Abstract
Background:
Liver resection surgery results in significant postoperative pain. However, it is still not clear which opioids used by patient-controlled analgesia (PCA) provides the best pain control and results in the least side effect in a patient with impaired liver function. Our hypothesis was that fentanyl is a better choice than morphine as it is a potent analgesic that its elimination half-life does not depend on the hepatic uptake and metabolism.
The Study Purpose:
Is to compare morphine and fentanyl PCA in liver resection patients as regards the degree of pain control, the consumption of opioids, and the side effects.
Methods:
A retrospective case–control study of hepatic resection patients who received postoperative morphine (Morph) or fentanyl (Fent) PCA. The study compared the pain scores, the morphine equivalent dose (MED), the number of demands requested as recorded by the PCA infusion pump, and the side effects every 12 h for 48 h.
Results:
This study yielded 40 patients; with the majority were living donor hepatic resection patients. There was no significant difference in the pain scores. However, the MED and the demands were significantly less in the Morph group. The P < 0.000, 0.0001, 0.0005, and 0.003, demands P < 0.002, 0.006, 0.014, and 0.013 at 12, 24, 48, and 36 h, respectively. The overall side effects were not different between the 2 groups at all time intervals measured; however, Morph patients were significantly more sedated in the first 12 h. There was one case of respiratory depression in the Morph group compared to two cases in the in the Fent group that needed treatment with naloxone.
Conclusions:
Although both groups had adequate pain control. The Morphine group reached faster pain control with less MED and PCA requests in liver resection patients, although it was more sedating in the first 12 h. However, fentanyl patients were less sedated; both drugs need close monitoring in the immediate postoperative period due to reported respiratory depressive effect and the need to use naloxone. The dosage of the PCA settings needs to be studied further to reach to the best dose with a reduced side effect. Further studies are recommended to reduce PCA dosages by introducing a multimodal approach of pain management relying on other methods with no additional sedative effects as regional anterior abdominal blocks.
Keywords: Fentanyl, living liver donors, morphine, patient-controlled analgesia, postoperative pain
Introduction
Hepatic resection surgeries have the potential to be very painful due to the proximity of the incision to the diaphragm and the extensive liver bed dissection and mobilization.[1] Good pain control is essential in avoiding postoperative complications and speeding up the overall recovery.[2] It also has a great impact on the patient's experience and potentially on the availability of living liver donors. Coagulopathy, impaired liver functions, and the unavailability of the oral route make pain control challenging in these types of surgeries.[3] Although thoracic epidural analgesia provides good pain relief, the small risk of an epidural hematoma has led several transplant centers to abandon its use in this group of patients.[4]
Intravenous patient-controlled analgesia (PCA) is an important option. It is effective in relieving pain, provides flexibility in dose adjustments, increases patient autonomy, reduces the burden on nurses, and increases patient satisfaction.[5] Morphine is considered the gold standard of analgesia; however, it is metabolized and conjugated by the liver and excreted by the kidney and in bile. The impaired postoperative liver functions may result in accumulation and potential side effects. In comparison, fentanyl is another strong opioid that does not depend on liver metabolism for initial clearance; hence, it seems ideal for the pain management in liver patients.[6,7,8]
Our hypothesis was that fentanyl PCA would provide better analgesia and less side effects for hepatic resection surgeries compared with morphine PCA because of its potency and lack of active metabolites. To the best of our knowledge, no study has compared morphine and fentanyl PCA in liver resection surgeries.
Methods
Following approval by the Institutional Review Board of King Abdul Aziz Medical City (KAMC) in Riyadh, Saudi Arabia, we conducted a retrospective case–control study of hepatic resection patients over 6 years. Selection criteria for the review included all postoperative hepatic resection patients, whether for graft donors or other reasons, who received PCA morphine (Morph-group) or fentanyl (Fent-group).
The records were divided into 2 groups based on the type of PCA received.
Exclusion criteria
American Society of Anesthesiologists (ASA) status above 3
Patients who received any form of regional anesthesia in the perioperative period
Patients who did not start the PCA within the first 6 h of surgery
Patients who had incisions other than subcostal incisions.
Because most of the liver donor patients in KAMC received epidural analgesia, a few anesthesiologists chose PCA for the postoperative pain management. After applying the exclusion criteria to the study patients, 40 patients (20 in each group) satisfied the inclusion criteria. All patients received general anesthesia and had morphine and/or fentanyl titrated intravenously intraoperatively. In the immediate postoperative period, they were loaded intravenously with morphine to a tolerable pain level – visual analog scale (VAS) score of 4 or less – and then patients were assigned to PCA morphine or fentanyl based on the anesthesiologist's preference. Patients had multimodal analgesia in the form of nonsteroidal anti-inflammatory analgesics that could be applied intravenously or orally whenever possible; they also had access through the nurse to intravenous rescue opioids for breakthrough pain, and they were encouraged to use their PCA before they could receive the rescue opioid medication. The rescue opioid dose was converted to a morphine equivalent dose (MED) and was added to the total MED dose consumed in each group. The PCA morphine was programmed initially to provide a 1–2 mg bolus every 6–10 min lockout intervals. The PCA fentanyl was programmed to a 15–20 mcg bolus every 6–10 min lockout intervals. All patients were started at the lower limit of PCA boluses. The PCA log was reviewed every 12 h or sooner if the pain control was inadequate, and the PCA program was changed accordingly.
The pain was assessed using the VAS; however, for statistical comparison the pain control was divided into good pain management (VAS <4) and suboptimal (VAS from 5 to 10). Indirect measures were further used to assess each method of pain control; to make the data uniform, all parameters were measured every 12 h for a total of 48 h even if the PCA was still ongoing after that.
Data about liver function tests (LFT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, and albumin were recorded every 12 h to establish any correlation between the occurrence of analgesic side effects and the LFT impairments.
Primary outcome
Pain scores ranged from mild (VAS <4) to suboptimal (VAS from) from 5 to 10)
The MED was used every 12 h
The number of demands requested and boluses delivered was recorded by the PCA pump every 12 h.
Secondary outcome
-
Sedations using University of Michigan sedation scale
0 = Awake/Alert
1 = Minimally sedated
2 = Moderately sedated
3 = Deeply sedated
4 = Unarousable.
Statistical analysis used for the sedation state awake, sedated, or unarousable
Other side effects included respiratory depression and bladder function.
Statistical analysis
All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). Demographics were summarized and reported across the PCA groups at baseline using descriptive statistics. Interval variables were summarized and reported in terms of mean (M) and standard deviation.
Categorical variables were compared statistically across the PCA groups using the Chi-square test of independence, Fisher exact test, and independent sample t-test. All statistical tests were declared significant at α level <0.05.
The difference in the average pain score between PCA groups for each 12-h period was compared using nonparametric Wilcoxon's ranked sum test. Repeated-measures generalized linear models analysis was used to test time and PCA effects on pain scores before and after adjusting for key demographics and clinical characteristics, taking into account the repeated scores of individual patients.
Results
Baseline demographic characteristics, the type of surgery, and the incision
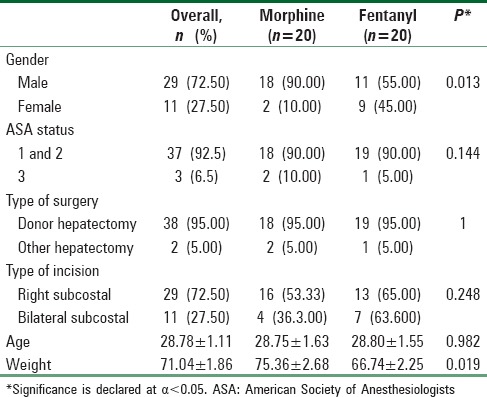
When the PCA groups were compared, there was no significant difference regarding age, ASA physical status, type of surgery, or type of incision. However, there was a significant difference in the categories of gender and weight which was adjusted for comparing the results [Table 1].
Table 1.
Baseline demographic characteristics and the type of surgery and the incision of the patient-controlled analgesia groups
Liver function tests
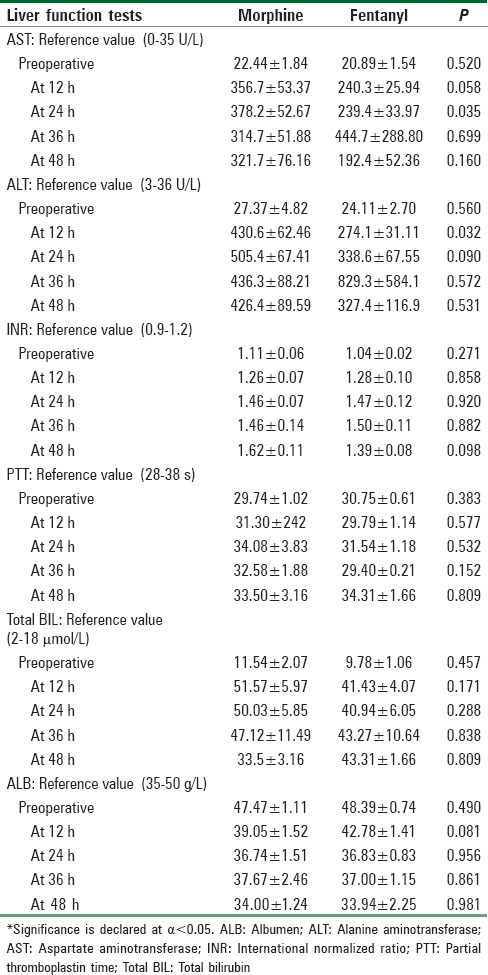
There was no significant difference between the two groups regarding their LFTs, except the AST and ALT in the first 12 h were significantly higher in the morphine group due to higher-than-average AST and ALT increases in 3 patients [Table 2].
Table 2.
Liver function tests analysis in patient-controlled analgesia groups every 12 h
Pain score analysis in patient-controlled analgesia groups
Pain control (mild vs. moderate to severe) was measured 4 times at 12-hour intervals during the first 48 h and then compared between study cohorts at each time interval using the Chi-square test. Morphine PCA was a better analgesic at the first and second 12-h time intervals, as the morphine group showed a lower median pain score (Md = 3, 2 sequentially; P = 0.002) compared with the fentanyl group (Md = 4, 4 sequentially; P = 0.011). The two groups became equal at the third and fourth-time interval (P = 0.282 and 0.792, respectively). However, there was no difference between the two groups after controlling for gender, weight, and type of incision (P = 0.095). There was no significant difference between study cohorts in terms of analgesic status (good/suboptimal) was noted at any of the 4-time intervals (P = 0.205, 1.000, 0.480, and 1.000).
Intraoperative and immediate postoperative morphine equivalent dose and duration of patient-controlled analgesia
There was no significant difference between the 2 groups with regard to the amount of MED used intraoperatively (61.81 ± 33.47 vs. 68.45 ± 18.55 mg and P = 0.444) and immediately postoperatively before the start of the PCA (4.8 ± 7.66 vs. 5.85 ± 9.97 mg and P =0.711) for the morphine and fentanyl groups, respectively.
Morphine equivalent dose and the number of the demands requested
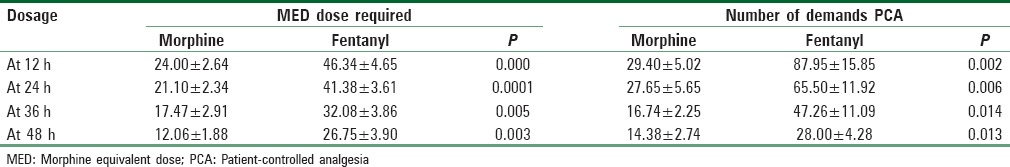
The total number of the MEDs received and the demands requested by pressing the PCA button were significantly higher at all time intervals in the fentanyl group compared with the morphine group [Table 3].
Table 3.
The total amount of the total morphine equivalent dose received and the demands requested by pressing the patient-controlled analgesia button
Opioid side effects
Sedation
There was a significant difference in the proportion of sedation levels between PCA groups at the first 12-hour interval when 100 percent of patients in the morphine group were light to deeply sedated compared with only 65 percent of patients in the fentanyl group who were light to deeply sedated (P = 0.008). However, the proportion of sedation levels did not differ by PCA group at the second, third, and fourth-time intervals (P = 0.197, 0.740, and 0.693, respectively) [Table 4].
Table 4.
Sedation level analysis in patient-controlled analgesia groups at different time intervals
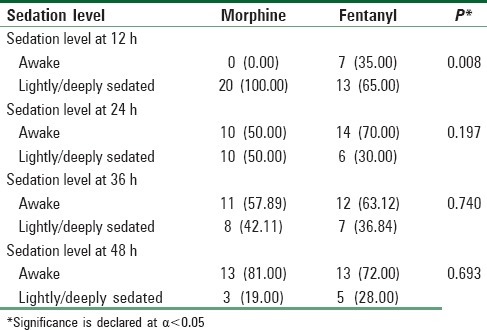
After controlling for weight and gender, the fully stratified model of sedation-level predictors showed that the morphine group was significantly more sedated, with an odds ratio of 3.43 and a confidence interval of 1.18–9.92 (P = 0.022). PCA group was a significant predictor of sedation level (P = 0.036).
Itching, nausea, and vomiting
The incidence of itching, nausea, and vomiting was low in the two groups; however, drawing statistical conclusions from these data were not possible because of the rarity of the occurrence.
Discontinuation of the Foley catheter
No morphine group patients had their Foley catheters discontinued in the first 12 h and eight (40 percent) had it until 48 h; trials for earlier discontinuation failed in three patients. In the fentanyl group, seven patients had their Foley catheters successfully removed in the first 12 h, whereas five (20 percent) needed the Foley catheter for 48 h or longer.
Respiratory depression
There was one case of respiratory depression in the morphine group. The respiratory rate dropped to 6 breaths/min, and it was treated with naloxone and discontinuation of the PCA. Meanwhile, two patients in the fentanyl group dropped their respiratory rate to between 8 and 9/min and were treated with naloxone and the reduction of PCA fentanyl boluses. All the patients were ASA 1, males, young, and of normal body mass index with no marked increase in their LFTs.
Discontinuation of patient-controlled analgesia due to side effects
One patient in the morphine group requested discontinuation of the PCA due to excessive sedation at 48 h, while another was discontinued following a respiratory depression in the first 24 h. However, one fentanyl PCA patient was switched to morphine PCA after 48 h due to inadequate pain relief. The pain markedly improved on morphine PCA, and the patient was very satisfied. Although two patients developed respiratory depression to a rate of 8–9 breaths/min and needed naloxone, the PCAs were continued at lower doses without problems.
Discussion
The living organ donors are generally healthy and undergo surgery for altruistic reasons. Good pain control that has the least side effects, avoids complications, and reduces the impact of the surgery on the patient's quality of life is extremely important.[9,10] Therefore, trying to find a better pain control regimen is of paramount importance.
Although the pain scores between the two groups were not different, the fentanyl PCA group consumed far more MEDs, registered more PCA demands, and needed frequent adjustments compared with the morphine PCA group. Although the overall side effects profile was not different, the fentanyl patients were less sedated in the first 12 h.
It was obvious from the results that more effort was needed to achieve adequate pain control in the fentanyl group. For instance, in the first 12 h the fentanyl patients demanded a PCA dose on average 7.3 times/h compared with 2.3 times/h in the morphine group. Although the demands decreased over time, the fentanyl group stayed in the higher demand category at all periods. We consider the MED and number of demands as more of a reflection of the severity of pain rather than the pain score itself. The pain needed to be controlled with whatever amount of medication was needed.
The AST and ALT values were significantly different at one period of time. Three patients in the morphine group and 1 patient in the fentanyl group had their AST and ALT moderately elevated. We do not think that affected either the analgesia or the side effect profile. None of the significant side effects were reported in those patients. Although morphine is metabolized primarily by the liver, it was found that in chronic liver disease or in the freshly transplanted liver, there is minimal effect on the metabolites.[11,12] Even in the absence of a functional liver, as in the anhepatic phase of liver transplantation, morphine metabolites were found in the serum and the urine because the kidneys or intestines assume the role of the extrahepatic conjugation.[13,14] However, morphine has to be used either minimally or not at all in severe hepatic disease because it is significantly affected by an impairment of the metabolism.[8]
Our study results were comparable to those of Howell et al.[15] who compared PCA morphine to fentanyl in postoperative pain control of cesarean section with doses comparable to those used in this study and found no differences in the quality of analgesia produced or in the incidence or severity of major side effects. Similarly, to maintain adequate analgesia with PCA fentanyl, more than 70% of patients required several readjustments to the PCA settings or supplemental boluses. PCA morphine provided more reliable analgesia and required little alteration or attention once established. Howell et al.[15] concluded that frequent readjustments can increase the incidence of the human error and can lead to inappropriate doses.
The fentanyl group needed more effort to attain pain control because of fentanyl's lipophilic nature and its tendency to redistribute after bolus administration in the adipose tissues.[16] After initial equilibration with adipose tissue, it is slowly released into the plasma. Because of its long half-time, fentanyl accumulates after one large or multiple smaller doses, and redistribution becomes less effective in removing fentanyl from its site of action in the brain.[7,16] Another explanation is that we might not have used a large enough bolus dose of fentanyl. Camu et al.[17] tried to find the best fentanyl dose that produced analgesia after major surgery. The authors found that a 40 mcg of fentanyl every 10 min was the most appropriate, as a 20 mcg dose every 10 min resulted in inadequate relief, and a 60 mcg dose produced respiratory depression. Another study examined the use of intravenous PCA during burn dressing changes and found a 30 mcg PCA bolus dose to be optimal.[18] Others have suggested PCA bolus doses between 20 mcg and 50 mcg for the acute pain management.[6] The fentanyl PCA bolus might be less than the morphine PCA bolus in terms of equivalency; however, the overall MED in the fentanyl group every 12 h was higher, and even with this dose, two patients developed a respiratory rate of 8 breaths/min.
The lockout interval was 5–10 min; the 10 min might have been too long for fentanyl. Shorter lockout intervals of 5–6 min or a basal continuous infusion were suggested to avoid excessive demands and to achieve a rapid steady state.[6,18,19] The active metabolites of morphine are known to have an analgesic effect and that might have played a role in the better pain control in the morphine group.[20]
The overall side effects were not different between the two groups except for sedation, which happened more in the first 12 h after surgery in the morphine group. However, we do not think that affected the outcome in any way. During the time when patients were in the intensive care unit or the liver stepdown unit, they were not required to participate in any activity such as ambulation or feeding.
The limitation of the study is its retrospective nature; a prospective randomized study would be ideal to confirm the results. However, the relative rarity of the donors would make such a study difficult to conduct. In addition, we had to limit our study to the first 48 h to make the data uniform because after that point some of the PCAs were discontinued based on the side effects or at the surgeon's request.
Although both groups had adequate pain control. The Morphine group reached faster pain control with less MED and PCA requests in liver resection patients, although it was more sedating in the first 12 h.
Conclusion
We conclude that morphine delivered by PCA provides better postoperative pain control with an acceptable side effects profile in the hepatic resection patients than fentanyl.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Heriot AG, Karanjia ND. A review of techniques for liver resection. Ann R Coll Surg Engl. 2002;84:371–80. doi: 10.1308/003588402760978148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui YL, Wu YW, Chen CL. Pain control for liver transplants. Acta Anaesthesiol Sin. 1994;32:61–4. [PubMed] [Google Scholar]
- 3.Borromeo CJ, Stix MS, Lally A, Pomfret EA. Epidural catheter and increased prothrombin time after right lobe hepatectomy for living donor transplantation. Anesth Analg. 2000;91:1139–41. doi: 10.1097/00000539-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Hwang GS, McCluskey SA. Anesthesia and outcome after partial hepatectomy for adult-to-adult donor transplantation. Curr Opin Organ Transplant. 2010;15:377–82. doi: 10.1097/MOT.0b013e3283387f75. [DOI] [PubMed] [Google Scholar]
- 5.Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2006;4:CD003348. doi: 10.1002/14651858.CD003348.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Peng PW, Sandler AN. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology. 1999;90:576–99. doi: 10.1097/00000542-199902000-00034. [DOI] [PubMed] [Google Scholar]
- 7.Mather LE. Clinical pharmacokinetics of fentanyl and its newer derivatives. Clin Pharmacokinet. 1983;8:422–46. doi: 10.2165/00003088-198308050-00004. [DOI] [PubMed] [Google Scholar]
- 8.Bosilkovska M, Walder B, Besson M, Daali Y, Desmeules J. Analgesics in patients with hepatic impairment: Pharmacology and clinical implications. Drugs. 2012;72:1645–69. doi: 10.2165/11635500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Cywinski JB, Parker BM, Xu M, Irefin SA. A comparison of postoperative pain control in patients after right lobe donor hepatectomy and major hepatic resection for tumor. Anesth Analg. 2004;99:1747–52. doi: 10.1213/01.ANE.0000136423.17446.5D. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Lim KC, Jeon MK, Kim IO, Jeong JS, Hong JJ, et al. Postoperative pain and influencing factors among living liver donors. Transplant Proc. 2012;44:363–5. doi: 10.1016/j.transproceed.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Mazoit JX, Sandouk P, Zetlaoui P, Scherrmann JM. Pharmacokinetics of unchanged morphine in normal and cirrhotic subjects. Anesth Analg. 1987;66:293–8. [PubMed] [Google Scholar]
- 12.Shelly MP, Cory EP, Park GR. Pharmacokinetics of morphine in two children before and after liver transplantation. Br J Anaesth. 1986;58:1218–23. doi: 10.1093/bja/58.11.1218. [DOI] [PubMed] [Google Scholar]
- 13.Bodenham A, Quinn K, Park GR. Extrahepatic morphine metabolism in man during the anhepatic phase of orthotopic liver transplantation. Br J Anaesth. 1989;63:380–4. doi: 10.1093/bja/63.4.380. [DOI] [PubMed] [Google Scholar]
- 14.Davis M. Cholestasis and endogenous opioids: Liver disease and exogenous opioid pharmacokinetics. Clin Pharmacokinet. 2007;46:825–50. doi: 10.2165/00003088-200746100-00002. [DOI] [PubMed] [Google Scholar]
- 15.Howell PR, Gambling DR, Pavy T, McMorland G, Douglas MJ. Patient-controlled analgesia following caesarean section under general anaesthesia: A comparison of fentanyl with morphine. Can J Anaesth. 1995;42:41–5. doi: 10.1007/BF03010570. [DOI] [PubMed] [Google Scholar]
- 16.Hug CC, Jr, Murphy MR. Tissue redistribution of fentanyl and termination of its effects in rats. Anesthesiology. 1981;55:369–75. doi: 10.1097/00000542-198110000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Camu F, Van Aken H, Bovill JG. Postoperative analgesic effects of three demand-dose sizes of fentanyl administered by patient-controlled analgesia. Anesth Analg. 1998;87:890–5. doi: 10.1097/00000539-199810000-00027. [DOI] [PubMed] [Google Scholar]
- 18.Prakash S, Fatima T, Pawar M. Patient-controlled analgesia with fentanyl for burn dressing changes. Anesth Analg. 2004;99:552–5. doi: 10.1213/01.ANE.0000125110.56886.90. [DOI] [PubMed] [Google Scholar]
- 19.Pasero C, Montgomery R. Intravenous fentanyl. Out of the operating room and gaining in popularity. Am J Nurs. 2002;102:73. doi: 10.1097/00000446-200204000-00027. 75, 76. [DOI] [PubMed] [Google Scholar]
- 20.Klimas R, Mikus G. Morphine-6-glucuronide is responsible for the analgesic effect after morphine administration: A quantitative review of morphine, morphine-6-glucuronide, and morphine-3-glucuronide. Br J Anaesth. 2014;113:935–44. doi: 10.1093/bja/aeu186. [DOI] [PubMed] [Google Scholar]


