Abstract
Despite increased use of zebrafish (Danio rerio) in biomedical research, consistent information regarding appropriate euthanasia methods, particularly for embryos, is sparse. Current literature indicates that rapid cooling is an effective method of euthanasia for adult zebrafish, yet consistent guidelines regarding zebrafish younger than 6 mo are unavailable. This study was performed to distinguish the age at which rapid cooling is an effective method of euthanasia for zebrafish and the exposure times necessary to reliably euthanize zebrafish using this method. Zebrafish at 3, 4, 7, 14, 16, 19, 21, 28, 60, and 90 d postfertilization (dpf) were placed into an ice water bath for 5, 10, 30, 45, or 60 min (n = 12 to 40 per group). In addition, zebrafish were placed in ice water for 12 h (age ≤14 dpf) or 30 s (age ≥14 dpf). After rapid cooling, fish were transferred to a recovery tank and the number of fish alive at 1, 4, and 12–24 h after removal from ice water was documented. Euthanasia was defined as a failure when evidence of recovery was observed at any point after removal from ice water. Results showed that younger fish required prolonged exposure to rapid cooling for effective euthanasia, with the required exposure time decreasing as fish age. Although younger fish required long exposure times, animals became immobilized immediately upon exposure to the cold water, and behavioral indicators of pain or distress rarely occurred. We conclude that zebrafish 14 dpf and younger require as long as 12 h, those 16 to 28 dpf of age require 5 min, and those older than 28 dpf require 30 s minimal exposure to rapid cooling for reliable euthanasia.
Abbreviations: dpf, days postfertilization; MS222, tricaine methanesulfonate
The use of zebrafish (Danio rerio) in biomedical research has expanded greatly in recent years, and this species increasingly replaces mammalian species in the study of human disease. In accordance with Russell and Burch's 3 Rs (replacement, refinement, and reduction),16 researchers attempt to use the least sentient species possible to address the question of interest. Some of the characteristics of zebrafish that make them an appealing model include their small size, ease of housing, large number of offspring, rapid and easily visualized development, short intergenerational time, and ease of genetic manipulation.5,12,19
The increasing interest in zebrafish comes with a need for information about the appropriate management and care of these animals, including humane euthanasia. The AVMA requires euthanasia methods that minimize pain and distress, lead to rapid loss of consciousness, are irreversible, and safe for staff. The AVMA also encourages consultation with veterinarians who possess professional experience in the aquatic species of interest to determine the appropriate method of euthanasia.1 However, euthanasia methods for zebrafish, particularly young zebrafish, remain controversial. Several techniques that are effective in adults are ineffective in larvae, leading to many variations in euthanasia protocols between institutions.3
Tricaine methanesulfonate (MS222) is by far the most common method of euthanasia for adult zebrafish (that is, older than 90 days postfertilization [dpf]).5 However, there is consistent literature reporting that zebrafish embryos (0 to 3 dpf) and larvae (3 to 29 dpf) are resistant to euthanasia with MS222, even with prolonged exposure times and at concentrations much higher than those necessary for euthanizing adults.3,9,18 Clove oil and its extracted chemical compound eugenol are acceptable with conditions as euthanasia agents,1 however, one study has shown that these methods are ineffective for the euthanasia of larval fish.18 Similarly, lidocaine—like MS222 and eugenol—is effective in adult but not larval zebrafish.3
According to the 2013 AVMA Guidelines for the Euthanasia of Animals, rapid cooling is an acceptable method of euthanasia for larvae and adult zebrafish.1 Studies have shown that, compared with MS222, rapid cooling is a faster, less stressful, and more consistent method of euthanasia in adult zebrafish,9,21 suggesting that rapid cooling provides benefits over the more commonly used methods. However, the study that helped refine the 2013 AVMA Guidelines for Euthanasia was performed on adult zebrafish older than 6 mo only and did not test the efficacy of rapid cooling in larval zebrafish.21
Only 3 published studies have examined rapid cooling in young zebrafish, with profoundly differing results. A study performed at the University of Oregon concluded that rapid cooling is an effective method of euthanasia when zebrafish larvae (age 4 to 7 dpf) are exposed to hypothermal shock for at least 20 min but that this method is ineffective for euthanizing 1 to 2 dpf zebrafish.9 In contrast, a recently published study found that rapid cooling was ineffective at euthanizing zebrafish up to 10 dpf even when exposed to hypothermia for up to 60 min.2 Finally, rapid cooling was a reliable method of euthanasia in 14 dpf zebrafish when exposed for at least 40 min.18 The varying results in the efficacy of rapid cooling for zebrafish younger than 10 dpf and the lack of information regarding the point at which young fish respond similarly to adults indicate a need for further investigation.
The current study was performed to distinguish the age at which rapid cooling is an effective method of euthanasia for zebrafish between 3 dpf and 90 dpf and the exposure times required to reliably euthanize zebrafish using this method. We hypothesized that younger fish need prolonged exposure to rapid cooling for effective euthanasia and the time of exposure would decrease as age increased.
Materials and Methods
Husbandry.
This study was performed in compliance with the Guide for the Care and Use of Laboratory Animals,7 with approval by the IACUC of the University of Pennsylvania, an AAALAC-accredited institution. All fish in the facility were observed daily by staff or veterinary technicians, and veterinary care was available at all times. The facility allows only bleached embryos to enter the main facility; no new adult zebrafish enter into the colony. All new fish enter a separate non-recirculating quarantine room, where they are bred; the resulting embryos are disinfected through a multistep bleaching process (described later) and then introduced into the system. Pseudoloma neurophila is present within the colony, but no other infectious diseases have been identified in the past 10 y. From the time fish are put into the recirculating water system at 6 dpf until they are euthanized at 1 to 1.5 y, the normal mortality rate within the facility is typically less than 10%.
Adult zebrafish (Danio rerio; age 6 to 10 mo) of the wildtype strain TU were bred to produce all study fish. Adult fish were housed in a recirculating system of mixed-sex, 14-L tanks (Aqua Schwartz, Göttingen, Germany) with approximately 45 fish per tank. Adults were fed once daily with tropical fish flakes (TetraMin Tropical Flakes, Tetra, Blacksburg, VA) and once daily with brine shrimp (Artemia sp.; 90% hatching grade, Sanders Great Salt Lake Artemia, Mountain Green, UT) hatched in the facility. Zebrafish bred in the facility typically reach spawning age by 3 to 4 mo, and the adults bred in this study were not set up for spawning prior to the study. A total of 60 paired matings in 5 separate spawning events with at least 14 d between spawnings produced the 1221 viable embryos that were used in this study. After paired mating, zebrafish embryos were removed from mating cages and maintained in dishes of standard E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4 in reverse-osmosis-purified water10). The embryos were bleached at 1 dpf in 0.004% sodium hypochlorite in E3 media (twice for 5 min each), with a 5 min wash in E3 between bleachings and 2 washes (5 min each) after the last bleaching step. The embryos remained until 3 dpf in 15-mL culture dishes containing E3 with 0.3 mg pronase to aid in softening the chorion (which hardens in the bleach) and subsequent hatching from the chorion. Embryos were defined as viable when they were fertilized, successfully completed gastrulation, and were morphologically normal at 3 dpf. Embryos that had not hatched at 3 dpf were manually freed from chorions, the E3 media was changed, and at 6 dpf the embryos were transitioned to 2.5-L tanks (Aqua Schwartz) containing system water.
Offspring were housed in 35 total tanks, with 15 to 60 fish per tank. All fish remained in 2.5-L tanks for the duration of the study, with approximately 9% mortality from 3 to 90 dpf. The facility follows standard operating procedures on the housing of young zebrafish, with a maximum density of 60 per tank, and has found no negative effect on growth or survival when fish are housed in this manner until 90 dpf. They were fed paramecia (100 mL at about 100 to 150 organisms/mL) 3 times daily until 13 dpf, after which time they were fed brine shrimp 3 times daily. Paramecia were raised in the facility in 20-L carboys in system water, fed brewers yeast and wheat kernels, and were checked daily under a microscope for density and potential contamination. Beginning at 45 dpf, zebrafish were fed dry flakes once daily and brine shrimp twice daily. Water flow rate began very slowly and was increased with age, with approximately 10% daily water exchange with fresh water. Beginning around 11 dpf, excess water was allowed to overflow from the tank, and the water from many tanks was collected and recirculated.
Water to all tanks in the facility was tap water that had been purified by reverse osmosis and treated by passage over a mechanical particle filter, a biologic filter, and 200 mJ/cm2 UV lights prior to recirculation. Room and water temperatures were both maintained at 26 to 28 °C and were controlled by temperature controllers and water heaters. Nitrate and nitrite levels were assayed by using test strips (Pentair Aquatic Eco-Systems, Apopka, FL) and were undetectable. The conductivity of the system water was maintained at 220 to 350 µS, and pH was maintained at 7.2 ± 0.1. Temperatures, conductivity, pH, nitrate, and nitrite levels were monitored daily and did not need adjustment during these studies. Room lights were maintained on a 13:11-h light:dark cycle.
Euthanasia.
All euthanasia was performed during the light phase, in a procedure room adjacent to the fish housing facility. For 3 to 28 dpf fish, a tea strainer (plastic, purchased locally in Mannheim, Germany; Figure 1) was placed into a holding tank containing a slurry of approximately equal amounts of ice and water such that fish would be placed into water maintained at 0 to 4 °C yet would not directly contact the ice; for 60 and 90 dpf fish, a slatted spawning box (Binsert2, Pentair Aquatic Eco-Systems) was used as described previously, instead of the tea strainer.21 Fish were transferred to the ice water bath by using a flexible pipette (at 3 and 4 dpf) or net (7 dpf and older). A camera was mounted above the euthanasia setup for recording to determine time to loss of righting reflex and monitor frequency of stressful behaviors. Zebrafish (3, 4, 7, 14, 16, 19, 21, 28, 60, and 90 dpf) were placed into the iced water bath for 5, 10, 30, 45, or 60 min (n = 12 to 40 per group). In addition, zebrafish 14 dpf and younger were exposed for 12 h, and zebrafish 14 dpf and older were tested at a 30 s exposure period. Fish were euthanized in groups of 4 to 40 within each age and time point (total euthanasia runs, 61). The 16 and 19 dpf groups were added after initial data were analyzed, to help clarify the age at which rapid cooling becomes effective. Therefore, except for the 16 and 19 dpf fish, all groups were represented by multiple breeding events.
Figure 1.
Setup for euthanasia of 3 to 28 dpf zebrafish. A tea strainer was placed inside a holding tank of approximately equal parts ice and water, so that fish did not have direct contact with ice. The temperature of water within the tea strainer was measured before and after each euthanasia run and was maintained at 0 to 4 °C.
Signs of pain and distress (piping, twitching, and erratic swimming)21 were evaluated in all fish. The time until the last fish in a group lost its righting reflex was recorded as an estimate of the time to loss of consciousness, after which point the fish are unable to experience pain or distress.3 The last fish to lose this reflex in each group was evaluated, because this fish was the most likely to experience distress. After the period of rapid cooling, the fish were transferred to a recovery tank of standard tank water at 26 to 28 °C. All materials were disinfected after use. Spawning boxes were washed in a dishwasher, tea strainers and fish nets were boiled for 1 h, and holding tanks were autoclaved.
Fish in the recovery tank were monitored at 3 time points (1, 4, and 12 to 24 h) after removal from ice water, and the number of fish that recovered from rapid cooling was documented. Euthanasia by rapid cooling was defined as a failure when evidence of recovery was observed (return of righting reflex, purposeful movements) at any point after removal from the ice-water bath. Maintenance of fish in the recovery tank for at least 12 h served as a confirmatory method of euthanasia for fish that were euthanized successfully by rapid cooling, and tissue decomposition was apparent at this time. Nevertheless, fish were transferred from the recovery tank to a freezer immediately after the 12 to 24 h check. Fish that recovered from the rapid cooling test were euthanized by replacing them in the ice-water bath for 12 h and then immediately transferring them to a freezer. Lack of opercular movement was not used as an indication of euthanasia, because the operculum is very difficult to see in subadult zebrafish and requires microscopic visualization. All fish 14 dpf and older were measured for standard length according to previously published methods immediately after placement into the recovery tank.13
Statistics.
The percentage of fish recovering from rapid cooling was documented for each age group and time point. Two separate Pearson chi-square tests were performed to determine whether 1) age and 2) exposure time significantly affected the proportion of fish that recovered from rapid cooling. When using chi-square testing, no posthoc test is available to determine which groups within the analysis differ significantly. Final recommendations regarding euthanasia methods require 100% euthanasia success with no recoveries. One-way ANOVA was used to determine significant differences between age and time to loss of righting reflex for fish 14 dpf and older—because the younger fish did not lose normal orientation, even after death—and to evaluate significant differences between age and standard length. When a significant effect was detected, a Tukey posthoc test was performed. A P value less than or equal to 0.05 was considered significant.
Results
Survival according to age and duration of exposure to rapid cooling.
Table 1 lists the percentage of fish recovering from rapid cooling by age and exposure time to the hypothermia and the results of the χ2 analyses. Recovery rate after different exposure times to rapid cooling for fish at all age groups can be found in Figure 2.
Table 1.
Percentages of fish recovering after removal from the cold water, according to age and exposure time
| 30 s | 5 min | 10 min | 30 min | 45 min | 60 min | 12 h | ||
| 3 dpf | 100% (n = 16) | 100% (n = 15) | 100% (n = 16) | 100% (n = 16) | 87% (n = 15) | 0% (n = 17) | P < 0.001 | |
| 4 dpf | 100% (n = 16) | 100% (n = 16) | 94% (n = 16) | 100% (n = 16) | 100% (n = 16) | 0% (n = 20) | P < 0.001 | |
| 7 dpf | 100% (n = 15) | 53% (n = 30) | 6% (n = 21) | 6% (n = 18) | 15% (n = 20) | 0% (n = 17) | P < 0.001 | |
| 14 dpf | 93% (n = 15) | 53% (n = 17) | 31% (n = 16) | 26% (n = 15) | 13% (n = 15) | 0% (n = 15) | 0% (n = 15) | P < 0.001 |
| 16 dpf | 60% (n = 15) | 0% (n = 18) | 0% (n = 12) | 0% (n = 12) | 0% (n = 15) | 0% (n = 15) | P < 0.001 | |
| 19 dpf | 0% (n = 15) | 0% (n = 15) | 0% (n = 14) | 0% (n = 14) | 0% (n = 14) | 0% (n = 14) | ||
| 21 dpf | 13% (n = 15) | 0% (n = 25) | 0% (n = 15) | 0% (n = 14) | 0% (n = 15) | 0% (n = 15) | P < 0.05 | |
| 28 dpf | 0% (n = 19) | 0% (n = 17) | 0% (n = 16) | 0% (n = 17) | 0% (n = 16) | 0% (n = 15) | ||
| 60 dpf | 0% (n = 35) | 0% (n = 40) | 0% (n = 34) | 0% (n = 35) | 0% (n = 36) | 0% (n = 40) | ||
| 90 dpf | 0% (n = 34) | 0% (n = 34) | 0% (n = 31) | 0% (n = 33) | 0% (n = 38) | 0% (n = 35) | ||
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
The P values from the χ2 analysis indicate significant differences within age (right column) and exposure time (bottom row) groups regarding recovery from rapid cooling. Cells without a P value indicate that no fish recovered; consequently a χ2 analysis could not be performed. Fish 7 dpf and younger were not tested at 30 s, and fish 16 dpf and older were not tested at 12 h. The analyses indicate that more fish recover at younger ages than at older ages and that fish are less likely to recover as exposure time increases.
Figure 2.
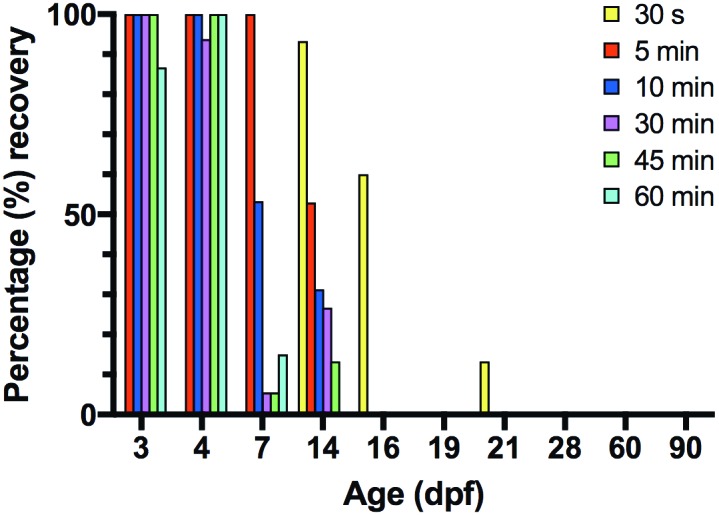
Percentage recovery, according to age and time of exposure to rapid cooling. Zebrafish (n = 1221; age 3 to 90 dpf) were placed in a rapid cooling euthanasia bath for 5 to 60 min. Additional groups of 14 to 90 dpf zebrafish were placed in a rapid cooling euthanasia bath for 30 s. The number of fish that recovered after transfer to a recovery bath of normal tank water was recorded. Except for 16 and 21 dpf fish exposed for 30 s, no fish 16 dpf or older recovered at any time point.
Survival according to duration of rapid cooling.
Except for 12 h, recovery from rapid cooling differed significantly (P < 0.001 for all exposure times) according to age. For each exposure time, younger fish were more likely to recover than older fish. There were no significant differences for fish exposed to rapid cooling for 12 h, as no fish recovered regardless of age.
Survival according to age.
Fish 3, 4, 7, 14, 16, and 21 dpf showed significant differences between exposure time and recovery (P < 0.001 for fish 16 dpf and younger and P < 0.05 for 21-dpf fish). For each of these age groups, the fish were less likely to recover as exposure time increased. No significant differences emerged for fish exposed to rapid cooling at 19, 28, 60, or 90 dpf, because no fish recovered regardless of exposure time. In addition, 21 dpf fish had a higher recovery rate than 19 dpf fish.
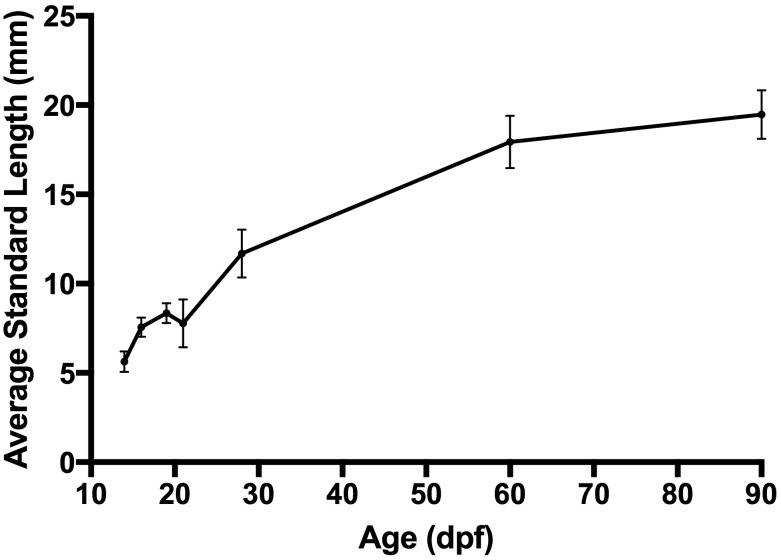
Effect of age on standard length.
As expected, standard length differed significantly (P < 0.001, one-way ANOVA; Figure 3) as the fish increased in age. A posthoc Tukey test showed that all comparisons were significant at P < 0.001, with the exception of 2 comparisons: 19 dpf fish were significantly (P = 0.018) longer than 21 dpf fish, and length did not differ (P = 0.899) between 16 and 21 dpf fish. Except for 21 dpf fish, all zebrafish grew as expected along a standard growth curve. No obvious cause for this discrepancy such as changes in temperature, water chemistry, or feeding was identified.
Figure 3.
Standard length of zebrafish, according to age. Standard length (mean ± 1 SD) was measured for all fish 14 dpf or older. All zebrafish grew as expected along a normal growth curve, except for 21 dpf fish, which were similar in length to 16 dpf fish.
Signs of pain and distress and time to loss of righting reflex.
Occurrences of twitching, piping, and erratic swimming were documented as signs of pain or distress. Twitching was seen in fish 7 dpf and younger only; within this age range, approximately 25% of fish displayed brief twitching. Piping and erratic swimming occurred in fish 60 dpf and older only, with 5 episodes of piping and one case of erratic swimming observed. In addition, some fish in each euthanasia group through 28 dpf displayed occasional lateral muscular contraction for as long as 5 min. Fish older than 28 dpf did not display this behavior and the last fish within each euthanasia group became immobile within an average of 9.2 s.
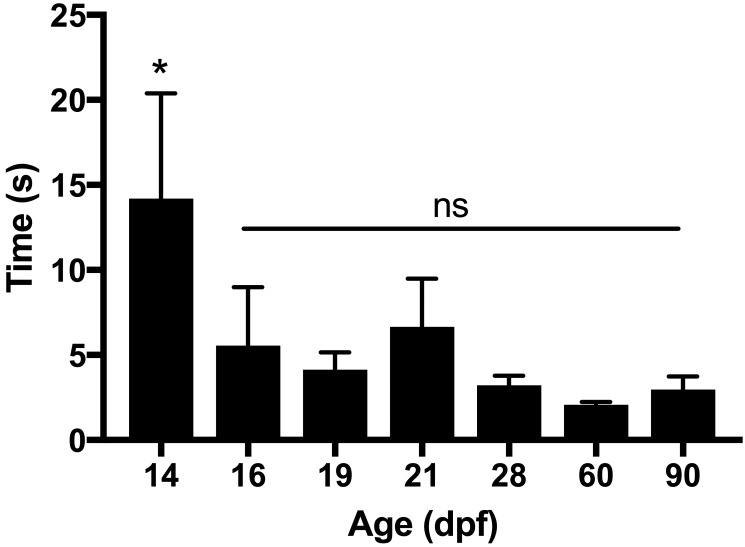
Loss of righting reflex was documented as the time at which the last fish in each euthanasia group became unable to maintain normal upright position. Zebrafish at 3 and 4 dpf, as well as some fish at 7 dpf, sank to the bottom of the euthanasia chamber but maintained normal position throughout the euthanasia periods. This behavior was likely due to the anatomic shape at larval stages, which is characterized by large eyes and the presence of the yolk sac, thus creating a wide base and preventing the fish from becoming lateral. Therefore these ages were not included in the analysis. Time to loss of righting reflex differed significantly (P < 0.001, one-way ANOVA) according to age (Figure 4). The posthoc test showed that 14 dpf zebrafish retained the righting reflex longer than fish 16 dpf and older.
Figure 4.
Time to loss of righting reflex, according to age. Video recordings were used to determine the time to loss of righting reflex (mean ± SE), which was defined as the time until the last fish in each euthanasia cohort lost normal ventral body orientation. All differences between 14 dpf fish and those 16 dpf or older were significant (P < 0.001), but time to loss of righting reflex did not differ between any groups 16 dpf or older.
Discussion
This study examined the age at which rapid cooling becomes an effective and humane method of euthanasia for zebrafish and the exposure times necessary to produce death in 3 to 90 dpf fish. Our data indicate that young zebrafish require prolonged exposure to rapid cooling and that the time required to effectively euthanize fish decreases as age increases. In our study, zebrafish 7 dpf and younger required exposure to hypothermal shock for as long as 12 h to achieve effective euthanasia, and many fish in this age range recovered after exposure to rapid cooling for 60 min. These data support a study that showed that rapid cooling is associated with little to no mortality when fish 10 dpf or younger are exposed to rapid cooling for 60 min or less.2 In addition, these data support a previous report regarding very young zebrafish but contradicts the findings that 4 to 7 dpf zebrafish need only 20 min of exposure to rapid cooling for effective euthanasia.9 These results may reflect differences in strain and development of the fish between studies. Here we provide conservative recommendations to maximize the likelihood of successful euthanasia of zebrafish up to 90 d of age.
Zebrafish at 14 dpf required 60 min of exposure to cold water before rapid cooling became a reliable method of euthanasia, with increasing recovery rates as the time of exposure decreased. As discussed later, although younger fish required long exposure times, animals were immobilized immediately on exposure to the cold water, and behavioral indicators of pain or distress rarely occurred. In a previous study, the heartbeat of 14 dpf zebrafish stopped after 40 min of exposure to rapid cooling and no animals recovered once returned to standard tank water,18 whereas 13% of 14-dpf fish in our study recovered after 45 min of exposure. Again, differences in background strain and developmental maturation might influence the results between studies. The current study did not test fish at 8 to 13 dpf, and because we do not know whether susceptibility to euthanasia by means of rapid cooling increases in a linear fashion, we recommend an exposure time of 12 h for zebrafish younger than 14 dpf to ensure successful euthanasia.
Zebrafish at 16 to 21 dpf required only 5 min of exposure for effective and reliable euthanasia by rapid cooling. At 30 s of exposure, 60% of fish at 16 dpf and 13% of fish at 21 dpf recovered, yet none at 19 dpf recovered. We hypothesize that this finding is due to differences in the developmental maturity of the 16, 19, and 21 dpf fish in the current study. A system for staging postembryonic zebrafish has been developed by using developmental milestones, such as fin length, ossification, and swim bladder characteristics, rather than age.13 The study found a high correlation between developmental milestones and the standard length of fish and introduced the use of a staging system to account for differences in growth that age alone does not encompass. For example, the musculoskeletal, neural, and digestive systems continue to develop in postembryonic zebrafish, with developmental progress better estimated by staging rather than age.13 In the current study, the differences in recovery after 30 s of rapid cooling are likely related to the fact that the 19 dpf fish were longer than both the 16 and 21 dpf fish (Figure 3). This feature indicates that the 21 dpf fish were less developmentally mature than the 19 dpf fish, resulting in more recoveries than expected at this age. This finding may reflect differences in stocking density between the cohorts of zebrafish, given that this variable was not controlled or recorded, whereas temperature and other rearing conditions remained constant. To our knowledge, this growth curve is the first one published based on standard length data for TU zebrafish. Additional studies should be conducted on changes in standard length by age for TU and other strains prior to extrapolation to other populations of zebrafish. However, the growth curve generated in the current study is comparable to previously reported standard length growth in AB zebrafish.17
Some 14 dpf fish recovered after 45 min of exposure to rapid cooling, yet none of the 16 dpf fish recovered after as little as 5 min of exposure. Rapid changes in developmental maturation are likely responsible for the shift in susceptibility to hypothermal shock between 14 and 21 dpf, given that all zebrafish at 19 dpf and at 28 dpf and older died after 30 s of exposure to hypothermal shock, whereas a small percentage of 21 dpf fish recovered. No fish at 28 dpf and older recovered after any of the tested exposure times and were euthanized effectively after a minimum of 30 s of exposure to rapid cooling. The finding that only minimal exposure to rapid cooling is necessary for effective euthanasia in older zebrafish is consistent with another study, which found that death (as defined as cessation of opercular movement) occurs within approximately 7 s of exposure to rapid cooling in adult zebrafish 6 mo and older.21
Many studies have shown that young animals are resistant to hypothermia.2,9,18 Hypothermic shock in zebrafish is thought to slow the rate of cellular activities and neural impulses.18 Because the central and peripheral nervous systems continue to develop in postembryonic development, this determinant is likely key to the success of rapid cooling as a method of euthanasia. Although the majority of the zebrafish nervous system develops within the first few days of life, some aspects of essential organ innervation take longer to refine. For example, the swim bladder becomes progressively innervated until 18 dpf.14 As the nervous system matures, fish are less able to recover from a rapid decrease in temperature. Any factors that delay developmental maturation, such as increased housing density, decreased food availability, temperature or water chemistry aberration, and genetic variation, likely will cause a need for longer exposure to rapid cooling to achieve effective euthanasia.
Rapid opercular movements, twitching, piping, and erratic swimming are commonly accepted behavioral responses to pain or distress in zebrafish.8,21 Due to the small size of larval and juvenile zebrafish, we included only twitching, piping, and erratic swimming in this study. Twitching occurred only in fish 7 dpf and younger, with approximately 25% of fish briefly displaying this behavior. The other behavioral indicators of pain or distress were demonstrated in only a very small percentage of zebrafish that were 60 dpf and older, with 5 occurrences of piping and 1 occurrence of erratic swimming. These findings are consistent with a previous study, which similarly showed that adult zebrafish exhibited few indicators of pain or distress when euthanized by rapid cooling.21
The fish rapidly lost consciousness after exposure to the cold water, fulfilling the AVMA's requirement of rapid death, with minimal pain and distress. Although all fish 14 dpf and older lost righting reflex in less than 30 s, 14 dpf zebrafish maintained the righting reflex significantly longer than fish that were 16 dpf and older (Figure 4). This finding mirrors the demarcation between prolonged exposure to rapid cooling required for 14 dpf fish and younger, compared with the brief exposure periods needed for fish 16 dpf and older. According to a previous study,4 loss of righting reflex is associated with stage III plane 1 anesthesia (that is, a light plane) in adult zebrafish. Surgical anesthesia is achieved when the righting reflex is lost and a noxious stimulus fails to elicit a response, such as twitching or swimming away.4 After the righting reflex was lost, some fish 28 dpf and younger experienced sporadic muscular contractions for as long as 5 min in iced water, which were not associated with purposeful movement. A lack of twitching or purposeful movement indicates that fish were at a surgical plane of anesthesia or deeper during this time. Because these muscular contractions were not indicative of recovery from rapid cooling, and given that no fish at 28 dpf or older recovered at any of the tested time points, we conclude that fish at 28 dpf and older can effectively be euthanized by exposure to rapid cooling for as little as 30 s. However, unless immediate removal of fish is indicated (such as sensitive tissue collection), we recommend that fish remain in iced water for 5 min to serve as a confirmation of euthanasia and to ensure no movement prior to disposal.
Overdose of an immersion anesthetic is often used for euthanasia of zebrafish.11 The approved immersion anesthetics are highly variable in time and depth of anesthesia, making exposure times for euthanasia highly variable as well.9,15 Fish should remain in anesthetic solution for at least 10 min after cessation of opercular movements.1 However, cessation of opercular movement does not guarantee that a fish has died, given that brain death, and successively cardiac arrest, lag behind respiratory arrest,11 making a confirmatory method of euthanasia an important step regardless of euthanasia method. Furthermore, when euthanizing young zebrafish, determining opercular movements without examining the fish under a microscope can be quite difficult. Therefore, determining appropriate euthanasia methods that reliably and efficiently produce death in zebrafish larvae and juveniles is quite important. In the current study, we observed the fish at 3 time points after removal from rapid cooling, with the last observation at least 12 h after removal. This monitoring served to confirm that no fish would recover from the rapid cooling event.
We did not include an objective measure of death such as visualization of heartbeat cessation in this study, given that the goal was to determine the exposure times necessary to ensure the absence of recovery from rapid cooling, rather than to determine the time to death at different ages. These data provide a practical guide for laboratory personnel who need to euthanize large numbers of fish and do not routinely check for the lack of opercular movement or heartbeat under a microscope during euthanasia. In addition, zebrafish younger than 14 dpf can maintain essential organ viability even after prolonged periods without opercular movement or heartbeat,18 demonstrating that these features are unreliable indicators of death. Using a second method to euthanize young fish more rapidly after the loss of consciousness, such as immersion in dilute sodium hypochlorite or freezing, are viable alternatives to the long times of exposure required for very young larvae. Regardless of age, zebrafish should be harvested or placed into a freezer for carcass disposal immediately after euthanasia by rapid cooling, to comply with the AVMA requirement for a secondary method to confirm death.1
In this study, the data support our hypotheses that younger fish would require prolonged exposure to rapid cooling for effective euthanasia and that the required exposure time would decrease with age. Because we evaluated wild-type TU fish only, care must be taken when euthanizing other strains of zebrafish or those with genetic modifications, because of potential differences in susceptibility to rapid cooling, especially in young fish, which may display dramatic differences in susceptibility to rapid cooling over short periods of time. In addition, zebrafish growth and maturation are influenced by many factors, including genetics and environmental factors such as fish stocking density, temperature, and water quality; all of these factors can affect developmental maturation and staging6,17,20 and, as the current study shows, may alter the exposure times necessary for reliable euthanasia at a particular age. Further studies are warranted to investigate the effects of strain and stocking density on the effectiveness of rapid cooling as a method of euthanasia.
In light of our current results, we make the following recommendations regarding the euthanasia of zebrafish by means of rapid cooling. First, zebrafish 14 dpf or younger should be exposed for 12 h to ensure death by rapid cooling. Second, zebrafish at 16 to 28 dpf should be exposed to rapid cooling for at least 5 min. Third, zebrafish that are at least 28 dpf can reliably be euthanized after exposure to hypothermal shock for as little as 30 s.
Acknowledgments
We thank the staff of the Cell and Developmental Biology Zebrafish Facility, University of Pennsylvania, for providing husbandry of the zebrafish during the study. The work in this publication was supported by NIH grant 1R01GM117981-03.
References
- 1.American Veterinary Medical Association. [Internet] 2013. AVMA guidelines for the euthanasia of animals: 2013 ed. [Cited 27 August 2017]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf. [Google Scholar]
- 2.Chen K, Wang CQ, Fan YQ, Xie YS, Yin ZF, Xu ZJ, Zhang HL, Cao JT, Han ZH, Wang Y, Song DQ. 2014. The evaluation of rapid cooling as an anesthetic method for the zebrafish. Zebrafish 11:71–75. [DOI] [PubMed] [Google Scholar]
- 3.Collymore C, Banks EK, Turner PV. 2016. Lidocaine hydrochloride compared with MS222 for the euthanasia of zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 55:816–820. [PMC free article] [PubMed] [Google Scholar]
- 4.Collymore C, Tolwani A, Lieggi C, Rasmussen S. 2014. Efficacy and safety of 5 anesthetics in adult zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 53:198–203. [PMC free article] [PubMed] [Google Scholar]
- 5.Harper C, Lawrence C. 2011. The laboratory zebrafish. Boca Raton (FL): CRC Press. [Google Scholar]
- 6.Hazlerigg CR, Lorenzen K, Thorbek P, Wheeler JR, Tyler CR. 2012. Density-dependent processes in the life history of fishes: evidence from laboratory populations of zebrafish Danio rerio. PLoS One 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 8.Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, Craddock C, Kyzar EJ, Roth A, Landsman S, Gaikwad S, Robinson K, Baatrup E, Tierney K, Shamchuk A, Norton W, Miller N, Nicolson T, Braubach O, Gilman CP, Pittman J, Rosemberg DB, Gerlai R, Echevarria D, Lamb E, Neuhauss SC, Weng W, Bally-Cuif L, Schneider H, Zebrafish Neuroscience Research Consortium 2013. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10:70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews M, Varga ZM. 2012. Anesthesia and euthanasia in zebrafish. ILAR J 53:192–204. [DOI] [PubMed] [Google Scholar]
- 10.Mullins MC, Hammerschmidt M, Haffter P, Nusslein-Volhard C. 1994. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol 4:189–202. [DOI] [PubMed] [Google Scholar]
- 11.Neiffer DL, Stamper MA. 2009. Fish sedation, analgesia, anesthesia, and euthanasia: considerations, methods, and types of drugs. ILAR J 50:343–360. [DOI] [PubMed] [Google Scholar]
- 12.Nusslein-Volhard C, Dahm R. 2002. Zebrafish: a practical approach. New York (NY): Oxford University Press. [Google Scholar]
- 13.Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. 2009. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn 238:2975–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson GN, McGee CA, Dumbarton TC, Croll RP, Smith FM. 2007. Development of the swimbladder and its innervation in the zebrafish, Danio rerio. J Morphol 268:967–985. [DOI] [PubMed] [Google Scholar]
- 15.Rombough PJ. 2007. Ontogenetic changes in the toxicity and efficacy of the anaesthetic MS222 (tricaine methanesulfonate) in zebrafish (Danio rerio) larvae. Comp Biochem Physiol A Mol Integr Physiol 148:463–469. [DOI] [PubMed] [Google Scholar]
- 16.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London (United Kingdom): Methuen [Google Scholar]
- 17.Singleman C, Holtzman NG. 2014. Growth and maturation in the zebrafish, Danio rerio: a staging tool for teaching and research. Zebrafish 11:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strykowski JL, Schech JM. 2015. Effectiveness of recommended euthanasia methods in larval zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 54:81–84. [PMC free article] [PubMed] [Google Scholar]
- 19.Tavares B, Santos Lopes S. 2013. The importance of zebrafish in biomedical research. Acta Med Port 26:583–592. [PubMed] [Google Scholar]
- 20.Vargesson N. 2007. Zebrafish. p 78–84, Chapter 12. In: Barnett SW. Manual of animal technology. Oxford (United Kingdom): Blackwell Publishing. [Google Scholar]
- 21.Wilson JM, Bunte RM, Carty AJ. 2009. Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 48:785–789. [PMC free article] [PubMed] [Google Scholar]