. Tukey box and whiskers plot; Kruskal–Wallis with Dunn multiple comparison test, P = 0.0046. †, P ≤ 0.01.
Sanitization methods.
All smartphones were left inside their cases during sanitization. Sanitization methods included 2 commercially available smartphone UVC-sanitizing devices (PS300, PhoneSoap, Provo, UT; FBM120, Flashbox mini, ClorDiSys Solutions, Branchburg, NJ; Figure 3), 70% ethanol spray, 0.55% sodium hypochlorite wipes (Bleach Germicidal Wipes, Clorox Healthcare, Oakland, CA), quaternary ammonium disinfectant spray (Quatricide PV, Pharmacal, Naugatuck, CT), and cleaning with a delicate-task wipe (KimWipe, Kimberly-Clark Professional, Roswell, GA). For the PS300, the smartphone was placed inside the device, screen-side up, and the lid was closed to activate the UV light, according to the manufacturer's instructions, for a set sanitizing time of 5 min. For the FBM120, the smartphone was placed screen-side up on the glass shelf set on the lowest height. The light mode was set to ‘all’ (for both top and bottom UV-light activation), and the time knob switched past 2 min while an independent timer was set for 2 min. For the spray sanitization methods of 70% ethanol and quaternary ammonium disinfectant, smartphones were sprayed twice (front and back) by using a squirt bottle at a distance of approximately 12 in. to mimic a realistic disinfection procedure. Smartphones were wiped immediately, front and combined back and sides, by using a clean paper towel in a single downward motion and were placed on top of a clean paper towel for sampling. For sanitization by using bleach wipes, smartphones were wiped front, sides, and back by using one wipe per smartphone and then allowed to dry completely on a clean paper towel prior to sampling. For sanitization by using a delicate-task wipe, the smartphone was wiped on the front, sides, and back by using a single wipe and placed on a clean paper towel for sampling. We evaluated 7 smartphones per method, except for the PS300 (n = 9). Two additional smartphones were tested by using the PS300 method because 3 phones tested had presanitization bacterial colony counts of 0 for the smartphone face sampling site.
Figure 3.
Characteristics of 254-nm UVC sanitizing devices.
Colony enumeration.
After overnight incubation at 37 °C with 5% CO2, aerobic colony counts were enumerated for each plate. For the purposes of statistical analysis, plates with colonies too numerous to count were designated with a count of 100. The same person performed colony enumeration (CMM) for all samples.
Statistical analysis.
Data were analyzed by using Prism 5.0 (GraphPad Software, La Jolla, CA). We compared the number of aerobic colonies prior to sanitization to determine the most likely contamination site on smartphones by using the Kruskal–Wallis test with Dunn multiple-comparison tests. The effect of a screen protector on number of aerobic colonies prior to sanitization of the smartphone face was evaluated by using the Mann–Whitney test. To evaluate each method of sanitization, we compared the numbers of aerobic colonies before and after sanitization by using Wilcoxon signed-rank tests. To normalize efficacy relative to the presanitization number of bacterial colonies and to compare efficacy between sanitization methods, percentage reduction in aerobic colony count was calculated by using the following equation:
Percentage reduction in aerobic colony count was evaluated for the combined smartphone face, smartphone–case junction, and smartphone case, unless otherwise indicated. Comparison in percentage reduction across different sanitization methods was evaluated by using the Kruskal–Wallis test with the Dunn multiple-comparison test. Smartphones with a presanitization colony count of 0 were excluded from percentage reduction analysis. For the smartphone that lacked a case, the back and sides of the smartphone were treated as the case sampling area. Categorical comparisons for a reduction in aerobic colony count to 0 were evaluated by using Pearson 2 analysis among sanitization methods. The significance level for all tests was set as an α value of 0.05.
A simple Bayesian estimation model was built to model the uncertainty surrounding the percentage reduction for all sampling sites, given the available data. Briefly, the presanitization and postsanitization colony counts were modeled by using Poisson likelihood, with a DiscreteUniform (0, 1000) prior placed on the Poisson rate parameter. The notation is as follows:
Percentage reduction, δp, was then computed deterministically from the estimated μ posterior distributions:
The Bayesian estimation model was implemented in PyMC3, version 3.0 (https://github.com/pymc-devs/pymc3/archive/v3.0.zip) in the Python programming language (version 3.5). Notebooks are available on GitHub (https://github.com/ericmjl/mia-stats/blob/master/sterilization/sterilization.ipynb) and are archived on Zenodo (DOI: http://doi.org/10.5281/zenodo.275624).
Results
Experiments were designed to mimic realistic conditions; consequently, smartphones were used ‘as-is’ and sanitized in a manner approximating a real-life scenario. We first determined overall colonization density at each smartphone sampling site and the efficacy of each sanitization method individually before comparing sanitization methods.
Contamination by site.
The median (range) for the number of aerobic bacterial colony-forming units present on the smartphone face, smartphone–case junction, and smartphone case prior to sanitization were 6.50 (0 to 100), 14.0 (0 to 86), and 12.0 (0 to 100) colonies, respectively, with the smartphone face having significantly fewer colonies present than the junction (P = 0.0046, Kruskal–Wallis; Figure 2 C). The number of aerobic colonies before sanitization did not differ between smartphone faces with a screen protector compared with those without a screen protector (P = 0.6827; Mann–Whitney test). The number of aerobic colonies present on smartphone cases before sanitization did not differ between the 3 most common case types—hard plastic, hard plastic–hard silicone, and hard silicone (P = 0.0532, Kruskal–Wallis). All cultures from the UVC devices were negative for aerobic growth.
Individual sanitization efficacy.
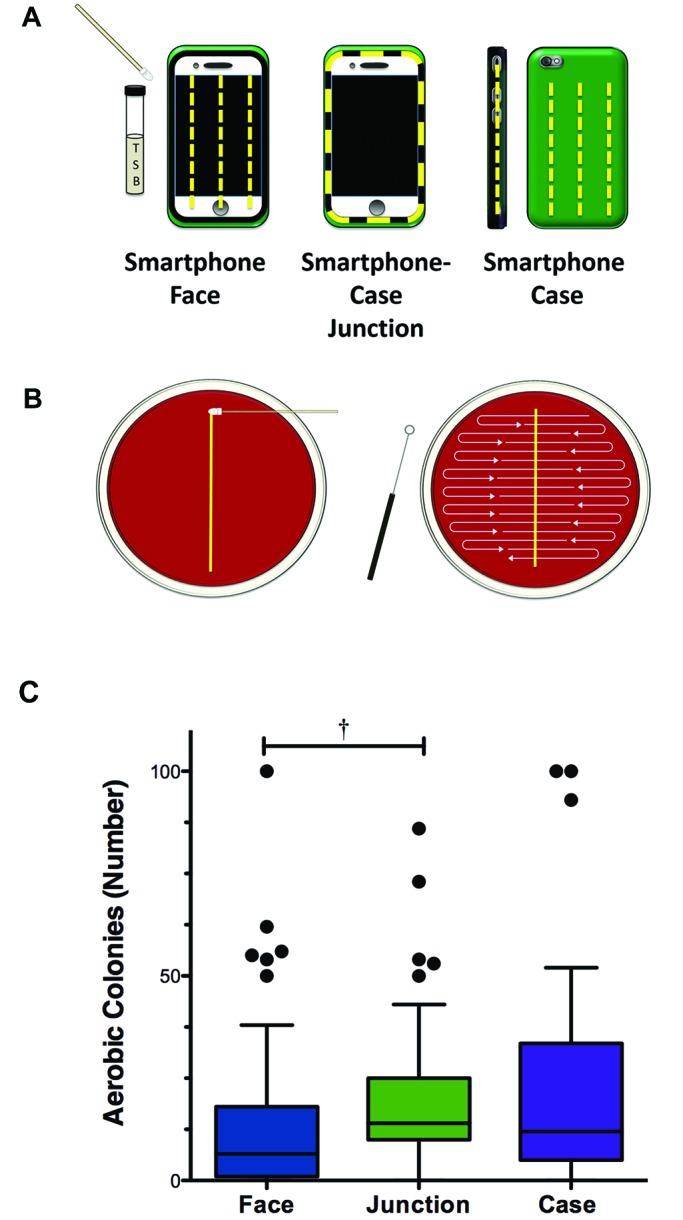
The number of colonies present before sanitization did not differ between methods tested (P = 0.5895, Kruskal–Wallis with Dunn multiple-comparison test). Each method tested was individually effective at reducing the number of aerobic colonies after sanitization (Figure 4).
Figure 4.
Efficacy of sanitization methods in reducing aerobic bacterial colony count. Tukey box and whiskers plot. Wilcoxon signed-rank test, with P values indicated above each treatment. Solid bars, presanitization colony counts; striped bars, postsanitization colony counts; †, P < 0.01; ‡, P < 0.001; BW, bleach wipe; ET, 70% ethanol; QA, quaternary ammonium disinfectant spray; KW, delicate-task wipe.
Percentage reduction in aerobic colony count.
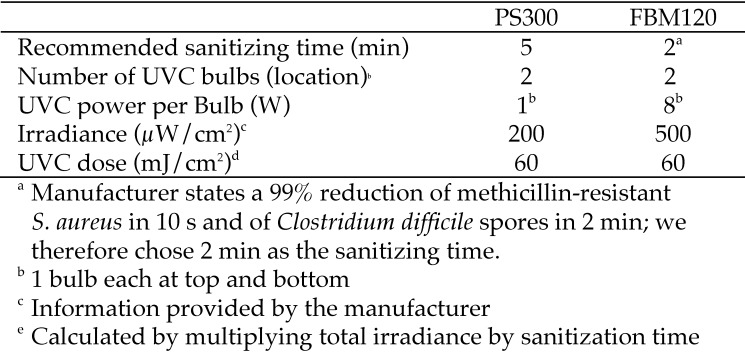
For all smartphone sampling sites combined, the FBM120 device achieved a significantly (P < 0.05) higher percentage reduction in colony count than all other sanitization methods except the PS300 device (Figure 5 A). The PS300 sanitization method showed a higher percentage reduction as compared with quaternary ammonium disinfectant and delicate-task wipes (Figure 5 A). When analyzed by sampling location, Kruskal–Wallis analysis found a significant difference in the percentage reduction at the smartphone face between sanitization methods (P = 0.0462); however, posthoc testing did not identify pairwise differences (Figure 5 B). For the smartphone–case junction, the FBM120 and PS300 devices showed higher percentage reductions in colony count compared with the delicate-task wipe (Figure 5 C). For the smartphone case, the FBM120 showed a higher percentage reduction in colony count than quaternary ammonium disinfectant (Figure 5 D). The Bayesian estimation model supported these results, revealing that the FBM120 and PS300 devices showed the most consistent percentage reduction in colony count with the narrowest 95% credible intervals (Figure 6).
Figure 5.
Percentage reductions in aerobic bacterial count for all sampling sites. Tukey box and whiskers plots. In this figure, maximum sanitization effectiveness is represented by100% reduction. (A) Combined colony count for all sampling sites. Kruskal–Wallis with Dunn multiple-comparison test, P < 0.0001; posthoc testing: *, P ≤ 0.05; †, P ≤ 0.01; ‡, P ≤ 0.001. (B) Smartphone face (P = 0.0462). (C) Smartphone–case junction (P = 0.0011). (D) Smartphone case (P = 0.0322); posthoc testing: *, P ≤ 0.05; †, P ≤ 0.01.
Figure 6.
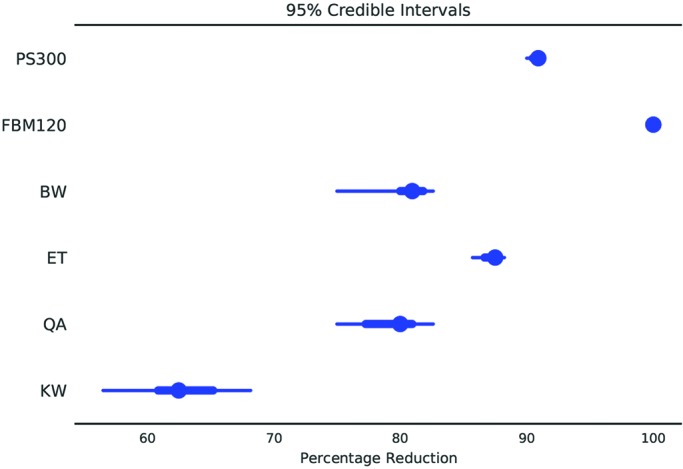
Bayesian estimation model of sanitization efficacy for all smartphone sampling sites combined. The posterior distribution is summarized in blue: the dot represents the median, the thick blue line indicates the interquartile range, and the thin blue line indicates the width of the 95% credible interval probability mass.
Efficacy in reducing aerobic bacterial colony count to 0.
We performed a χ2 test to evaluate the relationship between sanitization method and efficacy in reducing the aerobic bacterial colony count to 0 (that is, aerobic bacterial sterilization). The observed χ2 was 40.82 with 5 degrees of freedom and P < 0.0001, allowing rejection of the null hypothesis that all sanitization methods were equally likely to reduce the aerobic colony count to 0 (Figure 7). All 7 smartphones sanitized with the FBM120 demonstrated 100% reduction in aerobic bacterial colonies to 0 at all 3 sampling sites after sanitization.
Figure 7.
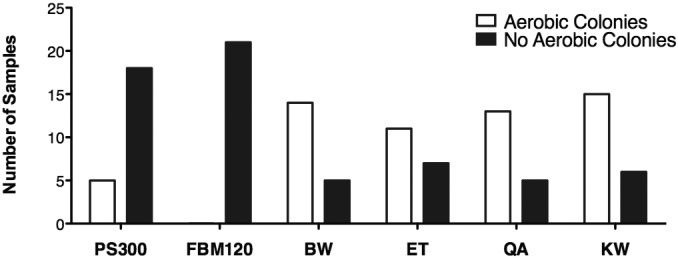
χ2 analysis of the efficacy in reducing aerobic bacterial colony count to 0 for all sampling sites combined. χ2 = 40.82; degrees of freedom, 5; P < 0.0001.
Discussion
In addition to efficacy in decreasing bacterial burden, several factors should be considered when establishing appropriate biosecurity protocols for smartphones and smart devices in the healthcare and preclinical research settings. To encourage compliance, sanitization methods should work rapidly (ideally, in the time it takes to wash hands or apply or remove personal protective equipment) and not damage mobile devices. Because of the risks of smartphone damage with exposure to liquid disinfectants, UVC sanitization methods are potentially superior to disinfection with bleach, ethanol, or quaternary ammonium solutions. None of the liquid disinfection methods used damaged the sampled smartphones in the current; however, we cannot comment on device impairment after repeated liquid disinfectant exposures. A previous study recovered pathogenic bacteria on 44 of 53 (83%) cell phones in a hospital environment; immediately after sanitization with an isopropanol-impregnated lens wipe, 4 of the 53 (8%) cell phones remained culture-positive for pathogenic bacteria.20 A second study found no bacterial growth on touchscreens after cleaning with an ethanol–isopropanol-impregnated lens wipe in 35% of phones (n = 20).7 Our results for sanitization with 70% ethanol are intermediate to these findings, with 39% of sampling sites (7 of 18 sites among 7 phones) showing no aerobic growth immediately after sanitization (Figure 7). Another publication reported that a 0.25% concentration of a detergent–disinfectant combination containing N-(3-aminopropyl)-N-dodecylpropane-1 and didecyldimethylammonium chloride reduced colony counts by approximately 50%; however only 25% (13 of 52) phones were free of colony growth following decontamination procedures.16 Our results for quaternary ammonium disinfection are in agreement, with 28% of sampling sites (5 of 18 sites among 7 phones) showing no aerobic growth after sanitization.
Our results indicated that the smartphone–case junction had significantly more contamination than the smartphone face. We hypothesize that this situation results from the persistence of debris and bacteria in the crevice between the smartphone and the case (Figure 2 C). UV sanitization methods had a smaller interquartile range for percentage reduction in aerobic colony counts for the smartphone–case junction as compared with liquid and wipe methods (Figure 5 C). We found it surprising that the case did not have a higher presanitization bacterial burden; this result may reflect the sample size and lack of standardization of case material and styles.
Physically wiping the phone is a variably effective method for decontamination when no other sanitization options are available. The delicate-task wipe was far less effective that UV-based methods when sanitizing the smartphone–case junction (Figure 5 C). A previous report found that 25% of touchscreens (n = 20) did not show any bacterial growth after cleaning with a new, dry, microfabric cloth.7 Our results found that the delicate-task wipe was effective at reducing bacterial burden by 55% to 70% overall, with no aerobic growth on 29% of sampling sites (6 of 21 sites among 7 phones; Figures 6 and 7).
During analysis of the results, there were several instances where the percentage reduction in colony count was negative, that is, more bacterial colonies were present on the smartphone after sanitization than before. Because no growth occurred after aerobic culture of the interior of the UV devices, we believe that increased postsanitization bacterial counts likely resulted from inconsistent speed when obtaining the swab samples. Specifically, a slower swabbing rate increases contact time between the phone and swab, allowing for more bacteria to be sampled. We also cannot rule out UVC bulb failure in the PS300, because the device design prohibits the UVC light from turning on when the device is open. One PS300 sanitization procedure yielded a marked increase in the case postsanitization case colony count, yet, the postsanitization smartphone face and phone–case junction colony counts decreased, suggesting either bulb malfunction or error during the postsanitization swabbing procedure. Another possible source for increased bacterial counts after spray sanitization methods with quaternary ammonium disinfectant or 70% ethanol are contamination of the spray nozzle or paper towel used for sanitization. Paper towels were removed directly from a shared paper towel dispenser to mimic everyday conditions.
Between the 2 commercially available 254-nm UVC devices evaluated in this study, we found that the FBM120 was superior to the PS300 for consistency in reducing the bacterial burden to 0 at all sampling sites and required only a 2-min sanitizing period. UVC kills cells through the induction of pyrimidine dimers in DNA, thus disrupting the DNA replication process.8 The effectiveness of UVC sterilization is dependent on the dose, which is defined as the amount of UV energy (mJ) per unit area (cm2) and sometimes expressed as irradiance (J/s/cm2 or W/cm2). Doses of 15 mJ/cm2 are able to achieve 3- to 4-log reductions in Klebsiella pneumoniae, Citrobacter spp., Escherichia coli, Salmonella typhi, Shigella sonnei, Staphylococcus aureus, and Enterococcus (Streptococcus) faecalis; however, higher doses are required for rotavirus and poliovirus (>30 mJ/cm2) and Bacillus subtilis spores (>60 mJ/cm2).2,10 The PS300 device contains two 1-W, 254-nm bulbs, 1 each located on the bottom and lid of the device and providing an output of 200 µW/cm2 each, at the distance to the phone (according to the manufacturer). This setup achieves a UVC dose of 12 mJ/cm2 for a 1-min exposure and a total experimental dose of 60 mJ/cm2 for the 5-min sanitization. The FBM120 device contains two 8-W bulbs, 1 each located on the ceiling and the floor of the sanitizing chamber, and provides a total output of 500 µW/cm2 at a 3-in. distance, equal to 30 mJ/cm2/min (according to the manufacturer) and a total experimental dose of 60 mJ/cm2 for the 2-min sanitization period.
Our results also indicated that the FBM120 device was the most effective and most consistent method for sanitizing smartphones. Although the PS300 device was highly effective in sanitizing the smartphone face and smartphone–case junction, it did not achieve 100% reduction in aerobic colonies to 0 after sanitization of the smartphone case.
We recognize that a limitation of this study is that we did not perform identification procedures on the bacterial colonies isolated from the smartphones. We did observe that the most common colony morphologies noted were consistent with Staphylococcus, Streptococcus, and Bacillus spp., and we acknowledge previous studies characterizing nonpathogenic and potentially pathogenic bacteria isolated from personal mobile devices.1,3,13,14,20,23,24 We also recognize that constraining the maximal number of colonies enumerated to 100 for statistical analysis potentially underestimates the calculation for percentage reduction in aerobic colony counts. The number of presanitization smartphone sampling sites designated as ‘too many to count’ represented a minority of our samples (3 of 131 samples; that is, 2% of swabs). In addition, reduction of aerobic bacteria count to 0 is unaffected by this constraint and is arguably the most important parameter when evaluating sanitization efficacy.
In the preclinical and laboratory animal research settings, other highly resistant pathogens of interest to exclude from rodent colonies include mouse parvovirus and pinworms, especially those of the genus Syphacia. Previous studies suggest that 254-nm UVC light is effective at inactivating porcine parvovirus19 as well as preventing hatching of Syphacia muris ova,6 although future studies should investigate the effectiveness of small, portable 254-nm UVC devices on these agents.
With increasing use of portable mobile devices in research and human and veterinary healthcare settings, appropriate biosecurity protocols must be established to prevent fomite transmission of bacterial pathogens. Our results indicate that UVC-based sanitization methods are effective in reducing bacterial burden, but not all devices are equivalent in their abilities to reduce aerobic bacterial colonization to 0. Our evaluation of 2 commercially available 254-nm UVC sanitizing devices suggests that sanitizing devices providing a total UVC dose of approximately 60 mJ/cm2 and UVC bulb exposure on both sides of the smartphone are effective in sanitizing smartphones and reducing the aerobic bacterial count to 0.
Acknowledgments
We acknowledge all participants willing to have their smartphone sanitized during this project and David Geisler for insightful discussions regarding energy calculations. This work was supported by the following grants from the National Institutes of Health: T32-OD010978 and P30-ES002109 (to JGF). UV sanitization devices were provided free of charge from the manufacturers. The manufacturers had no input or influence on study design or interpretation of results. The authors declare no conflicts of interest.
References
- 1.Brady RR, Hunt AC, Visvanathan A, Rodrigues MA, Graham C, Rae C, Kalima P, Paterson HM, Gibb AP. 2011. Mobile phone technology and hospitalized patients: a cross-sectional surveillance study of bacterial colonization, and patient opinions and behaviours. Clin Microbiol Infect 17:830–835. [DOI] [PubMed] [Google Scholar]
- 2.Chang JC, Ossoff SF, Lobe DC, Dorfman MH, Dumais CM, Qualls RG, Johnson JD. 1985. UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol 49:1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao Foong Y, Green M, Zargari A, Siddique R, Tan V, Brain T, Ogden K. 2015. Mobile phones as a potential vehicle of infection in a hospital setting. J Occup Environ Hyg 12:D232–D235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles River. [Internet] 2017. Routine health monitoring of isolator-reared immunodeficient rats and mice. [Cited 24 October 2017]. Available at: http://www.criver.com/files/pdfs/rms/rms_lad_td_health_monitoring_of_immunodeficient_mi.aspx.
- 5.Chatkupt TT, Horne DJ, Henderson KS, Albers T, Saunders KE. 2015. Infection of Corynebacterium bovis in a strain of hirsuite, immunologically altered murine model of neoplasia. Abstract presented at the American Association for Laboratory Animal Science AALAS National Meeting, Phoenix, Arizona 2015. J Am Assoc Lab Anim Sci 54:614. [Google Scholar]
- 6.Dix J, Astill J, Whelan G. 2004. Assessment of methods of destruction of Syphacia muris eggs. Lab Anim 38:11–16. [DOI] [PubMed] [Google Scholar]
- 7.Egert M, Spath K, Weik K, Kunzelmann H, Horn C, Kohl M, Blessing F. 2014. Bacteria on smartphone touchscreens in a German university setting and evaluation of 2 popular cleaning methods using commercially available cleaning products. Folia Microbiol (Praha) 60:159–164. [DOI] [PubMed] [Google Scholar]
- 8.Elmnasser N, Guillou S, Leroi F, Orange N, Bakhrouf A, Federighi M. 2007. Pulsed-light system as a novel food decontamination technology: a review. Can J Microbiol 53:813–821. [DOI] [PubMed] [Google Scholar]
- 9.Envigo. [Internet] 2017. Rodent health monitoring procedures. [Cited 24 October 2017]. Available at: http://www.envigo.com/resources/health-monitoring-reports/envigo-66-rodent-health-procedures_screen.pdf.
- 10.Giese N, Darby J. 2000. Sensitivity of microorganisms to different wavelengths of UV light: implications on modeling of medium pressure UV systems. Water Res 34:4007–4013. [Google Scholar]
- 11.Hirsch EB, Raux BR, Lancaster JW, Mann RL, Leonard SN. 2014. Surface microbiology of the iPad tablet computer and the potential to serve as a fomite in both inpatient practice settings as well as outside of the hospital environment. PLoS One 9:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.JAX. [Internet] 2017. JAX list of agents monitored and policy for communication of changes in health status. [Cited 24 October 2017]. Available at: https://www.jax.org/jax-mice-and-services/customer-support/animal-health/list-of-agents-monitored.
- 13.Lee YJ, Yoo CG, Lee CT, Chung HS, Kim YW, Han SK, Yim JJ. 2013. Contamination rates between smart cell phones and nonsmart cell phones of healthcare workers. J Hosp Med 8:144–147. [DOI] [PubMed] [Google Scholar]
- 14.Loyola S, Gutierrez LR, Horna G, Petersen K, Agapito J, Osada J, Rios P, Lescano AG, Tamariz J. 2016. Extended-spectrum β-lactamase–producing Enterobacteriaceae in cell phones of health care workers from Peruvian pediatric and neonatal intensive care units. Am J Infect Control 44:910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris TC, Moore LSP, Shaunak S. 2012. Doctors taking a pulse using their mobile phone can spread MRSA. BMJ 344:e412. [DOI] [PubMed] [Google Scholar]
- 16.Murgier J, Coste JF, Cavaignac E, Bayle-Iniguez X, Chiron P, Bonnevialle P, Laffosse JM. 2016. Microbial flora on cellphones in an orthopedic surgery room before and after decontamination. Orthop Traumatol Surg Res 102:1093–1096. [DOI] [PubMed] [Google Scholar]
- 17.Pew Research Center. [Internet] 2015. Technology device ownership: 2015. [Cited 24 October 2017]. Available at: http://www.pewinternet.org/2015/10/29/technology-device-ownership-2015/.
- 18.Pew Research Center. [Internet] 2016. Smartphone ownership and internet usage continues to climb in emerging economies. [Cited 24 October 2017]. Available at: http://www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climb-in-emerging-economies/.
- 19.Polo J, Rodríguez C, Ródenas J, Russell LE, Campbell JM, Crenshaw JD, Torrallardona D, Pujols J. 2015. Ultraviolet light (UV) inactivation of porcine parvovirus in liquid plasma and effect of UV-irradiated spray-dried porcine plasma on performance of weaned pigs. PLoS One 10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shakir IA, Patel NH, Chamberland RR, Kaar SG. 2015. Investigation of cell phones as a potential source of bacterial contamination in the operating room. J Bone Joint Surg Am 97:225–231. [DOI] [PubMed] [Google Scholar]
- 21.Taconic. [Internet] 2017. Taconic health standards. [Cited 24 October 2017]. Available at: http://www.taconic.com/quality/health-testing-program/health-standards/.
- 22.Tekerekoǧlu MS, Duman Y, Serindag A, Cuglan SS, Kaysadu H, Tunc E, Yakupogullari Y. 2011. Do mobile phones of patients, companions, and visitors carry multidrug-resistant hospital pathogens? Am J Infect Control 39:379–381. [DOI] [PubMed] [Google Scholar]
- 23.Ulger F, Esen S, Dilek A, Yanik K, Gunaydin M, Leblebicioglu H. 2009. Are we aware how contaminated our mobile phones with nosocomial pathogens? Ann Clin Microbiol Antimicrob 8:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ustun C, Cihangiroglu M. 2012. Healthcare workers’ mobile phones: a potential cause of microbial cross-contamination between hospitals and community. J Occup Environ Hyg 9:538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]