Abstract
This study presents recommendations for intramuscular injection into the caudal thigh muscle of mice according to analysis of in vivo imaging of intramuscularly injected iohexol, a radiocontrast agent commonly used in CT imaging. An experienced laboratory animal technician using a Hamilton syringe intramuscularly injected iohexol into isoflurane-anesthetized female and male BALB/c mice. Injected volumes (25, 50, 100, and 200 µL) underwent CT scanning at 9 time points over a 3-h period. The distribution of the injectate in the muscles of the rear leg was examined over time for each volume group. Results indicated that 25- and 50-µL volumes remain intramuscularly. At 100 µL, mild to moderate leakage into the extramuscular tissues occurred. At 200 µL, leakage into the extramuscular tissues was moderate to severe. Our results suggest volumes of 50 µL or less are recommended for the caudal thigh muscles of mice when intramuscular pharmacokinetics are needed; volumes greater than 50 µL display variable distribution into extramuscular tissues, thus potentially yielding different pharmacokinetic profiles.
Abbreviations: HU, Hounsfield units; ROI, region of interest
Intramuscular injection is a common route of administration for drugs and other substances in veterinary and human medicine. In rodent species, such as mice, intramuscular injection can be technically challenging, due to the small muscle mass available for injection. Although the intramuscular route of administration has been used for nearly 150 y, we still know little about this route.33 This is especially true in smaller rodent species, particularly in regard to the optimal administration volume for efficient dose delivery.
In mice, the thigh muscle, because of its relatively large size, is the most common route for intramuscular administration. The thigh muscles (defined for this study as distal to the gluteal muscles and proximal to the stifle) consist of 17 muscles, ranging from the thick rectus femoris to the thin strap-like semimembranosus muscle.20 We selected the caudal thigh muscle for the current study in light of common clinical practice: nonsedated mice can be manually restrained routinely for intramuscular injection to the caudal portion of the thigh. The caudal thigh muscles constitute roughly half of all the thigh muscles and are smaller strap-like muscles, compared with the cranial thigh muscles.
Numerous guidelines have been developed in terms of the best practice or upper limit volumes that can be administered to research animals by various routes, including intramuscular.9,10,38,15,26,35,36 These volume recommendations range from 1.5 to 100 µL for a 30-g mouse.9,15,26,33,36 With the exception of rats, most of the volumes recommended for small rodent species rely on expert opinion, common practice, or extrapolation from other species. These guidelines serve as a good starting point; however, an optimal intramuscular volume has not been definitively determined in mice.
Subtle differences in anatomy between species in addition to the nonlinear scaling of physiologic processes across the range of sizes of mammals may lead to over- or underdosing of animals, and the consequences to research can be profound. For example, when using an injected compound to study retrograde transport from the motor endplate of a muscle to the nerve cell bodies, any leakage or accidental delivery to surrounding tissue would lead to erroneous conclusions regarding how many and which nerve cells serve the target muscle.19 In the case of drug administration, accidental delivery to nontarget tissue might result in overdosage and associated toxicity if the substance were absorbed rapidly from the nontarget tissue or in underdosage with diminished or no effect if the nontarget tissue sequestered the compound and prevented absorption. In addition, studies in rats have demonstrated that the rate of absorption after intramuscular injection of a given substance is profoundly affected by the volume of the injectate, with both small and large volumes causing a delay in absorption.32
The intramuscular route of delivery is frequently used in biomedical research as a means of delivering anesthetics, analgesics, antibiotics, and infectious agents in addition to the delivery of test articles during preclinical testing for those compounds intended for intramuscular use in humans. Differences in the accuracy of delivery or rate of systemic absorption of substances delivered through intramuscular injection could lead to erroneous or misleading findings in a study or might complicate interpretation regarding the efficacy of a given therapeutic in human patients. For these reasons, it is essential to evaluate the range of volumes that might be administered intramuscularly to common laboratory species and how these volumes affect the accuracy of delivery and systemic absorption of injected compounds.
In this study, we administered intramuscular injections into the caudal thigh muscles of mice to determine which volumes could be delivered reliably, without inadvertent delivery or leakage into adjacent tissue compartments. Mice were injected intramuscularly with iohexol, a common, aqueous CT contrast agent, in a range of volumes that might be encountered in a research setting (25, 50, 100, 200 µL). Using in vivo CT imaging, we dynamically assessed the temporal biodistribution of the delivered volumes of iohexol in muscle. We hypothesized that as the injection volume increased, so would the risk of distribution of the injectate beyond the intended target tissue and of delayed systemic absorption from the muscle. The objectives of this study were limited to analysis of local tissue distribution. The results of this study lend support for, or allow for the better refinement of, the current guidelines for intramuscular injection volume for this commonly used species.
Materials and Methods
Animals.
BALB/c mice (age, 10 to 16 wk; weight, 17 to 30 g; 24 male and 24 female) were obtained from Charles River Laboratories (Frederick, MD) and used for this study. All procedures were approved by the United States Army Medical Research Institute of Infectious Diseases IACUC.
Housing parameters were in accordance with the Guide for the Care and use of Laboratory Animals,16 in an AAALAC-accredited facility. All mice were socially housed in same-sex groupings of 10 in solid-bottom polycarbonate cages (Allentown Caging, Allentown, NJ; Lab Products, Seaford, DE), which were either individually ventilated or fitted with a static filter-top. Mice were fed a pelleted diet (no. 2018 Teklad Global 18% Protein, Envigo, Frederick, MD) and municipal water (no further treatment) was provided without restriction. Food enrichment items, such as forage mix consisting of grain-based dry cereal and dry oatmeal, were provided weekly along with occupational enrichment in the form of compressed cotton squares (Nestlets, Ancare, Bellmore, NY) and paper nesting material (Enviro-Dri, Shepherd Specialty Papers, Watertown, TN). The cage bedding was cellulose (7070C Teklad Certified Diamond Dry Cellulose Bedding, Envigo, Indianapolis, IN). The room temperature range was maintained at 68 to 79 °F (20.0 to 26.1 °C) with a set point of 74.5 °F (23.6 °C), and relative humidity was kept between 30% to 70% on a 12:12-h light:dark cycle.
Serum samples from dirty-bedding sentinel mice were tested quarterly (VRL, Rockville, MD) for mouse parvovirus, mouse hepatitis virus, Clostridium piliforme, Theiler murine encephalomyelitis virus, epizootic diarrhea of infant mice, and Sendai virus. Additional quarterly testing included gross necropsy exams, PCR analysis of feces for Helicobacter spp., perianal tape testing for endoparasites, and fur plucks examination for ectoparasites. Once yearly, additional serology testing included pneumonia virus of mice, reovirus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus types 1 and 2, ectromelia, K virus, and polyoma virus and a complete necropsy with histopathology examination by a veterinary pathologist. All testing was negative throughout this study.
Drugs and materials.
Iohexol (240 mg/mL, Omnipaque 240, GE Healthcare, Chicago, IL) is an iodine-based radiopaque dye commonly used in radiography and CT imaging studies and is labeled for intravenous use. We chose this radiocontrast agent for off-label intramuscular use because it is sufficiently concentrated to produce reliable imaging results at low volumes (25 µL) and, due to various characteristics (pH, 6.8 to 7.7; osmolality of 520 mOsm/kg H2O), it is less likely to cause irritation than other contrast agents.1
Hamilton syringes and needles were used for all intramuscular injections. The small volumes we used could not accurately be achieved by using standard luer-tip or luer-lock 1-mL syringes fitted with 25-gauge hypodermic needles. To ensure accuracy and repeatability, specialty syringes (no. 7639-01, 725RN) and needles (no. 7806-03, RN, 26-gauge, 1 in., 12° bevel) were purchased (Hamilton, Reno, NV). This model of syringe is accurate in 5-µL increments. The syringes and needles were cleaned and autoclaved according to the manufacturer's recommendations.
Intramuscular injection of iohexol.
All mice received an intramuscular injection to the caudal thigh muscles. To prevent variation due to injection technique, a single, experienced veterinary technician performed the intramuscular injections for all mice. Mice were anesthetized with isoflurane (5% induction, 1% to 2% maintenance) delivered in oxygen by using an anesthetic vaporizer (Integrated Multi-Patient Anesthetic Center [IMPAC6], VetEquip, Piney River, VA). Once anesthetized, mice were weighed individually and placed sternally on a table top with a nose cone supplied with 1% to 2% isoflurane gas to maintain anesthetic depth. While anesthetized, each mouse underwent tail marking for identification, had the right pelvic leg shaved, and the site on the caudal thigh that was intended for intramuscular injection was circled in permanent marker. Once a mouse was prepared for injection, the technician advanced the needle through the skin, within the marked circle, and into the target muscle to a depth of approximately 2 to 4 mm. The syringe plunger was aspirated to check for inadvertent placement within a blood vessel, and then the injection was delivered.
Preparation for imaging.
The following text outlines the basic procedures used to prepare mice for scanning throughout this study. Once a mouse was injected intramuscularly with iohexol, it was positioned into the imaging trough and maintained on isoflurane (1% to 2%) mixed in oxygen via a nose cone. Mice were maintained under isoflurane anesthesia for the duration of the study (180 min) and were not moved or recovered between CT scans. During all imaging procedures, animal respiration rate and body temperature were monitored and maintained (M2M-BioVet Small Animal Physiology Monitoring System, M2M Imaging, Cleveland, OH). Animal warming was achieved by using a programmable electric heating element set to 40 °C and placed under the animals being scanned, and respirations were monitored by using a respiration pillow placed under each mouse; the movements of the animal were translated into a digital signal, which was displayed on the physiology monitoring system monitor. Isoflurane anesthesia was adjusted throughout the imaging session such that mice remained at 25 and 55 respirations per minute. The same 2 people positioned all mice for scanning throughout the study. Three mice were scanned during each session.
CT imaging.
We randomized the 48 mice into 4 groups (1 group per injection volume [25, 50, 100, and 200 µL]) and tracked them over time. After data analysis had begun, 2 mice were excluded, yielding a final sample size of 46 mice. In particular, all of the data generated from one mouse in the 100-µL group were excluded due to excessive movement from inadequate anesthesia which prevented uniform image analysis. In addition, all of the data generated from one mouse in the 200-µL group were excluded because the mouse died from anesthetic overdose and did not complete the full series of scans. The 25- and 50-µL groups each comprised 6 male and 6 female mice; the 100- and 200-µL groups each consisted of 6 male and 5 female mice. Mice were given intramuscular injections of iohexol and positioned for CT imaging as described earlier. Once a cohort of 3 mice was positioned at the start of a study, a short acquisition scan was completed to ensure correct placement in the scanner. The first mouse in a set of 3 that was injected defined as time 0. The first CT scan occurred at 10 min, meaning that it took no longer than 10 min to inject and position 3 mice and complete an acquisition scan prior to the start of study scans at 10 min after injection. Nine CT scans were completed for each group (10, 20, 30, 45, 60, 90, 120, 150, and 180 min after iohexol administration). When scanning was completed, mice were euthanized by carbon dioxide asphyxiation followed by cervical dislocation, in accordance with the AVMA Guidelines for the Euthanasia of Animals.22
CT image acquisition and reconstruction.
Each scan consisted of a single, approximately 4.5 min static frame (80 kV, 500 µA, 98 µm, 360° rotation in 220 steps) on a CT imaging system (Inveon, Siemens Medical Solutions, Knoxville, TN). All CT images were reconstructed into a 3D volume with 512-µm isotropic resolution by using the Inveon Acquisition Workplace version 2.0 software package (Siemens). Hounsfield unit (HU)-calibrated CT data were reconstructed by using a Feldkamp reconstruction algorithm, with a Shepp–Logan reconstruction filter, slight noise reduction, and beam-hardening correction applied.
Image analysis.
CT.
CT data were analyzed by using image processing software (VivoQuant version 2.5, inviCRO, Boston, MA) and results were reported in terms of mean HU per region of interest (ROI). For CT image analysis, the distribution and intensity of the iohexol signal were examined to determine tissue distribution over time for each volume group. Signal intensity was determined by quantifying mean HU within each ROI over time. Initial injection-volume ROI were automatically and objectively defined by placing a seed point within the hyperintense injection site on the first (time, 10 min) image for each mouse and then letting the analysis software define the borders of the injection volume by using a lower-limit thresholding algorithm that includes voxels into the ROI when they are contiguous with the seed point and have a value at or above 2 times muscle background plus 2 SD. After initial injection-volume ROI were defined, computed intramuscular injection volumes were within 10% of the known initial intramuscular injection volumes for all except 2 mice (1 each in the 50- and 100-µL groups), whose computed ROI injection volumes were within 15% of the known volume. Pelvic limb ROI were manually drawn on the last image in the time series (180 min) to include the hyperintense region of distributed iohexol within the pelvic limb and surrounding tissues. Pelvic limb ROI were drawn by 2 experienced data analysts, and each analyst confirmed the quality and consistency of the other analyst's ROI. Once completed, ROI for the 10- and 180-min time points were applied to each time point in the 9-image series, and average HU intensity was determined for each ROI over the duration of the study. Leakage of HU-intense iohexol away from the initial intramuscular injection site was measured as increases in mean HU intensity in the surrounding pelvic limb ROI over time.
To determine the amount of leakage from the initial intramuscular injection site and into potentially different surrounding tissue compartments, we considered many methods, understanding that no single method was perfect. We ultimately decided that the best way to capture leakage from the initial injection site was to define ROI for both the initial injection site (time, 10 min; illustrated as the blue ROI in Figure 1) and the final surrounding pelvic limb (time, 180 min; the yellow ROI in Figure 1). To measure leakage, the final ROI excluded the initial ROI HU value, to quantify changes in HU intensity across time. Increases in CT-measured HU value in the final ROI were interpreted as leakage of HU-intense iohexol from the injection site (blue ROI) into the surrounding tissue. For the larger injection volumes (100 and 200 µL), leakage from the injection site into adjacent tissues created pockets. These pockets (Figure 1) didn't exist prior to the large-volume injection and developed slowly over the 180-min course of the study. Taking this pocket formation into consideration, we decided that the most objective way to analyze the data and to best capture any leakage out of the initial intramuscular injection site was to create a final pelvic limb ROI that was based on the last time point (t = 180 min) for every animal, regardless of injection volume. Drawing the pelvic limb ROI on the final image was necessary to capture the pockets that didn't exist at the earlier time points and that swelled only as HU-intense iohexol leaked from the injection site and into the adjacent surrounding tissue (Figure 1). The pelvic limb ROI that was drawn according to the 180-min image for each mouse was then applied to all of the other imaging time points (10, 20, 30, 45, 60, 90, 120, and 150 min), to capture any leakage into this space throughout the study.
Figure 1.
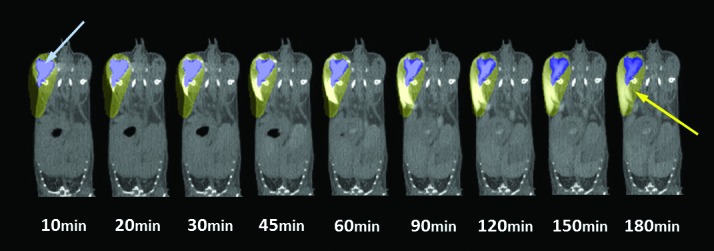
Region of interest (ROI) analysis. To quantify intramuscularly injected iohexol leakage from the injection site over time, 3D ROI were drawn for each mouse over the initial injection volume (blue arrow; blue ROI) at the beginning of the scan (10-min time point) and for the full pelvic limb region (yellow arrow; yellow ROI) at the end of the scan (180-min time point), to ensure that any leakage from the injection site over time is accounted for. For the purposes of this analysis, the volume of the yellow pelvic limb ROI does not include that of the blue, initial injection volume ROI. ROI were applied to each time point in the series, and average HU intensity in the pelvic limb ROI was determined for the duration of the study. The leakage of intramuscularly injected iohexol from the initial injection site over time is associated with increasing average HU intensity in the pelvic limb (yellow) ROI over time.
Clinical leakage assessment.
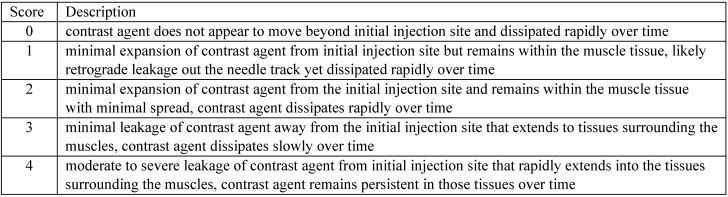
Once images were reconstructed, a slide deck consisting of the 9 serial CT scans was created for each mouse and placed in a time loop to act as a visual means to determine iohexol tissue distribution in and away from the initial site of injection. The 9 CT scans were sequentially compiled to compress the 180-min timeline of iohexol tissue distribution into 9 static images that were played in rapid succession. Each slide deck was placed in a time loop set to cycle repeatedly from 10 to 180 min over the course of 0.72 s, effectively creating a video image. Time-looped slide decks were randomized and compiled into a single, comprehensive slide deck. To test interrater variability in assessing the degree of iohexol leakage or tissue distribution in the injection site, the comprehensive slide deck was presented to 18 blinded veterinarian raters for scoring according to defined criteria (Figure 2). Raters received a visual example of each score by placing synchronized time-looped slide decks side by side for comparison (one time-looped slide deck for each score, 0 to 4). To test intrarater agreement, 22 individual mouse time-looped slide decks were repeated and randomly inserted into the compiled slide deck, for a total of 68 time-looped slide deck images (46 original slide decks with 22 repeat slide decks) for scoring. Raters scored all 68 time-looped slide decks at their own pace but completed scoring the comprehensive slide deck in a single session.
Figure 2.
Clinical leakage assessment scores and their descriptions were used to score 68 individual time-looped slide decks (46 original, 22 repeats).
Statistical analysis.
The association between injection volume and the resulting disparity in signal intensity between the initial and final pelvic limb ROI was analyzed. For each animal, an AUC was computed over the entire study period by application of a trapezoid rule quadrature. The mean AUC was compared across dose groups by one-way ANOVA. The effect of animal weight was analyzed by fitting the linear regression model having continuous predictors of volume, weight, and the product of these factors. All regression models included adjustment for the heteroscedastic residual to accommodate measures obtained at higher volumes that had greater variance than those obtained at lower injection volumes.
For each injection volume, image scores supplied by blinded veterinarians were summarized as the frequency and percentage of all scores having each of the 5 (0 to 4) possible values. The mean scores at each injection volume were estimated and compared by repeated-measures ANOVA, along with an estimate of the class correlation describing interrater reliability.28,29 Each veterinarian scored all available images. In addition, 22 of the images were reevaluated by each veterinarian under a novel ID, thus providing an estimate of the test–retest reliability of the scores, which was summarized by a weighted κ statistic.11 Only the first score obtained contributed to the subsequent analysis. Analysis was implemented in SAS version 9.4 (SAS Institute, Cary, NC).28
Results
Qualitative assessment of intramuscular iohexol leakage and tissue distribution.
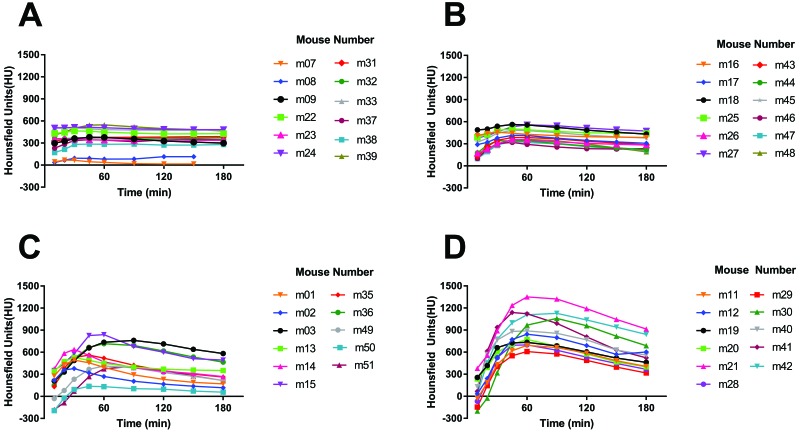
We injected 25, 50, 100, or 200 µL of iohexol (240 mg/mL) into the caudal thigh muscle of mice and tissue distribution was evaluated over a 180-min time course. For both the 25- and 50-µL groups, intramuscularly injected iohexol appeared to remain in the intramuscular space, because the injectate was absorbed centrally over time (Figure 3). For the 100- and 200-µL groups, intramuscularly injected iohexol appeared to leak from the injection site and into surrounding, extramuscular tissues. As the injection volume increased, the amount of iohexol that redistributed to surrounding tissues appeared to increase.
Figure 3.
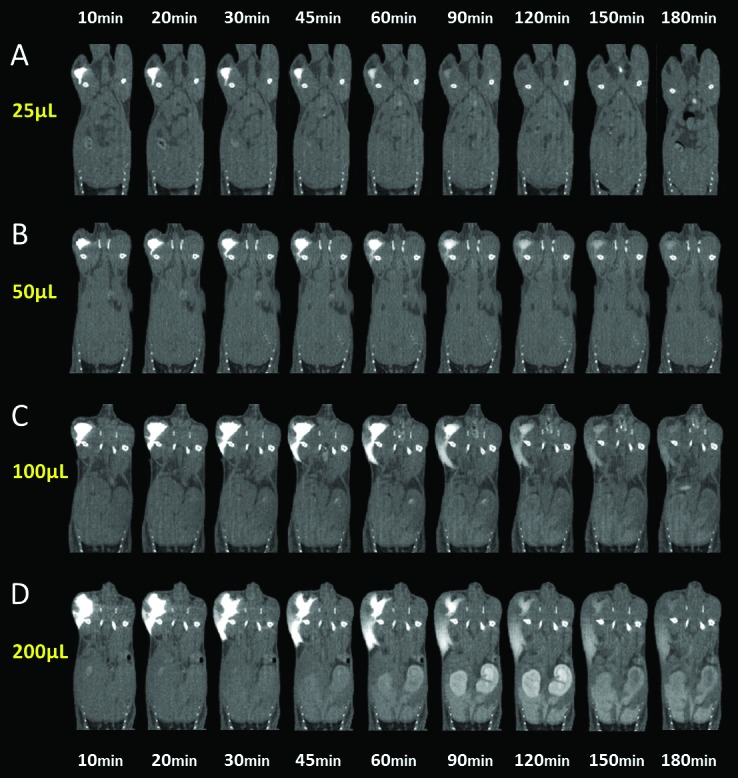
Iohexol (240 mg/mL; 25, 50, 100, or 200 µL) was injected intramuscularly into the caudal thigh muscles of mice, and tissue distribution was evaluated over time (10 through 180 min after injection). In the (A) 25-µL and (B) 50-µL injection groups, intramuscularly injected iohexol appears to remain exclusively in the intramuscular space throughout the study. In the (C) 100-µL and (D) 200-µL injection groups, a considerable portion of the intramuscularly injected iohexol appears to leak into the adjacent, extramuscular space over time. Representative animals from each volume group are shown.
Quantitative assessment of intramuscular iohexol leakage and tissue distribution.
To quantify the leakage of intramuscularly injected iohexol from the injection site in each mouse, 3D ROI were drawn over the initial injection volume at the beginning of the scan (time, 10 min after injection) and over the full pelvic limb region at the end of the scan (time, 180 min), to ensure that any leakage from the injection site over time was recorded (Figure 1). Leakage of HU-intense iohexol from the intramuscular injection site was measured as increases in mean HU intensity in the surrounding pelvic limb ROI over time. AUC analysis showed that as the initial intramuscular injection volume increased from 25 to 200 µL, mean HU intensity in the pelvic limb ROI increased over time (Figure 4). Although mean HU intensity in the pelvic limb increased slightly from 25 to 50 µL, the increase was not significant (P > 0.60) and the AUC for both volumes were similarly flat, indicating that the iohexol remained nearly exclusively intramuscularly at the injection site and did not markedly leak into the surrounding tissue over time (Figure 4 A and B; Table 1). Distribution of iohexol out of the initial target tissue did not differ significantly for the 25- and 50-µL volumes when compared with the 100-µL volume (P < 0.2 and 0.3, respectively), but variable distribution into extramuscular tissues is evident at 100 µL (Figures 3 C and 4 C). Distribution of iohexol in the initial target tissue differed significantly (P < 0.0003) for the 25-, 50-, and 100-µL volumes when compared with 200 µL (Table 1).
Figure 4.
These graphs depict the mean HU intensity in the pelvic limb ROI over time. Leakage of HU-intense iohexol from the initial intramuscular injection site is measured as increases in the mean HU intensity in the surrounding pelvic limb ROI over time. In the (A) 25-µL and (B) 50-µL injection groups, iohexol remains nearly exclusively intramuscularly at the injection site, as indicated by the relative lack of measureable increases in mean HU intensity into the surrounding pelvic limb ROI over time. In the (C) 100-µL and (D) 200-µL injection groups, considerable iohexol leakage from the initial injection site into the surrounding pelvic limb ROI is apparent.
Table 1.
Quantitative assessment of intramuscular iohexol leakage by AUC pairwise comparison analysis of volume groups with the 25µL group consisting of n= 12 (6 males, 6 females); the 50µL group consisting of n= 12 (6 males, 6 females); the 100µL group consisting of n= 11 (6 males, 5 females), and the 200µL group consisting of n= 11 (6 males, 5 females).
| Volumes (µL) compared | Increase in mean AUC (HU × min × 103) | 95% CI | P |
| 25 and 50 | 4.57 | −13.41 to 22.55 | 0.6001 |
| 25 and 100 | 14.11 | −7.27 to 35.5 | 0.1843 |
| 25 and 200 | 68.27 | 42.66 to 93.88 | <0.0001 |
| 50 and 100 | 9.54 | −8.48 to 27.57 | 0.2795 |
| 50 and 200 | 63.70 | 40.53 to 86.86 | <0.0001 |
| 100 and 200 | 54.16 | 28.55 to 79.77 | 0.0003 |
Clinical assessment of intramuscular iohexol leakage and tissue distribution.
We calculated the mean and standard error of the scores for each volume group, to quantify the subjective visual scores for the time-looped slide decks that were scored by blinded veterinarian raters. As injection volume increased, the score assigned by the raters also increased (Table 2). The mean score increased 1.09 levels (95% CI, 0.961 to 1.209) for each doubling of the injection volume. Interrater reliability quantified by intraclass correlation was 0.84 (95% CI, 0.786 to 0.901; Table 2); values near 1 indicate good agreement among raters. To determine intrarater reliability, 22 time-looped slide decks were repeated within the comprehensive slide deck. Intrarater reliability, a measure of how consistently a rater scored identical images, was 0.8503 (95% CI, 0.8219 to 0.8788).
Table 2.
Video analysis of 68 time-looped slide decks consisting of 46 original and 22 repeat slide decks
| Injection volume (µL) | |||||
| 25 | 50 | 100 | 200 | Overall | |
| No. (%) of ratings | |||||
| 0 (least severe) | 106 (49%) | 19 (9%) | 3 (2%) | 0 (0%) | 128 (15%) |
| 1 | 87 (40%) | 63 (29%) | 8 (4%) | 0 (0%) | 158 (19%) |
| 2 | 20 (9%) | 96 (44%) | 26 (13%) | 1 (1%) | 143 (17%) |
| 3 | 2(1%) | 37 (17%) | 115 (58%) | 31 (16%) | 185 (22%) |
| 4 (most severe) | 1 (0%) | 1 (0%) | 46 (23%) | 166 (84%) | 214 (26%) |
| Mean score (SE)a | 0.63 (0.137) | 1.71 (0.138) | 2.97 (0.145) | 3.83 (0.140) | |
| Mean change per 2-fold increase in injection volume (95% CI) | 1.09 (0.961, 1.209) | ||||
| Intraclass correlationb (95% CI) | 0.84 (0.786, 0.901) | ||||
| Intrarater agreementc (95% CI) | 0.8503 (0.8219, 0.8788) | ||||
Means, standard errors (SE), and regression parameters are estimated under linear mixed-effects model.
ICC measures interrater agreement; values near 1 indicate good agreement between raters.
cIntrarater agreement analysis conducted by weighted κ analysis demonstrated high fidelity in rater scoring (that is, raters agreed with themselves very well).
Assessment of intramuscular volume and weight.
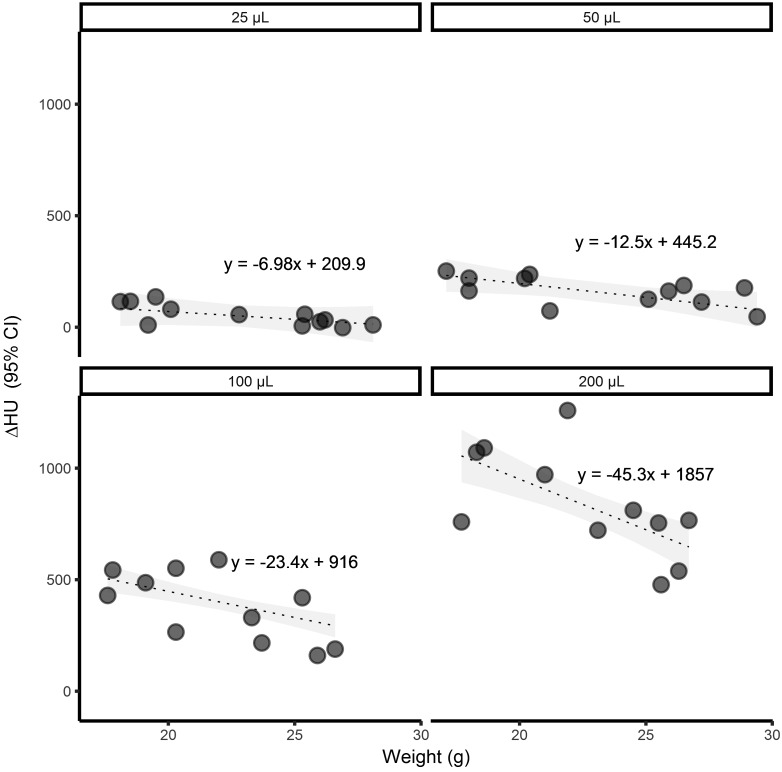
A linear regression model was used to determine whether weight influenced the amount of leakage per intramuscular volume. Leakage was determined by the relative change in HU over time. The effect of weight depended on the injection volume (P = 0.0054). The slope of each line was estimated to decrease by 2.2 HU/g (95% CI, 0.69 to 3.70) for each 10-µL increase in injected volume. As weight increased, the amount of leakage decreased across all volume groups (Figure 5).
Figure 5.
Effect of weight on change in pelvic limb ROI intensity according to injection volume. Dashed lines indicate level curves of the regression model Y = β1 × weight + β2 volume + β3 × weight × volume + α. The interaction between weight and volume effects is significant (P = 0.0054). The slope of the level curves decreased by an estimated 2.2 HU/g (95% CI, 0.68 to 3.70) for each 10-µL increase in the injected volume.
Discussion
Regarding the tissue distribution of an intramuscularly injected substrate, our findings suggest that the optimal volume for intramuscular injection of mice is 50 µL or less. The results of the analysis of the time-looped slide deck conducted by blinded veterinarian raters suggest that as the injection volume increased, extramuscular distribution increased. The AUC analysis revealed intramuscular injections of 200 µL do not remain in the target muscle tissue but instead distribute extramuscularly (Table 1). Extramuscular distribution did not differ significantly between 25 µL and 50 µL, 25 µL and 100 µL, or 50 µL and 100 µL (Table 1). Close examination of the 100-µL pelvic limb ROI plot (Figure 4 C) reveals how the signal intensity differed between individual mice (for example, compare m13—which had an absolute intensity change of 188 HU accompanied by a moderate rise in its curve before falling—with m51, which had an absolute intensity change of 589 HU and a steep rise), thus indicating variable amounts of extramuscular distribution of iohexol between mice. Clinically this lack of uniformity, although not statistically significant, should caution investigators to carefully consider the reliability of 100-µL intramuscular administration to a mouse caudal thigh muscle, given that the injected compound might variably distribute out of the muscle.
Mice are both the most common lab animal species used in research and one of the smallest. Consequently, even the large muscle groups—particularly the thin strap-like muscles that compose the caudal thigh muscle—are small in this species. The composition of the caudal thigh muscles needs to be considered, given the subtle differences between intramuscular injection compared with intermuscular injection. Clinically, the term ‘intramuscular injection’ is used synonymously with ‘intermuscular injection,’ despite the slight anatomic differences that distinguish these 2 injection approaches. In a true intramuscular injection, the needle is advanced into the belly of the muscle, and the injectate is delivered and contained within the muscle belly which is surrounded by fascia. A common side effect of intramuscular injection is retrograde leakage, or back flow, of the injectate along the needle track.14,19 This back flow may occur because the delivered injectate may exceed the elasticity of the muscle belly, which is confined by the surrounding fascia, resulting in retrograde flow along the needle tract to an area of lesser resistance. The primary mechanism for the absorption of injectate from muscle is vascular blood flow3 through the muscle belly perimysium. In intermuscular injection, the needle is advanced between a collection of muscle fibers that are surrounded by an epimysium and additional connective tissues, and the injectate is delivered in and around the epimysium of the muscle fibers. In intermuscular injection, the primary mode of injectate absorption is the lymphatic system, given that lymphatic tissue is more abundant in connective tissue2 than blood vessels. In intermuscular injection, the injectate can flow between facial planes and may distribute further than an intramuscular injection. Due to direct absorption into the bloodstream, injectate absorption is faster in intramuscular injection compared with intermuscular injection, which relies on passive diffusion of the lymphatic system for absorption.
From a clinical perspective, the number of areas appropriate for intramuscular injection in mice is limited. Although the large number of muscles makes the caudal thigh an appealing target for intramuscular injection, their strap-like nature makes mixed intermuscular–intramuscular injection likely, resulting in both vascular and lymphatic absorption. Perhaps that as the volume of the injection increased in our study, the amount of iohexol that was delivered intermuscularly increased, causing distribution of iohexol along and between fascial planes into the lymphatic compartment and ultimately into the subcutaneous compartment. This scenario may explain why iohexol persisted in the injected tissues for the duration of the 180-min CT scan in some of the 100-µL and most of the 200-µL studies.
Our study demonstrated that large volumes delivered intramuscularly display variable distribution within the caudal thigh tissues of mice. Moreover, in both the 100- and 200-µL volume groups, a portion of the iohexol dose remained within the caudal thigh for 180 min. Regarding tissue damage, intramuscular injections cause minimal tissue trauma due to the needle tract,34 whereas the properties of the injectate (pH, viscosity, temperature, and so forth) influence absorption at the site of injection17,18,37 and can cause additional tissue damage.4,12,30 Understanding the degree of pain and distress that a procedure might cause a lab animal is critical to implementing measures to mitigate that pain and distress. Although we did not evaluate the pain or distress our intramuscular injections might have caused, we speculate that the larger volumes (100 µL and 200 µL) might have induced some tissue damage at the injection site and thus caused pain or distress, however transiently, to the mice. Future studies are warranted to determine whether large intramuscular volumes cause pain or distress in mice. The rat paw-lick model has been used to study pain on injection associated with various parenteral compounds,5,7,13 and the rabbit lesion volume model has been used to measure muscle tissue damage from intramuscular administration of compounds.8,31 Classically, tissue damage has been determined through histopathology after completion of a study.8,24,27 A nonterminal method for assessing tissue damage that measures the serum creatinine kinase7,23 after intramuscular injection has been developed. This method might be used in mice to determine the degree of tissue damage due to the intramuscular injection of large volumes. From a behavioral standpoint, the mouse grimace scale21,25 might be used to measure pain or distress after large-volume intramuscular injections. In addition, other behavioral metrics, such as nest building, foraging, and presence or absence of other species-typical behaviors, could be assessed after injection to determine the effects of large-volume intramuscular injections on mice.
As the weight of the mouse increased, the amount of iohexol that distributed out of the initial injection site decreased across all volume groups (Figure 5). This outcome is understood intuitively, given that increasing weight implies greater muscle mass. As muscle mass increases, the injected compound has more muscle to traverse before it becomes extramuscular, thereby reducing the amount of leakage appreciated in heavier animals. Conversely, lighter animals have less muscle mass and experience more leakage from the injection site.
Although the small sample size precluded statistical analysis of a sex-associated effect, note that 20 of the 22 female mice weighed less than 22 g, whereas 22 of the 24 male mice weighed more than 22 g. This sex-associated difference in weight is expected and can be appreciated by reviewing the BALB/c growth chart.6 According to this chart, 15-wk-old female mice weigh 22 g maximally whereas 15-wk-old males weigh at least 22 g. If you apply the 22-g weight criterion to our data, approximately 90% of the data points at 22 g or less are female, and 91% of the data points above 22 g are from male mice. This pattern suggests weight and sex are closely linked in BALB/c mice and that lighter female mice experience more leakage from intramuscular injection sites than do heavier male mice.
Some readers may wonder why the initial HU values for the larger intramuscular injection volumes (100 and 200 µL) are much lower (Figure 4 C and D) than those for the smaller volumes (25 and 50 µL). The reason lies in the methodology we used to analyze the CT images and quantify leakage. The smaller volume groups (25 and 50 µL) had little or no iohexol leakage from the injection site and therefore few or no adjacent pockets were created. As we mentioned in the Methods section, the final pelvic limb ROI for mice in the 100- and 200-µL groups were based on the 180-min data. This ROI volume included all of the initial pelvic limb ROI plus that for the subcutaneous pocket that formed as HU-intense iohexol leaked from the injection site and into the subcutaneous pocket (Figure 1, yellow ROI, 180-min time point). When the resulting ROI is applied to the initial (10-min time point) image, a large part of this final pelvic limb ROI captures air, because the leakage from the larger intramuscular injection volume has yet to occur (Figure 1, blue ROI, 10-min time point). The HU value for air is –1000, distilled water has a defined HU value of 0, and tissue (mostly water, but also cells and bone) can range from >0 to approximately 3000. When we then calculate the average HU intensity in the pelvic limb ROI for the earlier time points of the larger-volume groups, we thus include numerous negative (–1000) values, resulting in an initial pelvic limb ROI with an average HU intensity of –300 to 300 HU. In the smaller volume groups without noteworthy leakage or pocketing, the 180-min pelvic limb ROI is nearly the same size and shape (and volume) of that at 10 min. Consequently, little or no air is captured even at the earliest time points (because there is little or no leakage) in the 25- and 50-µL groups, resulting in initial average HU values for these ROIs of 0 to 600 HU because they incorporate no –1000 (air) values.
One possible limitation of our study was the delay between injection and the initiation of CT scanning. Our timeline for CT scans started at 10 min after injection, to allow sufficient time to anesthetize, inject, and position 3 mice for a CT scan. As we became more efficient throughout the study, we needed much less time to prepare mice. Rather than change our start time, we chose to preserve consistency between groups and keep the first CT scan at 10 min rather than change the procedural paradigm in the middle of the study. This delay might have affected our data by overestimating the initial ROI we obtained at the 10-min time point. Had scanning started at 1 min after injection, we might have captured a more accurate value for the initial iohexol injection. If the initial measured iohexol injection volume (ROI) was smaller at 1 than 10 min, the disparity between the initial and final ROI might have increased in all volume groups tested, thereby further defining the significance of intramuscular and extramuscular distribution over time.
Another possible limitation was the choice of contrast agent. The form of iohexol we used in this study (Omnipaque 240) was hyperosmotic (520 mOsm/kg of water; plasma osmolality is 285 mOsm/kg), thus creating the potential for osmotic disparity in the local tissues. This disparity might have artifactually increased the amount of fluid redistribution. Second-generation nonionic monomer contrast agents such as iohexol are not highly osmotoxic,1 suggesting that using iohexol would not dramatically affect the data obtained in this study. We feel that the use of Omnipaque 240 does not negate the findings of this study.
In conclusion, when an injectate requires intramuscular distribution (such as vaccines and anesthetics), the volume of the injection delivered to the caudal thigh muscles of mice should be considered carefully. Our findings support our initial hypothesis: as intramuscular injection volume increased, distribution of the injectate outside of the target tissue also increased. We injected mice with 25, 50, 100, and 200 µL of iohexol and tracked the distribution of iohexol over time. Both the 25- and 50-µL volumes remained within the target muscle tissue, whereas 100 µL variably distributed and 200 µL markedly distributed into extramuscular tissues. Future studies should further refine intramuscular volume capacities and their potential for pain or tissue damage in mice and other commonly used laboratory animals.
Acknowledgments
We thank Ms. Nicole Roberts and Mr. Robert Stafford for their technical expertise in animal handling and imaging data collection and processing.
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the US Army. Research was conducted under an IACUC-approved protocol in compliance with the Animal Welfare Act, PHS Policy, and other Federal statutes and regulations relating to animals and experiments involving animals. The facility where this research was conducted is AAALAC-accredited and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
References
- 1.Almén T. 1990. Contrast media: the relation of chemical structure, animal toxicity, and adverse clinical effects. Am J Cardiol 66:2F–8F. [DOI] [PubMed] [Google Scholar]
- 2.Ballard BE. 1968. Biopharmaceutical considerations in subcutaneous and intramuscular drug administration. J Pharm Sci 57:357–378. [DOI] [PubMed] [Google Scholar]
- 3.Bederka J, Jr, Takemori AE, Miller JW. 1971. Absorption rates of various substances administered intramuscularly. Eur J Pharmacol 15:132–136. [DOI] [PubMed] [Google Scholar]
- 4.Beyers TM, Richardson JA, Prince MD. 1991. Axonal degeneration and self-mutilation as a complication of the intramuscular use of ketamine and xylazine in rabbits. Lab Anim Sci 41:519–520. [PubMed] [Google Scholar]
- 5.Celozzi E, Lotti VJ, Stapley EO, Miller AK. 1980. An animal model for assessing pain-on-injection of antibiotics. J Pharmacol Methods 4:285–289. [DOI] [PubMed] [Google Scholar]
- 6.Charles River Laboratories International. [Internet] 2017. BALB/c Growth chart. [Cited 15 October 2017. Available at: http://www.criver.com/products-services/basic-research/find-a-model/balb-c-mouse.
- 7.Chellman GJ, Faurot GF, Lollini LO, McCullough TE. 1990. Rat pawlick–muscle irritation model for evaluating parenteral formulations for pain-on-injection and muscle damage. Fundam Appl Toxicol 15:697–709. [DOI] [PubMed] [Google Scholar]
- 8.Comereski CR, Williams PD, Bregman CL, Hottendorf GH. 1986. Pain on injection and muscle irritation: a comparison of animal models for assessing parenteral antibiotics. Fundam Appl Toxicol 6:335–338. [DOI] [PubMed] [Google Scholar]
- 9.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C, European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alterative Methods 2001. A good-practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21:15–23. [DOI] [PubMed] [Google Scholar]
- 10.Flecknell P. 2009. Laboratory animal anaesthesia, 3rd ed. London (United Kingdom): Elsevier. [Google Scholar]
- 11.Fleiss JLC. 1969. Large-sample standard errors of κ and weighted κ. Psychol Bull 72:323–327. [Google Scholar]
- 12.Gaertner DJ, Boschert KR, Schoeb TR. 1987. Muscle necrosis in Syrian hamsters resulting from intramuscular injections of ketamine and xylazine. Lab Anim Sci 37:80–83. [PubMed] [Google Scholar]
- 13.Gupta PK, Patel JP, Hahn KR. 1994. Evaluation of pain and irritation following local administration of parenteral formulations using the rat pawlick model. J Pharm Sci Technol 48:159–166. [PubMed] [Google Scholar]
- 14.Houpert P, Combrisson H, Le Nain S, Autefage A, Toutain PL. 1993. Intra- vs intermuscular injections in swine. Vet Res 24:278–285. [PubMed] [Google Scholar]
- 15.Hull RM. 1995. Guideline limit volumes for dosing animals in the preclinical stage of safety evaluation. Toxicology Subcommittee of the Association of the British Pharmaceutical Industry. Hum Exp Toxicol 14:305–307. [DOI] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 17.Kakemi K, Sezaki H, Okumura K, Kobayashi H, Furusawa S. 1972. Absorption of drugs from the skeletal muscle of the rats. 3. Effect of water-soluble adjuvants and vehicles on the intramuscular absorption. Chem Pharm Bull (Tokyo) 20:443–451. [DOI] [PubMed] [Google Scholar]
- 18.Kakemi K, Sezaki H, Okumura K, Takada C, Furusawa S. 1971. Absorption of drugs from the skeletal muscle of the rats. Chem Pharm Bull (Tokyo) 19:2058–2064. [DOI] [PubMed] [Google Scholar]
- 19.Klueber KM, Ontell M. 1984. A new approach to intramuscular placement of horseradish peroxidase. Muscle Nerve 7:127–129. [DOI] [PubMed] [Google Scholar]
- 20.Komarek V. 2004. Gross anatomy, chapter 8 p 122 In: Hedrich H.The laboratory mouse (handbook of experimental animals), 1st ed. San Diego (CA): Elsevier Academic Press. [Google Scholar]
- 21.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. [DOI] [PubMed] [Google Scholar]
- 22.Leary S, Underwood CB, Gwaltney-Brant S, McCrakin MA, Meyer R, Miller D, Shearer J, Yanong R. [Internet]. 2013. AVMA guidelines for the euthanasia of animals: 2013 edition. [Cited 15 April 2017]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf.
- 23.Lefebvre HP, Laroute V, Braun JP, Lassourd V, Toutain PL. 1996. Noninvasive and quantitative evaluation of postinjection muscle damage by pharmacokinetic analysis of creatine kinase release. Vet Res 27:343–361. [PubMed] [Google Scholar]
- 24.Manthorpe M, Cornefert-Jensen F, Hartikka J, Felgner J, Rundell A, Margalith M, Dwarki V. 1993. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum Gene Ther 4:419–431. [DOI] [PubMed] [Google Scholar]
- 25.Miller AL, Leach MC. 2015. The mouse grimace scale: a clinically useful tool? PLoS One 10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton DB, Jennings M, Buckwell A, Ewbank R, Godfrey C, Holgate B, Inglis I, James R, Page C, Sharman I, Verschoyle R, Westall L, Wilson AB, Joint working group on refinement 2001. Refining procedures for the administration of substances. Lab Anim 35:1–41. [DOI] [PubMed] [Google Scholar]
- 27.Paget GE, Scott HM. 1957. A comparison of the local effects of various intramuscular injections in the rat. Br J Pharmacol Chemother 12:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SAS Institute. [Internet] 2013. SAS online doc 9.4. [Cited 01 May 2017]. Available at: http://support.sas.com/documentation/94/
- 29.Shrout PE, Fleiss JL. 1979. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428. [DOI] [PubMed] [Google Scholar]
- 30.Smiler KL, Stein S, Hrapkiewicz KL, Hiben JR. 1990. Tissue response to intramuscular and intraperitoneal injections of ketamine and xylazine in rats. Lab Anim Sci 40:60–64. [PubMed] [Google Scholar]
- 31.Sutton SC, Evans LA, Rinaldi MT, Norton KA. 1996. Predicting injection site muscle damage. I: evaluation of immediate release parenteral formulations in animal models. Pharm Res 13:1507–1513. [DOI] [PubMed] [Google Scholar]
- 32.Svedsen O. 1988. Studies of tissue injuries caused by intramuscular injection of drugs and vehicles. Methods for quantification and effects of concentration, volume, vehicle, injection speed, and intralipomatous injection. Olstykke (Denmark): Department of Science, Medical Faculty University of Copehnhagen. [Google Scholar]
- 33.Svendsen O, Andersen CB, Morkhoj CB, Lauritzen B. 2006. Spinal nociception induced by intramuscular injection of oxytetracycline preparations in rats and pigs. Basic Clin Pharmacol Toxicol 99:58–61. [DOI] [PubMed] [Google Scholar]
- 34.Thuilliez C, Dorso L, Howroyd P, Gould S, Chanut F, Burnett R. 2009. Histopathological lesions following intramuscular administration of saline in laboratory rodents and rabbits. Exp Toxicol Pathol 61:13–21. [DOI] [PubMed] [Google Scholar]
- 35.Tuffery AA. 1987. Laboratory animals: an introduction for new experiments, 3rd ed. Chichester (United Kingdom): John Wiley and Sons. [Google Scholar]
- 36.Turner PV, Brabb T, Pekow C, Vasbinder MA. 2011. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner JG. 1961. Biopharmaceutics: absorption aspects. J Pharm Sci 50:359–387. [DOI] [PubMed] [Google Scholar]
- 38.Waynforth HB, Brain P, Sharpe T, Stewart DF, Applebee KA, Darke PGG. 1998. Administration of substances (rat, mouse, guinea pig, rabbit). Good-practice guidelines. Staffordshire (United Kingdom): Laboratory Animal Science Association. [Google Scholar]