Abstract
Estrogen administration following menopause has been shown to support hippocampally-mediated cognitive processes. A number of previous studies have examined the effect of estrogen on hippocampal structure to determine the mechanism underlying estrogen effects on hippocampal function. However, these studies have been largely observational and provided inconsistent results. We examined the effect of short-term estradiol administration on hippocampal gray matter volume in a prospective study with multiple doses of estradiol (placebo, 1 mg, and 2 mg). Following three months of estradiol administration bilateral posterior hippocampal voxel-based gray matter volume was increased in women who received 2 mg estradiol. There were no significant differences in total hippocampal volume and no significant effects on gray matter volume in women who received placebo or 1 mg estradiol. These findings accord with previous animal studies and provide evidence of estrogen effects on hippocampal morphology that may represent a neurobiological mechanism for estrogen effects on cognition in post-menopausal women.
1. Introduction
Cognitive changes during the menopause-transition and following menopause in women are common (Halbreich et al., 1995; Weber et al., 2014; Woods et al., 2000). Reported changes during menopause include reductions in both delayed and immediate recall (Epperson et al., 2013) and minor decreases in concentration and processing speed (Kok et al., 2006). Additionally, cognitive changes in post-menopausal women are greater than expected from the effects of age alone (Halbreich et al., 1995), suggesting that the decline in estrogen during menopause may have negative consequences for cognitive performance (for review see: Maki and Dumas, 2009; Newhouse and Dumas, 2015; Sherwin and Henry, 2008). Past studies have also consistently observed cognitive decline and increased risk for dementia in women who undergo early menopause, thus experiencing a greater period of life with low ovarian hormone levels (Farrag et al., 2002; Nappi et al., 1999; Rocca et al., 2007). This further supports a negative impact of estrogen loss beyond the effects of age in older women.
The cognitive benefit of estrogen may be mediated in part by estrogen effects on the hippocampus. The hippocampus is implicated in memory function and has been shown to have an abundance of estrogen receptors (for review see Bean et al., 2014; Hara et al., 2015; Österlund et al., 2000; Woolley, 1998), suggesting that it may be a structure that is particularly sensitive to the effects of estrogen. Previous studies support that hormone replacement with estrogen has beneficial cognitive effects in postmenopausal women. In observational studies long-term hormone replacement is associated with better performance in a range of cognitive domains, including working and episodic memory, (Berent-Spillson et al., 2010; Maki et al., 2011; Phillips and Sherwin, 1992; Sherwin, 1997). Additionally, experimental administration of estrogen benefits hippocampally-mediated cognitive processes (Daniel et al., 2015; Jacobs et al., 1998; Verghese et al., 2000).
These benefits of estrogen on hippocampally-mediated cognitive processes may parallel positive effects of estrogen on hippocampal structure. Animal studies demonstrate that estrogen administration is associated with increased hippocampal dendritic density (Lewis et al., 1995; Woolley, 1998; Woolley and McEwen, 1992). The observed increases in dendritic density following estrogen administration is blocked by estrogen receptor antagonists (Lewis et al., 1995), supporting that estrogen has direct effects on morphology.
Previous human studies examining morphology changes with ovarian hormone replacement in postmenopausal women have reported inconclusive or conflicting results (Wnuk et al., 2012). Cross-sectional studies report greater hippocampal volume in women who previously received hormone replacement compared with never users (Boccardi et al., 2006; Lord et al., 2008), although others found no difference in hippocampal volume between past users and non-users of hormone replacement (Resnick et al., 2009). These studies are limited by being observational rather than prospectively administering estrogen or have included combined estrogen and progesterone hormone regimens that may have reduced effects compared with estrogen alone (Maki et al., 2007). Additionally, previous studies have not generally used voxel-based methods to assess changes in gray matter volume which may be more sensitive to the effects of short-term estrogen in the hippocampus than region of interest total volume measures (Mechelli et al., 2005).
The purpose of this study was to determine whether estradiol (E2) administration has an acute effect on hippocampal volume in post- menopausal women. Women completed an MRI scan before and after 3 months of receiving 17β-estradiol or placebo. We hypothesized that administration of E2 would result in greater increases in hippocampal gray matter volume than in women receiving placebo.
2. Methods
2.1. Participants
Seventy-five postmenopausal women were included in this study. Participants were recruited for two studies: one examining the effects of E2 administration on cognitive function (Dumas et al., 2013; Vega et al., 2016) and the other examining the effect of E2 administration on the stress response (unpublished). Participants completed the study at either Vanderbilt University Medical center or the University of Vermont. These studies were approved by the Vanderbilt University Medical Center or University of Vermont Institutional Review Boards and informed consent was obtained from all participants.
2.2. Cognitive Screening
All participants were cognitively assessed using the Mini Mental State Exam (MMSE) (Folstein et al., 1975), Brief Cognitive Rating Scale, and the Mattis Dementia Rating Scale (DRS-2) to establish a Global Deterioration Scale score (GDS) (Reisberg et al., 1982). Participants were required to have a GDS score of 1-2 and a MMSE score of greater than 26. No participant scored below 123 on the Mattis scale (Table 1).
Table 1.
Participant Age, Menopausal Symptoms, and Cognitive Assessment
| 17β-Estradiol | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| placebo (n = 21) | 1 mg (n = 21) | 2 mg (n = 33) | |||||||||||
|
| |||||||||||||
| Mean | SD | Min. | Max. | Mean | SD | Min. | Max. | Mean | SD | Min. | Max. | One-way ANOVA (E2 Groups) | |
| Age | 56.76 | 2.62 | 52.00 | 60.00 | 55.52 | 2.56 | 51.00 | 59.00 | 61.85 | 6.37 | 53.00 | 74.00 | F (2, 72) = 12.58, p < 0.001 |
| MSC | 19.29 | 10.18 | 6.00 | 49.00 | 15.71 | 9.00 | 4.00 | 37.00 | 14.72 | 9.61 | 1.00 | 41.00 | F (2,72) = 1.49, p = 0.23 |
| DRS | 141.24 | 2.12 | 137.00 | 144.00 | 141.24 | 1.92 | 137.00 | 144.00 | 139.97 | 8.90 | 92.00 | 144.00 | F (2,72) = 0.39, p = 0.67 |
| GDS | 1.52 | 0.51 | 1.00 | 2.00 | 1.48 | 0.51 | 1.00 | 2.00 | 1.73 | 0.45 | 1.00 | 2.00 | F (2,72) = 2.07, p = 0.13 |
| BCRS | 9.14 | 1.20 | 7.00 | 11.00 | 9.29 | 1.45 | 8.00 | 13.00 | 9.55 | 1.23 | 8.00 | 12.00 | F (2,72) = 0.68, p = 0.51 |
| MMSE | 28.90 | 1.41 | 26.00 | 30.00 | 29.10 | 1.04 | 26.00 | 30.00 | 29.27 | 0.98 | 26.00 | 30.00 | F (2,72) = 0.67, p = 0.51 |
Comparison across the three treatment groups (placebo, 1 mg, and 2 mg) for age, menopausal symptoms (MSC = Menopause Symptom Checklist total score) and cognitive assessments (DRS = Mattis Dementia Rating Scale 2; GDS = Global Deterioration Scale; BCRS = Brief Cognitive Rating Scale; MMSE = Mini Mental State Exam). Differences in scores for the treatment groups were assessed using one-way ANOVA; the only significant (α = 0.05) difference between groups was in age.
2.3. Estradiol Administration
All participants were postmenopausal, without menses for at least 1 year with FSH level greater than 27 mIU/ml. Women with bilateral oophorectomy were excluded from participation. None of the participants were taking ovarian hormones, hormone therapy, or hormonal contraception at enrollment and were at least one year without such treatment. Participants were physically healthy with a body mass index ≤33 kg/m2. Participants with major concomitant illnesses were excluded for abnormal findings on the basis of history, physical exam (including EKG), and laboratory tests assessing hematopoietic, renal, hepatic and hormonal function (complete blood count, serum chemistries, thyroid stimulating hormone, urinalysis). Participants were required to have had a normal mammogram within the last year.
Participants were excluded if they had specific contraindications for E2 treatment. Specific criteria for exclusion for the E2 treatment included contraindications for hormone replacement including history of breast cancer or E2-dependent neoplasia; blood pressure > 160/100 (untreated); history of deep vein thrombosis or other thromboembolic disease; hepatoma; severe migraines or stroke on oral contraceptives; diabetes; untreated thyroid disease; clinical osteoporosis; and severe menopausal symptoms. Menopausal symptoms were measured using a 60 item (somatic including vasomotor symptoms and mood symptoms) self-reported Menopause Symptoms Checklist, Each item on the checklist was rated from 0 (not at all) to 4 (extremely) for how much each symptom had bothered that participant in the last month, with a possible maximum total score of 240. All participants had a score below 50 on the Menopause Symptoms Checklist (Table 1).
Participants in the first study received double-blinded 1 mg oral 17β-estradiol or placebo daily for 3 months. Participants in the second study received open-label 1 mg oral 17β-estradiol daily for I month and then 2 mg oral 17β-estradiol daily for 2 months to limit side effects during treatment.
2.4. Imaging Procedures
Participants at both sites were scanned on a Philips 3.0 Tesla Achieva scanner, with an 8 channel head coil. Participants had repeated MRI sessions: once at enrollment before beginning E2 or placebo administration, following 3 months of treatment. All participants received the following MR sequences at each MRI session:
Sagittal T1-weighted 3D Turbo Field Echo Sensitivity Encoding (TFE SENSE) sequence perpendicular to the anterior commissure (AC) -posterior commissure (PC) line, repetition time (TR) of 9.9 ms, echo time (TE) of 4.6 ms, a flip angle of 8°, number signal averages (NSA) 1.0, a field of view (FOV) of 256 mm, a 256 × 256 matrix, and 1.0 mm slice thickness with no gap for 140 contiguous slices.
T2- weighted Gradient and Spin Echo (GRASE) sequence parallel to the AC-PC line, TR 2470 ms, TE 80 ms, NSA 3.0, FOV of 230 mm, 0.7 mm slice thickness with 5.0 mm gap for 28 slices
2.5. Hippocampal Gray Matter Volume Assessment
Morphological changes in the hippocampus were assessed by two methods: a region of interest (ROI) approach and modulated voxel-based morphometry (mVBM). The ROI-based method provides a measure of total hippocampal gray matter volume change with treatment while the mVBM approach allows for the assessment of gray matter volume change by voxel and the identification of clusters of voxels within the hippocampus that show changes in gray matter volume with treatment.
ROI Hippocampal Gray Matter Volume
ROI Hippocampal volume analysis was performed using the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/), including motion correction and averaging, removal of non-brain tissue, segmentation of the subcortical white matter and deep gray matter volumetric structures, automated topology correction and surface deformation. The FreeSurfer longitudinal stream (Reuter et al., 2012) was used to create an unbiased within participant template. This template was then used to run the standard “recon-all” procedure with default settings. Each scan was inspected to identify any areas where non-brain was included or brain was excluded. Manual corrections to the brain mask were made as appropriate and the recon procedure was run again with the corrected mask. The hippocampus segmentations were specifically inspected for errors but no manual corrections were required. We then analyzed the volume change of right and left hippocampal volume between time points (pre and post treatment). Hippocampal volumes were normalized by the estimated intracranial volume to account for variation in head size.
mVBM Hippocampal Gray Matter Volume
For each subject, a within subject average image was first created using the pairwise longitudinal registration function in SPM12. This method creates an unbiased average image along with a jacobian distance image. Each average T1 was segmented using the standard processes in SPM12. The gray matter tissue image for the average T1 was then multiplied by the jacobian distance image to create a gray matter change image for each subject. In this image, negative values show a decrease in gray matter volume from pre-treatment to post treatment, while positive values show increase from pre-treatment to time post treatment.
We then used the DARTEL tools in SPM12 to create a study-specific template, warps for each subject to this template, and the transform of the template to MNI space. The warps and transform were then applied to the gray matter change images to create images in MNI space for comparison. 2nd level analysis was conducted using SPM12 to identify clusters within the hippocampus (anatomical ROI from the human AAL atlas defined in WFU PickAtlas) where gray matter volume showed a significant change with E2 dose (with cluster threshold correction for multiple comparisons: uncorrected p = 0.05, k = 53, corrected p <0.05 voxel-based p = 0.001, T = 1.73). mVBM gray matter volume change values (pre to post treatment change in signal per voxel) within these clusters were extracted.
2.6. Statistical Analysis
Univariate ANCOVAs were conducted for E2 dose (placebo, 1 mg E2, 2 mg E2) effect on right and left hippocampal volume change (pre to post 17β-estradiol change in signal per voxel) and mVBM gray matter volume change within the clusters identified in the 2nd level analysis for E2 dose effects using SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). Age and intracranial volume were included as covariates in both ROI and mVBM analyses.
This study was conducted at two sites; the 0 and 1 mg groups had participants both at the University of Vermont and Vanderbilt University Medical Center while all of the 2 mg group participants completed the study at Vanderbilt University Medical Center. To assess a possible effect of study site, independent samples t-tests for baseline volume and mVBM measures were conducted. There were no significant differences in the baseline right (t (73) = 0.07, p = 0.95) or left (t (73) = 0.11, p = 0.92) hippocampal volumes or right (t (73) = 1.08, p = 0.28) and left (t (73) = 0.89, p = 0.38) cluster mVBM gray matter volume between study sites.
To address a possible relationship between E2 dose and age (due to differences in included age range in the studies) a univariate ANOVA examining mean age in the three E2 dose groups was conducted. There was a significant difference in age between the E2 dose groups (F (2, 72) = 12.58, p < 0.001). The 2 mg study included a wider age range in inclusion criteria so that the group that received 2 mg E2 had a significantly older mean than the 1 mg E2 and the placebo E2 group (Table 1). There was no significant difference in age between the 1 mg and placebo E2 groups (t (39) = 1.61, p = 0.12).
Secondary analyses were conducted including only participants 60 years of age and younger (the age range included in the placebo and 1 mg study) which brought the 2 mg group to n = 15.
3. Results
Participants were 75 postmenopausal women between the ages of 51 to 75, M=58.19 (SD= 5.42). Women were treated with placebo (n=21), 1 mg (n=21), or 2 mg (n=33) E2 for 3 months.
In the age-restricted (60 and younger) analysis a univariate ANOVA did not show a significant relationship between age and dose (F (2, 54) = 1.41, p = 0.25). Subsequent analyses were conducted including all participants and on an age-restricted subset of participants.
3.1. ROI Hippocampal Gray Matter Volume
Univariate ANCOVAs examining the effect of E2 dose in right and left hippocampal volume change (controlling for intracranial volume and age) showed no significant effect of E2 dose on hippocampal volume (right: F(2,70) = 1.36, p = 0.26; left: F(2,70) = 0.29, p = 0.75). There were no significant effects of intracranial volume or age. Restricting the analysis to participants less than 60 years of age did not result in any significant results in these analyses.
3.2. mVBM Hippocampal Gray Matter Volume
Linear regression analyses of mVBM hippocampal gray matter volume change (controlling for intracranial volume and age) demonstrated significant positive effects of E2 dose in bilateral posterior hippocampus clusters (right MNI: 30, -4, 3; Left MNI: -35, -39, -3) (Figure 1). There were no clusters that showed significant negative effects of E2 dose.
Figure 1. Estradiol Effects on Gray Matter Volume.
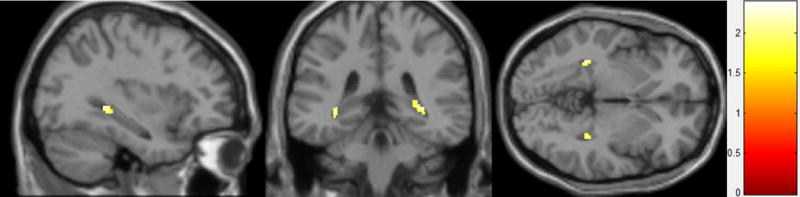
Clusters with significant (corrected p < 0.05) increase in gray matter volume with increasing estradiol dose.
In univariate ANCOVAs including all participants, there was a positive effect of E2 dose on mVBM gray matter volume change in both right (F(2,71) = 4.69, p < 0.012) and left (F(2,71) = 3.82, p < 0.027) posterior hippocampal clusters. Post hoc Tukey tests showed a significantly greater change in mVBM gray matter volume in the 2 mg group (right: M = 0.0013, SD = 0.0018; left: M = 0.0017, SD = 0.0029) compared to placebo (right: M = -0.0002, SD = 0.0022; left: M = -0.0002, SD = 0.0026) (right: p = 0.037, left: p = 0,030) and 1 mg (right: M = -0.0007, SD = 0.0022; left: M = -0.0001, SD = 0.0021) (right: p = 0.001, left: p = 0.036). Change in mVBM gray matter volume was not significantly different between the placebo and 1 mg groups. There were no significant effects of intracranial volume or age.
In univariate ANCOVAs including only participants 60 years of age and younger, we continued to observe a significant positive effect of E2 dose on mVBM gray matter volume change in both right (F (2, 57) = 3.69, p < 0.032) and left (F(2,57) = 3.43, p < 0.039) posterior hippocampal clusters. Post hoc Tukey tests showed a significantly greater change in mVBM gray matter volume in the 2 mg group (right: M = 0.0012, SD = 0.0020; left: M = 0.0020, SD = 0.0034) compared to the placebo (right: M = -0.0002, SD = 0.0022; left: M = -0.0002, SD = 0.0026) (right: p = 0.010, left: p = 0.030) and 1 mg (right: M = -0.0007, SD = 0.0022; left: M = -0.0001, SD = 0.0021) (right: p = 0.020, left: 0.026). Change in mVBM gray matter volume was not significantly different between the placebo and 1 mg groups.
4. Discussion
The main finding of the current study was that daily 2mg 17β-estradiol administered over three months was associated with increased bilateral posterior hippocampal gray matter volume. This effect was seen primarily in women who received 2 mg daily 17-β estradiol with no significant change in hippocampal gray matter volume in women who received placebo or 1 mg daily 17-β estradiol. We did not observe any effects of E2 administration on total hippocampal gray matter volume.
These results support that, similar to previous findings in animal studies, estrogen may have trophic effects in the human hippocampus. Estrogen has been shown to increase the density of dendritic spines on CA1 pyramidal neurons especially following neuronal damage or estrogen loss (Woolley, 1998; Woolley and McEwen, 1992) while estrogen receptor antagonists block this effect (Lewis et al., 1995). The presence of estrogen appears to prime the neuron for new synapse creation through an increase in dendritic spines, however these new spines are only maintained following synapse activation (Phan et al., 2015).
The morphologic effects of estrogen have generally been seen in young animals and proposed to diminish with time since estrogen deprivation (Smith et al., 2010; Vedder et al., 2014). However, Hao and colleagues have demonstrated estrogen-induced dendritic spine increases in the prefrontal cortex of aged female non-human primates (Hao et al., 2006) which accord with beneficial estrogen effects on working memory (Rapp et al., 2003) and executive function (Voytko et al., 2009) performance. These results suggest that older animals may remain sensitive to the effects of estrogen on brain morphology and function. The current results provide evidence that similar sensitivity may be seen in older postmenopausal women, as a significant portion of the 2 mg E2 group were over the age of 60 and there was no a significant effect of age on changes in gray matter volume. In the non-human primate studies, the morphological effects of estrogen were only seen in cyclic (as opposed to continuous) administration (Young et al., 2013). In contrast, this study utilized continuous administration. Whether there are differential effects of cyclic vs. continuous estrogen administration on hippocampal structure in post-menopausal women requires further study.
Paralleling these structural changes, estrogen therapy in animal models and human-studies improves performance on cognitive tasks that are hippocampally mediated (Daniel et al., 2015; Jacobs et al., 1998; Verghese et al., 2000). Our results may help explain those findings, as we observe the effect of E2 administration on gray matter volume in the posterior hippocampus. Posterior regions of the hippocampus are associated with cognitive processes including episodic and spatial memory, whereas the anterior hippocampus has been associated with emotional and stress response processes (Bannerman et al., 2004; Fanselow and Dong, 2010). Positive effects of E2 in the posterior hippocampus may indicate increased synaptic plasticity in regions of the hippocampus important for cognitive processes that benefit from estrogen administration. This change in plasticity may provide a mechanism through which estrogen administration improves hippocampally-mediated cognitive performance in post-menopausal women.
This study is distinct from past work as it included prospective experimental manipulation of E2 administration and examined the effect of multiple clinically relevant E2 doses on hippocampal gray matter volume through both ROI-based and mVBM analysis. Interpretation of this study should be considered in context of the small sample size. Additionally the study groups were not age matched and there was a confounding effect of age and E2 dose with the 2 mg treatment group including significantly older participants than the placebo and 1 mg group. However, restricting analysis with a limited age range to eliminate the age difference between treatment groups did not substantially change our results.
The effects of estrogen in older women may depend on the duration of treatment and whether estrogen is unopposed or administered with progesterone or progestins. Previous studies which found hormone therapy effects on hippocampal volume have included long-term estrogen use and combined estrogen-progesterone treatment (Boccardi et al., 2006; Lord et al., 2008). Total hippocampal volume and voxel-based measures of gray matter volume changes may be assessable on different time scales with voxel-based methods detecting changes after short-term use and total volume changes only seen after long-term use. Alternatively, hippocampal volume may be differentially affected by estrogen and progesterone. The use of short-term unopposed E2 in this study precludes the examination of the effects of long-term use or combined treatment.
This study did not include imaging approaches that allow examination of gray matter volume in specific hippocampal subfields. As hippocampal subfields have different roles in cognitive function, the ability to examine changes by subfield may provide additional information about the functional consequences of the structural changes seen in this study The cognitive assessment scores obtained at the screening visit for this study by design had very narrow ranges (due to inclusion and exclusion criteria requiring no evidence of mild cognitive impairment or dementia), and thus prevented meaningful analysis of the relationship between E2 effects on hippocampal structure and cognitive performance. Concurrent cognitive testing and imaging would likely contribute to the interpretation of these results and possible effects on cognitive performance.
5. Conclusions
In conclusion, postmenopausal women who were titrated to 2 mg of daily oral 17-β estradiol over 3 months exhibited increased posterior hippocampal gray matter volume. These findings support estrogen effects on hippocampal morphology that may provide a neurobiological basis for the beneficial effect of estrogen treatment on hippocampally-mediated cognitive function in postmenopausal women. Future work should examine whether the current findings of structural effects of E2 on the hippocampus are related to improvement in cognitive performance. This work may inform novel pharmacological and cognitive training approaches to maintaining cognitive function in aging women.
Acknowledgments
2R01 AG021476, R01 MH102246, K24 MH110598, UL1TR000445
References
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, Zhang WN, Pothuizen HHJ, Feldon J. Regional dissociations within the hippocampus - Memory and anxiety. Neurosci Biobehav Rev. 2004 doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist. 2014;20:534–45. doi: 10.1177/1073858413519865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berent-Spillson A, Persad CC, Love T, Tkaczyk A, Wang H, Reame NK, Frey KA, Zubieta JK, Smith YR. Early menopausal hormone use influences brain regions used for visual working memory. Menopause. 2010;17:692–699. doi: 10.1097/gme.0b013e3181cc49e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M, Ghidoni R, Govoni S, Testa C, Benussi L, Bonetti M, Binetti G, Frisoni GB. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a Voxel-based morphometry study. Menopause. 2006;13:584–91. doi: 10.1097/01.gme.0000196811.88505.10. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Witty CF, Rodgers SP. Long-term consequences of estrogens administered in midlife on female cognitive aging. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, McDonald BC, Naylor MR, Pfaff AC, Saykin AJ, Newhouse PA. Increased working memory-related brain activity in middle-aged women with cognitive complaints. Neurobiol Aging. 2013;34:1145–1147. doi: 10.1016/j.neurobiolaging.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: Findings from a longitudinal community cohort. J Clin Endocrinol Metab. 2013;98:3829–3838. doi: 10.1210/jc.2013-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrag AKF, Khedr EM, Abdel-Aleem H, Rageh TA. Effect of surgical menopause on cognitive functions. Dement Geriatr Cogn Disord. 2002;13:193–198. doi: 10.1159/000048652. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, Joe SH. Possible acceleration of age effects on cognition following menopause. J Psychiatr Res. 1995;29:153–163. doi: 10.1016/0022-3956(95)00005-P. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WGM, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–8. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol Rev. 2015;95:785–807. doi: 10.1152/physrev.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S. Sex steroids to maintain cognitive function in women after the menopause: A meta-analyses of treatment trials. Maturitas. 2010 doi: 10.1016/j.maturitas.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Tang MX, Stern Y, Sano M, Marder K, Bell KL, Schofield P, Dooneief G, Gurland B, Mayeux R. Cognitive function in nondemented older women who took estrogen after menopause. Neurology. 1998;50:368–373. doi: 10.1212/wnl.50.2.368. http://dx.doi.org/10.1212/WNL.50.2.368. [DOI] [PubMed] [Google Scholar]
- Kok HS, Kuh D, Cooper R, van der Schouw YT, Grobbee DE, Wadsworth ME, Richards M. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause. 2006;13:19–27. doi: 10.1097/01.gme.0000196592.36711.a0. [DOI] [PubMed] [Google Scholar]
- Lewis C, McEwen BS, Frankfurt M. Estrogen-induction of dendritic spines in ventromedial hypothalamus and hippocampus: effects of neonatal aromatase blockade and adult GDX. Dev Brain Res. 1995;87:91–95. doi: 10.1016/0165-3806(95)00052-F. [DOI] [PubMed] [Google Scholar]
- Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: A possible window of opportunity effect. Neurobiol Aging. 2008;29:95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Maki PM, Dennerstein L, Clark M, Guthrie J, Lamontagne P, Fornelli D, Little D, Henderson VW, Resnick SM. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 2011;1379:232–243. doi: 10.1016/j.brainres.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Dumas J. Mechanisms of action of estrogen in the brain: Insights from human neuroimaging and psychopharmacologic studies. Semin Reprod Med. 2009 doi: 10.1055/s-0029-1216278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: A randomized, double-blind trial. Neurology. 2007;69:1322–1330. doi: 10.1212/01.wnl.0000277275.42504.93. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-Based Morphometry of the Human Brain: Methods and Applications. Curr Med Imaging Rev. 2005;1:0–0. [Google Scholar]
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G. Memory functioning at menopause: Impact of age in ovariectomized women. Gynecol Obstet Invest. 1999;47:29–36. doi: 10.1159/000010058. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Dumas J. Estrogen-cholinergic interactions: Implications for cognitive aging. Horm Behav. 2015;74:173–185. doi: 10.1016/j.yhbeh.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Österlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha RNA. J Clin Endocrinol Metab. 2000;85:3840–3846. doi: 10.1210/jc.85.10.3840. [DOI] [PubMed] [Google Scholar]
- Phan A, Suschkov S, Molinaro L, Reynolds K, Lymer JM, Bailey CDC, Kow LM, MacLusky NJ, Pfaff DW, Choleris E. Rapid increases in immature synapses parallel estrogen-induced hippocampal learning enhancements. Proc Natl Acad Sci U S A. 2015;112:16018–16023. doi: 10.1073/pnas.1522150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-T. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, De Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Ockene J, Davatzikos C. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72:135–42. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, De Andrade M, Melton LJ. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, estrogen, and dementia: A 2014 update. Mol Cell Endocrinol. 2014 doi: 10.1016/j.mce.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen effects on cognition in menopausal women. Neurology. 1997;48:S21–6. doi: 10.1212/WNL.48.5_Suppl_7.21S. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: A critical review. Front Neuroendocrinol. 2008 doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen’s ability to enhance hippocampal synaptic physiology. Proc Natl Acad Sci. 2010;107:19543–19548. doi: 10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder LC, Bredemann TM, McMahon LL. Estradiol replacement extends the window of opportunity for hippocampal function. Neurobiol Aging. 2014;35:2183–2192. doi: 10.1016/j.neurobiolaging.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega JN, Zurkovsky L, Albert K, Melo A, Boyd B, Dumas J, Woodward N, McDonald BC, Saykin AJ, Park JH, Naylor M, Newhouse PA. Altered brain connectivity in early postmenopausal women with subjective cognitive impairment. Front Neurosci. 2016;10 doi: 10.3389/fnins.2016.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Kuslansky G, Katz MJ, Sliwinski M, Crystal HA, Buschke H, Lipton RB. Cognitive performance in surgically menopausal women on estrogen. Neurology. 2000;55:872–4. doi: 10.1212/WNL.55.6.872. [DOI] [PubMed] [Google Scholar]
- Lou Voytko M, Murray R, Higgs CJ. Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J Neurosci. 2009;29:10362–70. doi: 10.1523/JNEUROSCI.1591-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: a systematic review and meta-analysis. J Steroid Biochem Mol Biol. 2014;142:90–8. doi: 10.1016/j.jsbmb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wnuk A, Korol DL, Erickson KI. Estrogens, hormone therapy, and hippocampal volume in postmenopausal women. Maturitas. 2012 doi: 10.1016/j.maturitas.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NF, Mitchell Ellen S, Adams C. Memory Functioning Among Midlife Women: Observations from the Seattle Midlife Women’s Health Study. Menopause J North Am Menopause Soc. 2000;7:257–265. doi: 10.1097/00042192-200007040-00008. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–8. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1002/cne.903360210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Ohm DT, Janssen WGM, Gee NA, Lasley BL, Morrison JH. Continuously delivered ovarian steroids do not alter dendritic spine density or morphology in macaque dorsolateral prefrontal cortical neurons. Neuroscience. 2013;255:219–25. doi: 10.1016/j.neuroscience.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]


