INTRODUCTION
Along with the growth of patients at cardiovascular risk, a new wave is hitting the world of health care. Over the recent decade, noninvasive and invasive imaging technologies have been protagonists of a dramatic evolution. This process, as a Tsunami, may overwhelm the way the physicians were used to work until now [Figure 1]. The greater availability and reliability of current imaging modalities to reveal the details of various cardiac structures and pathophysiology has made the evaluation of cardiovascular disease multimodal. Hence, along with traditional imaging modalities, such as single photon computed emission tomography (SPECT) or tissue Doppler imaging and two-dimensional echocardiography (2DE), new technologies emerge in the scene such as deformation/speckle tracking imaging (strain and strain rate) and three-dimensional echocardiography (3DE); miniaturized or handheld cardiovascular ultrasound machine; positron emission tomography (PET) and computed tomography (CT), and cardiac magnetic resonance (CMR) as well as the combination of them, resulting in the so-called “fusion imaging.”
Figure 1.
Current Noah's ark where physicians may store old and new imaging modalities to avoid being overwhelmed by tsunami of the new technologies
It has been widely proven that new technologies may help clinicians to refine ability to diagnose disease, improve patient care, and advance biomedical research. Noninvasive imaging is central to clinical practice and research, irrespective of the disease entity or the area of interest of the cardiologist. From early detection of disease in primary prevention to guidance of therapy in hybrid operating suites, imaging is now ubiquitous and essential. However, which role will new technologies play in the next future? Hopefully, they will integrate conventional imaging modalities although CT and CMR constitute still a small fraction (0.1%–1%) of conventional echocardiography and nuclear imaging. Greater patient awareness also contributes to the demand for imaging technologies because many patients want state-of-the-art care, while others want the certainty that seems to be promised by technology. This process must be necessarily governed. The success of the application of imaging technologies will result from a virtuous integration with clinical evaluation and care. The possible decline of the physical examination with its automatic replacement by an imaging test must be absolutely avoided by both specialists and general practitioners. The ability to perform imaging based on outcomes and with the least amount of imaging, to avoid higher costs due to multiple and often redundant testing, and, more importantly, to add value in the decision-making process will guide clinicians [Figure 2]. An adequate training and knowing the strengths and limitations of all these imaging techniques will be required for all the professionals involved. It could be stated soon that possessing good skills in at least two modalities such as echocardiography and cardiac magnetic resonance imaging (MRI) or nuclear imaging or cardiac CT may represent the minimal standard requirement for all noninvasive cardiologists.[1] All international cardiac scientific societies are incorporating the study of cardiovascular imaging. Similarly, the Società Italiana di Ecocardiografia (SIEC), one of the largest and oldest subspecialty scientific societies in Europe, voted to change its name into SIEC e CardioVascular Imaging (SIECVI) in the last national meeting on April 7, 2017. Moreover, also the mission of the society has changed to “To gather all those who promote, study, and apply cardiovascular imaging in Italy,” incorporating at least one board member expert in MRI and CT. The present statement wants to underline the difficult mission that SIECVI is taking over the next years.
Figure 2.
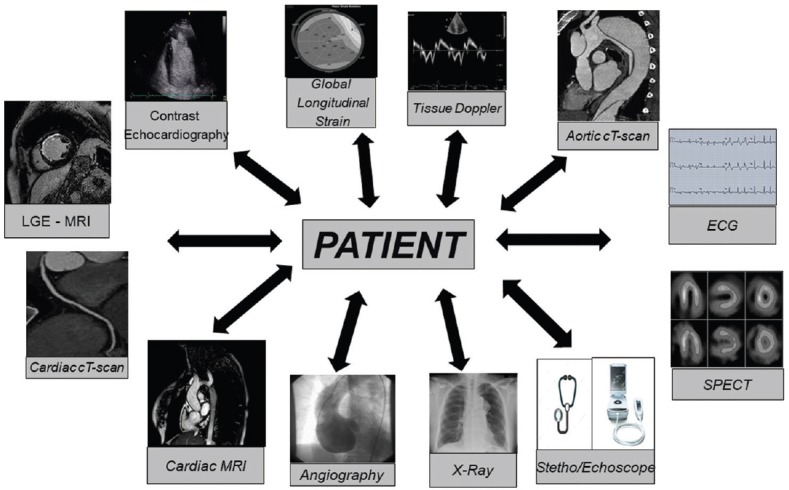
Patient plays a central role in the process of the application of new imaging technologies
ACUTE HEART FAILURE
Initial diagnosis of acute heart failure (HF) should be based on the assessment of signs/symptoms by physical examination and it should be further confirmed by standard additional investigations such as electrocardiography, chest X-ray, and laboratory assessment (with specific biomarkers). The European Association of Cardiovascular Imaging and the Acute Cardiovascular Care recommends to subject the dyspneic patient to ultrasound examination.[2]
In this clinical scenario, lung ultrasound examination is helpful in the differential diagnosis between respiratory and cardiological disease,[3] and pocket-size echocardiography may be used as an extension of the clinical examination.[2] Immediate comprehensive echocardiogram is mandatory in patients with hemodynamic instability (particularly in cardiogenic shock) and in patients suspected of acute life-threatening structural or functional cardiac abnormalities (mechanical complications, acute valvular regurgitation, and aortic dissection).[4] Transthoracic echocardiography (TTE) frequently requires nonstandardized echocardiographic views due to patient's filling status, eventual positive pressure ventilation, effects of sedation on myocardial function, and inotropic and/or metabolic status that can make acoustic window poor. In these cases and in the suspect of severe valve disease, aortic dissection, endocarditis, and for ruling out intracavitary thrombi in atrial fibrillation patients requiring cardioversion, transesophageal echocardiography (TOE) can integrate TTE.[5] Contrast echocardiography with second-generation contrast agents improves further the visualization of the endocardium[6] [Figure 3] or Doppler signal envelope in difficult patients with severe aortic valve (AV) stenosis.[7]
Figure 3.
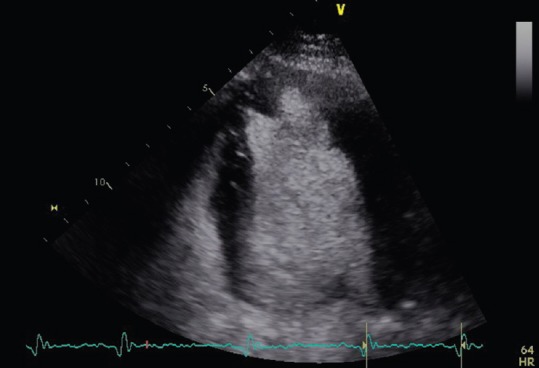
Apical left ventricle clot seen in a four chamber-view with contrast echocardiography
CMR has a limited role in the acute setting, due to the instability of patients and the time-consuming nature of the procedure. CT can be employed in selected cases for the differential diagnosis of unclear acute dyspnea (for excluding pulmonary embolism, aortic dissection, paracardiac compressive masses…).
CHRONIC HEART FAILURE
A major determinant of the prognosis is cardiac function. The two primary modalities for assessing function are echocardiography and CMR. Echocardiography is so far the more versatile of these techniques, and in addition, it is more widely available, less costly, and less time consuming.[8] TTE is the method of choice for the assessment of myocardial systolic and diastolic function of both ventricles.[9] The most commonly used parameters to describe left ventricle (LV) cavity size include linear internal dimensions and volumes. LV volumes are measured using 2DE or 3DE.[8] LV ejection fraction (LVEF), at rest, represents the most powerful prognostic and decision-making parameter in patients with systolic HF.[4] The European Heart Rhythm Association and the European Association of Cardiovascular Imaging recommend LVEF <35% at rest as the only imaging parameter for device implantation such as implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy regardless of the chosen modality (e.g., echocardiography, MRI, contrast ventriculography, or radionuclide angiography).[10] In the recent years, the latest approaches in tracking natural myocardial markers, or speckles, in 2DE images for computing myocardial deformation, provide increasing characterization of myocardial functional abnormalities beyond EF.[11] Global longitudinal strain (GLS) assessed using automated speckle-tracking echocardiography (STE) reflects the longitudinal contraction of the myocardium and it is an emerging technique for detecting and quantifying subtle disturbances in LV systolic function.[12] In chronic HF patients, GLS was shown to be a superior predictor of cardiac events and all-cause mortality compared to EF.[12] More recently, GLS was found to be a robust prognostic marker following myocardial infarction[13] either in patients with cardiomyopathy[14] or with aortic stenosis.[15] Early STE predicts LV remodeling after acute ST-segment elevation myocardial infarction.[16] Several recent studies have revealed the value of deformation imaging for the early detection of LV dysfunction secondary to cancer therapy[17] [Figure 4]. In the near future, technological improvements and standardization of the methodologies among industry vendors may create new opportunities for the use of speckle tracking into 3D echo. Cardiac MRI is a tomographic nonionizing technique that has an emerging role in the noninvasive diagnostic assessment of HF.[18] Cine and flow-sensitive CMR imaging allows to obtain images in all plane directions with good temporal and spatial resolutions, delivering reliable endocardial border detection and independency of geometrical assumptions.[19] It represents the current gold standard for quantification of cardiac chambers and EF of both right and LVs.[8] In addition, specific CMR sequences (T1, T2, or T2* weighted) can enable myocardial tissue characterization, based on the different relaxation properties of tissues such as fat, muscle, and areas of inflammation.[20] The extracellular substrate is studied by late gadolinium enhancement (LGE) which has the ability to identify myocardial scar/fibrosis [Figure 5]. Gadolinium is an extracellular agent, which enhances in necrotic or fibrotic myocardium, assuming a bright signal (hyperenhancement), opposed to dark viable myocardium. The pattern of LGE is useful to differentiate postinfarction necrosis from fibrosis in nonischemic cardiomyopathies or myocarditis.[21] Its characterization of myocardial tissue is unique among imaging technologies although software for the quantification of LGE have not been released yet in the market. However, diffuse myocardial fibrosis and inflammation could be invisible to LGE. Recent developments in CMR imaging involve myocardial perfusion and magnetic relaxation properties.[20] T1 and T2 mapping sequences generate a parametric map of perfusion or relaxation times through the acquisition of multiple images of the same myocardial region with different sensitivity to the parameter of interest. The signal intensities of these images are fit to a model which describes the underlying physiology or relaxation parameters. T1 mapping techniques performed both with and without contrast enable quantification of diffuse myocardial fibrosis and myocardial infiltration. Myocardial edema and inflammation can be evaluated using T2 mapping techniques.[22] Several studies proposed to measure the change in T1 time in precontrast versus postcontrast conditions, the information which allows us to calculate extracellular volume fraction (ECV). Myocardial ECV is defined as the ratio of the change in the myocardial T1 to the change in the blood T1. Recent studies have proven that increased ECV is associated with the severity of diffuse myocardial fibrosis in histology.[23] The widespread use of CMR in HF is limited by lack of portability, reduced availability, and higher costs. In addition, CMR cannot be performed in claustrophobic patients or in patients with severe renal impairment (>IV renal disease stage), in whom administration of gadolinium is contraindicated for the risk of nephrogenic systemic fibrosis. A multimodality imaging approach is fundamental in assessing patients with cardiomyopathies in order to define diagnosis and to establish diagnostic workup and therapeutic strategies. Although echocardiography is the first-line imaging for the evaluation of patients with dilated or hypertrophic cardiomyopathies, CMR provides additive information, especially about tissue characterization.[24] Specific patterns of contrast enhancement distribution as well as colorimetric myocardial mapp measured by native T1 signal seem to be associated with both infiltrative cardiomyopathies (amyloidosis and Gaucher disease) or storage cardiac diseases (Fabry disease, hemochromatosis, etc.) and are useful in the differential diagnosis of restrictive cardiomyopathy.[25,26] Although echocardiography is the first imaging tool to assess patients with suspected Takotsubo syndrome, cardiac MRI could be useful, especially in patients with uncommon presentation to confirm diagnosis and to exclude myocardial necrosis and myocarditis.[27,28] Being no single imaging modality pathognomonic of a specific cardiomyopathy, a combination of different complementary techniques should be encouraged in clinical practice. During last decades, percutaneous treatment of aortic and mitral valve (MV) disease represents a new therapeutic indication, especially in patients at high risk not amenable for surgery. A multimodality imaging approach has emerged useful for diagnosis, procedural planning, and monitoring the follow-up.[29]
Figure 4.
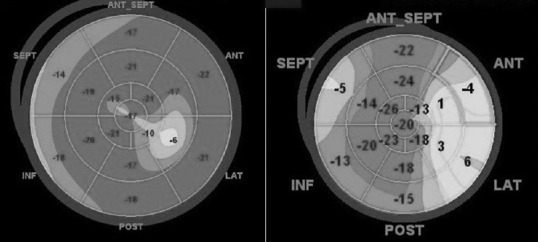
Worsening of the left ventricle global longitudinal strain, before (left) and after (right) 6 months of treatment with trastuzumab
Figure 5.
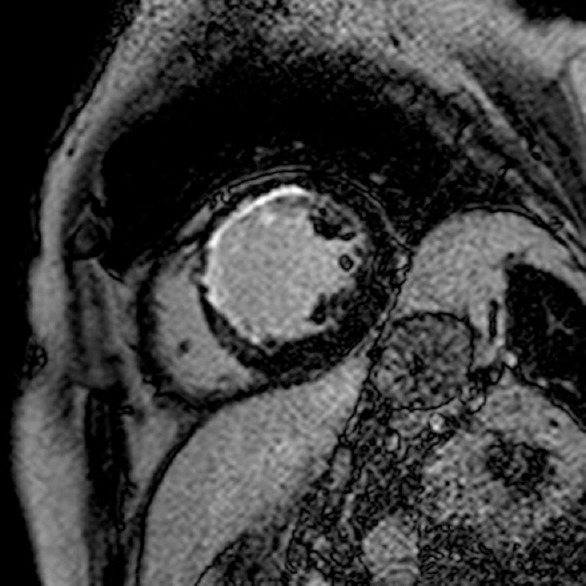
Myocardial fibrosis of the left ventricle seen with late gadolinium enhancement after anterior myocardial infarct (cardiovascular magnetic resonance)
CHRONIC ISCHEMIC HEART DISEASE
The spatial resolution of CT is particularly advantageous to visualize the coronary anatomy in patients with low-intermediate pretest probability of coronary artery disease (CAD) [Figure 6]. Diagnostic accuracy of CT for detection of significant coronary artery stenosis is high, with sensitivity of 91% and specificity of 92%.[30] However, coronary function assessed as inducible perfusion abnormalities by SPECT or wall motion abnormalities by echocardiography is a stronger predictor of clinical outcome than coronary anatomy assessed by internal carotid artery.[9,31] For this reason, the guidance regarding the assessment of stable CAD is that the presence of inducible ischemia should guide the need for revascularization, not just the degree of coronary stenosis.[32] Fractional flow reserve (FFR), which assesses the ratio of flow across a stenosis to putative flow in the absence of a stenosis, has been shown to be a powerful tool for detecting lesion-specific myocardial ischemia. FFR is the accepted reference standard for assessing the functional significance of CAD in a lesion-specific manner.[32] Recently, a method using computational fluid dynamics to calculate coronary blood flow, pressure, and FFR based on routinely acquired coronary CT datasets at rest combines anatomic and functional information to enable appropriate therapeutic decision-making.[33] A recent trial showed that noninvasive FFR derived from CT provides high diagnostic accuracy and discrimination for the diagnosis of hemodynamically significant CAD.[33] Other imaging modalities such as MRI can play a pivotal role in chronic ischemic heart disease. LGE-MRI can be extremely useful for differentiating between patients with potentially reversible ventricular dysfunction and those with irreversible dysfunction. In patients with ischemic heart disease, it is primarily those with reversible dysfunction who will benefit from coronary revascularization.[34] There is good concordance between perfusion CMR and FFR for the identification of myocardial ischemia in patients with multivessel disease.[35]
Figure 6.
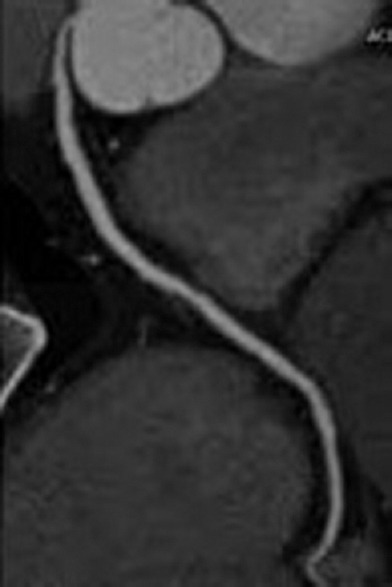
Normal right coronary artery seen at the computed tomography scan
INFECTIVE ENDOCARDITIS
Each imaging modality reviewed provides specific diagnostic information concerning sequelae of infective endocarditis.[36] Both TTE and TOE miss to detect infective endocarditis sequelae in 30% of patients, especially in patients with intracardiac prosthetic material.[36] Both CT and MRI provide high-quality anatomical information, whereas leukocyte scintigraphy and 18F-fluorodeoxyglucose PET/CT (18F-FDG PET/CT) provide functional data.[37] Leukocyte scintigraphy with SPECT/CT has added value in the diagnosis of prosthetic valve endocarditis because it is highly specific. However, leukocyte scintigraphy with SPECT/CT has insufficient sensitivity and has several limitations regarding preparation and patient comfort (laborious preparation and risk of missing small infectious foci).[38] The added value of this imaging technique has been demonstrated in cases with persisting diagnostic uncertainty for prosthetic valve endocarditis, ICD-related or pacemaker-related, and ventricular assist device-related infective endocarditis.[39] 18F-FDG PET/CT is evolving as an important supplementary method in difficult-to-diagnose cases of suspected infective endocarditis detecting also the foci of extracardiac infection. Whole-body imaging by18 F-FDG PET/CT can provide important clinical information concerning the presence of portal of entry and occult predisposing lesions such as primary tumors.[40]
HEART VALVES IN THE ERA OF INTERVENTIONAL CARDIOLOGY
Echocardiography is the predominant imaging modality for heart valve analysis.[41] Recent advances in cardiac magnetic resonance (CMR) and cardiac CT indicate that they have an important role for a better stratification of valve alteration severity, providing more and more precise details of their anatomical and functional abnormalities. Both echocardiography (specifically TOE) and CT have played an important role in the preprocedural planning for transcatheter AV implantation (TAVI) or MitraClip procedures. The multimodality cardia imaging has become an essential part of the pre-, intra-, and postprocedural planning for any successful transcatheter valvular procedures.
AORTIC STENOSIS
The size selection of the prosthetic AV for TAVI is crucial. It is currently based on 2DE-derived AV annulus measures; however, 2D planar imaging strategy measures the AV annulus, not taking into account its ellipsoid architecture.[42] CMR and CT allow routine assessment of AV annulus and aortic root morphology in multiple imaging planes, affording assessment of their ellipsoid nature, which may better guide TAVI size selection.[43] 3D TOE planimetry of AV annulus shows a better correlation with CT or CMR.[44] CT can quantify the calcium content in aortic walls and in valve cusps, where a “porcelain” aorta is suspected by 2DE or invasive coronary angiogram. Furthermore, CT could be useful after TAVI because it can detect the formation of AV pannus, so evaluating the prosthetic valve function. CMR, with the identification of flow turbulence on bright blood sequences within the LV outflow tract and into the ascending aorta, could help to better stratify the hemodynamics of the integrated anatomical complex constituted by AV and aortic root.[45]
MITRAL REGURGITATION
3D-TOE may provide additional anatomical and quantitative information, in comparison with 2D-TOE, in patients with complex MV lesions.[46] Exercise echocardiography may be useful in patients with discordant symptoms to provide information on changes in mitral regurgitation (MR) severity. CT and 3D-TOE are currently being used to anatomically select patients for MitraClip procedure (Abbott Vascular, Santa Clara, CA, USA). CMR is also promising in the assessment of regurgitant volume by both volumetric and velocity encoding methods, showing excellent reproducibility in the MitraClip settings. Direct MV annuloplasty is a very promising approach for transcatheter MV repair, since it closely reproduces a gold standard surgical method. The Cardioband (ValtechCardio Inc., Israel) is the closest transcatheter device to a surgical prosthetic ring. Today, the main concern about mitral direct annuloplasty repair is that these procedures are technically very challenging and they require advanced integrated imaging guidance. CT is important for several preprocedural steps of percutaneous MV repairing procedures, providing complementary information to echocardiography and fluoroscopy.[47] Additional information available from cardiac CT includes mitral annulus size, MV leaflet length and calcification, chordae tendineae thickening, left atrial size, and the detection of pulmonary edema. CMR could be useful for a complete anatomical and functional assessment of the MV regurgitation jets and help to localize dysfunctional MV leaflet scallops.
AORTA
In patients with aortic disease, imaging of thoracic aorta plays a major role in risk-stratifying individuals for life-threatening complications and in determining the time of surgical intervention. Optimal management of aortic aneurysms depends on the reported findings from echocardiography, CT, and MRI[48] [Figure 7]. The availability, cost/benefit ratio, and additive value of each technique determine its indications. TTE continues to be the technique most used in clinical practice for aortic root assessment. CT has the advantage of its high-resolution assessment of the entire aorta and excellent accuracy on size measurements. MRI offers the greatest morphologic and dynamic information of the aorta without radiation, although in clinical practice, it is less commonly available. The risk of radiation-induced malignancy is higher in neonates, children, and young adults.[49] For patients who require repeated imaging to follow an aortic abnormality, MR may be preferred to CT. However, a great variability in aortic size measurements is still widespread due to a lack of standardization in these procedures.[50,51] In the last few years, several reports have been published showing an effort by cardiovascular imagers to fill in the gap.[52,53] New techniques such as four-dimensional flow MRI are very promising and seem to allow the noninvasive measurement of cardiac flow dynamics in vivo.[54]
Figure 7.
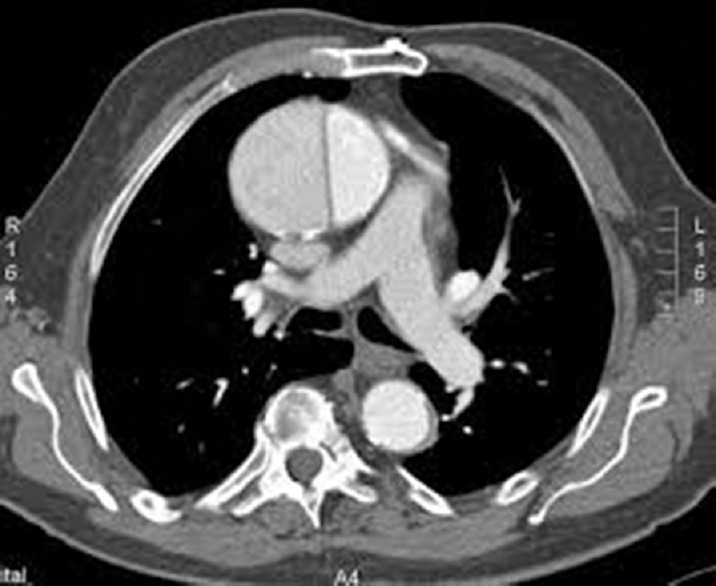
Axial view of ascending aorta dissection viewed at the computed tomography scan
CONGENITAL HEART DISEASE
As the majority of infants born with congenital heart disease now survive into adulthood, lifelong follow-up is an integral part of their ongoing care.[55] Adequate management of this group of adult congenital heart disease (ACHD) patients requires advanced imaging to assess the morphology and function of heart and vessels.[56] Echocardiography is the first-line cardiovascular imaging modality in patients with congenital heart disease, although suboptimal acoustic access can be problematic after previous cardiovascular surgery.[57] CMR plays a central role in ACHD patients. CMR can provide assessments of anatomical connections, biventricular function, myocardial viability, measurements of flow, angiography, and more, without ionizing radiation[58] [Figure 8]. 3D printing technology may give a further help to overcome the 3D visualization of cardiac defects.[59] Furthermore, there is a growing interest in using real-time MRI as an interactive guidance of complex procedures, thanks to its wide availability into device navigation.[60]
Figure 8.
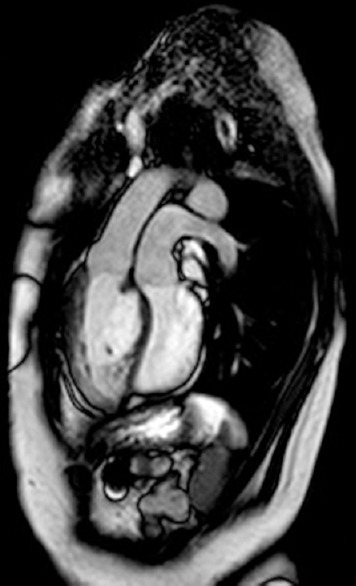
Imaging of transposition of the great arteries with steady-state free precession technique (cardiovascular magnetic resonance)
CONCLUSIONS
Noninvasive cardiac multimodality imaging has a pivotal role in the clinical practice and research and its successful application will result from the virtuous integration of the different techniques in the clinical care. The optimal use of each one of these imaging modalities for specific clinical scenarios, in terms of quality and cost-effectiveness, is still not completely defined. Cardiovascular imaging is at a crossroad; in the near future, it will be oriented to an integrated multimodality approach, comprehensive of the assessment and integration of morphology, pathophysiology, biology, risk stratification, prognosis, and guidance of therapy. At this regard, we might consider a more complex approach: translational multimodality cardiovascular imaging, redefining the role of cardiovascular imaging across the continuum of biomedical, bioengineering, statistical, epidemiological research, and clinical practice.[61]
There is a strong need for standardization, guided by clinically tailored “health technology assessment” studies, to obtain an appropriate utilization of the different available tools with greater efficiency and efficacy of care and better patient outcomes. This process will represent a challenge in the next years for all cardiovascular specialists and for the scientific societies of cardiac imaging. Provided all the important definitions in the present statement, the new name of SIECVI and the mission of the society “To gather all those who promote, study, and apply cardiovascular imaging in Italy,” with the new board, are in line with the new course of SIECVI, in order to be “on board” of the international world of cardiovascular multimodality imaging.
REFERENCES
- 1.Chandrashekhar Y, Dilsizian V, Kramer CM, Marwick T, Min JK, Shaw L, et al. Implementing multimodality imaging in the future. JACC Cardiovasc Imaging. 2016;9:91–8. doi: 10.1016/j.jcmg.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Lancellotti P, Price S, Edvardsen T, Cosyns B, Neskovic AN, Dulgheru R, et al. The use of echocardiography in acute cardiovascular care: Recommendations of the European association of cardiovascular imaging and the acute cardiovascular care association. Eur Heart J Cardiovasc Imaging. 2015;16:119–46. doi: 10.1093/ehjci/jeu210. [DOI] [PubMed] [Google Scholar]
- 3.Miglioranza MH, Gargani L, Sant’Anna RT, Rover MM, Martins VM, Mantovani A, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: A comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6:1141–51. doi: 10.1016/j.jcmg.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 5.Chambers JB, Garbi M, Nieman K, Myerson S, Pierard LA, Habib G, et al. Appropriateness criteria for the use of cardiovascular imaging in heart valve disease in adults: A European Association of Cardiovascular Imaging Report of literature review and current practice. Eur Heart J Cardiovasc Imaging. 2017;18:489–98. doi: 10.1093/ehjci/jew309. [DOI] [PubMed] [Google Scholar]
- 6.Kurt M, Shaikh KA, Peterson L, Kurrelmeyer KM, Shah G, Nagueh SF, et al. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol. 2009;53:802–10. doi: 10.1016/j.jacc.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Evangelista A, Flachskampf FA, Erbel R, Antonini-Canterin F, Vlachopoulos C, Rocchi G, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr. 2010;11:645–58. doi: 10.1093/ejechocard/jeq056. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 9.Gimelli A, Lancellotti P, Badano LP, Lombardi M, Gerber B, Plein S, et al. Non-invasive cardiac imaging evaluation of patients with chronic systolic heart failure: A report from the European Association of Cardiovascular Imaging (EACVI) Eur Heart J. 2014;35:3417–25. doi: 10.1093/eurheartj/ehu433. [DOI] [PubMed] [Google Scholar]
- 10.Blomström Lundqvist C, Auricchio A, Brugada J, Boriani G, Bremerich J, Cabrera JA, et al. The use of imaging for electrophysiological and devices procedures: A Report from the First European Heart Rhythm Association Policy Conference, Jointly Organized with the European Association of Cardiovascular Imaging (EACVI), the Council of Cardiovascular Imaging and the European Society of Cardiac Radiology. Europace. 2013;15:927–36. doi: 10.1093/europace/eut084. [DOI] [PubMed] [Google Scholar]
- 11.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–69. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: Comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–64. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 13.Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2013;61:2365–73. doi: 10.1016/j.jacc.2013.02.061. [DOI] [PubMed] [Google Scholar]
- 14.Saito M, Okayama H, Yoshii T, Higashi H, Morioka H, Hiasa G, et al. Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2012;13:617–23. doi: 10.1093/ejechocard/jer318. [DOI] [PubMed] [Google Scholar]
- 15.Bartko PE, Heinze G, Graf S, Clavel MA, Khorsand A, Bergler-Klein J, et al. Two-dimensional strain for the assessment of left ventricular function in low flow-low gradient aortic stenosis, relationship to hemodynamics, and outcome: A substudy of the multicenter TOPAS study. Circ Cardiovasc Imaging. 2013;6:268–76. doi: 10.1161/CIRCIMAGING.112.980201. [DOI] [PubMed] [Google Scholar]
- 16.Mele D, Nardozza M, Chiodi E. Early speckle-tracking echocardiography predicts left ventricle remodeling after acute ST-segment elevation myocardial infarction. J Cardiovasc Echogr. 2017;27:93–8. doi: 10.4103/jcecho.jcecho_2_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamorano JL, Lancellotti P, Muñoz DR, Aboyans V, Asteggiano R, Galderisi M, et al. ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines. Kardiol Pol. 2016;74:1193–233. doi: 10.5603/KP.2016.0156. [DOI] [PubMed] [Google Scholar]
- 18.Salerno M, Sharif B, Arheden H, Kumar A, Axel L, Li D, et al. Recent advances in cardiovascular magnetic resonance: Techniques and applications. Circ Cardiovasc Imaging. 2017;10:pii: e003951. doi: 10.1161/CIRCIMAGING.116.003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maceira AM, Prasad SK, Khan M, Pennell DJ. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur Heart J. 2006;27:2879–88. doi: 10.1093/eurheartj/ehl336. [DOI] [PubMed] [Google Scholar]
- 20.Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging. 2013;6:806–22. doi: 10.1016/j.jcmg.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 22.Greupner J, Zimmermann E, Grohmann A, Dübel HP, Althoff TF, Borges AC, et al. Head-to-head comparison of left ventricular function assessment with 64-row computed tomography, biplane left cineventriculography, and both 2- and 3-dimensional transthoracic echocardiography: Comparison with magnetic resonance imaging as the reference standard. J Am Coll Cardiol. 2012;59:1897–907. doi: 10.1016/j.jacc.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 23.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: Evaluation of an automated method. J Cardiovasc Magn Reson. 2012;14:63. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardim N, Galderisi M, Edvardsen T, Plein S, Popescu BA, D’Andrea A, et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: An expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging. 2015;16:280. doi: 10.1093/ehjci/jeu291. [DOI] [PubMed] [Google Scholar]
- 25.Shah R, Nucifora G, Perry R, Selvanayagam JB. Noninvasive imaging in cardiac deposition diseases. J Magn Reson Imaging. 2018;47:44–59. doi: 10.1002/jmri.25720. [DOI] [PubMed] [Google Scholar]
- 26.Habib G, Bucciarelli-Ducci C, Caforio AL, Cardim N, Charron P, Cosyns B, et al. Multimodality imaging in restrictive cardiomyopathies: An EACVI expert consensus document in collaboration with the “Working group on myocardial and pericardial diseases” of the European Society of Cardiology Endorsed by the Indian Academy of Echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18:1090–121. doi: 10.1093/ehjci/jex034. [DOI] [PubMed] [Google Scholar]
- 27.Citro R, Pontone G, Pace L, Zito C, Silverio A, Bossone E, et al. Contemporary imaging in Takotsubo syndrome. Heart Fail Clin. 2016;12:559–75. doi: 10.1016/j.hfc.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Citro R, Lyon AR, Meimoun P, Omerovic E, Redfors B, Buck T, et al. Standard and advanced echocardiography in Takotsubo (stress) cardiomyopathy: Clinical and prognostic implications. J Am Soc Echocardiogr. 2015;28:57–74. doi: 10.1016/j.echo.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 appropriate use criteria for multimodality imaging in valvular heart disease: A Report of the American College of Cardiology Appropriate use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;70:1647–72. doi: 10.1016/j.jacc.2017.07.732. [DOI] [PubMed] [Google Scholar]
- 30.Neglia D, Rovai D, Caselli C, Pietila M, Teresinska A, Aguadé-Bruix S, et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging. 2015;8:pii: e002179. doi: 10.1161/CIRCIMAGING.114.002179. [DOI] [PubMed] [Google Scholar]
- 31.Olmos LI, Dakik H, Gordon R, Dunn JK, Verani MS, Quiñones MA, et al. Long-term prognostic value of exercise echocardiography compared with exercise 201Tl, ECG, and clinical variables in patients evaluated for coronary artery disease. Circulation. 1998;98:2679–86. doi: 10.1161/01.cir.98.24.2679. [DOI] [PubMed] [Google Scholar]
- 32.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 33.Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease using CT angiography: Next steps) J Am Coll Cardiol. 2014;63:1145–55. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 34.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 35.Hussain ST, Chiribiri A, Morton G, Bettencourt N, Schuster A, Paul M, et al. Perfusion cardiovascular magnetic resonance and fractional flow reserve in patients with angiographic multi-vessel coronary artery disease. J Cardiovasc Magn Reson. 2016;18:44. doi: 10.1186/s12968-016-0263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 37.Gomes A, Glaudemans AW, Touw DJ, van Melle JP, Willems TP, Maass AH, et al. Diagnostic value of imaging in infective endocarditis: A systematic review. Lancet Infect Dis. 2017;17:1–4. doi: 10.1016/S1473-3099(16)30141-4. [DOI] [PubMed] [Google Scholar]
- 38.Hyafil F, Rouzet F, Lepage L, Benali K, Raffoul R, Duval X, et al. Role of radiolabelled leucocyte scintigraphy in patients with a suspicion of prosthetic valve endocarditis and inconclusive echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:586–94. doi: 10.1093/ehjci/jet029. [DOI] [PubMed] [Google Scholar]
- 39.Erba PA, Sollini M, Conti U, Bandera F, Tascini C, De Tommasi SM, et al. Radiolabeled WBC scintigraphy in the diagnostic workup of patients with suspected device-related infections. JACC Cardiovasc Imaging. 2013;6:1075–86. doi: 10.1016/j.jcmg.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Dell’Aquila AM, Mastrobuoni S, Alles S, Wenning C, Henryk W, Schneider SR, et al. Contributory role of fluorine 18-fluorodeoxyglucose positron emission tomography/computed tomography in the diagnosis and clinical management of infections in patients supported with a continuous-flow left ventricular assist device. Ann Thorac Surg. 2016;101:87–94. doi: 10.1016/j.athoracsur.2015.06.066. [DOI] [PubMed] [Google Scholar]
- 41.Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS) Vahanian A, Alfieri O, Andreotti F, Antunes MJ, et al. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–96. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 42.Otto CM, Pearlman AS, Comess KA, Reamer RP, Janko CL, Huntsman LL, et al. Determination of the stenotic aortic valve area in adults using Doppler echocardiography. J Am Coll Cardiol. 1986;7:509–17. doi: 10.1016/s0735-1097(86)80460-0. [DOI] [PubMed] [Google Scholar]
- 43.Shah RG, Novaro GM, Blandon RJ, Whiteman MS, Asher CR, Kirsch J, et al. Aortic valve area: Meta-analysis of diagnostic performance of multi-detector computed tomography for aortic valve area measurements as compared to transthoracic echocardiography. Int J Cardiovasc Imaging. 2009;25:601–9. doi: 10.1007/s10554-009-9464-z. [DOI] [PubMed] [Google Scholar]
- 44.Willson AB, Webb JG, Labounty TM, Achenbach S, Moss R, Wheeler M, et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: A multicenter retrospective analysis. J Am Coll Cardiol. 2012;59:1287–94. doi: 10.1016/j.jacc.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 45.John AS, Dill T, Brandt RR, Rau M, Ricken W, Bachmann G, et al. Magnetic resonance to assess the aortic valve area in aortic stenosis: How does it compare to current diagnostic standards? J Am Coll Cardiol. 2003;42:519–26. doi: 10.1016/s0735-1097(03)00707-1. [DOI] [PubMed] [Google Scholar]
- 46.Zeng X, Nunes MC, Dent J, Gillam L, Mathew JP, Gammie JS, et al. Asymmetric versus symmetric tethering patterns in ischemic mitral regurgitation: Geometric differences from three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr. 2014;27:367–75. doi: 10.1016/j.echo.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Alkadhi H, Wildermuth S, Bettex DA, Plass A, Baumert B, Leschka S, et al. Mitral regurgitation: Quantification with 16-detector row CT – Initial experience. Radiology. 2006;238:454–63. doi: 10.1148/radiol.2381042216. [DOI] [PubMed] [Google Scholar]
- 48.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, et al. 2010. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:27–129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Brenner DJ, Hall EJ. Computed tomography – An increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 50.Mongeon FP, Marcotte F, Terrone DG. Multimodality noninvasive imaging of thoracic aortic aneurysms: Time to standardize? Can J Cardiol. 2016;32:48–59. doi: 10.1016/j.cjca.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez-Palomares JF, Teixidó-Tura G, Galuppo V, Cuéllar H, Laynez A, Gutiérrez L, et al. Multimodality assessment of ascending aortic diameters: Comparison of different measurement methods. J Am Soc Echocardiogr. 2016;29:819–26. doi: 10.1016/j.echo.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Park JY, Foley TA, Bonnichsen CR, Maurer MJ, Goergen KM, Nkomo VT, et al. Transthoracic echocardiography versus computed tomography for ascending aortic measurements in patients with bicuspid aortic valve. J Am Soc Echocardiogr. 2017;30:625–35. doi: 10.1016/j.echo.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Muraru D, Maffessanti F, Kocabay G, Peluso D, Dal Bianco L, Piasentini E, et al. Ascending aorta diameters measured by echocardiography using both leading edge-to-leading edge and inner edge-to-inner edge conventions in healthy volunteers. Eur Heart J Cardiovasc Imaging. 2014;15:415–22. doi: 10.1093/ehjci/jet173. [DOI] [PubMed] [Google Scholar]
- 54.Binter C, Gotschy A, Sündermann SH, Frank M, Tanner FC, Lüscher TF, et al. Turbulent kinetic energy assessed by multipoint 4-dimensional flow magnetic resonance imaging provides additional information relative to echocardiography for the determination of aortic stenosis severity. Circ Cardiovasc Imaging. 2017;10:pii: e005486. doi: 10.1161/CIRCIMAGING.116.005486. [DOI] [PubMed] [Google Scholar]
- 55.Boris JR, Béland MJ, Bergensen LJ, Colan SD, Dangel J, Daniels CJ, et al. 2017 AHA/ACC key data elements and definitions for ambulatory electronic health records in pediatric and congenital cardiology: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards. J Am Coll Cardiol. 2017;70:1029–95. doi: 10.1016/j.jacc.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 56.Orwat S, Diller GP, Baumgartner H. Imaging of congenital heart disease in adults: Choice of modalities. Eur Heart J Cardiovasc Imaging. 2014;15:6–17. doi: 10.1093/ehjci/jet124. [DOI] [PubMed] [Google Scholar]
- 57.Bulbul Z, Issa Z, Siblini G, Moiduddin N, Di Salvo G. Normal range of left ventricular strain, dimensions and ejection fraction using three-dimensional speckle-tracking echocardiography in neonates. J Cardiovasc Echogr. 2015;25:67–71. doi: 10.4103/2211-4122.166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kilner PJ, Geva T, Kaemmerer H, Trindade PT, Schwitter J, Webb GD, et al. Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European Society of Cardiology. Eur Heart J. 2010;31:794–805. doi: 10.1093/eurheartj/ehp586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faganello G, Campana C, Belgrano M, Russo G, Pozzi M, Cioffi G, et al. Three dimensional printing of an atrial septal defect: Is it multimodality imaging? Int J Cardiovasc Imaging. 2016;32:427–8. doi: 10.1007/s10554-015-0801-0. [DOI] [PubMed] [Google Scholar]
- 60.Campbell-Washburn AE, Tavallaei MA, Pop M, Grant EK, Chubb H, Rhode K, et al. Real-time MRI guidance of cardiac interventions. J Magn Reson Imaging. 2017;46:935–50. doi: 10.1002/jmri.25749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Bello V, Pugliese NR, Liga R, Barletta V, Santini V, Conte L, et al. Translational cardiovascular imaging: A new integrated approach to target myocardial fibrosis turnover in different forms of cardiac remodeling. J Cardiovasc Echogr. 2017;27:30–1. doi: 10.4103/2211-4122.199066. [DOI] [PMC free article] [PubMed] [Google Scholar]


