Abstract
Importance
Prenatal antidepressant exposure has been associated with adverse outcomes. Previous studies, however, may not have adequately accounted for confounding.
Objective
To evaluate alternative hypotheses for associations between first-trimester antidepressant exposure and birth and neurodevelopmental problems.
Design, Setting, and Participants
This retrospective cohort study included Swedish offspring born between 1996 and 2012 and followed through 2013 or censored by death or emigration. Analyses controlling for pregnancy, maternal, and paternal covariates, as well as sibling comparisons, timing of exposure comparisons, and paternal comparisons, were used to examine the associations.
Exposures
Maternal self-reported first-trimester antidepressant use and first-trimester antidepressant dispensations.
Main Outcomes and Measures
Preterm birth (< 37 gestational weeks), small for gestation age (birth weight < 2 SDs below the mean for gestational age), and first inpatient or outpatient clinical diagnosis of autism spectrum disorder and attention-deficit/hyperactivity disorder in offspring.
Results
Among 1,580,629 offspring (mean gestational age 279 days; 48.6% female; 1.4% [n = 22,544] with maternal first-trimester self-reported antidepressant use) born to 943,776 mothers (mean age at childbirth 30 years), 7.0% of exposed vs. 4.8% of unexposed offspring were preterm, 2.5% of exposed vs. 2.2% of unexposed were small for gestational age, 5.3% of exposed vs. 2.1% of unexposed were diagnosed with autism spectrum disorder by age 15, and 12.6% of exposed vs. 5.5% of unexposed were diagnosed by attention-deficit/hyperactivity disorder by age 15. At the population level, first-trimester exposure was associated with all outcomes, compared with unexposed offspring (preterm birth: OR = 1.5, 95% CI, [1.4, 1.6]; small for gestational age: OR = 1.2, 95% CI, [1.1, 1.3]; autism spectrum disorder: HR = 2.0, 95% CI, [1.8, 2.3]; attention-deficit/hyperactivity disorder: HR = 2.2, 95% CI, [2.0, 2.4]). However, in models that compared siblings while adjusting for pregnancy, maternal, and paternal traits, first-trimester antidepressant exposure was associated with preterm birth (OR = 1.3, 95% CI [1.2, 1.5]) but not with small for gestational age (OR = 1.0, 95% CI [0.8, 1.3]), autism spectrum disorder (HR = 0.8, 95% CI [0.6, 1.1]), or attention-deficit/hyperactivity disorder (HR = 1.0, 95% CI [0.8, 1.3]). Results from analyses assessing associations with maternal dispensations before pregnancy and paternal first-trimester dispensations were consistent with findings from the sibling comparisons.
Conclusion and Relevance
Among offspring born in Sweden, after accounting for confounding factors, first-trimester antidepressant exposure, compared to no exposure, was associated with a small increased risk of preterm birth but no increased risk of small for gestational age, autism spectrum disorder, or attention-deficit/hyperactivity disorder.
Given the increasing prevalence of antidepressant use among pregnant women,1 gaining knowledge on the safety of their use during pregnancy is a public health priority. Prenatal antidepressant exposure is associated with birth and neurodevelopmental problems, including shorter gestation,2 reduced fetal growth,2 autism spectrum disorder,3–6 and attention-deficit/hyperactivity disorder.7 These associations may be due to causal mechanisms (e.g., dysfunctional serotonin signaling8). However, there are alternative explanations for the associations. Maternal depression and stress are associated with birth9 and neurodevelopmental10 problems, suggesting that antidepressant associations could be attributable to confounding by indication for such treatment. Furthermore, autism spectrum disorder and attention-deficit/hyperactivity disorder have strong genetic influences,11 and these influences partially overlap with genetic contributions to depression.12,13 Thus, genetic transmission of shared risk for neurodevelopmental problems and depression could explain the associations (i.e., passive gene-environmental correlation). Other factors, such as poor health practices during pregnancy, could also account for the associations.14
Randomized clinical trials have not been able to test the safety of antidepressant use during pregnancy because pregnant women are typically excluded from these studies. Thus, researchers must use observational designs to rule out alternative explanations for the associations.15 The present study used four such designs to explore associations between first-trimester antidepressant exposure (assessed via both maternal self-report and registered medication dispensations) and offspring birth and neurodevelopmental problem (i.e., preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder. In addition to (1) statistical controls to adjust for measured pregnancy, maternal, and paternal characteristics, this study used (2) sibling comparisons to account for unmeasured genetic and environmental factors that make siblings similar, (3) timing-of-exposure comparisons to account for selection factors related to maternal antidepressant treatment around the time of pregnancy, and (4) paternal comparisons to further account for familial confounding.
Methods
The institutional review board at Indiana University and the Regional Ethical Review Board in Stockholm approved this study. By Swedish law informed consent was not necessary because the study used data available from national registers.
Sample
We obtained a population-based dataset by linking information from the following Swedish registers: (1) the Multi-Generation Register, which included biological relationships for all individuals residing in Sweden since 1961, (2) the Prescribed Drug Register, which included prescription medication dispensation records since 2006, (3) the Medical Birth Register, which included information on 96-99% of births since 1973, (4) the National Patient Register, which included diagnoses from all hospital admissions since 1987 and specialist outpatient care since 2001, (5) the National Crime Register, which included criminal convictions since 1973, and (6) the Education Register, which included highest level of completed formal education through 2013.
Measures
Antidepressant exposure
The main exposures evaluated were first-trimester exposure to (1) any antidepressants (medications with Anatomical Therapeutic Chemical Classification [ATC] codes beginning with N06A) and (2) Selective Serotonin Reuptake Inhibitors (SSRIs; medications with ATC codes beginning with N06AB). Exposure was defined according to two sources of information: (1) maternal self-reports (available for offspring born between 1996 and 2012) and (2) dispensation records (available for both parents of offspring born between 2006 and 2012).
Information on maternal self-reported medication use during the first trimester of pregnancy came from the Medical Birth Register, which contains information obtained from standardized interviews conducted by midwives at the first antenatal visit. Medication reported in these interviewers is presumed to represent first-trimester use because interviews typically occur between week 10 and 12 of pregnancy.
Information on medication use based on dispensation records came from the Prescribed Drug Register, which covers all medication dispensations and accompanying prescriptions made in Sweden since July 2005. The only medication use not covered by the register is medication administered while in hospital, purchased over the counter, or obtained on the black market. The Prescribed Drug Register was used to obtain information on maternal antidepressant dispensations that covered the periods before pregnancy and during the first trimester of pregnancy and paternal antidepressants that covered the period during the first trimester of pregnancy. First-trimester exposure was defined as having at least one dispensation between 90 days before estimated conception and 90 days after estimated conception (see eFigure 1). The window included 90 days before conception because chronic disease medication is typically prescribed for at least 3-month periods in Sweden. Use before pregnancy only was defined as having at least one dispensation between 270 and 90 days before estimated conception and no dispensations during pregnancy or during the 180 days after delivery.
Main Outcomes
The birth outcomes were preterm birth (< 37 gestational weeks) and small for gestational age (birth weight < 2 SDs below the mean for gestational age). The neurodevelopmental outcomes were first diagnosis of autism spectrum disorder and attention-deficit/hyperactivity disorder, which were identified using inpatient and outpatient diagnoses made by specialists according to International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 criteria. Previous research has validated these diagnoses in the Swedish registers.16,17 Participants were followed through 2013 or were censored because of death or emigration. More details about the registers and variables are available in previous publications.e.g.,18,19
Covariates
Pregnancy covariates included parity (categorized as first, second, third, or fourth or higher) and year of birth. Maternal and paternal covariates included country of birth (Sweden or outside Sweden), age at childbearing (categorized into six levels), highest level of completed education (categorized into seven levels), history of any criminal conviction, history of severe psychiatric problems (inpatient diagnosis of ICD-8, ICD-9, or ICD-10 schizophrenia, bipolar disorder, or other non-drug-induced psychoses), and history of any suicide attempts (definite or uncertain). History of criminal convictions is commonly used in Swedish register studies to index problems with behavior regulation.e.g.,20,21
Analyses
We performed a complete-case analysis. We managed and analyzed data in SAS 9.4 and STATA 13.1 and calculated 95% confidence intervals based on two-sided hypothesis testing.
Descriptive statistics
We provided the distribution of covariates and outcomes in the whole sample and in the subsamples of exposed an unexposed offspring. In addition, we provided the occurrence of the outcomes and covariates in differentially exposed and unexposed siblings. For the birth outcomes, we presented proportions and unadjusted risk differences. We presented Kaplan Meier estimates of the probability of the neurodevelopmental diagnoses because follow-up time was censored.
Population-wide associations and within-family comparisons
Logistic regression was used to estimate the model-based associations for the two birth (i.e., binary response) outcomes. Cox proportional hazards regression (using calendar age in years as the timescale) was used to estimate the associations for the two neurodevelopmental outcomes to account for censored observations in the data. We examined the associations between antidepressant exposure and outcomes by estimating a sequence of three models with increasing degree of control for potential confounding factors. First, the baseline models assessed population-wide associations while only adjusting for pregnancy covariates (parity and year of birth). Second, the population-wide associations were further adjusted for all maternal and paternal covariates. These population models used robust standard errors to account for clustering of individuals (i.e., siblings) within nuclear families bound by the same biological mother. Third, sibling comparison models compared exposure and outcome discordant offspring within families and included covariates that could vary among siblings born to the same mother. By design, these models accounted for all factors that made siblings similar (e.g., shared genetic and early environmental influences), as well as measured covariates that vary within families, thereby producing a stronger test of the associations than the adjusted population models.22 As recommended,23 we fit fixed-effects models using conditional logistic and stratified Cox regression to make purely within-family comparisons.
Comparisons of timing of maternal use and paternal use
To explore whether intrauterine exposure was specifically associated with outcomes over and above maternal depression treatment around the time of pregnancy, we compared associations for maternal first-trimester antidepressant dispensations with associations for dispensations before pregnancy, while adjusting for measured pregnancy, maternal, and paternal covariates. We evaluated whether these associations differed statistically using Wald χ2 tests. We also compared the fit of models that included separate parameters for before pregnancy dispensations and first-trimester dispensations to models that included one parameter for both dispensation windows. In addition, paternal first-trimester antidepressant dispensations were used as a negative control to further explore the role of familial confounding. We first assessed the association between maternal and paternal first-trimester dispensations. We then estimated associations between paternal first-trimester antidepressant dispensations and the four outcomes while adjusting for the pregnancy covariates.
Sensitivity analyses
First, to evaluate the influence of exposure misclassification, we examined adjusted associations with five additional exposure definitions in the cohort with exposure information from both maternal self-reports and dispensations (i.e., the cohort born 2006 to 2012). The four additional definitions included: (a) first-trimester exposure defined as use according to either self-reports or dispensation records, (b) first-trimester exposure defined as use according to both self-reports and dispensation records, (c) a narrower first-trimester dispensation window of 30 days before conception to 90 days after conception, and (d) at least two dispensations during the original first-trimester exposure window. Second, given that single-offspring families cannot contribute to sibling-comparison analyses, we reassessed the population models in the subsample of offspring with siblings to evaluate the generalizability of sibling-comparison results. Third, to assess if exposure to other psychotropic medications confounded the associations, we restricted the analyses to offspring not exposed to other psychotropic medications. Fourth, given that prior to 2001 outpatient psychiatric diagnoses were not included in the National Patient Register, we conducted analyses on a subsample of offspring born after 2000 to assess whether left censoring of the neurodevelopmental outcomes biased the findings. These analyses also enabled us to explore whether cohort effects influenced the results. Fifth, we estimated the associations with the neurodevelopmental outcomes in subsamples excluding offspring with diagnoses before age 2 to address concerns about the validity of early neurodevelopmental diagnoses. Sixth, because the main analyses focused on first-trimester exposure, we examined the association between dispensations during the second and/or third trimester and each outcome in the subsample of offspring whose mothers had a dispensation during the first trimester.
Results
The target sample included 1,670,237 offspring born 1996-2012. Multiple births (48,979 offspring), cases with missing father identifier (16,295), missing or invalid responses on covariates (20,118), and missing on the small for gestational age variable (4,216) were sequentially dropped. The final analytic cohort of 1,580,629 offspring (48.6% female) represented 95% of target singleton births and included 943,776 distinct mothers and 946,579 distinct fathers. According to maternal self-reports, 22,544 (1.4%) of the offspring in the final cohort were exposed to any antidepressant during the first trimester, and of these, 82% (18,470) were exposed to SSRIs.
The timing of exposure and paternal comparisons were conducted on the subsample of 708,450 offspring (born between 2006 and 2012) with dispensation-based exposure data. There were 26,477 (3.7%) offspring with first-trimester maternal antidepressant dispensations. Of these, 84% (22,125) had first-trimester maternal SSRI dispensations specifically. There were 8,203 (1.2%) offspring who had mothers with antidepressant dispensations before pregnancy only. Of these, 81% (6,674) had mothers who were specifically dispensed SSRIs before pregnancy. There were 18,727 (2.6%) offspring who had fathers with first-trimester antidepressant dispensations. Of these, 72% (13,521) had fathers with first-trimester SSRI dispensations specifically.
The same pattern of results was observed for associations with first-trimester exposure to any antidepressant as first-trimester exposure to SSRIs specifically. Therefore, results for exposure to any antidepressant are presented in the text and tables. The results for SSRIs can be found in the tables and online supplement.
Descriptive Statistics Stratified By Maternal Self-reported Antidepressant Use
In the whole sample, 7.0% of exposed and 4.8% of unexposed offspring were preterm (Table 1), which equates to 220 (95% CI [187, 254]) additional preterm birth cases per 10,000 offspring. Approximately 2.5% of exposed and 2.2% of unexposed offspring were born small for gestational age, (risk difference = 35 additional cases per 10,000 offspring; 95% CI [14, 56]). Compared to unexposed offspring, exposed offspring also had a higher probability of the neurodevelopmental diagnoses (see Figure 1a and 1c for the Kaplan Meier estimates and confidence intervals). For example, by age 15, Kaplan Meier estimates indicated a cumulative risk of autism spectrum disorder of 5.3% for exposed and 2.1% for unexposed offspring. By age 15, the cumulative risk of attention-deficit/hyperactivity disorder was 12.6% for exposed and 5.5% for unexposed offspring. See eSupplement A for more descriptive information.
Table 1.
Descriptive statistics in the whole sample and stratified by maternal self-reported first-trimester use of any antidepressant
| Whole sample (n=1,580,629) |
Exposed offspring (n=22,544) |
Unexposed offspring (n=1,558,085) |
|
|---|---|---|---|
|
|
|||
| No. (%) | No. (%) | No. (%) | |
|
|
|||
| Offspring outcomes | |||
| Preterm birth | 76061 (4.81) | 1574 (6.98) | 74487 (4.78) |
| Small for gestational age | 34728 (2.20) | 573 (2.54) | 34155 (2.19) |
| Autism spectrum disordera | 14617 (2.16) | 299 (5.28) | 14318(2.14) |
| Attention-deficit/hyperactivity disordera | 32924 (5.51) | 613 (12.63) | 32311(5.46) |
| Pregnancy covariates | |||
| First born | 693070 (43.85) | 10467 (46.43) | 682603 (43.81) |
| Second born | 585619 (37.05) | 6891 (30.57) | 578728 (37.14) |
| Third born | 213382 (13.50) | 3463 (15.36) | 209919 (13.47) |
| Fourth born or higher | 88558 (5.60) | 1723 (7.64) | 86835 (5.57) |
| Born 1996 to 1999b | 333791 (21.12) | 1649 (7.31) | 332142 (21.32) |
| Born 2000 to 2003b | 349143 (22.09) | 3004 (13.33) | 346139 (22.22) |
| Born 2004 to 2007b | 386511 (24.45) | 6349 (28.16) | 380162 (24.40) |
| Born 2008 to 2012b | 511184 (32.34) | 11542 (51.20) | 499642 (32.07) |
| Maternal covariates | |||
| Age at birth | |||
| < 20 years | 25637 (1.62) | 327 (1.45) | 25310 (1.62) |
| 20 to 24 years | 210552 (13.32) | 2636 (11.69) | 207916 (13.34) |
| 25 to 29 years | 495050 (31.32) | 6124 (27.16) | 488926 (31.38) |
| 30 to 34 years | 544746 (34.46) | 7599 (33.71) | 537147 (34.47) |
| 35 to 39 years | 254771 (16.12) | 4730 (20.98) | 250041 (16.05) |
| ≥ 40 years | 49873 (3.16) | 1128 (5.00) | 48745 (3.13) |
| Education | |||
| Primary and lower secondary, < 9 years | 33648 (2.13) | 180 (0.80) | 33468 (2.15) |
| Primary and lower secondary, 9 years | 107953 (6.83) | 2684 (11.91) | 105269 (6.76) |
| Upper secondary, 1-2 years | 246415 (15.59) | 3852 (17.09) | 242563 (15.57) |
| Upper secondary, 3 years | 414949 (26.25) | 6053 (26.85) | 408896 (26.24) |
| Post-secondary, < 3 years | 224706 (14.22) | 3012 (13.36) | 221694 (14.23) |
| Post-secondary, ≥ 3 years | 533710 (33.77) | 6585 (29.21) | 527125 (33.83) |
| Postgraduate | 19248 (1.22) | 178 (0.79) | 19070 (1.22) |
| Nationality (Swedish) | 1281142 (81.05) | 20361 (90.32) | 1260781 (80.92) |
| Criminal convictions (any) | 173631 (10.98) | 3973 (17.62) | 169658 (10.89) |
| Severe psychiatric problemc | 16736 (1.06) | 1734 (7.69) | 15002 (0.96) |
| Suicide attempt (definite or uncertain) | 66655 (4.22) | 3251 (14.42) | 63404 (4.07) |
| Paternal covariates | |||
| Age at birth | |||
| < 20 years | 7789 (0.49) | 134 (0.59) | 7655 (0.49) |
| 20 to 24 years | 100339 (6.35) | 1543 (6.84) | 98796 (6.34) |
| 25 to 29 years | 364992 (23.09) | 4815 (21.36) | 360177 (23.12) |
| 30 to 34 years | 547663 (34.65) | 7118 (31.57) | 540545 (34.69) |
| 35 to 39 years | 355300 (22.48) | 5380 (23.86) | 349920 (22.46) |
| ≥ 40 years | 204546 (12.94) | 3554 (15.76) | 200992 (12.90) |
| Education | |||
| Primary and lower secondary, < 9 years | 29369 (1.86) | 257 (1.14) | 29112 (1.87) |
| Primary and lower secondary, 9 years | 153577 (9.72) | 2672 (11.85) | 150905 (9.69) |
| Upper secondary, 1-2 years | 403919 (25.55) | 5647 (25.05) | 398272 (25.56) |
| Upper secondary, 3 years | 381746 (24.15) | 6271 (27.82) | 375475 (24.10) |
| Post-secondary, < 3 years | 233560 (14.78) | 2930 (13.00) | 230630 (14.80) |
| Post-secondary, ≥ 3 years | 348397 (22.04) | 4404 (19.54) | 343993 (22.08) |
| Postgraduate | 30061 (1.90) | 363 (1.61) | 29698 (1.91) |
| Nationality (Swedish) | 1273973 (80.60) | 19699 (87.38) | 1254274 (80.50) |
| Criminal convictions (any) | 582002 (36.82) | 9313 (41.31) | 572689 (36.76) |
| Severe psychiatric problemc | 10373 (0.66) | 321 (1.42) | 10052 (0.65) |
| Suicide attempt (definite or uncertain) | 64879 (4.10) | 1364 (6.05) | 63515 (4.08) |
All percentages are based on the number of offspring.
Age 15 Kaplan Meier estimates.
Year of birth is presented in bins in Table 1 but was not binned when used as a covariate in models.
Severe psychiatric problem was defined as an inpatient or outpatient diagnosis of schizophrenia, bipolar disorder, or other non-drug induced psychosis.
Figure 1. Risk of Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder by Maternal Self-reported First-trimester Exposure.
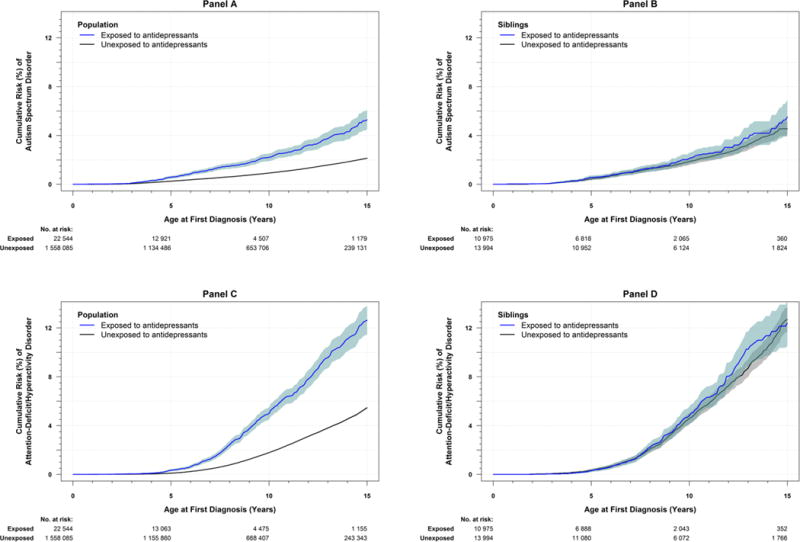
The figure shows Kaplan-Meier estimates of cumulative risk (i.e., proportion diagnosed with) the neurodevelopmental outcomes (y-axis) by age (x-axis) among offspring with and without maternal self-reported first-trimester antidepressant exposure. The blue line shows exposed offspring. The black line shows unexposed offspring. Shaded areas around the blue and black lines are pointwise 95% confidence intervals. Top panels (A and B) show risk for autism spectrum disorder. Bottom panels (C and D) show risk for attention-deficit/hyperactivity disorder. Left panels (A and C) include the full cohort. Right panels (B and D) include siblings discordant for first-trimester antidepressant exposure. The median and interquartile range (IQR) follow-up time in the study (i.e., age since birth in years) were estimated separately for each exposure group. The median follow-up for autism spectrum disorder in the full cohort was 8.71 y [IQR: (4.69, 13.19)] for the unexposed group, and 5.82 y [IQR: (3.32, 9.08)] for the exposed group. The median follow-up for autism spectrum disorder in the sample of discordant siblings was 9.24 y [IQR: (5.54, 12.96)] for the unexposed group, and 6.27 y [IQR: (3.77, 9.02)] for the exposed group. The median follow-up for attention-deficit/hyperactivity disorder in the full cohort was 8.54 y [IQR: (4.54, 13.54)] for the unexposed group, and 5.54 y [IQR: (3.54, 9.54)] for the exposed group. The median follow-up for attention-deficit/hyperactivity disorder in the sample of discordant siblings was 9.54 y [IQR: (5.54, 13.54)] for the unexposed group, and 6.54 y [IQR: (3.54, 9.54)] for the exposed group.
Among differentially exposed siblings, 6.2% of exposed and 5.1% of unexposed siblings were born preterm. However, 1.9% of exposed and 2.0% of unexposed siblings were small for gestational age. See Figure 1b and 1d for the probabilities of the neurodevelopmental diagnoses among differentially exposed siblings through age 15. By age 15, the cumulative risk for autism spectrum disorder was 5.5% for exposed and 4.6% for unexposed siblings; the cumulative risk for attention-deficit/hyperactivity disorder was 12.4% for exposed and 12.7% for unexposed siblings.
Population-wide Associations and Sibling Comparisons
In the baseline models (Table 2), maternal self-reported first-trimester antidepressant use was associated with preterm birth (OR = 1.5, 95% CI [1.4, 1.6]), small for gestational age (OR = 1.2, 95% CI [1.1, 1.3]), autism spectrum disorder (HR = 2.0, 95% CI [1.8, 2.3]), and attention-deficit/hyperactivity disorder (HR = 2.2, 95% CI [2.0, 2.4]). In the adjusted models, first-trimester exposure to antidepressants was also statistically significantly associated with all outcomes (preterm birth OR = 1.4, 95% CI [1.3, 1.4]; small for gestational age OR = 1.1, 95% CI [1.0, 1.2]; autism spectrum disorder HR = 1.6, 95% CI [1.5, 1.8]; attention-deficit/hyperactivity disorder HR = 1.6, 95% CI [1.5, 1.7]).
Table 2.
Baseline, adjusted, and sibling comparison associations between maternal self-reported first-trimester use and birth and neurodevelopmental outcomes
| Baseline Model | Adjusted Model | Sibling Comparison | ||||
|---|---|---|---|---|---|---|
| Any antidepressant | ||||||
|
| ||||||
| OR | 95% CI | OR | 95% CI | OR. | 95% CI | |
|
|
||||||
| Preterm birth | 1.47 | 1.40-1.55 | 1.35 | 1.28-1.42 | 1.34 | 1.18-1.52 |
| Small for gestational age | 1.15 | 1.06-1.25 | 1.12 | 1.03-1.22 | 1.01 | 0.81-1.25 |
|
|
||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
|
|
||||||
| Autism spectrum disorder | 2.02 | 1.80-2.26 | 1.64 | 1.46-1.83 | 0.83 | 0.62-1.13 |
| Attention-deficit/hyperactivity disorder | 2.21 | 2.04-2.39 | 1.58 | 1.46-1.71 | 0.99 | 0.79-1.25 |
|
SSRIs | ||||||
| OR | 95% CI | OR | 95% CI | OR. | 95% CI | |
|
|
||||||
| Preterm birth | 1.38 | 1.30-1.46 | 1.27 | 1.20-1.35 | 1.33 | 1.16-1.53 |
| Small for gestational age | 1.11 | 1.01-1.21 | 1.09 | 0.99-1.20 | 0.88 | 0.70-1.12 |
|
|
||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
|
|
||||||
| Autism spectrum disorder | 2.04 | 1.80-2.32 | 1.66 | 1.46-1.89 | 0.81 | 0.58-1.14 |
| Attention-deficit/hyperactivity disorder | 2.25 | 2.06-2.46 | 1.60 | 1.47-1.75 | 0.94 | 0.73-1.22 |
OR = odds ratio. HR = hazard ratio. CI = confidence interval. Baseline and adjusted models were fit in a sample of 1,580,629 offspring. See eSupplement A for information about offspring who could be informative in sibling comparisons. Baseline models controlled for parity and year of birth. Adjusted models controlled parity and year of birth and maternal and paternal country of birth, age at childbearing, highest level of completed education, history of any criminal convictions, history of severe psychiatric problems, and history of any suicide attempts. Sibling comparisons controlled for parity and year of birth, paternal country of birth, age at childbearing, highest level of completed education, history of any criminal convictions, history of severe psychiatric problems, and history of any suicide attempts, and maternal age at childbearing.
In the sibling comparison models, first-trimester exposure was associated with preterm birth (OR = 1.3, 95% CI [1.2, 1.5], p < 0.0001). However, it was not associated with small for gestational age (OR = 1.0, 95% CI [0.8, 1.3]), autism spectrum disorder (HR = 0.8, 95% CI [0.6, 1.1]), or attention-deficit/hyperactivity disorder (HR = 1.0, 95% CI [0.8, 1.3]). See eSupplement A for information on offspring who could contribute to sibling comparison analyses.
Comparisons of Timing of Maternal Use and Paternal Use
Dispensation data was used in timing of exposure and paternal comparisons (see eSupplement B for more information). For preterm birth, the association with maternal dispensations before pregnancy but not during or after pregnancy (OR = 1.2, 95% CI [1.1, 1.3]; Table 3) was statistically significantly weaker than the association with first-trimester maternal dispensations (OR = 1.4, 95% CI [1.3, 1.5]). For all other outcomes, the associations with maternal dispensations before but not during or after pregnancy did not statistically significantly differ from the associations with first-trimester maternal dispensations.
Table 3.
Adjusted associations between maternal antidepressant dispensations before pregnancy and during the first trimester of pregnancy and birth and neurodevelopmental outcomes
| Before pregnancy | 1st trimester | |||
|---|---|---|---|---|
|
| ||||
| Any antidepressant | ||||
| OR | 95% CI | OR | 95% CI | |
|
|
||||
| Preterm birth | 1.17 | 1.07-1.28 | 1.40 | 1.33-1.47 |
| Small for gestational age | 1.07 | 0.93-1.24 | 1.12 | 1.03-1.21 |
|
|
||||
| HR | 95% CI | HR | 95% CI | |
|
|
||||
| Autism spectrum disorder | 1.40 | 1.02-1.93 | 1.75 | 1.49-2.07 |
| Attention-deficit/hyperactivity disorder | 2.09 | 1.53-2.86 | 1.85 | 1.55-2.20 |
|
| ||||
| SSRIs | ||||
|
| ||||
| OR | 95% CI | OR | 95% CI | |
|
|
||||
| Preterm birth | 1.13 | 1.02-1.25 | 1.37 | 1.30-1.45 |
| Small for gestational age | 1.09 | 0.94-1.28 | 1.13 | 1.03-1.23 |
|
|
||||
| HR | 95% CI | HR | 95% CI | |
|
|
||||
| Autism spectrum disorder | 1.49 | 1.06-2.10 | 1.72 | 1.43-2.06 |
| Attention-deficit/hyperactivity disorder | 1.93 | 1.35-2.74 | 1.81 | 1.50-2.19 |
OR = odds ratio. HR = hazard ratio. CI = confidence interval. All models were fit in a sample of 708,450 offspring. Models controlled parity and year of birth and maternal and paternal country of birth, age at childbearing, highest level of completed education, history of any criminal convictions, history of severe psychiatric problems, and history of any suicide attempts.
Paternal first-trimester antidepressant dispensations were associated with maternal first-trimester antidepressant dispensations (OR = 3.4, 95% CI [3.3, 3.6]). Paternal first-trimester antidepressant dispensations (Table 4) had very modest associations with preterm birth (OR = 1.1, 95% CI [1.1, 1.2]) and small for gestational age (OR = 1.1, 95% CI [1.0, 1.2]), with the latter not being statistically significant. Paternal dispensations during pregnancy were associated with autism spectrum disorder (HR = 1.3, 95% CI [1.1, 1.6]), and attention-deficit/hyperactivity disorder (HR = 1.7, 95% CI [1.4, 2.2]).
Table 4.
Baseline associations between paternal first-trimester antidepressant dispensations and birth and neurodevelopmental outcomes
| Any Antidepressant | SSRIs | |||
|---|---|---|---|---|
|
|
||||
| OR | 95% CI | OR | 95% CI | |
|
|
||||
| Preterm birth | 1.13 | 1.05-1.20 | 1.13 | 1.05-1.22 |
| Small for gestational age | 1.06 | 0.96-1.17 | 1.00 | 0.89-1.13 |
|
|
||||
| HR | 95% CI | HR | 95% CI | |
|
|
||||
| Autism spectrum disorder | 1.31 | 1.05-1.62 | 1.27 | 0.98-1.65 |
| Attention-deficit/hyperactivity disorder | 1.73 | 1.38-2.17 | 1.71 | 1.31-2.23 |
OR = odds ratio. HR = hazard ratio. CI = confidence interval. All models were fit in a sample of 708,450 offspring. Models controlled for parity and year of birth. Analyses compared offspring of fathers with first trimester antidepressant dispensations to offspring of fathers who were not dispensed antidepressants before pregnancy, during the second and third trimester of pregnancy, and after pregnancy (eFigure 1 shows dispensation windows).
Sensitivity Analyses
Sensitivity analyses showed a consistent pattern of results across analyses using stricter criteria for exposure and narrower exposure windows, suggesting that exposure misclassification was not responsible for the pattern of findings (eSupplement C). Results from population models conducted on a subsample that excluded offspring who did not have siblings also were essentially identical to the main results (eSupplement D). These results provide support for the generalizability of sibling comparison results. Sensitivity analyses also suggested that confounding by exposure to other psychotropic medications (eSupplement E); left censoring of the neurodevelopmental outcomes and cohort effects (eSupplement F); and measurement error of the neurodevelopmental outcomes (eSupplement G) had very little influence on the results. In addition, among offspring whose mothers had a dispensation during the first trimester, a dispensation during the second or third trimester was associated with increased risk of the pregnancy outcomes, though the associations with the neurodevelopmental diagnoses were not statistically significant (eSupplement H).
Discussion
The present study found that, after accounting for measured pregnancy, maternal, and paternal traits, as well as all (unmeasured) stable familial characteristics shared by siblings, maternal antidepressant use during the first trimester of pregnancy, compared to no exposure, was associated with a small increased risk of preterm birth but no increased risk of small for gestational age, autism spectrum disorder, or attention-deficit/hyperactivity disorder. That is, unexposed siblings were at equal risk for small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder as their exposed siblings. These results are consistent with the hypothesis that genetic and/or familial environmental factors account for the population-wide associations between first-trimester antidepressant exposure and these outcomes. Moreover, results from analyses examining timing of exposure were consistent with the interpretation of the sibling-comparison findings. Specifically, the strength of the associations between antidepressant dispensations before pregnancy and small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder did not statistically significantly differ from that of associations for first-trimester antidepressant dispensations, suggesting that the underlying condition, rather than exposure to antidepressants during the first trimester, explained the associations. Paternal first-trimester antidepressant dispensations were also associated with the neurodevelopmental disorders. Because paternal antidepressant use during the first trimester is unlikely to contribute to intrauterine exposure, these findings provide further support that associations between first-trimester antidepressant exposure and offspring neurodevelopmental problems may, at least partially, be explained by familial confounding.
The results also showed that, across multiple designs that account for familial confounding factors, first-trimester antidepressant exposure was associated with a slightly elevated risk of preterm birth. Although these results may be consistent with the hypothesis that prenatal antidepressant exposure could lead to a small increased risk of preterm birth, other possible explanations for the findings need to be considered. Most important, the potential role of confounding by maternal depression should be noted because both the existence and severity of depression symptoms in the mother could potentially influence the risk of preterm birth.24
The results of the population-wide models were consistent with numerous observational studies that have demonstrated associations between prenatal antidepressant exposure and birth and neurodevelopmental problems.2–7 The results of the sibling comparisons were also consistent with the limited previous sibling-comparisons studies that have examined associations between prenatal antidepressant exposure and birth and neurodevelopmental problems. A sibling comparison study using dispensation data from the Swedish registers found a statistically significant associations between prenatal antidepressant dispensations and shorter gestation.25 Another sibling comparison reported that prenatal antidepressant exposure was not associated with autism spectrum disorder,26 although confidence intervals were too wide to draw strong conclusions.
The current study had several strengths. First, the study analyzed a large, population-based sample, which provided statistical power to examine rare-yet-serious outcomes. Second, the conclusions were based on converging evidence from multiple research designs that accounted for both measured and unmeasured confounding factors. Third, first-trimester antidepressant use was indexed by both maternal self-report and dispensations. Fourth, the study included four outcomes, two pregnancy-related and two neurodevelopmental problems, all of which are associated with significant morbidity and mortality. Fifth, sensitivity analyses suggested that misclassification of antidepressant use, several assumptions of sibling-comparison analyses, confounding by other psychotropic medications, and misclassification of the neurodevelopmental problems were unlikely to influence the overall conclusions.
The findings from the present study should be considered in light of several limitations. First, and most important, observational designs such as these cannot fully rule out all sources of confounding. In particular, like other register-based approaches,26 this study could not comprehensively assess maternal depression or its severity,27 nor could it compare different antidepressant treatment regimes. Thus, associations could have been influenced by confounding by antidepressant indication. In order to address this limitation, the study used multiple designs, each of which could help rule out some but not all sources of confounding, to provide complementary evidence. For example, sibling comparisons ruled out all stable confounders (e.g., chronic maternal depression), but that design may not have been able to account for confounding from maternal depression that varied across pregnancies.28 Thus, the within-family associations with preterm birth may plausibly be driven by unmeasured time-varying maternal depression rather than by antidepressant use.29
Second, this study focused on first-trimester exposure. Whereas one recent study found an association between antidepressant dispensations late—but not early—in pregnancy and autism spectrum disorder,3 there has been considerable debate regarding the role of timing.30–32 In fact, several studies have found stronger associations with first-trimester antidepressant use than with use later in pregnancy.4,6 Supplemental analyses indicated that among offspring whose mothers had a dispensation during the first trimester, a dispensation during the second or third trimester was associated with greater risk of offspring being born preterm and small for gestational age. These associations could be due to intrauterine exposure to antidepressants later in pregnancy, increased severity of depression (i.e., confounding by indication), or other unmeasured confounding. Future studies are, therefore, needed to explicitly examine whether timing of exposure moderates the preterm birth association or whether exposure later in pregnancy is more strongly associated with other outcomes.
Third, the vast majority of antidepressant exposure (82% according to maternal reports) was to SSRIs. Future research should explore class- and drug-specific associations. Fourth, analyses were conducted on a Swedish sample, and it is not known if results would generalize to other countries. Although the population-wide associations in the present study were commensurate with those from other countries, future research should use designs that help account for unmeasured confounders to explore associations with prenatal antidepressant exposure in the United States and elsewhere. Fifth, sibling comparisons require large samples to have adequate statistical power.33 Although the large Swedish sample ensured fairly precise parameter estimates in sibling comparisons, small effects of antidepressant exposure cannot be ruled out. However, their magnitudes, particularly for the neurodevelopmental outcomes, would be much smaller than those suggested by population-wide associations.
Conclusion
Among offspring born in Sweden, after accounting for confounding factors, first-trimester exposure to antidepressants, compared to no exposure, was associated with a small increased risk of preterm birth but no increased risk of small for gestational age, autism spectrum disorder, or attention-deficit/hyperactivity disorder.
Supplementary Material
Key Points.
Question
Is first-trimester maternal antidepressant use related to offspring birth and/or neurodevelopmental problems?
Findings
In this retrospect cohort study of 1,580,629 Swedish offspring using multiple statistical and methodical approaches to adjust for confounding, first-trimester antidepressant exposure was significantly associated with preterm birth (OR = 1.3 in a sibling comparison analysis) but not with risk of being born small for gestational age or later autism spectrum disorder or attention-deficit/hyperactivity disorder.
Meaning
After accounting for confounding factors, first-trimester antidepressant exposure, compared with no exposure, was associated with a small increased risk of preterm birth but no increased risk of small for gestational age, autism spectrum disorder, or attention-deficit/hyperactivity disorder.
Acknowledgments
A. Sujan and M. Rickert (Indiana University – Bloomington) conducted the data analyses. A. Sujan and M. Rickert had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analyses.
H. Larsson has served as a speaker for Eli-Lilly and Shire and has received a research grant from Shire; all outside the submitted work. S. Hernández-Díaz received salary support from the North American AED Pregnancy Registry; received research funding from GSK, Lilly and Pfizer; and consulted for UCB, Teva, and Boehringer-Ingelheim. Paul Lichtenstein has served as a speaker for Medice.
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number T32MH103213 and the National Institute on Drug Abuse of the National Institutes of Health under Award Number K99DA040727. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by a National Science Foundation Graduate Research Fellowship [1342962], Indiana Clinical and Translational Sciences Institute: Pediatric Project Development Team, the Swedish Initiative for Research on Microdata in the Social and Medical Sciences (SIMSAM) framework [340-2013-5867], the Swedish Research Council for Health, Working Life and Welfare (FORTE) [50623213], and the Swedish Research Council [2014-38313831]. The funders of the study had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript or the decision to submit for publication.
Footnotes
All other authors declared no conflicts of interest.
References
- 1.Bakker MK, Kolling P, van den Berg PB, de Walle HEK, van den Berg L. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. British journal of clinical pharmacology. 2008 Apr;65(4):600–606. doi: 10.1111/j.1365-2125.2007.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang H, Coleman S, Bridge JA, Yonkers K, Katon W. A meta-analysis of the relationship between antidepressant use in pregnancy and the risk of preterm birth and low birth weight. Gen Hosp Psychiatry. 2014;36(1):13–18. doi: 10.1016/j.genhosppsych.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boukhris T, Sheehy O, Mottron L, Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr. 2015:1–8. doi: 10.1001/jamapediatrics.2015.3356. [DOI] [PubMed] [Google Scholar]
- 4.Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68(11):1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- 5.El Marroun H, White TJ, van der Knaap NJ, et al. Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children. Br J Psychiatry. 2014;205(2):95–102. doi: 10.1192/bjp.bp.113.127746. [DOI] [PubMed] [Google Scholar]
- 6.Harrington RA, Lee L-C, Crum RM, Zimmerman AW, Hertz-Picciotto I. Prenatal SSRI Use and Offspring With Autism Spectrum Disorder or Developmental Delay. Pediatrics. 2014 doi: 10.1542/peds.2013-3406. 2014-04-01 00:00:00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry. 2015 Jun;20(6):727–734. doi: 10.1038/mp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479–485. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 9.Jarde A, Morais M, Kingston D, et al. Neonatal outcomes in women with untreated antenatal depression compared with women without depression: A systematic review and meta-analysis. JAMA Psychiatry. 2016;73(8):826–837. doi: 10.1001/jamapsychiatry.2016.0934. [DOI] [PubMed] [Google Scholar]
- 10.Talge NM, Neal C, Glover V. Antenatal maternal stress and long‐term effects on child neurodevelopment: how and why? Journal of Child Psychology and Psychiatry. 2007;48(3‐4):245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. American Journal of Psychiatry. 2010;167(11):1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 12.Scherff A, Taylor M, Eley TC, Happé F, Charman T, Ronald A. What Causes Internalising Traits and Autistic Traits to Co-occur in Adolescence? A Community-Based Twin Study. Journal of Abnormal Child Psychology. 2014;42(4):601–610. doi: 10.1007/s10802-013-9796-y. [DOI] [PubMed] [Google Scholar]
- 13.Cole J, Ball HA, Martin NC, Scourfield J, McGuffin P. Genetic Overlap Between Measures of Hyperactivity/Inattention and Mood in Children and Adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(11):1094–1101. doi: 10.1097/CHI.0b013e3181b7666e. [DOI] [PubMed] [Google Scholar]
- 14.Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. General Hospital Psychiatry. 2009;31(5):403–413. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Academy of Medical Sciences Working Group. Identifying the environmental causes of disease: How should we decide what to believe and when to take action? London: Academy of Medical Sciences; 2007. [Google Scholar]
- 16.Larsson H, Ryden E, Boman M, Langstrom N, Lichtenstein P, Landen M. Risk of bipolar disorder and schizophrenia in relatives of people with attention-deficit hyperactivity disorder. British Journal of Psychiatry. 2013;203(2):103–106. doi: 10.1192/bjp.bp.112.120808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundström S, Reichenberg A, Anckarsäter H, Lichtenstein P, Gillberg C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. The BMJ. 2015;350:h1961. doi: 10.1136/bmj.h1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P. Preterm birth and mortality and morbidity: A population-based quasi-experimental study. JAMA Psychiatry. 2013;70(11):1231–1240. doi: 10.1001/jamapsychiatry.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Onofrio BM, Rickert ME, Frans E, et al. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry. 2014;71(4):432–438. doi: 10.1001/jamapsychiatry.2013.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bramson LM, Rickert ME, Class QA, et al. The association between childhood relocations and subsequent risk of suicide attempt, psychiatric problems, and low academic achievement. Psychological Medicine. 2016;46(5):969–979. doi: 10.1017/S0033291715002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kendler KS, Lönn SL, Morris NA, Sundquist J, Långström N, Sundquist K. A Swedish national adoption study of criminality. Psychological Medicine. 2014;44(9):1913–1925. doi: 10.1017/S0033291713002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Onofrio BM, Class QA, Rickert ME, et al. Translational Epidemiologic Approaches to Understanding the Consequences of Early-Life Exposures. Behav Genet. 2016;46(3):315–328. doi: 10.1007/s10519-015-9769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allison PD. Fixed effects regression models. Washington DC: Sage; 2009. [Google Scholar]
- 24.Huybrechts KF, Sanghani RS, Avorn J, Urato AC. Preterm birth and antidepressant medication use during pregnancy: a systematic review and meta-analysis. PLoS ONE. 2014;9(3):e92778. doi: 10.1371/journal.pone.0092778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viktorin A, Lichtenstein P, Lundholm C, et al. Selective serotonin re-uptake inhibitor use during pregnancy: association with offspring birth size and gestational age. Int J Epidemiol. 2016;45(1):170–177. doi: 10.1093/ije/dyv351. [DOI] [PubMed] [Google Scholar]
- 26.Sørensen MJ, Grønborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–459. doi: 10.2147/CLEP.S53009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmsten K, Hernández-Díaz S. Can non-randomized studies on the safety of antidepressants during pregnancy convincingly beat confounding, chance, and prior beliefs? Epidemiology (Cambridge, Mass) 2012;23(5):686–688. doi: 10.1097/EDE.0b013e318258fbb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frisell T, Oberg AS, Kuja-Halkola R, Sjolander A. Sibling comparison designs: Bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- 29.Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry. 2007;164(8):1206–1213. doi: 10.1176/appi.ajp.2007.06071172. [DOI] [PubMed] [Google Scholar]
- 30.Boukhris T, Bérard A. Selective Serotonin Reuptake Inhibitor Use during Pregnancy and the Risk of Autism Spectrum Disorders: A Review. J Pediatr Genet. 2015;04(02):084–093. doi: 10.1055/s-0035-1556744. 31.07.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan Y, Keskin-Arslan E, Acar S. Prenatal antidepressant use and risk of autism spectrum disorders in children. JAMA Pediatrics. 2016;170(7):712. doi: 10.1001/jamapediatrics.2016.0727. [DOI] [PubMed] [Google Scholar]
- 32.Fombonne E. Prenatal antidepressant use and risk of autism spectrum disorders in the children. JAMA Pediatrics. 2016;170(7):711–712. doi: 10.1001/jamapediatrics.2016.0745. [DOI] [PubMed] [Google Scholar]
- 33.Gauderman WJ, Witte JS, Thomas DC. Family-based association studies. J Natl Cancer Inst Monogr. 1999(26):31–37. doi: 10.1093/oxfordjournals.jncimonographs.a024223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


