Abstract
A common method for urine collection from rats requires the use of a metabolic cage, thus exposing animals to extended periods of isolation in an unfamiliar cage with a wire-mesh floor. A new method involving hydrophobic sand, a material more similar to bedding, has become available recently but has not been extensively compared with metabolic cages in regard to collection efficiency or stress. Using a within-subjects crossover design, we examined differences in stress markers, urinary markers, and urine volume of clinically healthy male Sprague–Dawley rats during 2-, 4-, and 6-h collection sessions in hydrophobic sand and metabolic cages. Stress response markers of weight loss, fecal pellet output, or corticosterone did not differ between hydrophobic sand and metabolic cages, and observed behavior suggested that sand may be less stressful than metabolic cages. All clinically relevant urinary markers examined were normal, with no differences between collection methods. Total urine volume collected was greater from the metabolic cage than sand in 3 of the 5 sessions, but the volume collected during the shortest session (2 h) did not differ between methods and accounted for 62% of the total volume collected during the longest session (6 h). Our results suggest that hydrophobic sand is a refinement of urine collection methods for rats that decreases isolation time, risk of injury, and stress and maintains the integrity of urine samples.
Collecting urine samples from rodents in a volume sufficient for standard urinary testing protocols usually involves single housing the animals in metabolic cages for 16 to 24 h. Although this confinement is not considered overly stressful for the animal,5,6 habituation to the metabolic cage is recommended,9 and the collection procedure requires removing the animal from its homecage environment. Animal ethics review guidelines recommend that animals should not be housed in metabolic cages without express permission of the Animal Ethics Committee of the institution, and all efforts to enrich the cage and provide rats with visual, auditory, and olfactory contact with other rats should be provided as far as possible.1 Recently, a product developed to support nonstressful urine collection from cats was proposed as a potentially useful way to collect urine from rodents. Hydrophobic sand (for example, LabSand, Kit4Cat), a biodegradable material with a nontoxic urine-repelling coating, replaces the bedding in a normal cage during the urine collection period. After collection is complete, the rats can be returned to their normal homecage environment, and the used hydrophobic sand is discarded as laboratory waste.
Although no reports in the peer-reviewed literature discuss the use of this material for rodent urine collection, a poster presentation13 compared metabolic cages and hydrophobic sand in regard to urine collection volume and urinalysis integrity in mice. The authors found that 3-h collections from the sand yielded their necessary volume (0.2 mL) in 85% of mice, and 10 urinalysis markers showed no significant difference between 3-h collections from sand and 16-h collections from metabolic cages. However, the authors did not measure any stress markers in the mice, nor did they directly compare the same collection duration between sand and metabolic cages. Our literature search also returned an abstract from a JAALAS conference11 that compared collection volumes from hydrophobic sand and metabolic cages at various time points in both mice and rats. For mice, urine volume was significantly less in sand than metabolic cage only at the 24-h collection. For rats, urine volume was significantly less in sand than metabolic cages at the 2-, 4-, and 6-h collections. The cited abstract did not report the actual urine volumes collected and lacked any comparisons of stress or urinalysis assays between collection methods.
Corticosterone is a key glucocorticoid hormone that is produced in the adrenal gland of rodents and serves as a primary stress response; the human equivalent is cortisol.14 Urinary corticosterone levels are an accepted marker of stress response in rodents.2,8 The number of fecal pellets expressed during urine collection is another marker of stress.3,4,12 The goal of the current study was to determine whether the use of hydrophobic sand provides a useful urine sample from rats and reduces various stress markers measured during single housing in metabolic cages. We hypothesized that markers of stress during urine collection by using hydrophobic sand would be similar to or significantly less than those collected by using metabolic cages. In addition, we hypothesized that using the sand would not introduce deleterious contaminants that would alter clinically relevant urine marker measurements and properties in future studies.
Materials and Methods
Test subjects and housing conditions.
Experiments in this study were conducted at the Armed Forces Radiobiology Research Institute (Bethesda, MD). Male Sprague–Dawley rats (Rattus norvegicus; n = 8; age, approximately 30 d; weight, 75 to 100 g) were purchased from Envigo (Barrier 208A, Frederick, MD). Rats were allowed to acclimate in the vivarium for at least 2 wk prior to the start of experiments. The room was maintained at standard temperature (21 ± 2 °C) and humidity (30% to 70%), with a 12:12-h light:dark cycle (lights on, 0600) and restricted access to food (Teklad Global Rodent Diet 8604, Envigo) and water. Cages were changed 2 or 3 times weekly. Rats were pair-housed in plastic microisolation cages (23.8 × 45.4 cm) on bedding (Teklad Sani-Chips, Envigo) as home cages and individually in metabolic cages (Nalgene, Thermo Fisher, Pittsburgh, PA) or smaller (mouse) plastic microisolation cages (described following) containing hydrophobic sand during urine collection. All procedures involving animals were conducted to achieve maximal possible wellbeing of the rats, were IACUC-approved (protocol no. 2016-05-006) prior to the start of the study, and were performed in compliance with the guidelines set forth in the Guide for the Care and Use of Laboratory Animals7 in an AAALAC-accredited facility.
Urine collection apparatus.
For both collection methods, rats were individually housed for the duration of the session and immediately returned to pair housing in their home cages at the end of the session. Rats had free access to water replacement pouches (HydroGel, Clear H2O, Westbrook, ME) instead of water bottles, to avoid dilution of urine droplets in the hydrophobic sand. All cages were cleaned thoroughly with detergent (Contrex, Decon Labs, King of Prussia, PA) and water between sessions.
Metabolic cage.
The metabolic cages (Nalgene, Thermo Fisher) consist of a circular upper portion, which houses the rat; a wire-grid floor (diameter, 21.5 cm; approximate surface area, 363 cm2; opening, 1 × 3.1 cm); and a lower collection chamber with a specialized funnel that separates fecal pellets and urine that fall through the grid floor for their collection into 2 separate tubes (diameter, 4 cm; Nalgene, Thermo Fisher).
Hydrophobic sand.
For each rat, we spread a single 300-g (single pack) of hydrophobic sand (LabSand, Coastline Global, Palo Alto, CA) on the bottom of a mouse plastic microisolation cage (15.2 × 25.4 cm, surface area 386 cm2) with a filtered lid. Urine pooled on top of the sand and was collected at specific time intervals (see Group assignment and schedule of collection) by using a pipette.
Group assignment and schedule of collection.
In a within-subjects crossover design (Figure 1), rats were randomly assigned to either group A (metabolic cage followed by hydrophobic sand, n = 4) or group B (hydrophobic sand followed by metabolic cage, n = 4). Because a habituation period is highly recommended when using metabolic cages, both methods followed the procedure used for our previous studies10 that involved urine collection. Groups A and B were run simultaneously in the same testing room. For each method, urine was collected for a 2-h session, a 4-h session, and 3 sessions of 6 h each. Consecutive sessions were separated by a rest period of at least 48 h and occurred over a period of 2 wk, at which point the session schedule was repeated but by using the other collection method. Sessions began at 0800 h each day, and testing room lighting and temperature were maintained as in the regular housing room.
Figure 1.
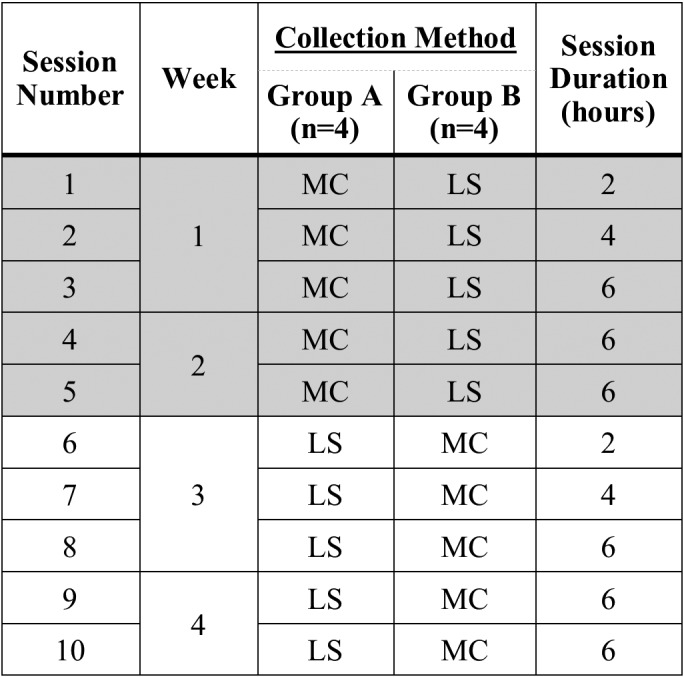
Experimental design. Rats were randomly assigned to either group A (metabolic cage followed by hydrophobic sand, n = 4) or group B (hydrophobic sand followed by metabolic cage, n = 4), and experiments were run simultaneously in a within-subjects crossover design. Five collection sessions with increasing length of time (2, 4, 6, 6, and 6 h, respectively) were performed over a period of 2 wk before the crossover, at which time the collection schedule was repeated.
Rats were weighed prior to and after each session, and each rat's total fecal pellets were counted for each session. In the metabolic cages, the urine collection tube is graduated in 2-mL increments, but urine volume can only be determined at the end of the collection period. For the hydrophobic sand, urine can be collected at any time; output volume was determined every 30 min during the session and pooled at the end. Refractometer and test strip analyses were completed immediately on all pooled urine samples before their storage at –80 °C until further analysis. All frozen samples were analyzed in a single session.
Urinalysis.
Multiple methods were used to assess routine urinary markers of general health and stress. A digital refractometer (model 300027, Kernco, El Paso, TX) was used to determine urine specific gravity (detection range, 1.000 to 1.050) and refractive index (detection range, 1.3330 to 1.3900). Clinically relevant urine markers were assessed by using test strips (catalog no. URS-10T, HealthyWiser, Eastleigh, Hampshire, United Kingdom). Each test strip consists of colorimetric reaction spots for 10 markers: leukocytes (range, negative to 500 cells/µL), nitrite (negative or positive), urobilinogen (3.2 to 125 µmol/L), protein (negative to >20.2 g/L), pH (5.0 to 8.5), blood (negative; trace of nonhemolyzed; or hemolyzed, 10 to 220 cells/µL), specific gravity (1.000 to 1.030), ketones (negative to 16 mmol/L), bilirubin (negative to 100 µmol/L), and glucose (negative to 110 mmol/L). Each square was wet with a droplet of urine, and the marker value was determined through comparison with a standard association chart after the required reaction time (30 to 120 s). The urine sticks were evaluated by eye by 2 technicians.
Creatinine.
Urine creatinine levels were determined by using a colorimetric creatinine assay kit (catalog no. CR01, Oxford Biomedical Research, Oxford, MI) and were read on a spectrophotometer (SpectraMax 190 with SoftMax Pro 2.0 software, Molecular Devices, Sunnyvale, CA). Briefly, urinary creatinine produces an orange color when it reacts with picric acid under alkaline conditions. This reaction also occurs with other components in biologic fluids, but the specific color produced by creatinine degrades rapidly under acidic conditions. Urine samples were diluted and placed in a 96-well plate; picric acid was added and the color reaction read at 490 nm. An acid reagent was then added and the reaction read again at 490 nm. The difference in absorbance reading was calculated, and samples values were determined against a creatinine standard curve (0 to 10.0 mg/dL) and corrected for dilution.
Corticosterone.
Urine corticosterone levels were determined by using a colorimetric corticosterone ELISA kit (catalog no. ab108821, Abcam, Cambridge, MA; minimal detectable dose, 0.28 ng/mL). Briefly, diluted urine samples were added to a 96-well plate precoated with a corticosterone-specific antibody. Biotinylated corticosterone was added to each well, which then was washed with wash buffer. Streptavidin–peroxidase conjugate was added to each well, and unbound conjugates were washed away with wash buffer. A chromogen substrate was added to each well to produce a blue color, which changed to yellow after the addition of an acidic stop solution. The plate was then read at 450 nm on a spectrophotometer (Spectramax 190, Molecular Devices); corticosterone concentrations were determined according to a standard curve (0 to 100 ng/mL) and corrected for dilution.
Statistical analysis.
Animal growth over time was determined as a line of best fit for each group's growth, and curves were compared between groups. The decrease in urine corticosterone over time was determined by generating a line of best fit for each group's growth, and curves were compared between collection methods (metabolic cage compared with hydrophobic sand). Unless specifically noted, all other data were analyzed as within-subjects 2-tailed t test comparisons between collection methods for each session time. All analyses were performed by using Prism (version 7.01, GraphPad Software, La Jolla, CA), and P values less than 0.05 were considered significant.
Results
Urinalysis.
Neither refractive index nor specific gravity differed between collection methods during any session (Table 1). All common urinalysis clinical markers assessed by using test strips were within normal ranges for all animals; there was no variability in results for nitrate (all tests negative), urobilinogen (all tests 3.2 µmol/l), or blood (all negative). Bilirubin was negative for 72 of the 80 tests; all 8 exceptions yielded 17 µmol/L, with no discernable pattern by group. Glucose was negative for all 80 tests except 1, which yielded a result of 15 mmol/L. Leukocyte counts, protein, pH, ketones, and creatinine were similar between collection methods and sessions (Table 1).
Table 1.
Analysis of common, clinically relevant urine markers and properties
| Collection method | Session | |||||
| 2 h | 4 h | first 6 h | second 6 h | third 6 h | ||
| Refractive index | MC | 1.343 ± 0.003 | 1.343 ± 0.003 | 1.342 ± 1.343 | 1.346 ± 0.004 | 1.344 ± 0.003 |
| HS | 1.340 ± 0.007 | 1.342 ± 0.004 | 1.343 ± 0.003 | 1.344 ± 0.005 | 1.344 ± 0.004 | |
| Specific gravity | MC | 1.028 ± 0.011 | 1.030 ± 0.009 | 1.026 ± 0.006 | 1.039 ± 0.011 | 1.033 ± 0.010 |
| HS | 1.026 ± 0.007 | 1.029 ± 0.012 | 1.030 ± 0.006 | 1.035 ± 0.013 | 1.032 ± 0.011 | |
| Leukocytes (no. of cells/µL) | MC | 90.63 ± 40.92 | 123.8 ± 154.8 | 83.75 ± 48.75 | 135.6 ± 153.3 | 81.88 ± 42.84 |
| HS | 83.75 ± 48.75 | 70.00 ± 41.58 | 54.38 ± 50.81 | 104.4 ± 40.92 | 73.13 ± 51.82 | |
| Protein (g/L) | MC | 0.78 ± 0.97 | 0.74 ± 0.99 | 0.37 ± 0.41 | 1.06 ± 1.22 | 0.58 ± 0.46 |
| HS | 1.42 ± 1.38 | 1.45 ± 1.38 | 0.74 ± 0.99 | 0.81 ± 0.94 | 0.56 ± 0.36 | |
| pH | MC | 8.25 ± 0.38 | 8.13 ± 0.44 | 8.00 ± 0.38 | 8.13 ± 0.52 | 7.50 ± 0.00 |
| HS | 8.19 ± 0.26 | 8.00 ± 0.53 | 8.00 ± 0.27 | 7.75 ± 0.46 | 7.69 ± 0.37 | |
| Ketones (mmol/L) | MC | 0.63 ± 0.74 | 0.12 ± 0.26 | 0.75 ± 0.80 | 0.20 ± 0.25 | 0.63 ± 0.35 |
| HS | 0.63 ± 0.74 | 0.44 ± 0.50 | 0.75 ± 0.80 | 0.46 ± 0.48 | 0.50 ± 0.46 | |
| Creatinine | MC | 0.63 ± 0.23 | 0.58 ± 0.19 | 0.64 ± 0.27 | 0.55 ± 0.26 | 0.56 ± 0.27 |
| HS | 0.59 ± 0.15 | 0.59 ± 0.36 | 0.68 ± 0.23 | 0.74 ± 0.26 | 0.57 ± 0.24 | |
HC, hydrophobic sand; MC, metabolic cage
Data are presented as mean ± 1 SD for each group (collection method and individual session) and analyzed by paired within-subjects t test for each collection session. All values are within normal reference ranges for rats; no parameter differed significantly between groups during the same session.
Urine collection.
The total volume of urine collected in metabolic cages and hydrophobic sand did not differ significantly by the end of the 2-h session (t7 = 1.002, P = 0.35) or the second 6-h session (t7 = 1.07, P = 0.32) but was greater in the metabolic cages by the end of the 4-h session (t7 = 4.43, P < 0.01) and first and third 6-h sessions (t7 = 4.47, P < 0.01; t7 = 4.47, P < 0.01, respectively; Figure 2 A).
Figure 2.

Urine volume collection. (A) Pooled urine output (mL) for each rat at the end of each session. Data presented as individual sample values and within-subjects comparison for each session. †, P < 0.01. (B) Urine collected only during the sessions using hydroscopic sand. Urine was collected every half hour and the volume determined. Data are presented as the mean ± SEM of the cumulative volume for each subject across each session.
Due to the style of the collection tube in the metabolic cages, we were unable to assess urine output throughout each session. However, we collected urine from hydrophobic sand every 30 min, and the collection is graphed as cumulative urine volume over time (Figure 2 B) to assess the pattern of urine output. The rate of urine output slowed over time and did not yield a linear accumulation: more than half of the total volume was collected during the first 2 h. The cumulative volume (mean ± 1 SD) collected at 2 h (all 5 sessions) was 1.15 ± 0.62 mL and represents 70% ± 30% of the total volume collected during all 5 sessions). Excluding the 2-h session, the 2-h cumulative volume for the other 4 sessions was 62% ± 29% of the total volume.
Stress assessment.
Initial weight (mean ± 1 SD) did not differ between the 2 groups of rats (group A, 236.4 ± 2.5 g; group B, 240.2 ± 2.0 g; t6 = 2.43, P = 0.05). Weight gain in animals over the course of the entire experiment appeared normal. Changes in rat weight (in grams) over time did not differ between the groups (group A: y-intercept, 235.3 g; slope, 4.0 g/d; R2 = 0.971; group B: y-intercept, 239.7 g; slope, 4.2 g/d; R2 = 0.974; comparison of fits: F1,76 = 1.491, P = 0.24). Weight loss during each session did not differ between the metabolic cage and hydrophobic collection methods for any session (2-h: t7 = 0.86, P = 0.42; 4-h: t7 = 1.65, P = 0.14; first 6-h: t7 = 0.29, P = 0.78; second 6-h: t7 = 0.97, P = 0.36; third 6-h: t7 = 0.35, P = 0.73; Figure 3 A).
Figure 3.
Stress indicators. (A) Each animal was weighed at the beginning and the end of each session and the difference calculated as weight lost during the session. (B) Fecal pellets were counted for each animal at the end of each session. (C) Corticosterone concentration was determined by ELISA for each animal's pooled urine sample for each session. For panels A through C, all data are presented as individual sample values and within-subjects comparison for each session. †, P < 0.01. (D and E) Representative images of ambulatory behavior during the collection sessions. (D) Rat in metabolic cage (E) on hydrophobic sand. (F and G) Representative images of sleeping behavior during the collection sessions. (F) Metabolic cage. (G) Hydrophobic sand.
Total fecal pellet counts did not differ between collection methods during the 4-h session (t7 = 1.02, P = 0.34) or any of the 6 h sessions (t7 = 1.02, P = 0.33; t7 = 1.08, P = 0.32; t7 = 0.47, P = 0.66). In the 2-h session, the count (mean ± 1 SD) was significantly higher on hydrophobic sand (4.5 ± 3.5 pellets) than in metabolic cages (1.5 ± 2 pellets; t7 = 3.31, P = 0.01; Figure 3 B). However, this difference is due to a single rat on hydroscopic sand that had a much higher pellet count than the rest of the group (Dixon test for a single outlier, P < 0.05).
Urine corticosterone concentrations did not differ between metabolic cages and hydrophobic sand in any of the sessions (2-h: t7 = 0.70, P = 0.51; 4-h: t7 = 0.20, P = 0.85; first 6-h: t7 = 1.07, P = 0.32; second 6-h: t7 = 0.71, P = 0.50); third 6-h: t7 = 0.71, P = 0.50; Figure 3 C). In addition, corticosterone levels decreased for all subjects over subsequent sessions, but with no significant difference in concentration between collection methods (metabolic cage: y-intercept, 20.77 ng/mL; slope, –1.421 ng/mL/h; R2 = 0.285; hydrophobic sand: y-intercept, 23.78 ng/mL; slope, –1.575 ng/mL/h; R2 = 0.369; comparison of fits: F1,76 = 0.087, P = 0.77).
During each session, animal behavior was observed but not quantified. Rats in metabolic cages did not exhibit overt signs of stress but, compared with those on hydrophobic sand, appeared less ambulatory and had greater difficulty walking due to the wire grid floor (Figure 3 D). Rats in the cages containing hydrophobic sand appeared more relaxed than those in metabolic cages, exhibiting normal exploratory and grooming behavior similar to that seen in home cages with the usual bedding (Figure 3 E). In the metabolic cage, rats often slept or rested with their heads tucked under their chests (Figure 3 F); in the cages containing hydrophobic sand, rats rested curled in a C shape. (Figure 3 G). In addition, all rats consumed some of the available hydrocup and, in the later sessions, were observed to flip it over and stand on it. All rats displayed normal behaviors after returning to their home cage.
Discussion
Currently metabolic cages are one of the few IACUC-approved—and the most commonly used—methods of collecting urine from laboratory rodents. Although effective, their use must be justified due to the potential for the prolonged isolation, unfamiliar enclosure shape, and wire flooring to cause stress or injury to animals. A new, alternative urine collection method, hydrophobic sand, has recently become commercially available, but little research has been published on its use, effectiveness, or capacity to induce stress in rodents. To our knowledge, this study represents the first peer-reviewed publication to compare hydrophobic sand with metabolic cages as a potential refinement of urine collection methods in rats.
Our main goal was to determine whether hydrophobic sand would be a successful alternative method of urine collection in rats. Our condition for hydrophobic sand qualifying as ‘successful’ was a minimum of not differing significantly from metabolic cages in regard to normal urinary markers and properties, volume of urine collected, and measures of stress. Given the many small pieces in the metabolic cages that must be assembled, disassembled, and cleaned between uses, the ability to use an alternate method with a faster set-up, easier clean-up, and no additional stress to rats for the same quantity and quality of urine collection is highly desired. If hydrophobic sand proved to be less stressful or more efficient (or both), these characteristics would provide even more reason to use hydrophobic sand instead of metabolic cages. To accomplish this evaluation, we used a within-subjects crossover design so each rat served as its own control for comparing the 2 collection methods, thus increasing the statistical power while minimizing the number of animals needed for the study.
The most important of the criteria for using hydrophobic sand instead of metabolic cages in future studies is ensuring that the sand does not induce greater stress in the rats. Neither a study evaluating mice only13 nor one involving mice and rats11 analyzed stress-specific differences associated with the collection methods. Hydrophobic sand and metabolic cages present 2 structurally different environments for the rat. The mouse cages used for the sand provided 386 cm2 of floor space in a familiar rectangular shape, and the texture of the sand was similar to that of regular bedding and poses no risk of injury to small feet. In contrast, rats had less floor space (363 cm2) in metabolic cages, an unfamiliar circular shape lacking corners to huddle in, and a wide wire-mesh floor they had to learn to navigate or risk getting a foot caught in the grid. For both collection methods, hydrocups were included during every session to serve as sources of hydration instead of water bottles that might potentially dilute urine. The cups also served as a form of enrichment to counteract the isolation required for both methods. From the exploratory and resting behavior we observed and based on our experience, the rats were more comfortable and relaxed in the sand environment than in metabolic cages.
In addition to observed behaviors, we quantified 3 common measures of stress response: weight loss, fecal pellet count, and urinary corticosterone. Both crossover groups had the same initial weight and the same weight gain over the course of the entire experiment. All animals displayed normal behaviors and were naïve to any treatment. Neither method induced rapid weight loss during any session. Although the 2-h session had significantly higher fecal pellet counts in the sand group, this difference was due to a single outlier, and fecal pellet count did not differ between sand and metabolic cage for any other session. Corticosterone, a hormone produced by the adrenal gland, is recognized as positively correlating to stress level in rats. However, urinary corticosterone concentrations did not differ between collection methods for any session. We also noted that corticosterone decreased over time (across repeated exposure and longer session times) for both groups, with no significant difference in slope, indicating that rats habituated equally to each urine collection method. Our results suggest that using hydrophobic sand as a urine collection method induces no more stress than metabolic cages and, on the basis of our subjective and unquantified assessment of the rats’ behavior, potentially provides a less stressful environment that is insufficient to elicit a change in urine corticosterone concentrations.
The next important comparison involves the quality of the urine collected, to ensure that clinically relevant urinary markers and properties do not differ between methods and thus create a confounding variable in future studies. A previous study in mice11 did not examine any urine markers and another that involved both mice and rats13 reported no significant differences between sand and metabolic cage collection methods in 10 basic urinary markers. We compared several urinary properties (refractive analysis and specific gravity) and markers (creatinine, leukocytes, protein, pH, ketones, nitrate, urobilinogen, blood, bilirubin, and glucose) and found no significant differences between using hydrophobic sand or metabolic cages for urine collection for any session. Although the urine test strips are not considered as accurate as more advanced diagnostic techniques, our results suggest that the use of hydrophobic sand does not introduce any contaminants or alter urine properties in any way that would be relevant to future studies using urine for analysis.
The final determination of the success of hydrophobic sand as an alternative method for urine collection is its efficiency compared with the metabolic cage procedure, that is, the ability to collect a useful volume of urine in the same amount of time as metabolic cages. We examined this characteristic in 2 ways: by comparing the total volume collected for each session across methods and by calculating the cumulative volume of urine collected over time within each session. When comparing across methods within a session, we found significantly higher total urine volume from metabolic cages than sand in 3 of the 5 sessions (4 h, and the first and third 6 h sessions); urine volume did not differ between methods for the other 2 sessions. The structure of the metabolic cage and its urine collection tube does not accommodate determination of the urine volumes at any time other than the end of the experiment; therefore, no cumulative totals over time are reported. However, urine is easily collected from hydrophobic sand at any time because it pools on the surface and can be removed by using a pipette. We collected urine from sand subjects every half hour for the duration of each session and reported this value as the cumulative volume collected for each session. Although the total volume is greater for the longer sessions than the shorter ones, as expected, we found that more than half (almost 62%) of the total urine is collected within the first 2 h of the session, providing us with an average of 1.2 mL of urine per subject. Depending on the volume needed for subsequent analyses, a single 2-h session, or several 2-h sessions spaced over several days, likely would allow collection of a sufficient amount of urine from each rat yet minimize the time spent in isolation and away from the home cage, especially given that total urine volume collected did not differ between sand and metabolic cages for the 2-h session. Similarly, a previous study11 reported that in mice lower total urine volumes were collected from sand than metabolic cages during a 24-h session but not during 3- and 6-h sessions, whereas rats had lower total urine volumes from sand during all sessions examined (2, 4, and 6 h). However, the investigators in the cited study collected urine only at the end of each session, not every half hour as we did, and they noted that they observed rats drinking urine droplets. We similarly observed rats ingesting urine between the half-hour collection times, whereas the rats in the metabolic cages have no access to excreted urine. Therefore, had urine been collected from the sand as it was deposited rather than at set times, we expect that the total urine volume collected during a session would have been higher than what we report here.
Together, our data suggest that hydrophobic sand is a viable alternative method for collecting urine from laboratory rats that does not alter important urine properties or induce any more stress than does the use of metabolic cages. In addition, the use of hydrophobic sand is advantageous for studies that require greater volumes of urine because collection can be repeated more easily over multiple days in shorter sessions rather than as a single, continuous session in a metabolic cage, thus decreasing periods of isolation. Investigations involving urinary metabolites that exhibit diurnal variation would also benefit from using hydrophobic sand, because sample collections can be timed more precisely. In addition, hydrophobic sand is easily discarded as laboratory waste and does not require extensive disassembly and cleaning, as do metabolic cages, and therefore may prove to be more cost-effective than purchasing a large number of metabolic cages.
Acknowledgments
The project described was supported by the grant Assessing the Health Effects of Blast Injuries and Embedded Metal Fragments (W81XWH-16-2-0058) from the Congressionally Directed Medical Research Programs (CDMRP) Peer-reviewed Medical Research Program.
All procedures involving animals were (a) conducted with maximal possible well-being of the rats, (b) approved by the AFRRI IACUC prior to the start of the study under protocol 2016-05-006, and (c) performed in compliance with the guidelines set forth in the Guide for the Care and Use of Laboratory Animals in an AAALAC-accredited facility.
The use of the LabSand brand of hydrophobic sand in this work does not represent an endorsement of the product or company by the US Government.
The views expressed in the paper are those of the authors and do not reflect the official policy or position of the Armed Forces Radiobiology Research Institute, Uniformed Services University, Department of Defense, or US Government.
References
- 1.Animal Research Review Panel 2007. [Internet]. Guideline 20: guidelines for the housing of rats in scientific institutions. [Cited 26 May 2017]. Available at http://www.animalethics.org.au/__data/assets/pdf_file/0014/222512/housing-rats-scientific-institutions.pdf
- 2.Bamberg E, Palme R, Meingassner JG. 2001. Excretion of corticosteroid metabolites in urine and faeces of rats. Lab Anim 35:307–314. [DOI] [PubMed] [Google Scholar]
- 3.Barone FC, Barton ME, White RF, Legos JJ, Kikkawa H, Shimamura M, Kurtani K, Kinoshita M. 2007. Inhibition of phosphodiesterase type 4 decreases stress-induced defecation in rats and mice. Pharmacology 81:11–17. [DOI] [PubMed] [Google Scholar]
- 4.Cavigelli SA, Guhad FA, Ceballos RM, Whetzel CA, Nevalainen T, Lang CM, Klein LC. 2006. Fecal corticoid metabolites in aged male and female rats after husbandry-related disturbances in the colony room. J Am Assoc Lab Anim Sci 45:17–21. [PubMed] [Google Scholar]
- 5.Eriksson E, Royo F, Lyberg K, Carlsson HE, Hau J. 2004. Effect of metabolic cage housing on immunoglobulin A and corticosterone excretion in faeces and urine of young male rats. Exp Physiol 89:427–433. [DOI] [PubMed] [Google Scholar]
- 6.Gil MC, Aguirre JA, Lemoine AP, Segura ET, Barontini M, Armando I. 1999. Influence of age on stress responses to metabolic cage housing in rats. Cell Mol Neurobiol 19:625–633. [DOI] [PubMed] [Google Scholar]
- 7.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 8.Jorgensen A, Maigaard K, Wortwein G, Hageman I, Henriksen T, Weimann A, Moller P, Loft S, Hau J, Poulsen HE, Jorgensen MB. 2013. Chronic restraint stress in rats causes sustained increase in urinary corticosterone excretion without affecting cerebral or systemic oxidatively generated DNA/RNA damage. Prog Neuropsychopharmacol Biol Psychiatry 40:30–37. [DOI] [PubMed] [Google Scholar]
- 9.Kurien BT, Everds NE, Scofield RH. 2004. Experimental animal urine collection: a review. Lab Anim 38:333–361. [DOI] [PubMed] [Google Scholar]
- 10.Mak TD, Tyburski JB, Krausz KW, Kalinich JF, Gonzalez FJ, Fornace AJ., Jr 2014. Exposure to ionizing radiation reveals global dose- and time-dependent changes in the urinary metabolome of rat. Metabolomics 11:1082–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinkus CA, Pereira A, Nguyen A, Debrue MC, Menendez LG. 2016. Use of hydrophobic sand as a refinement for urine collection in mice and rats. Abstracts presented at the American Association for Laboratory Animal Science National Meeting, Charlotte, North Carolina, October 30–3 November 2016. J Am Assoc Lab Anim Sci 55:607. [Google Scholar]
- 12.Smith AL, Leung J, Kun S, Zhang R, Karagiannides I, Raz S, Lee U, Golovatscka V, Pothoulakis C, Bradesi S, Mayer EA, Rodriguez LV. 2011. The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology 78:967.e1–967.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JC, Lancaster H, Ridley D, McClure F, Whelan G. [Internet]. 2015. 3Rs refinement–use of hydrophobic sand in collection of analytical urine samples [Cited 26 May 2017]. Available at: http://labsand.com/wp-content/uploads/2015/05/GSK-poster.pdf
- 14.Tulane University. [Internet] 2014. The hormones: corticoids. [Cited 26 May 2017]. Available at: http://e.hormone.tulane.edu/learning/corticoids.html [Google Scholar]



