Abstract
Despite few published studies that assess the accuracy of glucometers in laboratory animals, glucometers are commonly used in animal research. We set out to determine the accuracy of 5 point-of-care glucometers (POCG) when used to evaluate murine whole blood, plasma, and serum samples. The POCG tested included one veterinary device (POCG A) and 4 human-use instruments (POCG B through E). Whole blood, plasma, and serum samples from 50 female C57BL/6J mice were analyzed on all POCG, and serum was analyzed on a reference biochemical analyzer. The mean blood glucose concentration (BGC) measured in whole blood by using POCG A was greater than that on the biochemical analyzer, whereas the mean BGC in whole blood according to POCG B through E did not differ significantly from that on the biochemical analyzer. Mean BGC in plasma and serum did not differ between POCG B and E and the biochemical analyzer, whereas the plasma and serum BGC values from POCG C and D were greater than the mean BGC from the biochemical analyzer. The accuracy of each POCG for each sample type was evaluated by analyzing mean differences, correlations, and Bland–Altman graphs. We found that the 4 human-use POCG are appropriate for use with whole blood from female C57BL/6J mice, whereas only 2 of the evaluated POCG were sufficiently accurate for use with plasma or serum.
Abbreviations: BGC, blood glucose concentration; LOA, limits of agreement; POCG, point-of-care glucometer
Point-of-care glucometers (POCG) are used in the clinical management of diabetes in both humans and animals as well as in biomedical research with animals. The ease of use, low cost, effectiveness with small blood volumes, and rapid feedback as compared with biochemical analyzers make POCG a convenient choice for determining blood glucose concentration (BGC).11,39-41 The International Standards Organization updated the requirements for human-use POCG used to manage human diabetes in 2013, requiring 95% of BGC measurements to be within 15 mg/dL of the true value as measured by the reference method when less than 100 mg/dL and within 15% of the true value when 100 mg/dL or higher.23 No standard guidelines regarding the accuracy of glucometers for use in veterinary medicine are available, and little literature regarding POCG accuracy in various animal species has been published; it seems that glucometers are tested for validation in each species as the need arises. Published guidelines for quality assurance of glucometers in veterinary medicine have been endorsed by the American Society for Veterinary Clinical Pathology.17 These guidelines suggest that the user correct for pre- and postanalytical errors of POCG, compare results of POCG with those of a reference laboratory, and harmonize all POCG used within a single veterinary hospital.17
Particular models of POCG have proven to be inaccurate in some species, including dogs,11,12,25 cats,25 ferrets,32,40 white-tailed deer,7 Hispaniolan Amazon parrots,2 alpacas,5 sheep,20 cows,30 horses,19 and a variety of NHP species.10 Other studies have determined that various specific glucometers are accurate in alpacas,5 horses,18,19 dogs,14,24,45 cats,14,44 cattle,26,30,46 sheep,26 rabbits,37 ferrets,32 and NHP.10 However, only 2 abstracts and 2 peer-reviewed papers have examined the use of POCG in laboratory rodents.3,15,27,42 The comparison of 3 human-use POCG with a biochemical analyzer revealed that only 2 of the 3 POCG were acceptable for use in rats.15 Another group tested 11 human-use POCG relative to a biochemical analyzer for evaluating samples from male C57BL/6J mice and found that 10 of the POCG yielded higher BGC and 1 yielded a lower BGC than the biochemical analyzer.42 The authors of the previous study also noted that BGC in hyperglycemic mice tended to be higher when using POCG than the biochemical analyzer.42
Several companies have recently developed veterinary POCG that are calibrated for use in animals, including dogs, cats, horses, ferrets, and rodents. These devices have been marketed to the companion animal community, and recent literature has revealed their increased use over the past few years in a variety of species, with some studies showing their accuracy18,24,25,27,31,32 and others indicating that they are not appropriate for the particular species under study.2,3,10,11,37 One device's user's manual indicates that it is calibrated for mice and rats,47 and the manufacturer has published an abstract indicating its accuracy in these species.27 However, only one peer-reviewed study has evaluated this device in rodents, and the regression analysis did not support the device's use with murine whole blood.3
Although human-use POCG are designed to analyze whole blood, several reports indicate that POCG are more accurate with serum or plasma in alpacas,5 horses,19 dogs,41 and cats.5,19,41 For example, values obtained for equine plasma samples were more accurate than those from whole blood when one of the POCG included in the current study was used.19 No studies evaluating POCG accuracy for use with rodent plasma or serum have yet been published.
The purpose of the current study was to determine the accuracy of 5 POCG in determining BGC in female C57BL/6J mice and to determine whether whole blood, plasma, or serum is the most appropriate sample type for each glucometer. We tested serum samples in a biochemical analyzer, and whole blood, plasma, and serum samples on 5 POCG. The differences between values obtained for each sample tested in each of the POCG and the biochemical analyzer were determined and, along with Pearson correlations and Bland–Altman graphs, used to determine accuracy.
Materials and Methods
Animals.
Subjects were 50 experimentally naïve female C57BL/6J mice (age, 10 to 28 wk; weight, 19 to 31 g; The Jackson Laboratory, Bar Harbor, ME). Female mice were chosen because of their ability to withstand greater volumes of blood loss than males.34 Mice were fed a commercially available irradiated, balanced mouse diet (no. 5058, LabDiet, St Louis, MO) and maintained on corncob bedding, with a cotton square (Ancare, Bellmore, NY) for enrichment. Mice were group-housed under a 12:12-h light:dark cycle in IVC or static cages, which were changed once or twice weekly, respectively. The housing facility excludes ectromelia virus, epizootic diarrhea of infant mice, lymphocytic choriomeningitis virus, mouse adenoviruses 1 and 2, mouse hepatitis virus, mouse parvovirus, minute virus of mice, polyomavirus, pneumonia virus of mice, reovirus 3, Theiler meningoencephalomyelitis virus, Sendai virus, cilia-associated respiratory bacillus, Mycoplasma pulmonis, Clostridium piliforme, Encephalitozoon cuniculi, Myocoptes spp., Radfordia spp., Myobia spp., Aspicularis tetraptera, Syphacia muris, and Syphacia obvelata. This research was approved by the Animal Welfare Committee (IACUC) of The University of Texas Health Science Center at Houston (UTHealth); all procedures were performed in AAALAC-accredited facilities and in accordance with the Guide for the Care and Use of Laboratory Animals.22
Equipment.
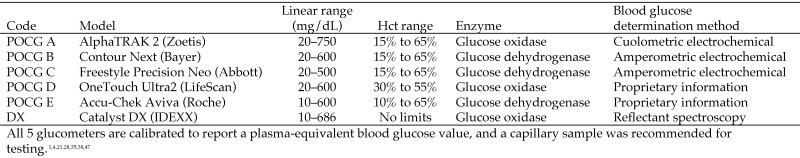
One veterinary glucometer and 4 human-use glucometers were chosen on the basis of a literature search of 162 studies designed to determine which brands and models were used most frequently. The POCG evaluated were: A, AlphaTRAK2 (Zoetis, Parsippany, NJ [originally manufactured by Abbott]); B, Contour Next (Bayer, Parsippany, NJ); C, Freestyle Precision Neo (Abbott, Alameda, CA); D, OneTouch Ultra2 (LifeScan, Milpitas, CA); and E, Accu-Chek Aviva (Roche, Indianapolis, IN). Selected parameters of these POCG are summarized in Figure 1. The POCG accept blood samples via capillary action into the test strips and use various methods to determine BGC (Figure 1), which is displayed as a plasma-equivalent blood glucose concentration. As recommended by the manufacturers and according to guidelines from the American Society for Veterinary Clinical Pathology, POCG were calibrated with the manufacturer's control sample every time a new package of test strips was opened.1,4,17,28,35,47 Glucometers and test strips were stored and used at an appropriately controlled temperature and humidity for the duration of the study. POCG A was set to ‘dog,’ as recommended for use in C57BL/6J mice.47 POCG A produced error messages when analyzing plasma and serum, consistent with manufacturer specifications; therefore, no plasma or serum BGC are reported for POCG A.
Figure 1.
Parameters of the point-of-care glucometers and biochemical analyzer used.
The biochemical analyzer used as the reference method was a Catalyst DX (IDEXX, Westbrook, ME). Serum samples were chosen as the reference sample because of the blood volume required (60 µL of serum compared with 600 µL of whole blood). The analyzer received monthly cleaning as recommended by the manufacturer and was calibrated by using a standard provided by IDEXX. The results were within normal limits.
Experimental design.
Mice were assigned in approximately equal numbers to 3 groups to obtain samples with a wide range of BGC: one group was given streptozotocin, another group was administered insulin, and another group received no intervention.
Streptozotocin was administered at a dose of 50 mg/kg IP once daily for 5 d to mice on unrestricted feeding; this approach was based on a 2013 study, which indicated that fasting may cause additional stress and is not necessary to induce diabetes.9,43 Blood samples from diabetic mice were collected 4 wk after streptozotocin administration. Humulin R insulin (Lilly, Indianapolis, IN) was administered at a dose of 1 to 4 U/kg IP, and blood samples were collected within 30 min to 2 h after insulin administration.8
Mice were acclimated to a procedure room for 1 to 3 h prior to 2 survival and 1 terminal blood collections of approximately 300 µL each at 2- to 4-wk intervals. A minimum of 180 µL of whole blood was collected at each time point to provide serum for the biochemical analyzer and the POCG. Another 75 µL was collected into a heparinized hematocrit tube for plasma testing on the POCG, and approximately 30 µL was collected into a plain hematocrit tube for whole blood testing on the POCG. Mice were euthanized by carbon dioxide inhalation followed by cervical dislocation immediately after the third blood collection.
After each survival blood collection, mice received fluid support with 0.9% saline solution IP (approximately 3 times blood loss volume), and hypoglycemic animals also were given a gel diet (ClearH2O, Westbrook, ME). Although serial blood samples for glucose testing are generally collected through tail-vein puncture, this study required a larger blood volume than can be obtained from the tail vein consistently. Blood initially was collected from the retroorbital sinus under isoflurane anesthesia in an O2 vehicle delivered by a precision vaporizer. However, isoflurane anesthesia was found to increase the BGC of nearly all samples collected. To get sufficient numbers of hypoglycemic and euglycemic samples, blood was then collected from the facial vein puncture of nonanesthetized mice by using Goldenrod animal lancets (MEDIPoint, Mineola, NY). A small subset of mice (n = 10) was fasted once, for less than 12 h, to obtain blood glucose values within target ranges. Two animals showed clinical signs of hypoglycemia after insulin administration and were treated with 50% dextrose diluted in 0.9% saline solution (0.5 mg/kg IP). All animals recovered from insulin administration and blood collection, exhibiting normal behavior within 1 to 5 h. All blood samples were collected by the same person, and 3 people were trained to use the glucometers, for consistency throughout the study.
Sample processing.
Whole blood was collected into a nonheparinized hematocrit tube (Jorgensen Laboratories, Loveland, CO) and tested on each of the 5 POCG immediately. Additional whole blood was collected into a heparinized hematocrit tube (Jorgensen Laboratories) and underwent centrifugation (ReadACrit, Becton Dickinson, Parsippany, NJ) to obtain a plasma sample, which was used for a PCV reading and tested on POCG B through E. The remaining blood was collected in a serum separator tube (Becton Dickenson, Franklin Lakes, NJ) and allowed to clot on ice for 18 to 22 min prior to centrifugation (StatSpin, IDEXX) at 12,000 × g for 5 min. Serum samples were then tested on POCG B through E and on the biochemical analyzer. POCG testing order was rotated every 3 samples to control for time to POCG testing.
Chemical preparation.
On each day of injection, streptozotocin (Sigma, St Louis, MO) was prepared by using deionized water and a 0.2-µm pore filter (Braun, Ann Arbor, MI).16,43 Immediately before use, Humulin R insulin was diluted into sterile vials by using 0.9% saline to achieve concentrations of 0.1 to 2.0 U/mL.
Statistical analyses.
Concentrations of glucose and the differences in the concentration of glucose between each POCG and the biochemical analyzer were analyzed by using the MIXED procedure of SAS (SAS Institute, Cary, NC). The model included the fixed effect of the method used to determine the concentration of glucose. Each mouse was considered as a random effect and was specified in the RANDOM statement. Data from all 146 samples were pooled for analysis, and a P value of less than 0.05 was considered significant. Correlation coefficients were generated with the CORR procedure to evaluate the relation between the BGC measured by each POCG and that measured by the biochemical analyzer. Data are presented as least-squares means ± SEM. In addition, Bland–Altman analysis was used to assess the level of agreement between each POCG and the biochemical analyzer.6 The bias was calculated as the mean difference in glucose concentration determined in whole blood, plasma, and serum by each POCG and the serum glucose concentration determined by the biochemical analyzer. The 95% limits of agreement (LOA) were calculated as the mean difference ± 1.96 SD. In general, a Bland–Altman plot that supports a good level of agreement between the 2 methods shows narrower LOA, with differences between the methods lying close to the bias line, which ideally is close to 0.
Results
A total of 146 blood samples were collected, and the BGC obtained ranged from 17 to 498 mg/dL as determined by the biochemical analyzer.
Whole blood samples.
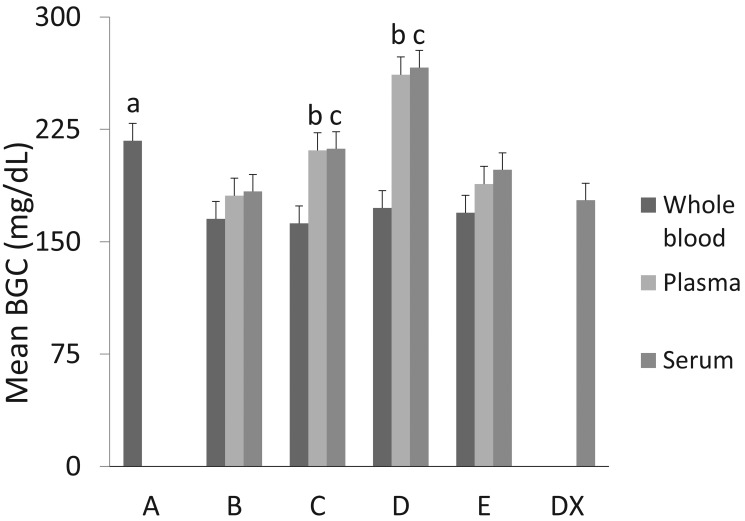
The mean BGC of whole blood samples measured on POCG A were significantly different from that measured in serum by the biochemical analyzer, averaging 39.4 mg/dL greater than the BGC from the analyzer (P = 0.0066; Figure 2). The mean BGC of whole blood measured in POCG B through E did not differ significantly from the mean BGC measured in the biochemical analyzer; the mean differences were: B, –12.7 mg/dL (P = 0.38); C, –15.8 mg/dL (P = 0.27); D, –5.5 mg/dL (P = 0.70); and E, –8.7 mg/dL (P = 0.55).
Figure 2.
Blood glucose concentrations (BGC; mean ± 1 SD) from whole blood, plasma, and serum for 5 point-of-care glucometers (POCG A through E) and the mean BGC from serum derived from the same whole-blood sample and analyzed on the biochemical analyzer (DX). a, significant (P = 0.0066) difference between BGC in whole blood measured on POCG A and that in serum measured on the biochemical analyzer; b, significant differences between BGC in plasma measured on POCG C and D and that of serum measured on the biochemical analyzer (P = 0.0466 and P < 0.0001, respectively); c, significant differences between BGC in serum measured on POCG C and D and that in serum measured on the biochemical analyzer (P = 0.0340 and P < 0.0001, respectively).
The BGC for whole blood measured on POCG A through E increased directly with the BGC measured on the biochemical analyzer for serum from samples obtained during the same blood collection. Pearson correlation coefficients (r) for POCG A (r = 0.98; P < 0.0001), POCG B (r = 0.96; P < 0.0001), POCG C (r = 0.98; P < 0.0001), POCG D (r = 0.98; P < 0.0001), and POCG E (r = 0.98; P < 0.0001) showed strong positive correlation for each POCG and the biochemical analyzer (data not shown).
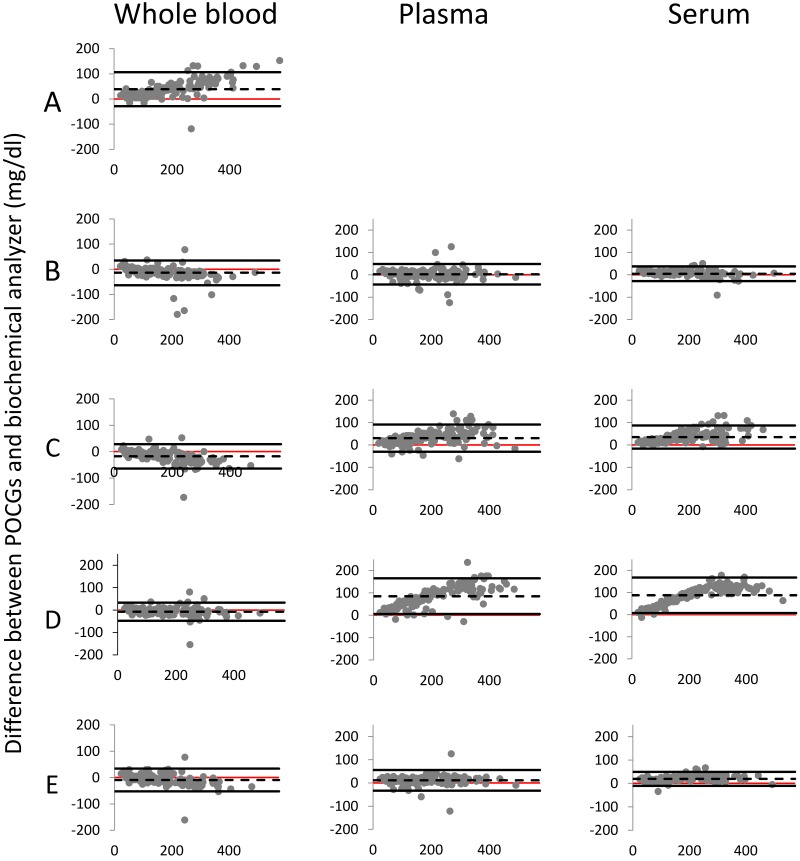
Test agreement between whole blood, plasma, and serum glucose concentrations determined by each POCG and those determined by using the biochemical analyzer is shown in Figure 3. Bland–Altman plots for whole blood showed that POCG A had a much wider 95% LOA of –28.4 to 106.8 mg/dL and a bias of 39.2 mg/dL greater than the biochemical analyzer. The remaining whole blood Bland–Altman plots were very similar to each other. POCG B had a 95% LOA of –63.4 to 34.8 mg/dL and a bias of –13.3 mg/dL relative to data from the biochemical analyzer. POCG C had a 95% LOA of –63.5 to 28.4 mg/dL and a bias of –17.5 mg/dL compared with values from the biochemical analyzer. POCG D had the narrowest 95% LOA, –47.6 to 33.5 mg/dL, with a bias of –7.1 mg/dL relative to measurements from the biochemical analyzer. POCG E had a 95% LOA of –52.4 to 34.0 mg/dL and a bias of –9.2 mg/dL lower than those of the biochemical analyzer.
Figure 3.
Bland–Altman plots of blood glucose concentration (BGC) values obtained from whole blood, plasma, and serum samples on each of 5 point-of-care glucometers (POCG). Differences in BGC between the POCG and the biochemical analyzer are plotted against the mean BGC of that POCG and the biochemical analyzer (n = 146 for each). Dashed lines represent the mean difference (bias) of the POCG reading from that of the biochemical analyzer: for whole blood: A, 39.2 mg/dL; B, –13.3 mg/dL; C, –17.5 mg/dL; D, –7.1 mg/dL; and E, –9.2 mg/dL; for plasma: B, 2.8 mg/dL; C, 32.3 mg/dL; D, 85.1 mg/dL; and E, 11.3 mg/dL; and for serum: B, 4.9 mg/dL; C, 35.2 mg/dL; D, 87.8 mg/dL; and E, 19.6 mg/dL. Solid black lines above and below the dashed line indicate the 95% confidence interval (that is, ± 1.96 SD). The red line is the x-axis at 0 and represents the reading from biochemical analyzer for each data point.
Plasma samples.
Four POCG, B through E, were tested with plasma samples. The mean BGC for plasma measured on POCG B and E were not significantly different from the mean BGC for serum measured on the biochemical analyzer (Figure 2), with POCG B having a mean difference of 2.1 mg/dL (P = 0.8953) and POCG E a mean difference of 10.0 mg/dL (P = 0.5384). The mean BGC of plasma measured on POCG C and D differed significantly from that for serum measured on the biochemical analyzer, with POCG C having a mean difference of 32.3 mg/dL (P = 0.0466) and POCG D a mean difference of 83.0 mg/dL (P < 0.0001).
BGC for plasma measured on POCG B through E increased directly with that for serum measured on the biochemical analyzer for samples obtained during the same blood collection. Pearson correlation coefficients for POCG B (r = 0.97; P < 0.0001), POCG C (r = 0.96; P < 0.0001), POCG D (r = 0.96; P < 0.0001), and POCG E (r = 0.98; P < 0.0001) showed strong positive correlation for each POCG and the analyzer (data not shown).
Bland–Altman plots for plasma varied, with POCG E having the narrowest LOA range and POCG D the largest (Figure 3). POCG B had a 95% LOA of –42.9 to 48.5 mg/dL, with a bias of 2.8 mg/dL greater than the value obtained by using the biochemical analyzer. POCG C had a 95% LOA of –30.5 to 91.1 mg/dL, with a bias of 30.3 mg/dL greater than that from the biochemical analyzer. POCG D had a 95% LOA of 5.9 to 165.4 mg/dL, with a bias of 85.1 mg/dL greater than values from the biochemical analyzer. POCG E had a 95% LOA of –33.3 to 55.5 mg/dL, with a bias of 11.3 mg/dL greater than those for the biochemical analyzer.
Serum samples.
Of the 4 POCG tested with serum samples, POCG B and E produced mean BGC that did not differ significantly from that for serum measured on the biochemical analyzer (Figure 2), with POCG B having a mean difference of 7.3 mg/dL (P = 0.65) and POCG E a mean difference of 20.3 mg/dL (P = 0.21). The mean BGC for serum measured on POCG C and D were significantly different from that for serum measured on the biochemical analyzer, with POCG C having a mean difference of 34.4 mg/dL (P = 0.034) and POCG D a mean difference of 88.6 mg/dL (P < 0.0001).
BGC for POCG B through E increased directly with those for the biochemical analyzer for serum samples obtained from the same blood collection. Pearson correlation coefficients for POCG B (r = 0.99; P < 0.0001), POCG C (r = 0.98; P < 0.0001), POCG D (r = 0.98; P < 0.0001), and POCG E (r = 0.99; P < 0.0001) show strong positive correlation for each POCG and the biochemical analyzer (data not shown).
Bland–Altman plots for serum samples were similar to those for plasma samples, with POCG E having the narrowest 95% LOA range and POCG D having the largest (Figure 3). POCG B had a 95% LOA of –27.7 to 37.6 mg/dL, with a bias of 5.0 mg/dL greater than values obtained by using the biochemical analyzer. POCG C had a 95% LOA of –16.4 to 86.8 mg/dL, with a bias of 35.2 mg/dL greater than the data from the biochemical analyzer. POCG D had a 95% LOA of 7.6 to 168.1 mg/dL with a bias of 87.8 mg/dL greater than measurements from the biochemical analyzer. POCG E had a 95% LOA of –10.3 to 49.5 mg/dL, with a bias of 19.6 mg/dL greater than the data from the biochemical analyzer.
PCV.
To ensure that PCV values were within the manufacturer's guidelines for POCG accuracy, we measured PCV by using the plasma samples contained in the hematocrit tube before these samples were tested on the POCG. The PCV values from the mice ranged from 35% to 55%, which were within all of the recommended ranges for glucometer accuracy (Figure 1).
Discussion
The accuracy of POCG in humans has been under investigation since they were first introduced in the 1970s. The most recent literature suggests that, when used appropriately, cleaned and calibrated regularly, and kept at appropriate temperature and humidity, these devices are accurate for daily glucose control but not for diagnostic or research purposes.36 However, many researchers are using POCG for measuring BGC in mice with little literature to support instrument accuracy. We therefore designed this study to validate 5 widely used POCG.
For whole blood samples, POCG B through E yielded BGC results consistent with those for serum BGC on the biochemical analyzer. For these glucometers, the values fell within a tight range, and mean differences (bias) from the biochemical analyzer were not significantly different; of these 4 POCG, POCG D had the narrowest range and the smallest bias. POCG A yielded a much wider confidence interval and a significantly greater mean difference from the biochemical analyzer. These results indicate that any of the 4 POCG B through E are appropriate choices when measuring BGC in whole blood samples from female C57BL/6J mice, whereas POCG A is not.
The mean BGC measured from plasma and serum samples for POCG B and E were not significantly different from the mean BGC measured in serum by the biochemical analyzer. Bland–Altman plots indicated that POCG E had the narrowest range for both plasma and serum, and POCG B likewise had a narrow range as well as the smallest bias from the biochemical analyzer for both plasma and serum. This finding indicates that when plasma or serum is used, POCG E or B is an appropriate choice.
Pearson correlation coefficients showed a strong positive correlation between BGC measured by the POCG and by the biochemical analyzer. These r values suggest that a correction equation could be derived for the POCG that yielded values significantly different from the biochemical analyzer. Similarly, one group determined that one brand of glucometer tended to have lower readings for cattle and sheep than a benchtop method and calculated an appropriate reference range to account for the difference in readings.26 However, calculating either a new set of reference ranges or a correction equation for each inaccurate glucometer was beyond the scope of this study.
Although the International Standards Organization recommends that 95% of human-use POCG results lie within 15 mg/dL of the true value determined by the reference method when less than 100 mg/dL or within 15% when greater than 100 mg/dL, we did not calculate the statistics for this study on that basis. The mean difference for POCG C was –15.8 mg/dL with whole blood; this value was not significantly different from that measured by the biochemical analyzer. Whether, if the ISO standards had been applied, POCG C would have had more than 95% of samples fall within the recommended range is unknown.
Differences in RBC size and shape among species have been indicated as potential reasons for the inaccuracy of human-use POCG in animals.5,19 Mouse RBC are approximately 6 µm in diameter, which is relatively close in size to human RBC (6 to 8 µm) and canine RBC (7 µm). This similarity suggests that both human-use and veterinary POCG might be accurate for murine whole blood samples. Others suggest that differences in the distribution of glucose between plasma and RBC among species cause the observed variations in results.10,17,47 Rats have 84% of blood glucose in plasma, whereas dogs have 87.5%, cats 93%, and humans only 58%.13,29 For example, POCG A performs different calculations depending on which ‘code’ is used to account for the differences in blood glucose distributions of dogs and cats. Assuming that mice have a distribution similar to that of rats, the manufacturer recommends using the dog code for mice. Why POCG A had poor performance in a previous study3 and the current one is unknown
In humans, BGC obtained from plasma are 10% to 15% higher than those from whole blood, but most POCG filter the RBC and run the subsequent test on the remaining plasma.17 Those that do not filter to obtain plasma perform a calculation to adjust for the difference. All of the POCG we used in this study report ‘plasma-equivalent’ values, according to the manufacturers, but they did not always describe exactly how this value is obtained. This factor may explain why the 4 human-use POCG gave higher BGC values when used with samples of plasma and serum than when used with whole blood, because they analyze the sample as though it were whole blood and then formulate an increase of 10% to 15% to report the plasma-equivalent value. Even the POCG whose results did not differ significantly from those of the biochemical analyzer (that is, POCG B and E) yielded values that were slightly higher than those from the analyzer, confirming that this difference could be due to a programmed calculation. However, POCG C and D had elevated values well beyond the 10% to 15% compensatory calculation for whole blood to plasma; therefore, the significant differences that we revealed in this study cannot be entirely explained by conversion to a plasma-equivalent value.
POCG work by using chemical enzymatic reactions, including those mediated by hexokinase, glucose oxidase, and glucose dehydrogenase. These reactions take place on the strip, where the sample mixes with dehydrated enzymes, resulting in either a color change or the production of an electrical current. The glucometer then uses either optical (spectroscopy or photometry) or electrochemical (potentiometry, amperometry, or cuolometry) methods to determine the BGC, which is then displayed. Biochemical analyzers generally use either the hexokinase or glucose oxidase method.17,33 Figure 1 shows the method used by each of the POCG and the biochemical analyzer in this study.
In conclusion, our results indicate that the 4 human-use POCG tested (B through E) are accurate for testing BGC in whole blood from female C57BL/6J mice. Although we did not evaluate other brands and models of glucometers for human use, we expect, considering that all 4 of the human-use POCG tested in this study showed sufficient accuracy with whole blood, that the same would be true for many other available POCG. When using a plasma or serum sample is necessary, we recommend validation of the POCG against a reference method. The veterinary POCG proved to be inaccurate when used on the dog setting for whole blood samples from female C57BL/6 mice. An alternative study would be to attempt to use the veterinary POCG on the cat setting for analysis of mouse whole blood.
Acknowledgments
We thank UTHealth Center for Laboratory Animal Medicine and Care for funding this project. We also thank Sarah Frazier, AAS, LVT, LATg, and Margaret Horscroft, AAS, LVT, for assistance with sample testing throughout this study; Vihang Narkar, PhD, and Ya-Ping Lin, MS, for providing materials and use of equipment; and the Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, for editing assistance.
References
- 1.Abbott 2014. FreeStyle Precision Neo blood glucose monitoring system user's manual. Mississauga, Ontario (Canada): Abbott Diabetes Care. [Google Scholar]
- 2.Acierno MJ, Schnellbacher R, Tully TN., Jr 2012. Measuring the level of agreement between a veterinary and a human point-of-care glucometer and a laboratory blood analyzer in Hispaniolan Amazon parrots (Amazona ventralis). J Avian Med Surg 26:221–224. [DOI] [PubMed] [Google Scholar]
- 3.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP, NIH Mouse Metabolic Phenotyping Center Consortium. 2010. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 3:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer Healthcare 2012. Contour Next blood glucose monitoring system user guide. Toronto, Ontario (Canada): Bayer. [Google Scholar]
- 5.Beemer O, Byers S, Bohn A. 2013. Evaluation of 4 point-of-care glucose meters in alpacas. J Vet Intern Med 27:990–995. [DOI] [PubMed] [Google Scholar]
- 6.Bland JM, Altman DG. 1986. Statistical methods for assessing agreement between 2 methods of clinical measurement. Lancet 327:307–310. [PubMed] [Google Scholar]
- 7.Burdick S, Mitchell MA, Neil J, Heggem B, Whittington J, Acierno MJ. 2012. Evaluation of 2 point-of-care meters and a portable chemistry analyzer for measurement of blood glucose concentrations in juvenile white-tailed deer (Odocoileus virginianus). J Am Vet Med Assoc 240:596–599. [DOI] [PubMed] [Google Scholar]
- 8.Canada SE, Weaver SA, Sharpe SN, Pederson BA. 2011. Brain glycogen supercompensation in the mouse after recovery from insulin-induced hypoglycemia. J Neurosci Res 89:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhry ZZ, Morris DL, Moss DR, Sims EK, Chiong Y, Kono T, Evans-Molina C. 2013. Streptozotocin is equally diabetogenic whether administered to fed or fasted mice. Lab Anim 47:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemmons EA, Stovall MI, Owens DC, Scott JA, Jones-Wilkes AC, Kempf DJ, Ethun KF. 2016. Accuracy of human and veterinary point-of-care glucometers for use in rhesus macaques (Macaca mulatta), sooty mangabeys (Cercocebus atys), and chimpanzees (Pan troglodytes). J Am Assoc Lab Anim Sci 55:346–353. [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen TA, Nelson RW, Kass PH, Christopher MM, Feldman EC. 2009. Evaluation of 6 portable blood glucose meters for measuring blood glucose concentration in dogs. J Am Vet Med Assoc 235:276–280. [DOI] [PubMed] [Google Scholar]
- 12.Cohn LA, McCaw DL, Tate DJ, Johnson JC. 2000. Assessment of 5 portable blood glucose meters, a point-of-care analyzer, and color test strips for measuring blood glucose concentration in dogs. J Am Vet Med Assoc 216:198–202. [DOI] [PubMed] [Google Scholar]
- 13.Coldman MF, Good W. 1967. The distribution of sodium, potassium, and glucose in the blood of some mammals. Comp Biochem Physiol 21:201–206. [DOI] [PubMed] [Google Scholar]
- 14.Domori A, Sunahara A, Tateno M, Miyama TS, Setoguchi A, Endo Y. 2014. The clinical utility of 2 human portable blood glucose meters in canine and feline practice. Vet Clin Pathol 43:55–62. [DOI] [PubMed] [Google Scholar]
- 15.Fowler S, Slocum N, Prescott J, Boysza J. 2011. Validation of portable blood glucose monitoring systems for use in rats. Abstracts presented at the AALAS National Meeting, San Diego, California, 2–6 October 2011. J Am Assoc Lab Anim Sci 50:812. [Google Scholar]
- 16.Furman BL. 2015. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 70:5.471 –20. [DOI] [PubMed] [Google Scholar]
- 17.Gerber KL, Freeman KP. 2016. ASVCP guidelines: quality assurance for portable blood glucose meter (glucometer) use in veterinary medicine. Vet Clin Pathol 45:10–27. [DOI] [PubMed] [Google Scholar]
- 18.Hackett ES, McCue PM. 2010. Evaluation of a veterinary glucometer for use in horses. J Vet Intern Med 24:617–621. [DOI] [PubMed] [Google Scholar]
- 19.Hollis AR, Dallap Schaer BL, Boston RC, Wilkins PA. 2008. Comparison of the Accu-chek Aviva point-of-care glucometer with blood gas and laboratory methods of analysis of glucose measurement in equine emergency patients. J Vet Intern Med 22:1189–1195. [DOI] [PubMed] [Google Scholar]
- 20.Hornig KJ, Byers SR, Callan RJ, Holt T, Field M, Han H. 2013. Evaluation of a point-of-care glucose and β-hydroxybutyrate meter operated in various environmental conditions in prepartum and postpartum sheep. Am J Vet Res 74:1059–1065. [DOI] [PubMed] [Google Scholar]
- 21.IDEXX. [Internet] 2017. Catalyst DX operator's guide. [Cited 01 June 2016]. Available at: https://www.idexx.com/resource-library/smallanimal/catalyst-dx-operators-guide-en.pdf. [Google Scholar]
- 22.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 23.International Organization for Standardization Technical Committee 212. [Internet] 2013. ISO 15197:2013 In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. [Cited 15 March 2017]. Available at: https://www.iso.org/standard/54976.html [Google Scholar]
- 24.Johnson BM, Fry MM, Flatland B, Kirk CA. 2009. Comparison of a human portable blood glucose meter, veterinary portable blood glucose meter, and automated chemistry analyzer for measurement of blood glucose concentrations in dogs. J Am Vet Med Assoc 235:1309–1313. [DOI] [PubMed] [Google Scholar]
- 25.Kang MH, Kim DH, Jeong IS, Choi GC, Park HM. 2015. Evaluation of 4 portable blood glucose meters in diabetic and nondiabetic dogs and cats. Vet Q 36:2–9. [DOI] [PubMed] [Google Scholar]
- 26.Katsoulos PD, Minas A, Karatzia MA, Pourliotis K, Christodoulopoulos G. 2011. Evaluation of a portable glucose meter for use in cattle and sheep. Vet Clin Pathol 40:245–247. [DOI] [PubMed] [Google Scholar]
- 27.Krecic M, Clark P, Apgar S. 2015. Use of an animal-specific glucose meter for mice and rats and its potential impact with future research. Abstracts presented at the AALAS National Meeting, Phoenix, Arizona, 1–5 November 2015. J Am Assoc Lab Anim Sci 54:576. [Google Scholar]
- 28.Lifescan 2009. OneTouch Ultra2 blood glucose monitoring system user guide. (Switzerland): LifeScan Europe. [Google Scholar]
- 29.MacKay EM. 1932. Distribution of glucose in human blood. J Biol Chem 97:685–689. [Google Scholar]
- 30.Mair B, Drillich M, Klein-Jobstl D, Kanz P, Borchardt S, Meyer L, Schwendenwein I, Iwersen M. 2016. Glucose concentration in capillary blood of dairy cows obtained by a minimally invasive lancet technique and determined with 3 different handheld devices. BMC Vet Res 12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul AE, Shiel RE, Juvet F, Mooney CT, Mansfield CS. 2011. Effect of hematocrit on accuracy of 2 point-of-care glucometers for use in dogs. Am J Vet Res 72:1204–1208. [DOI] [PubMed] [Google Scholar]
- 32.Petritz OA, Antinoff N, Chen S, Kass PH, Paul-Murphy JR. 2013. Evaluation of portable blood glucose meters for measurement of blood glucose concentration in ferrets (Mustela putorius furo). J Am Vet Med Assoc 242:350–354. [DOI] [PubMed] [Google Scholar]
- 33.Quimby F. 1999. The mouse, p. 3–32. In: Loeb WF, Quimby F. The clinical chemistry of laboratory animals. Ann Arbor (MI): Taylor and Francis. [Google Scholar]
- 34.Raabe BM, Artwohl JE, Purcell JE, Lovaglio J, Fortman JD. 2011. Effects of weekly blood collection in C57BL/6 mice. J Am Assoc Lab Anim Sci 50:680–685. [PMC free article] [PubMed] [Google Scholar]
- 35.Roche 2016. Accu-Chek Aviva Connect blood glucose monitoring system user's manual. Indianapolis (IN): Roche Diabetes Care. [Google Scholar]
- 36.Salacinski AJ, Alford M, Drevets K, Hart S, Hunt BE. 2014. Validity and reliability of a glucometer against industry reference standards. J Diabetes Sci Technol 8:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selleri P, Di Girolamo N, Novari G. 2014. Performance of 2 portable meters and a benchtop analyzer for blood glucose concentration measurement in rabbits. J Am Vet Med Assoc 245:87–98. [DOI] [PubMed] [Google Scholar]
- 38.Siska WD, Rosen NK, Christian JA, Taddeo DA, DeNicola DB. 2011. Performance of the IDEXX Catalyst DX chemistry analyzer for inhouse biochemical analysis. [Cited 26 April 2017]. Available at: https://www.idexx.com/pdf/en_us/landing-pages/cdx-white-paper.pdf. [Google Scholar]
- 39.Stein JE, Greco DS. 2002. Portable blood glucose meters as a means of monitoring blood glucose concentrations in dogs and cats with diabetes mellitus. Clin Tech Small Anim Pract 17:70–72. [DOI] [PubMed] [Google Scholar]
- 40.Summa NM, Eshar D, Lee-Chow B, Larrat S, Brown DC. 2014. Comparison of a human portable glucometer and an automated chemistry analyzer for measurement of blood glucose concentration in pet ferrets (Mustela putorius furo). Can Vet J 55:865–869. [PMC free article] [PubMed] [Google Scholar]
- 41.Tauk BS, Drobatz KJ, Wallace KA, Hess RS. 2015. Correlation between glucose concentrations in serum, plasma, and whole blood measured by a point-of-care glucometer and serum glucose concentration measured by an automated biochemical analyzer for canine and feline blood samples. J Am Vet Med Assoc 246:1327–1333. [DOI] [PubMed] [Google Scholar]
- 42.Togashi Y, Shirakawa J, Okuyama T, Yamazaki S, Kyohara M, Miyazawa A, Suzuki T, Hamada M, Terauchi Y. 2016. Evaluation of the appropriateness of using glucometers for measuring the blood glucose levels in mice. Sci Rep 6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.University of Michigan Medical Center, Brosius F. [Internet] 2015. Low-dose streptozotocin induction protocol (mouse). [Cited 30 May 2016]. Available at: https://diacomp.org/shared/showFile.aspx?doctypeid=3&docid=19.
- 44.Wess G, Reusch C. 2000. Assessment of 5 portable blood glucose meters for use in cats. Am J Vet Res 61:1587–1592. [DOI] [PubMed] [Google Scholar]
- 45.Wess G, Reusch C. 2000. Evaluation of 5 portable blood glucose meters for use in dogs. J Am Vet Med Assoc 216:203–209. [DOI] [PubMed] [Google Scholar]
- 46.Wittrock JA, Duffield TF, LeBlanc SJ. 2013. Short communication: validation of a point-of-care glucometer for use in dairy cows. J Dairy Sci 96:4514–4518. [DOI] [PubMed] [Google Scholar]
- 47.Zoetis 2015. AlphaTRAK2 blood glucose monitoring system user guide and package insert. Parsippany (NJ): Zoetis Services. [Google Scholar]