Abstract
Azo dyes are widely used in dye manufacturing, paper printing, textile industries, and as tattoo pigmentation. Since intestinal and skin bacteria can metabolize certain azo dyes to carcinogenic compounds, many researchers have studied the azoreductases of these bacteria. In this study, we used a microarray method to identify the intestinal bacterial species from cultured fecal samples in Brain Heart Infusion (BHI) broth with or without azo dyes that may be involved in azo dye reduction. The microarray was designed to identify 40 bacterial species that are reported in the literature to be predominant in human feces. Results from this study showed 26–30 species are present in the cultured fecal samples. The representative bacteria were then examined for the azo dye reduction activity.
Keywords: Microarray, Human intestinal microflora, Azo dye reduction
1. Introduction
Azo dyes are compounds consisting of a diazotized amine coupled to an amine or a phenol containing one or more azo linkages. There are at least 3000 azo dyes used in food, pharmaceutical, and other industries (Chung et al., 1992). Some azo dyes have also been used in tattoo pigmentation (Baumler et al., 2000). Previously studies have shown that some intestinal and skin bacteria can metabolize certain azo dyes to carcinogenic compounds (Chung et al., 1992; Cerniglia et al., 1982; Rafii et al., 1992). However, only a limited number of bacterial species were studied individually for the presence of azoreductases. Early studies led us to ask several questions: what effects do azo dyes have on the intestinal microflora? How many intestinal bacterial species are involved in azoreduction? Since we cannot use human subjects for the in vivo study, human fecal samples were used to test the azoreductase activity in a complex intestinal bacterial mixture and a microarray method was used to identify the bacterial species present in the cultured fecal samples.
Intestinal bacteria are important for human health because they can digest dietary components, metabolize xenobiotics and drugs, and provide short chain fatty acids and vitamins that are later absorbed into the system (Cerniglia et al., 1984; Chadwick et al., 1992). The intestinal population also acts as a barrier against colonization of the gastrointestinal tract by pathogenic bacteria (Vollaard and Clasener, 1994). The human gastrointestinal tract contains over 1011 bacterial cells per gram of fecal content consisting of more than 400 bacterial species (Carman et al., 1993; Drasar and Duerden, 1991; Moore and Holdeman, 1974; Cerniglia and Kotarski, 1999; Drasar and Roberts, 1990; Moore and Moore, 1995). The predominant intestinal bacteria are Bacteroides spp., Clostridium spp., Eubacterium spp., Bifidobacterium spp., Fusobactertium spp., Ruminococcus spp., Enterococcus spp., and Peptostreptococcus spp.
Traditionally, the human intestinal bacteria population was characterized by microscopic, biochemical, physiological, and culture methods (Drasar and Roberts, 1990; Moore and Moore, 1995). Culture methods are time consuming and many intestinal bacteria are not easily cultured or isolated from the complex mixture. In recent years, many molecular methods have been used for identification of intestinal bacteria; however, many of these methods are group-specific for bacteria, but not species-specific (Langendijk et al., 1995; Harmsen et al., 2002; Kageyama and Benno, 2001; Jansen et al., 1999; Matsuki et al., 2002; Sghir et al., 2000). The complex microflora of the human gut is difficult to study with only species-specific primers because of the diversity of this ecosystem (Matsuki et al., 2002). Previously, we developed polymerase chain reaction (PCR) methods for the detection and quantitation of anaerobic bacteria in human and animal feces (Wang et al., 1994, 1996, 1997). A limitation of this methodology was that each bacterial species was assayed separately.
Microarray technology is a powerful tool used for the simultaneous detection of thousands of genes or target DNA sequences on one glass slide (Chizhikov et al., 2001; Wu et al., 2001). Microarrays were initially used for gene expression studies. However, microarrays can also be used for the detection of bacteria or for performing DNA-based typing of specific pathogenic bacterial strains (Chizhikov et al., 2001; Call et al., 2001; Wang et al., 2002a,b). We developed an oligonucleotide microarray method on the species-level for detecting and identifying 40 intestinal bacterial species on one slide (Wang et al., 2003). In this study, we used this microarray method to identify the intestinal bacterial species involved in azo dye reduction.
2. Materials and methods
2.1. Source of bacterial strains and culture conditions
Reference strains for 40 human intestinal bacterial species (Table 1) were obtained from American Type Culture Collection (ATCC). Anaerobic bacteria were cultured at 35 °C in either prereduced anaerobically sterilized (PRAS) Brain Heart Infusion (BIH) broth supplemented with vitamin K and hemin (Remel, Lenexa, KS, USA), inoculated under an oxygen-free cannula using 85% nitrogen, 10% hydrogen and 5% carbon dioxide, or on PRAS brucella blood agar plates supplemented with vitamin K and hemin (Remel).
Table 1.
Bacteria and the probe numbers in the microarray
| Number | Bacterial species and strain | Probe number |
|---|---|---|
| 1 | B. thetaiotaomicron ATCC 29148 | 1, 2, 3 |
| 2 | B. vulgatus ATCC 8482 | 4, 5, 6 |
| 3 | B. fragilis ATCC 23745 | 7, 8, 9 |
| 4 | B. distasonis ATCC 8503 | 10, 11, 12 |
| 5 | C. clostridioforme ATCC 29084 | 13, 14, 15 |
| 6 | C. leptum ATCC 29065 | 16, 17, 18 |
| 7 | F. prausnitzii ATCC 27768 | 19, 20, 21 |
| 8 | P. productus ATCC 27340 | 22, 23, 24 |
| 9 | R. obeum ATCC 29174 | 25, 26, 27 |
| 10 | R. bromii ATCC 27255 | 28, 29, 30 |
| 11 | R. callidus ATCC 27760 | 31, 32, 33 |
| 12 | R. albus ATCC 27210 | 34, 35, 36 |
| 13 | B. longum ATCC 15707 | 37, 38, 39 |
| 14 | B. adolescentis ATCC 15703 | 40, 41, 42 |
| 15 | B. infantis ATCC 15697 | 43, 44, 45 |
| 16 | E. biforme ATCC 27806 | 46, 47, 48 |
| 17 | E. aerofaciens ATCC 25986 | 49, 50, 51 |
| 18 | L. acidophilus ATCC 4356 | 52, 53, 54 |
| 19 | E. coli ATCC 25922 | 55, 56, 57 |
| 20 | E. faecium ATCC 19434 | 58, 59, 60 |
| 21 | B. uniformis ATCC 8492 | 61, 62, 63 |
| 22 | B. ovatus ATCC 8483 | 64, 65, 66 |
| 23 | B. caccae ATCC 43185 | 67, 68, 69 |
| 24 | C. perfringens ATCC 13124 | 70, 71, 72 |
| 25 | C. butyricum ATCC 19398 | 73, 74, 75 |
| 26 | C. ramosum ATCC 25582 | 76, 77, 78 |
| 27 | C. difficile ATCC 9689 | 79, 80, 81 |
| 28 | C. indolis ATCC 25771 | 82, 83, 84 |
| 29 | F. russii ATCC 25533 | 85, 86, 87 |
| 30 | F. nucleatum ATCC 25586 | 88, 89, 90 |
| 31 | B. catenulatum ATCC 27539 | 91, 92, 93 |
| 32 | B. angulatum ATCC 27535 | 94, 95, 96 |
| 33 | E. rectale ATCC 33656 | 97, 98, 99 |
| 34 | E. eligens ATCC 27750 | 100, 101, 102 |
| 35 | E. limosum ATCC 8486 | 103, 104, 105 |
| 36 | E. lentum ATCC 25553 | 106, 107, 108 |
| 37 | L. fermentum ATCC 9338 | 109, 110, 111 |
| 38 | E. faecalis ATCC 27274 | 112, 113, 114 |
| 39 | P. magnus ATCC 14955 | 115, 116, 117 |
| 40 | R. gnavus ATCC 291492 | 118, 119, 120 |
2.2. Microarray method
The assay was previously described by Wang et al. (2003). Briefly, the method was developed to detect and identify 40 common intestinal bacterial species. These 40 species were reported to be predominant in the human gastrointestinal tract, primarily using on culture methods (Carman et al., 1993; Drasar and Duerden, 1991; Moore and Holdeman, 1974; Cerniglia and Kotarski, 1999; Drasar and Roberts, 1990; Moore and Moore, 1995). Three 40-mer oligos specific for each bacterial species (a total of 120 probes) were designed based on a comparison of the 16S rRNA gene sequences available in the GenBank database. The oligo DNA-array was made on epoxy slides (MWG-biotech, High Point, NC, USA). The 120 oligonucleotide probe numbers and the corresponding bacterial species are listed in Table 1. Using two universal primers (Amp-F and Amp-R), the 16S rRNA gene from all bacterial DNA isolated from fecal samples was amplified and labeled with Cyanine5-dCTP by PCR.
Method for PCR amplification of cyanine5 (CY5)-labeled 16S rDNA: 25 µl of PCR mixture was made by combining 15.6µl of water; 2.5 µl of 10 × BSA-buffer (1 ml 10 × buffer is composed of 0.5 ml 1M Tris–HCl, pH 8.5, 0.2 ml 1M KCl, 30µl of 1M mgCl2, 0.27 ml of water, BSA 5 mg); 2.3µl of dNTP (2.5mM each of dATP, dTTP, dGTP, and 1.7mM of dCTP, Invitrogen, Carlsbad, CA); 1.2 µl of 1mM of CY5-dCTP (Perkin-Elmer Life and Analytical Sciences, Boston, MA, USA); 1.2 µl of primers Amp-F and Amp-R (50 ng/µl each); 0.3 µl of Taq DNA polymerase (5 unit/µl, Invitrogen, Carlsbad, CA); and 2 µl of bacterial DNA or fecal DNA (1–10 ng/µl). The Amp-F and Amp-R primer sequences are GAGAGTTTGATYCTGGCTCAG and AAGGAGGTGATCCARCCGCA, respectively (Y is C or T; R is A or G). PCR was performed in a 9700 GeneAmp PCR System (Perkin-Elmer Life and Analytical Sciences, Boston, MA, USA), using thin-walled 0.2 ml tubes. The amplification conditions were incubation at 95 °C for 3 min, then 35 cycles of 95 °C for 10 s, 53 °C for 10 s, and 72 °C for 70 s, followed by one cycle at 72 °C for 4 min and a cool down to 4 °C.
The PCR product was purified with a Centri-Spin column (Princeton Separations, Adelphia, NJ, USA) following the manufacturer instructions. The purified CY5-labeled PCR products were dried by Speed-Vac centrifugation (Savant, Farmingdale, NY, USA) and dissolved in 13 µl of hybridization buffer (MWG-biotech). The tube was heated for 3 min in a boiling water bath, then immediately placed into ice-water for 2 min. The solution was collected by brief centrifugation and applied onto the microarray. A small glass cover slip that was autoclaved and dried was used to cover the hybridization solution on the array area. The slide was placed into a hybridization chamber (Corning Inc., Corning, NY, USA) and then immersed in a water bath for hybridization overnight at 42 °C. After hybridization, the cover slip was removed by washing the slides for 5 min with 0.5 × SSC, 0.1% SDS. The slides were then washed for 5 min with 0.1 × SSC, 0.1% SDS, followed by a 5 min wash with 0.1 × SSC only. All of the washing steps were conducted at room temperature. The slides were dried by centrifugation for 1 min at 3000 × g in an IEC clinical centrifuge with IEC CAT 801 rotor (International Equipment Company, Needham Heights, MA, USA) and scanned with the ScanArray Express Microarray Scanner (Packard BioScience-Perkin-Elmer, USA). Potential cross-reactivity problems for the species-specific probes were resolved by designing three probes for each species. Positive reactions for at least two out of the three probes for a species were required to consider an identification of the species as positive.
2.3. Human fecal culture for the azo dye reduction study
Brain Heart Infusion broth (100 ml) with a stirrer in a 500 ml beaker was autoclaved and placed into an anaerobic hood for prereducing anaerobically. Fresh human fecal sample (10 g) was added into this 100 ml BHI broth, and then stirred until completely mixed in the anaerobic hood. Five milliliter each of the mixed BHI-fecal sample was aliquoted into 15 ml sterile tubes, then 0.17 ml of sterile azo dyes (10mM of Direct Blue 15, 50mM of Orange II or 50mM of Methyl Red) or 0.17 ml of control (distilled water) was added into different tubes. The tubes were incubated at 37 °C in an anaerobic hood for 16 h without shaking. One milliliter of the upper phase from each tube were transferred into 1.5 ml tubes and centrifuged at 14,000 × g for 10 min. The supernatant was used to measure the azo dye reduction as described below. The pellet was washed with 1ml of 0.85% NaCl and used for isolation of the genomic DNA by using the Easy-DNA kit (Invitrogen, Carlsbad, CA, USA) as described in the earlier publications (Wang et al., 2002b, 2003). The DNA was used for PCR amplification and labeling with Cynine5-dCTP. The labeled PCR products were used to hybridize with the oligo-array on epoxy slides.
For azo dye reduction determination, 0.2 ml each of the supernatant was diluted with 1.8 ml water (1:10). However, for the Methyl Red detection, 0.2 ml of the supernatant was diluted with 1.6 ml water and 0.2 ml of 100% acetic acid. The samples were measured using appropriate wavelengths in a Hewlett-Packard 8453 UV-Visible Spectrophotometer (A601 for Direct Blue 15; A482 for Orange II; A430 for Methyl Red) with 1:10 water diluted supernatant from BHI-feces-water as a blank.
2.4. Examination of azo dye reductive activity by different bacterial pure cultures
Seventeen bacterial species that were strong positive in microarray test were examined for azo dye reduction activity by pure culture of ATCC strains. Each species was inoculated into 5ml BHI broth supplemented with vitamin K and hemin (Ruminococcus and Eubacterium spp. were cultured with additional Rumen Fluid supplements) under an oxygen-free cannula (85% N2, 10% H2 and 5% CO2). The cultures were incubated at 37 °C for 17 h or 48 h until visible growth of the bacteria were observed. An azo dye solution of Direct Blue 15 (3.3 mg/ml in water), sterilized by using a 0.2 µm syringe filter (Gelman Sciences, Ann Arbor, MI, USA), was added for 0.5 ml each into the culture tubes under anaerobic conditions. The cultures were incubated at 37 °C for 24 h. One ml of the mixture from each tube was transferred into 1.5 ml tubes and centrifuged at 14,000 × g for 10 min. The supernatant was diluted (1:10) and assayed for azo dye reduction by measuring the A601 using 1:10 diluted PRAS BHI broth as a blank.
3. Results and discussion
After 16 h incubation of the mixtures of the BHI-fecal sample-azo dyes, the blue, orange, or red color from three different azo dyes (Direct Blue 15, Orange II and Methyl Red) completely disappeared. Table 2 shows the results of azo dye reduction. The concentration of the individual dye was totally reduced, but the fecal sample had some background absorbance for Orange II and Methyl Red samples. To determine which bacterial species in the fecal culture were the major sources of the azoreductase, two methods were used in this study: first, we used the microarray method to determine which bacterial species were present in the cultured fecal samples. Then, we examined the potential of azo dye reduction activity using pure cultures of the selected bacterial species that showed strong positive microarray results.
Table 2.
Azo dye reduction of cultured fecal sample, n = 1 (the remained absorbance was background of fecal sample at this wavelengths)
| Before culture (0 h) | After culture (16 h) | |
|---|---|---|
| A601 for Direct-Blue 15 | 1.364 | 0.051 |
| A482 for Orange II | 1.912 | 0.120 |
| A430 for Methyl Red | 1.596 | 0.216 |
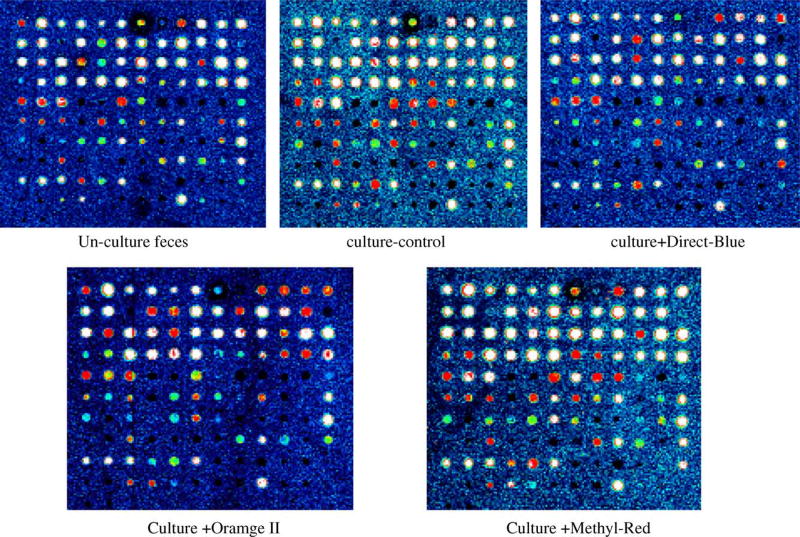
The microarray method was used to determine the bacterial species in original fecal and cultured fecal samples with or without azo dyes. The fecal cultures were incubated without shaking; therefore the upper phase should contain most of the actively growing bacteria. Fig. 1 shows the microarray test results. To read this figure, one should compare Table 1 for probe number and the corresponding bacterial species. For example, since three separate probes were used for each bacterial species, so four different species are distributed in the 12 spaces across top row, with species number 1–4 planted on the top row. Table 3 is the result read from Fig. 1. Comparing un-cultured or cultured fecal sample, the only difference is that after culture, Escherichia coli and Enterococcus faecalis became weakly positive from negative. This indicated that these two species were enriched under these culture conditions and present in lower numbers in the original fecal sample. We calculated the dilution factor as described below: 10 g feces was placed into 100 ml medium (1:11), then 1ml was used for isolation of DNA. The DNA (total 200 µl) was 1:10 diluted and 2µl of the dilution was used for PCR-microaray (1:1000). So the total dilution folds are 1.1 × 104. The minimum cell number for positive microarray result is approximately 10 cells (Wang et al., 2003). Therefore, if the original cell number for certain bacterial species in feces are more than 1.1 × 105 per gram fecal sample (wet weight), that species can give a positive microarray result. The predominant anaerobic bacterial species in feces are more than 109 cells per gram feces (Carman et al., 1993; Drasar and Duerden, 1991; Moore and Holdeman, 1974), hence many bacterial species gave very strong positive microarray test results. However, the initial published literature based the predominant intestinal bacterial species on-culture results, therefore easy to grow bacterial species may be reported as predominant but their actual cell numbers in fecal sample might be low. The microarray method should be a more accurate representation of the predominant fecal microflora.
Fig. 1.
Microarray test results for cultured feces with azo dyes and the same control fecal sample without culture (un-culture feces) or after culture (culture-control). The position of the oligo probes on the array from top-left to the right-bottom are 1–120, 12 per lane total 10 lanes. The probe number and the corresponding bacterial species are listed in Table 1. The positive signal intensities from strongest to weakest are white, red, yellow, green and blue (see web version for color picture).
Table 3.
Microarray test results read from Fig. 1
| Bacterial species | Un-culture feces | Culture-control | Culture + Direct-Blue | Culture + Orange II | Culture + Methyl Red |
|---|---|---|---|---|---|
| B. thetaiotaomicron | + | + | + | + | + |
| B. vulgatus | + | + | + | + | + |
| B. fragilis | + | + | + | + | + |
| B. distasonis | + | + | + | + | + |
| B. uniformis | + | + | + | + | + |
| B. ovatus | + | + | + | + | + |
| B. caccae | + | + | + | + | + |
| C. clostridioforme | + | + | + | + | + |
| C. leptum | + | + | + | + | + |
| C. perfringens | + | + | + | − | + |
| C. butyricum | + | + | − | + | + |
| C. ramosum | + | + | + | + | + |
| C. difficile | − | − | − | − | − |
| C. indolis | + | + | − | − | + |
| R. obeum | + | + | + | + | + |
| R. bromii | + | + | + | + | + |
| R. callidus | + | + | + | + | + |
| R. albus | + | + | + | + | + |
| R. gnavus | − | − | − | − | − |
| B. longum | + | + | + | + | + |
| B. adolescentis | + | + | + | + | + |
| B. infantis | + | + | + | + | + |
| B. catenulatum | + | + | + | + | + |
| B. angulatum | + | + | + | + | + |
| E. biforme | + | + | + | + | + |
| E. aerofaciens | + | + | + | + | + |
| E. rectale | + | + | + | + | + |
| E. eligens | + | + | + | + | + |
| E. limosum | − | − | − | − | − |
| E. lentum | − | − | − | − | − |
| F. prausnitzii | + | + | + | + | + |
| F. russii | − | − | − | − | − |
| F. nucleatum | − | − | − | − | − |
| P. productus | + | + | + | + | + |
| P. magnus | − | − | − | − | − |
| L. acidophilus | − | − | − | − | − |
| L. fermentum | − | − | − | − | − |
| E. faecium | − | − | − | − | − |
| E. faecalis | − | + | − | + | + |
| E. coli | − | + | − | − | + |
| Total (+) | 28 | 30 | 26 | 27 | 30 |
It is possible that Direct Blue 15 and Orange II may have some toxicity to some bacterial species, because the microarray test results showed only 26 and 27 positive species, respectively, but the culture-control and culture with Methyl Red have 30 species positive each (Table 3). However, this did not affect the overall azo dye reduction because the colors from these two dyes were also completely disappeared.
To determine what species are the major sources of azoreductase activity, 17 selected bacterial species that showed strong positive microarray results were examined for azo dye reduction activity by pure culture of the selected ATCC strains incubated with Direct Blue 15 (Table 4). Among these 17 species, Clostridium perfringens, Clostridium clostridioforme, Enterococcus faecalis, Ruminococcus obeum, and Bifidobacterium adolescentis showed the highest azo dye reduction activity. In a separate study, we have cloned, over-expressed, and purified the azoreductase from E. faecalis (unpublished data), which confirms that this bacterial species may play an important role for the azo reduction in the gastrointestinal tract.
Table 4.
Azo dye (Direct Blue 15) reduction activity of 17 bacterial species in pure culture
| Bacterial species | Remained percentage after azo dye (Direct Blue 15) reduction (%) |
|---|---|
| C. perfringens ATCC 3624 | 2.3 |
| C. clostridioforme ATCC 25537 | 4.1 |
| C. leptum ATCC 29065 | 49.5 |
| C. ramosum ATCC 25582 | 68.6 |
| B. vulgatus ATCC 8482 | 56.2 |
| B. distasonis ATCC 8503 | 70.7 |
| B. thetaiotaomicron ATCC 29148 | 62.2 |
| B. uniformis ATCC 8503 | 75.3 |
| B. longum ATCC 15707 | 70.6 |
| B. adolescentis ATCC 15703 | 15.6 |
| E. biforme ATCC 27806 | 62.1 |
| E. aerofaciens ATCC 25986 | 70.8 |
| E. eligens ATCC 27750 | 75.6 |
| P. productus ATCC 27340 | 72.8 |
| R. obeum ATCC 29174 | 8.2 |
| R. bromii ATCC 27255 | 49.8 |
| E. faecalis ATCC 19433 | 7.7 |
Bacteria can lose or gain genetic materials mostly via plasmids. The azoreductase genes are not located on plasmids but are chromosomal. Isolates from fecal samples were difficult to identify that behave identical to an ATCC strain, even at the species level. So, it is valid at least partly to demonstrate biological activity in a mix of wild bacterial strains found in a clinical fecal samples then switch to pure ATCC cultures. The experimental results demonstrated which species are most prevalent in G–I tract and most likely have the azoreductase gene. Then separate studies can be continued to clone, isolate or characterize the azoreductase gene. One example is that we have already cloned, characterized and purified the azoreductase from E. faecalis ATCC 19433.
This study is an example of the application of microarray method for detection and identification of bacteria in azo dye research. Actually, the microarray method we developed has many more potential applications; for example, the method could be used for examining intestinal bacterial species in various patients clinically treated for intestinal diseases and for experimental animal studies to determine the effect of food additives, antimicrobial residues, and xenobiotics on the intestinal microflora.
Acknowledgments
We thank Drs. Mark Hart and Rajesh Nayak for critical review of the manuscript.
References
- Baumler W, Eibler ET, Hohenleutner U, Sens B, Sauer J, Landthaler M. Q-switched laser and tattoo pigment: first results of the chemical and photophysical analysis of 41 compounds. Lasers Surg. Med. 2000;26:13–21. doi: 10.1002/(sici)1096-9101(2000)26:1<13::aid-lsm4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Call DR, Brockman FJ, Chandler DP. Detection and genetyping Escherichia coli O157:H7 using multiplexed PCR and nucleic acid microarrays. Int. J. Food Microbiol. 2001;67:71–80. doi: 10.1016/s0168-1605(01)00437-8. [DOI] [PubMed] [Google Scholar]
- Carman RJ, Van Tassell RL, Wilkins TD. The normal intestinal microflora: ecology, variability and stability. Vet. Hum. Toxicol. 1993;35(Suppl. 1):11–14. [PubMed] [Google Scholar]
- Cerniglia CE, Freeman JP, Franklin W, Pack LD. Metabolism of azo dyes derived from benzidine 3,3′-dimethylbenzidine and 3,3′-dimethoxybenzidine to potentially carcinogenic aromatic amines by intestinal bacteria. Carcinogenesis. 1982;3:1255–1260. doi: 10.1093/carcin/3.11.1255. [DOI] [PubMed] [Google Scholar]
- Cerniglia CE, Kotarski S. Evaluation of veterinary drug residues in food for their potential to affect human intestinal microflora. Regul. Toxicol. Pharmacol. 1999;29:238–261. doi: 10.1006/rtph.1999.1300. [DOI] [PubMed] [Google Scholar]
- Cerniglia CE, Howard PC, Fu PP, Franklin W. Metabolism of nitropolycyclic aromatic hydrocarbons by human intestinal microflora. Biochem. Biophys. Res. Comm. 1984;123:262–270. doi: 10.1016/0006-291x(84)90407-8. [DOI] [PubMed] [Google Scholar]
- Chadwick RW, George SE, Claxton LR. Role of gastrointestinal mucosa and microflora in the, bioactivation of dietary and environmental mutagens or carcinogens. Drug Metab. Rev. 1992;24:425–492. doi: 10.3109/03602539208996302. [DOI] [PubMed] [Google Scholar]
- Chizhikov V, Rasooly A, Chumakov K, Levy DD. Microarray analysis of microbial virulence factors. Appl. Environ. Microbiol. 2001;67:3258–3263. doi: 10.1128/AEM.67.7.3258-3263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KT, Stevens SE, Jr, Cerniglia CE. The reduction of azo dyes by the intestinal microflora. Crit. Rev. Microbiol. 1992;18:175–190. doi: 10.3109/10408419209114557. [DOI] [PubMed] [Google Scholar]
- Drasar BS, Duerden BI. Anaerobes in the normal flora of man. In: Duerden BI, Drasar BS, editors. Anaerobes in Human Disease. Wiley-Liss; New York, NY: 1991. pp. 162–179. [Google Scholar]
- Drasar BS, Roberts AK. Control of the large bowel microflora. In: Hill MJ, Marsh BS, editors. Human Microbial Ecology. CRC Press, Inc.; FL: 1990. pp. 95–100. [Google Scholar]
- Harmsen HJ, Raangs GC, He T, Degener JE, Welling GW. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 2002;68:2982–2990. doi: 10.1128/AEM.68.6.2982-2990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen GJ, Wildeboer-Veloo AC, Tonk RH, Franks AH, Welling GW. Development and validation of an automated, microscopy-based method for enumeration of groups of intestinal bacteria. J. Microbiol. Methods. 1999;37:215–221. doi: 10.1016/s0167-7012(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Kageyama A, Benno Y. Rapid detection of human fecal Eubacterium species and related genera by nested PCR method. Microbiol. Immunol. 2001;45:315–318. doi: 10.1111/j.1348-0421.2001.tb02624.x. [DOI] [PubMed] [Google Scholar]
- Langendijk PS, Schut F, Jansen GJ. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus specific 16S rRNA targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 1995;61:3069–3075. doi: 10.1128/aem.61.8.3069-3075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki T, Watanabe K, Fujimoto J, Miyamoto Y, Takada T, Matsumoto K, Oyaizu H, Tanaka R. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 2002;68:5445–5451. doi: 10.1128/AEM.68.11.5445-5451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WEC, Holdeman LV. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl. Microbiol. 1974;27:961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WEC, Moore LH. Intestinal floras of populations that have a high risk of colon cancer. Appl. Environ. Microbiol. 1995;61:3202–3207. doi: 10.1128/aem.61.9.3202-3207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii F, Smith DB, Benson RW, Cerniglia CE. Immunological homology among azoreductases from Clostridium and Eubacterium strains isolated from human intestinal microflora. J. Basic Microbiol. 1992;32:99–105. doi: 10.1002/jobm.3620320204. [DOI] [PubMed] [Google Scholar]
- Sghir A, Gramet G, Suau A, Rochet V, Pochart P, Dore J. Quantification of bacterial groups within human fecal flora by oligonucleotide probe hybridization. Appl. Environ. Microbiol. 2000;66:2263–2266. doi: 10.1128/aem.66.5.2263-2266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob. Agents Chemother. 1994;38:409–414. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R-F, Cao WW, Campbell WL, Hairston L, Franklin W, Cerniglia CE. The use of PCR to monitor the population abundance of six human intestinal bacterial species in an in vitro semicontinuous culture system. FEMS Microbiol. Lett. 1994;124:229–237. doi: 10.1016/0378-1097(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Wang R-F, Cao WW, Cerniglia CE. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl. Environ. Microbiol. 1996;62:1242–1247. doi: 10.1128/aem.62.4.1242-1247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R-F, Cao WW, Cerniglia CE. PCR detection of Ruminococcus spp. in human and animal fecal samples. Mol. Cell. Probes. 1997;11:259–265. doi: 10.1006/mcpr.1997.0111. [DOI] [PubMed] [Google Scholar]
- Wang RF, Beggs ML, Erickson BD, Cerniglia CE. Abstract of the 103rd General Meeting of the American Society for Microbiology 2003. American Society for Microbiology; Washington, DC: 2003. DNA microarray analysis of predominant human intestinal bacteria in fecal samples, N-193. [Google Scholar]
- Wang R-F, Beggs ML, Robertson LH, Cerniglia CE. Design and evaluation of oligonucleotide-microarray method for the detection of human intestinal bacteria in fecal samples. FEMS Microbiol. Lett. 2002a;213:175–182. doi: 10.1111/j.1574-6968.2002.tb11302.x. [DOI] [PubMed] [Google Scholar]
- Wang RF, Kim SJ, Robertson LH, Cerniglia CE. Development of a membrane-array method for the detection of human intestinal bacteria in fecal samples. Mol. Cell. Probes. 2002b;16:341–350. doi: 10.1006/mcpr.2002.0432. [DOI] [PubMed] [Google Scholar]
- Wu L, Thompson DK, Li G, Hurt RA, Tiedje JM, Zhou J. Development and evaluation of functional gene arrays for detection of selected genes in the environment. Appl. Environ. Microbiol. 2001;67:5780–5790. doi: 10.1128/AEM.67.12.5780-5790.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]