Abstract
All currently accepted methods of euthanasia for laboratory mice involve some degree of stress, fear, anxiety, or pain. We evaluated the voluntary oral administration of a euthanasia drug in 99 male and 81 female mice of various strains. We first explored the palatability of sugar-cookie dough with various flavorings added. We placed the cookie dough in the cage with an adult mouse and recorded the amount ingested after 1 h. Mice readily ingested all flavors of sugar-cookie dough. We then added a euthanasia solution containing pentobarbital and phenytoin to all flavors of cookie dough and placed a small bolus in the cage of each mouse or mouse pair. We observed the mice for 1 h for clinical signs of pentobarbital intoxication and then weighed uneaten dough to determine the dose of pentobarbital ingested. Palatability declined sharply when euthanasia solution was present. Mice ingested higher doses of pentobarbital in cookie dough during the dark phase and after fasting. Ingestion caused ataxia in some mice but was not sufficient to cause loss of righting reflex, unconsciousness, or death in any mouse. We successfully identified sugar cookie dough as a drug vehicle that was readily and rapidly eaten by mice without the need for previous exposure. Additional research is needed to identify euthanasia compounds for mice that do not affect the palatability of cookie dough.
In planning a study, investigators are mandated to consider alternatives that avoid or minimize discomfort, distress, and pain.3,30 This mandate includes handling, restraint, surgical and nonsurgical procedures, and euthanasia. The Guide for the Care and Use of Laboratory Animals defines euthanasia as “the act of humanely killing animals by methods that induce rapid unconsciousness and death without pain or distress.”24 The Guide also recommends that researchers follow the AVMA guidelines27 when selecting euthanasia methods. The AVMA considers the ability to induce loss of consciousness and death while pain and distress are minimized to be of high importance and places the responsibility on veterinarians to use “humane techniques to induce the most rapid and painless and distress-free death possible.”27
All acceptable euthanasia methods (and those acceptable with conditions) for laboratory mice in the AVMA guidelines27 involve some degree of stress, fear, anxiety, or pain. Euthanasia methods are often categorized by methodology: injectable, physical, or inhalant. Both injectable and physical euthanasia methods require animal restraint, which is a stressor in mice.10,23 Parenteral injections elicit a physiologic stress response and momentary pain.28,34 Inhalant agents such as carbon dioxide cause anxiety and breathlessness,5,11 and inhalant anesthetics produce a stress response.4,6 A possible alternative method for euthanasia is voluntary ingestion. Voluntary ingestion of effective euthanasia agents would alleviate all of the previously mentioned concerns, because it does not involve restraint, injection, or the inhalation of noxious substances. The AVMA currently considers oral administration of euthanasia agents to be unacceptable, due to a lack of established dosages and drugs, concerns about bioavailability and absorption, potential aspiration, vomiting or regurgitation of drug (in species able to do so), and administration difficulty.27 If these disadvantages are addressed, then voluntary ingestion of an oral euthanasia agent is more likely to be considered acceptable.
The goal of the current study was to identify a method of presenting an orally effective euthanasia drug that mice ingest voluntarily. The ideal drug will be easily delivered, be reliably and quickly ingested, and result in rapid death consistently without pain or distress. Voluntary ingestion of an oral euthanasia drug might improve the welfare of the animal during the procedure and might also be useful during emergencies when rapid depopulation is required. To identify a potential suitable voluntary euthanasia drug requires 2 steps: 1) identification of a highly palatable drug delivery vehicle, and 2) identification of a euthanasia agent that is efficacious when delivered orally. To address the first step, we evaluated the palatability of variously flavored cookie dough as a vehicle for oral euthanasia drug delivery. For the second step, we tested a commercially available pentobarbital–phenytoin euthanasia solution added to cookie dough. We hypothesized that voluntary ingestion of a lethal dose of a pentobarbital-containing solution would result in sedation, followed by unconsciousness and death, without the development of negative affective states.
Materials and Methods
Mice.
We used 180 mice (99 male, 81 female; mean weight, 27.0 ± 5.6 g; median age, 96 d), including inbred strains, outbred lines, and transgenics. The majority of mice used in the study were transgenic on a C57BL/6 background. All mice were drug- and test-naïve and healthy at the time of testing. Transgenic mice were phenotypically normal, with no known effects of the altered genetics on appetite. Except where noted, food (Envigo Teklad, Madison, WI) and tap water were provided without restriction, with water removed at the time of the experiment. Aspen chip bedding (Envigo Teklad) and nesting material (Nestlets, Ancare, Bellmore NY) were provided. Due to variation in the manner which specific strains were housed prior to experiments, some mice had additional enrichment items, such as cardboard tubes (Jonesville Paper Tube, Jonesville, MI) and plastic huts (Alternative Design, Siloam Springs, AR) in their cages. Lighting was maintained on a 12:12-h light:dark cycle (lights on, 0630), and ambient temperature maintained at 23.3 ± 2.2 °C. Most mice were socially housed prior to experimental use, but tests were conducted with a single mouse present in each cage, unless otherwise indicated (additional mice were removed from the cage 30 min prior to experimental activities).
All mice were negative for the following pathogens: Mycoplasma pulmonis, mouse parvovirus, minute virus of mice, mouse hepatitis virus, Theiler murine encephalomyelitis virus, epizootic diarrhea of infant mice virus, pneumonia virus of mice, reovirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse adenovirus 1 and 2, polyoma virus, and Sendai virus (IDEXX RADIL, Columbia, MO). All procedures were reviewed and approved by the IACUC at Wright State University. Animals were housed in an AAALAC-accredited facility, and all procedures were completed in accordance with federal guidelines and regulations.
Experimental procedures.
Except where noted, experimental procedures occurred during the light phase (between 1300 and 1600) in nonfasted mice that remained in their home cages during the experiment (standard conditions). Cages were transferred to a procedure room within the facility, and mice were allowed to acclimate for 30 min prior to experimental procedures. Experimental group size was 10, with approximate equal numbers of male and female mice in each group. Group size was determined through a power analysis (α, 0.05; β, 0.2; estimated effect size, 0.25; and estimated standard deviation, 0.2). Pair-housed mice were tested, with 10 pairs in each group. No adverse events occurred during any experimental procedure. At the conclusion of each experiment, all mice were euthanized with carbon dioxide gas administered at 30% chamber replacement rate, according to AVMA guidelines.27
Palatability of sugar-cookie dough.
We first evaluated the palatability of sugar-cookie dough (Pillsbury, General Mills, Minneapolis, MN) in 4 groups of mice (40 total; Figure 1). Cookie dough with or without flavor enhancers was formed into 225-mg spherical boluses, and one was offered to each mouse by either placing the bolus on the wire bar feeder over the nest or by dropping directly into the nest, to minimize bedding adherence. Group treatments included sugar cookie dough and cookie dough with one of the following additions: peanut butter powder (Honeyville, Brigham City, UT), berry-flavored gelatin powder (Kraft Heinz, Glenview IL), and finely minced bacon crumbles (Hormel Foods, Austin, MN). The flavor enhancers and cookie dough were mixed in the following amounts: 1.8 g cookie dough mixed with 0.45 g berry-flavored gelatin powder or bacon crumbles, or 2.0 g cookie dough mixed with 0.25 g peanut butter powder. The amount of cookie dough remaining after 1 h was weighed and percentage consumption determined. Latency to ingest the cookie dough was recorded for mice that ingested the entire bolus.
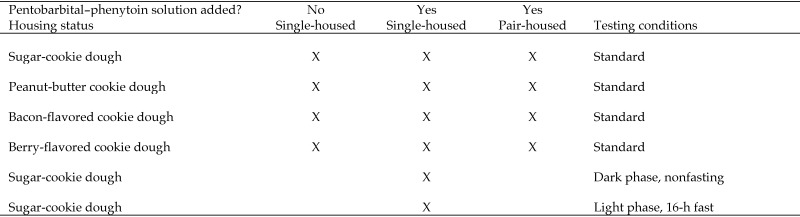
Figure 1.
Experimental groups. Standard conditions involved testing during the light phase and without fasting.
Oral ingestion of pentobarbital–phenytoin.
Ten experimental groups (total, 140 mice) were evaluated to determine whether mice voluntarily eat a treat containing euthanasia solution (Figure 1). Four groups of single-housed mice and 4 groups of pair-housed mice were offered the treats under standard conditions. In addition, one group of single-housed mice was tested 30 min after onset of the dark phase, and another group was tested during the light phase after a 16-h fast. For single-housed mice, 0.14 mL Euthasol solution (390 mg/mL pentobarbital and 50 mg/kg phenytoin, Virbac, St Louis, MO) was added to sugar-cookie dough with and without flavor enhancers in the proportions described previously; the resulting compound was divided into 10 equal doses. For single-housed mice, each dose was formulated to contain pentobarbital equivalent to approximately 200 mg/kg for a 25- to 30-g mouse. When offered to pair-housed mice, two 750-mg cookie-dough boluses were placed in each cage, with the same concentration of pentobarbital in the cookie dough (0.02 mg pentobarbital/mg cookie dough) as previously. The size of the bolus was increased for pair-housed mice to allow both mice to ingest portions of the same bolus and to accommodate increases in the amount ingested that might occur under pair-housed conditions. Euthanasia solution was added on the day of testing, and unused cookie dough discarded daily. Cookie dough was presented to single-housed mice either on the wire bar lid or directly into the nest. For pair-housed mice, each bolus was placed on the cage wall adjacent to the nest, to minimize the adherence of bedding and nesting material to the larger bolus, thus facilitating accurate quantification of remaining dough at the conclusion of the study. Mice were monitored for 1 h, and clinical effects (for example, lethargy, ataxia, recumbency) were recorded. At the conclusion of 1 h, any remaining cookie dough was removed from the cage and weighed. The ingested dose of pentobarbital was calculated for each mouse or mouse pair by multiplying the amount of cookie dough ingested by the concentration of pentobarbital in the cookie dough and then dividing by the body weight of the single-housed mouse or the combined weight of the mouse pair.
Results
Palatability of cookie dough.
All flavors of cookie dough were well accepted, with a mean of 82% to 94% of boluses ingested within 1 h, depending on flavor (Table 1). In both the sugar-cookie and bacon-flavored dough groups, all mice except 1 ingested the entire cookie dough bolus. In addition, 7 of the 10 mice receiving berry-flavored dough ingested the entire bolus, as did 8 of the 10 mice receiving peanut-butter cookie dough. The latency (mean ± 1 SD) to eat the entire dough bolus was lowest with plain sugar-cookie dough (8.4 ± 11.0 min) and longest with berry-flavored dough (29.0 ± 18.0 min).
Table 1.
Ingestion of sugar-cookie dough with and without flavorings and pentobarbital–phenytoin solution
| Amount of dough ingested (%, mean ± 1 SD) | Latency to ingest (min, mean ± 1 SD) | Amount of pentobarbital–phenytoin-containing dough ingested (%, mean ± 1 SD) | |
| Sugar-cookie dough | 91 ± 29 | 8.4 ± 11.0 | 42.1 ± 30.5 |
| Peanut-butter cookie dough | 82 ± 38 | 17.0 ± 10.7 | 30.0 ± 37.4 |
| Bacon-flavored cookie dough | 94 ± 19 | 16.1 ± 16.1 | 19.5 ± 29.1 |
| Berry-flavored cookie dough | 88 ± 21 | 29.0 ± 18.0 | 26.3 ± 13.7 |
Each group contained 10 mice.
Oral ingestion of pentobarbital–phenytoin.
The ingestion of cookie dough decreased sharply when pentobarbital– phenytoin solution was added (Table 1). Under single-housed standard conditions, the mean dose of pentobarbital ingested was highest for unflavored cookie dough (83.2 ± 52.0 mg/kg) and lowest for bacon-flavored cookie dough (45.1 ± 71.4 mg/kg; Table 2). Only 4 of the 40 mice in this experiment ingested the entire bolus, and 12 mice ingested less than 10% of the bolus. Of mice that ingested the entire bolus, 2 developed symptoms of moderate to severe ataxia and reduced activity, whereas the other 2 mice showed no identifiable signs of pentobarbital intoxication. In addition to the mice that ingested the entire bolus, 6 others developed ataxia after ingesting a portion of the bolus. The lowest ingested dose of pentobarbital that caused clinically apparent (mild) ataxia in a mouse was 59 mg/kg; however other mice ingested higher doses and did not develop ataxia. Under standard conditions, single-housed mice appeared to ingest higher doses of pentobarbital than paired mice when offered 3 of the flavors of cookie dough but ate less of the fourth flavor; these perceptions were not confirmed statistically (Table 2). Of the 80 mice tested under pair-housed conditions, 12 became mildly ataxic, but no other clinical signs were apparent.
Table 2.
Pentobarbital dose ingested by single- and pair-housed mice
| Pentobarbital dose ingested (mg/kg, mean ± 1 SD) | ||
| Single-housed mice | Pair-housed mice | |
| Sugar-cookie dough | 83.2 ± 52.0 | 79.6 ± 28.5 |
| Peanut-butter cookie dough | 64.1 ± 83.1 | 61.5 ± 46.0 |
| Bacon-flavored cookie dough | 45.1 ± 71.4 | 69.7 ± 35.7 |
| Berry-flavored cookie dough | 56.6 ± 34.5 | 43.5 ± 29.2 |
| Sugar-cookie dough, dark phase | 149.1 ± 94.7 | not tested |
| Sugar-cookie dough, 16-h fast | 194.3 ± 70.3 | not tested |
For single-housed mice, each group contained 10 mice; for pair-housed mice, each group contained 10 pairs of mice (total, 20)
Mice that were offered cookie dough containing euthanasia solution appeared to ingest (albeit not confirmed statistically) higher doses of pentobarbital during the dark phase (149.1 ± 94.7 mg/kg) than during the light phase (83.2 ± 52.0 mg/kg; Table 2). After a 16-h fast, mice ingested even larger doses of pentobarbital (194.3 ± 70.3 mg/kg). Three of the 10 mice in each of these 2 groups ingested the entire bolus of cookie dough. Two of the 10 mice that were tested during the dark phase became ataxic, whereas all 10 of the mice tested after a 16-h fast rapidly became ataxic. One mouse that was tested after a 16-h fast also became less responsive to stimuli (gentle touch), although it never became recumbent. Regardless of experimental group, no mouse that ingested pentobarbital–phenytoin-containing cookie dough lost the righting reflex, became unconscious, or died.
Discussion
The euthanasia solution used in these experiments contained pentobarbital (390 mg/mL) and phenytoin (50 mg/mL) and typically is administered parenterally. Pentobarbital is a barbiturate that may be used clinically as a sedative, anesthetic, and euthanasia agent, whereas phenytoin is used therapeutically in animals as an anticonvulsant.32 Phenytoin is often added to euthanasia solutions because of its additional cardiodepressant effects, and its addition changes the solution from a Schedule II to a Schedule III controlled substance.32 Parenteral administration of a lethal dose of pentobarbital–phenytoin solution to an animal leads to depression of the respiratory and vasomotor centers, resulting in death.32 The LD50 of oral pentobarbital sodium in rats is 118 mg/kg,35 and because the standard parenteral pentobarbital euthanasia dosage for mice at our institution is 150 mg/kg, we chose 200 mg/kg as our target dose for single-housed mice.
Although pentobarbital-containing euthanasia solutions are designed to be administered parenterally, pentobarbital is absorbed rapidly from the gut.13,25,33 Pentobarbital is described as the “best euthanasia drug” for human suicide,31 and oral administration is a frequent route of administration for this purpose.7-9,20,29 In a study evaluating treatments for pentobarbital toxicity, rats administered 40 mg/kg pentobarbital by oral gavage lost their righting reflex within 8 min, and remained anesthetized for 1.5 to 2 h, with peak plasma concentration reached within 1 h.13 Pentobarbital administered orally to fasted dogs at a dose of 63 mg/kg caused lateral recumbency in 6 of 7 dogs.33 In addition to those studies, numerous fatalities or near-fatalities in wildlife and companion animals have been reported after ingestion of incorrectly discarded carcasses or contaminated food containing pentobarbital residues.2,16,19 Given these studies and reports, we considered oral ingestion of a pentobarbital-containing solution a possible method for euthanizing mice.
The first step in trying to have mice voluntarily eat the euthanasia solution was to find a highly palatable food to potentially mask the bitter taste31 of the pentobarbital–phenytoin combination. Previous studies in rodents evaluated various food items as vehicles for oral drug delivery.1,12,14,15,21 Successful oral administration of medications including losartan,14 buprenorphine,1,15,21 and fluoxetine,12 has been achieved by using flavored gelatin,15 chocolate–hazelnut spread,1,21 sugar paste14 and sugar cookie dough,12 We chose sugar-cookie dough as the vehicle for the pentobarbital–phenytoin solution because of its availability and semisolid form, which accommodated both liquid and solid additives. We anticipated that adding pentobarbital–phenytoin solution would have a negative effect on palatability, so we evaluated cookie dough both with and without flavor additives. All flavors of cookie dough were well accepted by mice, with bacon-flavored cookie dough having the highest ingestion (mean, 94%). Many mice ingested the entire cookie dough bolus. Of these mice, latency to ingest was 30 min or less for all flavors tested. Plain sugar-cookie dough was ingested most rapidly, with a mean of 8.4 min before complete ingestion. According to these findings, cookie dough was considered an excellent potential vehicle for the oral delivery of euthanasia solution to mice.
Results of the palatability testing of cookie dough indicated that all flavors evaluated were well accepted. Mice ingested nearly all of the cookie dough relatively quickly. We therefore included all flavors in the next phase of testing, the evaluation of cookie dough to which pentobarbital–phenytoin solution had been added. When pentobarbital–phenytoin solution was added to the cookie dough, the amount eaten declined sharply. Despite the subeffective dose, clinical signs of pentobarbital–phenytoin intoxication occurred in 8 of the 40 single-housed mice tested under standard conditions. Two mice that ingested at least 180 mg/kg pentobarbital were notably affected: one mouse became lethargic and less reactive, whereas the other was severely ataxic. The remaining mice most commonly demonstrated mild to moderate ataxia, but no mouse lost the righting reflex, became unconscious, or died.
We considered 2 aspects of mouse behavior in the experimental design: social tendencies and nocturnal nature. Group-housed mice develop social hierarchies and may display dominance behaviors, and we considered that hoarding or gluttonous ingestion of a limited resource such as cookie dough might occur. However, the amount of pentobarbital–phenytoin-containing cookie dough ingested was lower in pair-housed mice for all varieties except bacon-flavored cookie dough, of which the paired mice ingested more. Although unexpected, this result may reflect empathy in the mice. Studies have found that mice show empathy toward other mice when they are familiar with that mouse, but mice do not have empathy toward unfamiliar mice.22,26 We tested pairs of mice in addition to single mice to determine whether social condition affected the amount of pentobarbital ingested. All pairs of mice in our study were familiar to each other, because they had been housed together for at least 3 wk prior to the experiment. As clinical signs took effect, the observing mouse may have changed its behavior, effectively reducing the intake of cookie dough. To determine whether the nocturnal nature of mice affected ingestion of cookie dough, we gave sugar-cookie dough containing pentobarbital–phenytoin solution to single-housed mice at 30 min after the beginning of the dark phase and found that the mean dose of pentobarbital ingested was nearly twice the amount ingested during the light phase. However, only 2 of these 10 mice became ataxic.
Studies of the efficacy and pharmacokinetics of oral pentobarbital have used fasted animals;13,25,33 however the majority of mice in the present study were not fasted prior to experimental use, because we wanted to simulate conditions under which an oral euthanasia drug might be used. After oral administration, pentobarbital undergoes pronounced intestinal metabolism,25 which might be affected by stomach ingesta. Variability in stomach content volume could account for the observed differences in clinical signs in mice ingesting similar pentobarbital dosages. To determine the effects of stomach ingesta on clinical efficacy, we tested one group of mice after a 16-h fast. All 10 mice in that group ingested some or all of the cookie dough containing pentobarbital–phenytoin, and all mice developed subsequent clinical signs of intoxication. Although all of these mice became ataxic, none died as a result of the cookie dough ingestion.
United States regulations for extralabel drug use and compounding drugs limit prescribers’ options regarding formulating an oral euthanasia agent for use in mice. The Animal Medicinal Drug Use Clarification Act permits extralabel drug use under specific conditions.18 However, drugs that are compounded from unapproved drugs or bulk drugs are considered adulterated and may not be administered.18 We chose Euthasol as our euthanasia agent because it is an approved drug and can therefore be compounded legally. Although the addition of Euthasol decreased the palatability of cookie dough, an oral euthanasia drug compounded from bulk pentobarbital likely would be better accepted by rodents, given that Euthasol contains as much as 10% ethyl alcohol and as much as 10% benzyl alcohol in its formulation.35 The compounding of animal drugs from bulk ingredients is currently illegal in the United States, although the FDA recognizes that doing so may be appropriate in limited situations. This topic is under active consideration by the FDA, and further guidance is expected.17 Additional research is needed to evaluate oral compounds containing bulk-sourced pentobarbital, as well as other anesthetic or barbiturate compounds that might be effective for euthanasia.
We identified several limitations in this study. The poor palatability of the pentobarbital–phenytoin solution limited its ingestion by mice, and mice therefore ingested a sublethal dose. We were therefore unable to fully evaluate its use as an oral euthanasia drug. Another limitation was that assessing mice for gait abnormalities such as mild ataxia is highly subjective, and more specific assessments for clinical effects are needed. The person who performed these assessments was not blinded to the procedures, and unintentional bias might have occurred. Finally, it was sometimes difficult to collect all of the remaining cookie dough bolus from the bedding at the conclusion of the testing period, and this difficulty may have affected the measurement of amount ingested.
We successfully identified a drug vehicle that consistently was readily and rapidly eaten by mice without the need for previous exposure or acclimation. We consider the use of sugar-cookie dough as a carrier for an oral euthanasia drug to be a prudent choice. However, we failed to identify a method for presenting a pentobarbital-containing euthanasia solution that resulted in voluntary ingestion and subsequent death in mice. The results of this study indicate that Euthasol is distasteful to mice, thus precluding its voluntary ingestion in amounts sufficient to cause death. In the few instances in which enough agent was ingested to yield doses sufficient to cause death of rats, mouse awareness showed little effect. We found that mice ingested higher dosages of pentobarbital during the dark phase and after a 16-h fast and therefore recommend further evaluation of voluntarily ingested oral euthanasia agents under these conditions. Future research also is needed to identify alternative compounds that might be useful as oral euthanasia agents in mice.
Acknowledgments
The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
References
- 1.Abelson KSP, Jacobsen KR, Sundbom R, Kalliokoski O, Hau J. 2012. Voluntary ingestion of nut paste for administration of buprenorphine in rats and mice. Lab Anim 46:349–351. [DOI] [PubMed] [Google Scholar]
- 2.American Veterinary Medical Association. [Internet] 2002. Euthanatized animals can poison wildlife: veterinarians receive fines. [Cited 02 May 2017]. Available at: https://www.avma.org/News/JAVMANews/Pages/s011502d.aspx. [PubMed]
- 3.Animal and Plant Health Inspection Service. [Internet] 2017. §2.31(d)(1)(i) (2013). Institutional animal care and use committee (IACUC). p 56–60. [Cited 10 May 2017]. Available at: https://www.aphis.usda.gov/animal_welfare/downloads/ AC_BlueBook_AWA_FINAL_2017_508comp.pdf. [Google Scholar]
- 4.Arras M, Rettich A, Seifert B, Kasermann HP, Rulicke T. 2007. Should laboratory mice be anaesthetized for tail biopsy? Lab Anim 41:30–45. [DOI] [PubMed] [Google Scholar]
- 5.Beausoleil NJ, Mellor DJ. 2014. Introducing breathlessness as a significant animal welfare issue. N Z Vet J 63:44–51. [DOI] [PubMed] [Google Scholar]
- 6.Boivin GP, Bottomley MA, Schiml PA, Goss L, Grobe N. 2017. Physiologic, behavioral, and histologic responses to various euthanasia methods in C57BL/6NTac male mice. J Am Assoc Lab Anim Sci 56:69–78. [PMC free article] [PubMed] [Google Scholar]
- 7.Bosshard G, Jermini D, Eisenhart D, Bar W. 2003. Assisted suicide bordering on active euthanasia. Int J Legal Med 117:106–108. [DOI] [PubMed] [Google Scholar]
- 8.Brandt-Casadevall C, Krompecher T, Giroud C, Mangin P. 2003. A case of suicide disguised as natural death. Sci Justice 43:41–43. [DOI] [PubMed] [Google Scholar]
- 9.Cantrell FL, Nordt S, McIntyre I, Schneir A. 2010. Death on the doorstep of a border community—intentional self-poisoning with veterinary pentobarbital. Clin Toxicol (Phila) 48:849–850. [DOI] [PubMed] [Google Scholar]
- 10.Cinelli P, Rettich A, Seifert B, Burki K, Arras M. 2007. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim 41:174–184. [DOI] [PubMed] [Google Scholar]
- 11.Concas A, Sanna E, Cuccheddu T, Mascia MP, Santoro G, Maciocco E, Biggio G. 1993. Carbon dioxide inhalation, stress and anxiogenic drugs reduce the function of GABAA receptor complex in the rat brain. Prog Neuropsychopharmacol Biol Psychiatry 17:651–661. [DOI] [PubMed] [Google Scholar]
- 12.Corbett A, McGowan A, Sieber S, Flannery T, Sibbitt B. 2012. A method of reliable voluntary oral administration of a fixed dosage (mg/kg) of chronic daily medication to rats. Lab Anim 46:318–324. [DOI] [PubMed] [Google Scholar]
- 13.Curd-Sneed CD, Bordelon JG, Parts KS, Stewart JJ. 1987. Effects of activated charcoal and sorbitol on sodium pentobarbital absorption in the rat. J Toxicol Clin Toxicol 25:555–566. [DOI] [PubMed] [Google Scholar]
- 14.Diogo LN, Faustino IV, Afonso RA, Pereira SA, Monteiro EC, Santos AI. 2015. Voluntary oral administration of losartan in rats. J Am Assoc Lab Anim Sci 54:549–556. [PMC free article] [PubMed] [Google Scholar]
- 15.Flecknell PA, Roughan JV, Stewart R. 1999. Use of oral buprenorphine (‘buprenorphine jello’) for postoperative analgesia in rats—a clinical trial. Lab Anim 33:169–174. [DOI] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. [Internet] 2017. FDA cautions pet owners and caretakers not to feed certain Evanger's or Against the Grain canned pet foods. [Cited 02 May 2017]. Available at: https://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm542265.htm.
- 17.Food and Drug Administration. [Internet] 2017. FDA announces withdrawal of draft guidance for industry #230 regarding animal drug compounding. [Cited 11 December 2017]. Available at: https://www.fda.gov/AnimalVeterinary/NewsEvents/ CVMUpdates/ucm580525.htm.
- 18.Food and Drug Administration. [Internet] 2017. Resources for you: the ins and outs of extralabel drug use in animals: a resource for veterinarians. [Cited 30 March 2017]. Available at: https://www.fda.gov/animalveterinary/resourcesforyou/ucm380135.htm.
- 19.Fucci V, Monroe WE, Riedesel DH, Jackson LL. 1986. Oral pentobarbital intoxication in a bitch. J Am Vet Med Assoc 188:191–192. [PubMed] [Google Scholar]
- 20.Giroud C, Augsburger M, Horisberger B, Lucchini P, Rivier L, Mangin P. 1999. Exit association-mediated suicide. Am J Forensic Med Pathol 20:40–44. [DOI] [PubMed] [Google Scholar]
- 21.Goldkuhl R, Hau J, Abelson KSP. 2010. Effects of voluntarily ingested buprenorphine on plasma corticosterone levels, body weight, water intake, and behavior in permanently catheterized rats. In vivo 24:131 –135. [PubMed] [Google Scholar]
- 22.Gonzalez-Liencres C, Juckel G, Tas C, Friebe A, Brune M. 2014. Emotional contagion in mice: the role of familiarity. Behav Brain Res 263:16–21. [DOI] [PubMed] [Google Scholar]
- 23.Gregory NG. 2004. Stress. p 12–21. In: Kirkwood JK, Hubrecht RC, Roberts EA. Physiology and behavior of animal suffering. Oxford (United Kingdom): Blackwell Publishing. [Google Scholar]
- 24.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 25.Knodell RG, Spector MH, Brooks DA, Keller FX, Kyner WT. 1980. Alterations in pentobarbital pharmacokinetics in response to parenteral and enteral alimentation in the rat. Gastroenterology 79:1211–1216. [PubMed] [Google Scholar]
- 26.Langford DJ, Crager SE, Shezhad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. 2006. Social modulation of pain as evidence of empathy in mice. Science 312:1967–1970. [DOI] [PubMed] [Google Scholar]
- 27.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R, [Internet] 2013. AVMA guidelines for the euthanasia of animals: 2013 ed. [Cited 30 March 2017]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf. [Google Scholar]
- 28.Meijer MK, Lemmens AG, Van Zutphen BF, Baumans V. 2005. Urinary corticosterone levels in mice in response to intraperitoneal injections with saline. J Appl Anim Welf Sci 8:279–283. [DOI] [PubMed] [Google Scholar]
- 29.Melo P, Costa P, Quintas MJ, Castro A, Tarelho S, Franco JM, Teixeira HM. 2017. Pentobarbital in the context of possible suicides: analysis of a case. Forensic Sci Int 274:109 –112. [DOI] [PubMed] [Google Scholar]
- 30.National Institutes of Health Office of Animal Care and Use. [Internet] 1985. US government principles for the utilization and care of vertebrate animals used in testing, research, and training. [Cited 10 May 2017]. Available at: https://grants.nih.gov/grants/olaw/references/PHSPolicyLabAnimals.pdf
- 31.Nitschke P, Stewart F. 2009. Drug options, p 152–223. The peaceful pill handbook. Bellingham (WA): Exit International USA. [Google Scholar]
- 32.Plumb DC. 2015. Plumb's veterinary drug handbook, 8th ed. Stockholm (WI): PharmaVet. [Google Scholar]
- 33.Ramsay EC, Wetzel RW. 1998. Comparison of 5 regimens for oral administration of medication to induce sedation in dogs prior to euthanasia. J Am Vet Med Assoc 213:240–242. [PubMed] [Google Scholar]
- 34.Siswanto H, Hau J, Carlsson HE, Goldkuhl R, Abelson KSP. 2008. Corticosterone concentrations in blood and excretion in faeces after ACTH administration in male Sprague–Dawley rats. In Vivo 22:435 –440. [PubMed] [Google Scholar]
- 35.Virbac 2016. Safety data sheet. Euthasol euthanasia solution, Australia: Chemwatch. [Google Scholar]