This cohort study identifies the hemodynamic characteristics and outcomes of pulmonary hypertension associated with heart failure with preserved ejection fraction.
Key Points
Question
What are the hemodynamic characteristics and outcomes for pulmonary hypertension associated with heart failure with preserved ejection fraction?
Findings
In a cohort study of 10 023 individuals undergoing right heart catheterization at a single center between 2005 and 2012, 2587 (25.8%) had PH-HFpEF. Mortality was 23.6% at 1 year and 48.2% at 5 years, and cardiac hospitalizations occurred in 28.1% at 1 year and 47.4% at 5 years.
Meaning
Pulmonary hypertension associated with heart failure with preserved ejection fraction is common in individuals undergoing right heart catheterization and is associated with poor outcomes; this heart failure with preserved ejection fraction phenotype requires further study and new therapies.
Abstract
Importance
Heart failure with preserved ejection fraction (HFpEF) is highly prevalent, yet there are no specific therapies, possibly due to phenotypic heterogeneity. The development of pulmonary hypertension (PH) in patients with HFpEF is considered a high-risk phenotype in need of targeted therapies, but there have been limited hemodynamic and outcomes data.
Objective
To identify the hemodynamic characteristics and outcomes of PH-HFpEF.
Design, Setting, and Participants
Cohort study of participants who had a right heart catheterization from January 2005 to September 2012 (median [interquartile range] follow-up time, 1578 [554-2513] days) were analyzed. Hemodynamic catheterization data was linked to the clinical data repository of all inpatient and outpatient encounters across a health system. Single tertiary referral center for heart failure and PH within a large health care network using a common clinical data repository was studied. There were 19 262 procedures in 10 023 participants.
Exposures
Participants were classified as having no PH, precapillary PH, or PH in the setting of left heart disease (reduced or preserved ejection fraction). Pulmonary hypertension associated with HFpEF was defined as mean pulmonary artery pressure of 25 mm Hg or more, pulmonary artery wedge pressure of 15 mm Hg or more, and left ventricular ejection fraction of 45% or more. Pulmonary hypertension severity was quantified by the hemodynamic parameters transpulmonary gradient, pulmonary vascular resistance, and diastolic pulmonary gradient.
Main Outcomes and Measures
The primary outcome was time to all-cause mortality. Secondary outcomes were time to acute hospitalization and cardiovascular hospitalization.
Results
The mean (SD) of all study individuals was 65 (38) years. Of 10 023 individuals, 2587 (25.8%) had PH-HFpEF. Mortality was 23.6% at 1 year and 48.2% at 5 years. Cardiac hospitalizations occurred in 28.1% at 1 year and 47.4% at 5 years. The frequency of precapillary PH using clinically defined cut-offs for transpulmonary gradient (>12 mm Hg), pulmonary vascular resistance (3 Woods units), and diastolic pulmonary gradient (≥7 mm Hg) were 12.6%, 8.8%, and 3.5%, respectively. Transpulmonary gradient, pulmonary vascular resistance, and diastolic pressure gradient were predictive of mortality and cardiac hospitalizations.
Conclusions and Relevance
In a large cohort referred for invasive hemodynamic assessment, PH-HFpEF was common. Transpulmonary gradient, pulmonary vascular resistance, and diastolic pulmonary gradient are all associated with mortality and cardiac hospitalizations.
Introduction
Heart failure is one of the largest public health problems, affecting 5.7 million patients with an estimated cost of $30.7 billion in the United States.1 Left ventricular (LV) diastolic dysfunction is present in approximately 28% of asymptomatic and 81% of symptomatic patients with heart failure, referred to as having heart failure with preserved ejection fraction (HFpEF).2,3 There are no specific therapies for HFpEF, with now multiple randomized clinical trials with negative results in large part thought to be due to substantial clinical heterogeneity and leading to a progressive call for deeper phenotyping to better target randomized clinical trials.4 One such phenotype is pulmonary hypertension (PH) associated with HFpEF (PH-HFpEF). Many patients with LV diastolic dysfunction have chronically elevated pulmonary venous filling pressures leading to pathologic pulmonary vascular remodeling, PH, and eventual right ventricular (RV) dysfunction.5,6 Increased mortality in HFpEF correlates with increased systolic RV pressure and reduced RV function.7,8,9,10,11,12 As with HFpEF in general, there are no known therapies for PH-HFpEF, owing in large part to the phenotypic heterogeneity confounding interventional trials to date.13 Thus, better phenotypic and outcomes data are greatly needed.
Pulmonary hypertension in the setting of left heart disease (PH-LHD) is the most common form of PH and encompasses a heterogeneous patient population. Left ventricular systolic dysfunction, LV diastolic dysfunction, valvular disease, as well as metabolic dysregulation are all contributing factors. Our group has studied the association of complex metabolic dysregulation with PH-HFpEF in preclinical models.14 The prevalence of PH has been variably described as between 23% to 83% of the patients with HFpEF.13 Further, although there is histologic evidence of pulmonary vascular remodeling in these patients,5,15 the hemodynamic phenotype that defines PH-HFpEF and precapillary involvement remains controversial with limited published data to date and to our knowledge. The severity of precapillary involvement can be assumed by the measured pulmonary vascular resistance (PVR), the transpulmonary gradient (TPG), and/or the diastolic pulmonary gradient (DPG) obtained from right heart catheterization, which we and others have used to study outcomes in PH.16,17,18,19,20 Clinically used cut-off values include PVR of more than 2.5 to 3.0 Woods units, TPG of more than 12 mm Hg, and/or DPG of 7 mm Hg or more5,21,22,23,24,25; however, questions remain about the application in the PH-HFpEF population.
Pulmonary hypertension associated with HFpEF is a large public health problem with a lack of diagnostic and prognostic consensus for health care professionals. Our large-scale clinical data repository presents an opportunity to study this patient population. Such approaches have been useful in other diseases, such as end-stage renal disease,26 and applying to PH-HFpEF may greatly affect future clinical trial design and phenotypically specific therapies in this large, heterogenous population.
The aim of this study was to test the hypothesis that precapillary involvement, as assessed by hemodynamic measures in a large data set, increases risk of mortality and hospitalization in individuals with HFpEF.
Methods
Development of a Clinical Data Repository Enhanced Right Heart Catheterization Database
In collaboration with the University of Pittsburgh, Department of Medicine, Analytics Center, the hemodynamic database was enhanced with data extracted from the University of Pittsburgh Medical Center (UPMC) data repository, including clinical, administrative, and financial data (eFigure 1 in the Supplement).27 Structured data was extracted from the data repository, and postprocessing scripts were used to extract relevant clinical facts from clinical reports.
The Right Heart Catheterization database includes all the right heart catheterization procedures that were performed at UPMC Presbyterian Hospital, a 795-bed acute tertiary care hospital with a specialty in cardiovascular and pulmonary medicine. For each participant, administrative and charge data was obtained from December 13, 1990, through October 15, 2014 (eAppendix 1 and eFigure 2 in the Supplement).
Study Population
All patients who underwent a right heart catheterization between January 19, 2005, and September 26, 2012, were included in the cohort. In patients undergoing multiple right heart catheterizations, only the first right heart catheterization was included in the analysis (eAppendix 1 in the Supplement). The University of Pittsburgh institutional review board approved this study, and informed patient consent was waived.
Definition of PH-HFpEF
Participants were initially stratified into those with no PH (mean pulmonary artery pressure [mPAP], <25 mm Hg), precapillary PH (mPAP, ≥25 mm Hg; pulmonary artery wedge pressure [PAWP], ≤15 mm Hg), and PH-LHD (mPAP, ≥25 mm Hg; PAWP, >15 mm Hg). Participants with PH-LHD were further stratified by LV ejection fraction (LVEF) into HFpEF or heart failure with reduced ejection fraction (HFrEF) using the cutpoint of 45% based on the American College of Cardiology/American Heart Association 2014 heart failure guidelines (eMethods in the Supplement).28,29
Hemodynamic Markers of Precapillary Involvement
The prevalence of precapillary involvement in participants with PH-HFpEF was estimated using hemodynamic markers including TPG (mPAP–PAWP), PVR (PVR = TPG/cardiac output), and DPG (diastolic pulmonary artery [PA] pressure–PAWP). Participants were dichotomized based on proposed values from the literature including: TPG of more than 12 mm Hg, PVR of 3 Woods units or more, and DPG of 7 mm Hg or more.5,13,30
Outcomes
The primary outcome was time to all-cause mortality. To determine mortality, we identified those who died in a UPMC hospital at any time, then examined each patient’s last health system encounter to determine if they were alive at the end of the study period. The patients who did not have a visit in the follow-up period were compared with the United States Social Security Death Index to determine if death occurred. Secondary outcomes were time to acute hospitalization and cardiovascular hospitalization after index procedure, identified via International Classification of Diseases, Ninth Revision coding (eAppendix 1 in the Supplement).
Statistics Analysis
Kaplan-Meier survival curves were constructed and the log-rank test was used to compare either death or cardiac hospitalizations as events in patients based on type of PH (precapillary PH, PH-LHD, PH-HFrEF, and PH-HFpEF compared with no PH). Cox proportional hazards models were used to model time to event data for the outcomes. All Cox models were assessed for proportional hazards violation. In the case of mortality as an outcome, age, PA compliance, PVR, and arterial elastance in the RV violate this assumption. For age, an interaction term of time was added to the model. For PA compliance, PVR and arterial elastance in the RV, a spline model (B-spline with 1 knot) was created, and final spline models were selected based on model fitness using Bayesian Information Criteria. To assess the increased association between precapillary pulmonary vascular disease in PH-HFpEF, TPG, PVR, and DPG were also turned into categorical variables using the standard clinical cut-offs of 12 mm Hg, 3 Woods units, and 7 mm Hg, respectively. Time-dependent receiver operator characteristic curves were used to determine specificity and sensitivity for TPG, PVR, and DPG. Optimal cut-off values for TPG, PVR, and DPG were determined for predicting 1-year survival based on specificity and sensitivity. Data preparation and analyses were conducted using Stata, version 14.0 (StataCorp) and R, version 3.1.1 (R Foundation).
Results
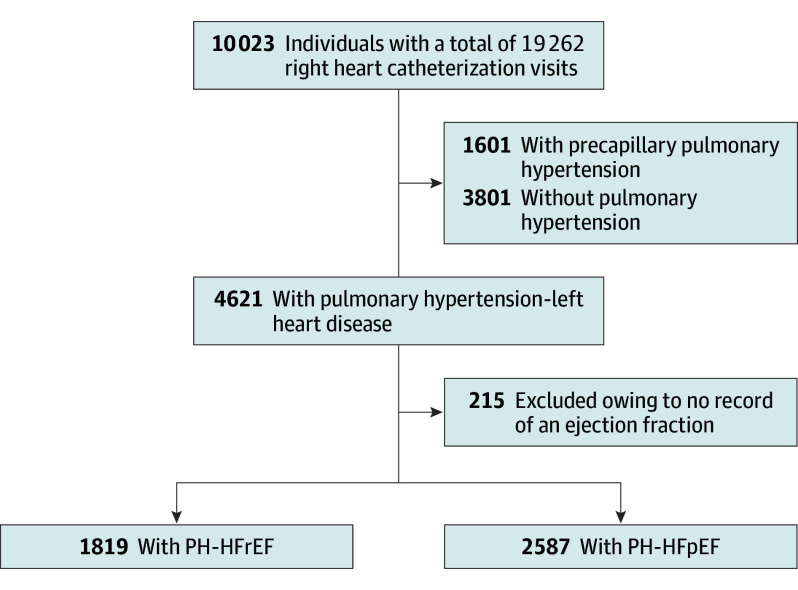
Between January 19, 2005, and September 26, 2012, 19 262 right heart catheterization procedures were performed at UPMC Presbyterian University Hospital among 10 023 unique participants (Figure 1). Based on the index right heart catheterization, 3801 participants (37.9%) were classified as having no PH, 1601 (16.0%) with precapillary PH, and 4621 (46.1%) with PH-LHD (Figure 1). Left ventricular ejection fraction was identified via linking hemodynamic catheterization data to the system-wide clinical data repository and searching (eAppendix 1 and eAppendix 2 in the Supplement) in 9427 participants (94.1%). The PH-LHD group was subdivided as follows: 2587 (25.8%) with PH-HFpEF, 1819 (18.1%) with PH-HFrEF, and 215 (2.1%) with no EF recorded. The final analysis focused on participants in the PH-HFpEF group. Characteristics of the cohort are presented in Table 1.
Figure 1. Flow Diagram of the Cohort.
Participants with a documented ejection fraction of 45% or more were classified as having a preserved ejection fraction or pulmonary hypertension in the setting of heart failure preserved ejection fraction (PH-HFpEF). Participants with an left ventricular ejection fraction of less than 45% had a reduced ejection fraction (PH-HFrEF).
Table 1. Characteristics of the Study Cohort.
| Characteristica | Total (N = 10 023) |
No PH (n = 3801) |
Precapillary PH (n = 1601) |
PH-LHD (n = 4621) |
PH-LHD: PH-HFrEF (n = 1819) |
PH-LHD: PH-HFpEF (n = 2587) |
|---|---|---|---|---|---|---|
| Sex, No. (%) | ||||||
| Female | 4312 (43.0) | 1571 (41.3) | 797 (49.8) | 1944 (42.1) | 538 (29.6) | 1298 (45.4) |
| Male | 5706 (56.9) | 2227 (58.6) | 804 (50.2) | 2675 (57.9) | 1281 (70.4) | 1287 (45.0) |
| Unknown | 5 (0) | 3 (0) | 0 | 2 (0) | 0 | 2 (0) |
| Age, y | 62 (23) | 61 (14) | 62 (13) | 64 (30) | 63 (15) | 65 (38) |
| Heart rate, bpm | 77 (18) | 74 (18) | 78 (17) | 78 (19) | 81 (21) | 77 (18) |
| BMI | 29 (8) | 28 (7) | 29 (8) | 31 (8) | 29 (8) | 31 (9) |
| Body surface area, m2 | 1.95 (0.28) | 1.93 (0.27) | 1.91 (0.27) | 1.99 (0.29) | 1.99 (0.29) | 1.98 (0.28) |
| LVEF, No. (%) | 51 (17) | 56 (15) | 57 (13) | 46 (18) | 26 (8) | 60 (7) |
| Right atrial pressure, mm Hg | 10 (7) | 5 (4) | 8 (5) | 14 (7) | 14 (6) | 14 (7) |
| Systolic pulmonary artery pressure, mm Hg | 47 (19) | 30 (6) | 54 (19) | 58 (16) | 57 (14) | 58 (18) |
| Diastolic pulmonary artery pressure, mm Hg | 18 (10) | 10 (4) | 20 (9) | 24 (8) | 25 (7) | 23 (8) |
| mPAP, mm Hg | 30 (12) | 19 (4) | 34 (11) | 38 (10) | 39 (9) | 38 (10) |
| PAWP, mm Hg | 17 (12) | 10 (4) | 11 (3) | 25 (14) | 26 (7) | 24 (15) |
| Cardiac output, L/min | 5.45 (1.94) | 5.68 (1.85) | 5.49 (1.72) | 5.25 (2.05) | 4.59 (1.77) | 5.69 (2.11) |
| Cardiac index, L/min | 2.8 (0.92) | 2.95 (0.9) | 2.88 (0.83) | 2.64 (0.95) | 2.31 (0.84) | 2.87 (0.95) |
| TPG, mm Hg, median (IQR) | 11 (8 to 16) | 8 (6 to 11) | 18 (14 to 28) | 12 (9 to 17) | 11 (8 to 15) | 12 (9 to 18) |
| Pulmonary vascular resistance, mm Hg/L/min, median (IQR) | 2.1 (1.4 to 3.3) | 1.5 (1.1 to 2.1) | 3.6 (2.5 to 5.6) | 2.4 (1.6 to 3.7) | 2.6 (1.7 to 3.9) | 2.3 (1.5 to 3.6) |
| DPG, mm Hg, median (IQR) | 0 (−3 to 4) | 0 (−3 to 2) | 6 (2 to 13) | −1 (−4 to 3) | −1 (−4 to 2) | −1 (−4 to 3) |
| Pulmonary artery pulse pressure, mm Hg | 29 (12) | 21 (6) | 35 (13) | 34 (12) | 32 (10) | 35 (13) |
| Stroke volume, mL | 76 (38) | 82 (40) | 74 (30) | 72 (38) | 62 (40) | 79 (36) |
| PA compliance, mL/mm Hg | 3.12 (2.18) | 4.29 (2.38) | 2.43 (1.94) | 2.39 (1.57) | 2.11 (1.53) | 2.56 (1.57) |
| RV SW, mm Hg × mL | 1521 (1022) | 1096 (640) | 1881 (1015) | 1748 (1155) | 1525 (1167) | 1884 (1138) |
| Arterial elastance in the right ventricle, mm Hg/mL | 0.76 (0.56) | 0.43 (0.2) | 0.88 (0.58) | 1 (0.62) | 1.15 (0.65) | 0.9 (0.58) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BPM, beats per minute; DPG, diastolic pulmonary gradient; IQR, interquartile range; LHD, left heart disease; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary artery pressure; PA, pulmonary artery; PAWP, mean pulmonary artery wedge pressure; PH, pulmonary hypertension; PH-HFpEF, pulmonary hypertension associated with heart failure and preserved ejection fraction; PH-HFrEF, pulmonary hypertension associated with heart failure and reduced ejection fraction; RV SW, right ventricular stroke work; TPG, transpulmonary gradient.
Values are reported as mean (SD) unless otherwise noted.
Association Between PH Classifications and Adverse Outcomes
During a median (interquartile range [IQR]) follow-up time of 1578 (554-2513) days, there were 4925 deaths. Event-free curves for mortality and cardiac hospitalizations are presented in Figure 2 and eTables 1 and 2 in the Supplement. Survival was significantly worse in the precapillary PH and PH-LHD group than the group without PH, as were hospitalizations, which were substantially worse in patients with PH-LHD than precapillary PH. Splitting the PH-LHD group into those with PH-HFrEF and PH-HFpEF (Figure 2C and 2D) showed that mortality for patients with PH-HFrEF was the poorest and similar in patients with PH-HFpEF and precapillary PH. Hospitalizations were markedly greater for patients with PH-HFrEF, and patients with PH-HFpEF had more hospitalizations than those with precapillary PH.
Figure 2. Survival Probability and Freedom From Cardiac Hospitalizations in Participants Undergoing a Right Heart Catheterization.
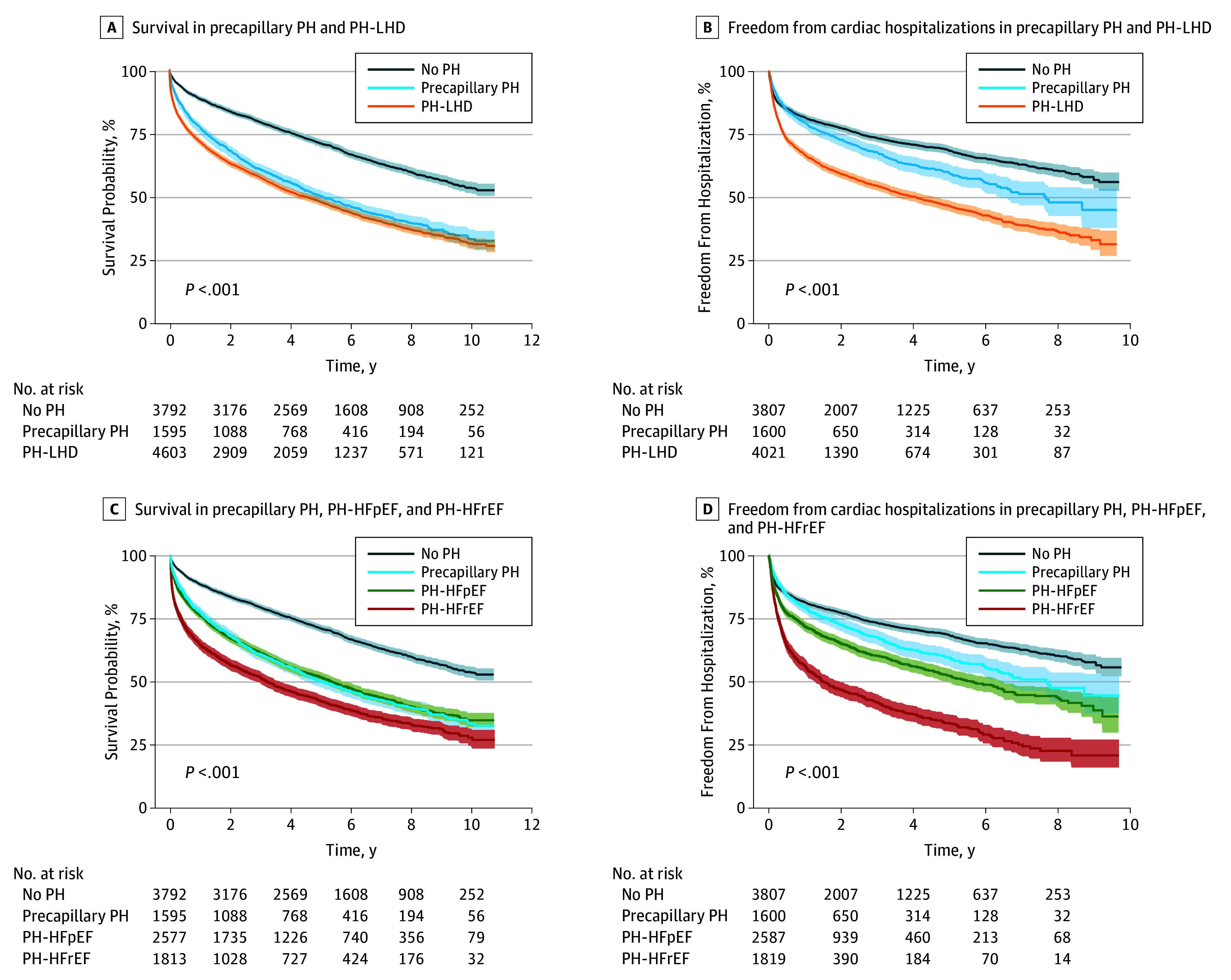
A, Precapillary pulmonary hypertension (PH) and PH in the setting of left heart disease (PH-LHD) both increase the risk of mortality in participants that have undergone a right heart catheterization. B, Participants with PH-LHD have more acute cardiac hospitalizations than participants without PH and precapillary PH. C, Participants in the PH-LHD group that have a reduced ejection fraction (PH-HFrEF) have increased mortality compared with those with preserved ejection fraction (PH-HFpEF) and precapillary PH. D, Both PH-HFrEF and PH-HFpEF groups have more acute cardiac associated hospitalizations than precapillary PH. Shaded areas indicate 95% CI.
Prevalence of Precapillary Involvement in PH-LHD and PH-HFpEF Based on Hemodynamic Markers
In patients with PH-LHD, the median (IQR range) TPG, PVR, and DPG was 12 (9-17) mm Hg, 2.4 (1.6-3.7) Woods units, and –1 (–4 to 3) mm Hg, respectively. In patients with PH-HFpEF, the median (IQR) TPG, PVR, and DPG was 12 (9-18) mm Hg, 2.3 (1.5-3.6) Woods units, and −1 (−4 to 3) mm Hg, respectively (Table 1). Because a negative DPG is nonphysiological, we systematically reviewed 200 randomly selected participants for a nested case study of DPG. Using a Bland-Altman analysis, there is a systematic bias of +4 mm Hg in DPG (ie, DPG calculated from the nonadjudicated retrospective data set was underestimated by a mean of 4 mm Hg). The bias for diastolic PA pressure was +5 mm Hg, and the bias for PAWP was +1 mm Hg. The results showed that catheter whip (with underestimation of PA diastolic pressure) was the most common cause of a negative DPG.
To better understand the prevalence of PH within the PH-LHD and PH-HFpEF cohorts, participants were segmented into several groupings (eFigure 3 in the Supplement) based on TPG (≤6, 7-9, 10-12, 13-15, and >15 mm Hg), PVR (<1, 1.01-2, 2.01-3, 3.01-4, and >4 Woods units), and DPG (<1, 1-2, 3-4, 5-6, and ≥7 mm Hg). Using clinically defined cut-offs for TPG (>12 mm Hg), PVR (≥3 Woods units), and DPG (≥7 mm Hg) as various indices of precapillary pulmonary vascular involvement, yielded prevalences within PH-LHD of 45.9%, 36.2%, and 11.7%, respectively. Corresponding prevalences within PH-HFpEF were 48.9%, 34.2%, and 13.7%. Participants had significant overlap in elevated TPG, PVR, and DPG (eTable 3 in the Supplement). The number of participants that met the recent European Society of Cardiology and the European Respiratory Society definition of combined precapillary and postcapillary PH (PVR, ≥3 Woods units and DPG, ≥7 mm Hg) was 531 (11.5%) within PH-LHD and 354 (13.7%) within PH-HFpEF groups.
Stratifying the PH-HFpEF cohort by the 3 hemodynamic markers of precapillary involvement identified different phenotypes (eTable 4 in the Supplement). A TPG of more than 12 mm Hg (n = 1265) was associated with increased body mass index (calculated as weight in kilograms divided by height in meters squared), left atrial size, right atrial pressure, pulmonary pressures, resistance, RV stroke work, and decreased PA compliance. Participants with a PVR of 3 Woods units or more (n = 884) had somewhat lower body mass index, increased right atrial and PA pressure, RV stroke work, and decreased cardiac index and PA compliance. In contrast, participants with a DPG of 7 mm Hg or more (n = 354) were younger with increased right atrial and PA pressures, lower PA compliance, slightly lower cardiac index with higher heart rate, but higher LVEF with no difference in body mass index or left atrial size.
Association Between Hemodynamic Markers of Precapillary Involvement and Mortality in PH-HFpEF
The median (IQR) follow-up time was 1383 (419-2348) days and a total of 1406 deaths. When modeled as continuous variables, age, right atrial pressure, mPAP, PAWP, PA pulse pressure, PA compliance, RV arterial elastance, TPG, PVR, and DPG significantly associated with mortality (Table 2). Using common clinically defined cut-off values, a PVR of 3 Woods units or more carried the highest risk for mortality (hazard ratio [HR], 1.54; 95% CI, 1.39-1.72; P < .001) compared with TPG of more than 12 mm Hg (HR, 1.41; 95% CI, 1.27-1.56; P < .001) and DPG of 7 mm Hg or more (HR, 1.44; 95% CI, 1.25-1.66; P < .001). From a time-dependent receiver operating characteristic analysis, the optimal cut-offs for TPG, PVR, and DPG to predict mortality in this data set were 16 mm Hg, 2.3 Woods units, and 4.7 mm Hg, respectively, with sensitivities of 38%, 63%, and 43% and specificities of 73%, 49%, and 74%, respectively (TPG: HR, 1.57; 95% CI, 1.40-1.75; PVR: HR, 1.45; 95% CI, 1.30-1.61; and DPG: HR, 1.42; 95% CI, 1.25-1.60).
Table 2. Cox Univariate Regression Analysis of the Association of Parameters With Mortality, All-Cause Hospitalization, and Cardiac Hospitalization in Participants With PH-HFpEF.
| Parameter | Mortality HR (95% CI) | P Value | All-Cause Hospitalization HR (95% CI) |
P Value | Cardiac Hospitalizationa HR (95% CI) |
P Value |
|---|---|---|---|---|---|---|
| Age, y | 1.03b (1.02-1.03) | <.001 | 1.00 (1.00-1.01) | .46 | 1.01 (1.01-1.02) | <.001 |
| Male/female | 1.07 (0.97-1.19) | .18 | 0.97 (0.88-1.07) | .60 | 0.87 (0.76-1.00) | .04 |
| Ejection fraction, % | 0.99 (0.99-1.00) | .12 | 1.01 (1.00-1.01) | .05 | 0.99 (0.98-1.00) | .01 |
| Left atrial size, cm | 1.06 (1.00-1.13) | .07 | 1.03 (0.98-1.09) | .25 | 1.19 (1.11-1.28) | <.001 |
| Right atrial pressure, mm Hg | 1.04 (1.03-1.05) | <.001 | 1.01 (1.00-1.02) | .09 | 1.02 (1.01-1.03) | .002 |
| mPAP, mm Hg | 1.02 (1.01-1.02) | <.001 | 1.00 (1.00-1.01) | .41 | 1.02 (1.01-1.02) | <.001 |
| PAWP, mm Hg | 1.02 (1.01-1.02) | .006 | 1.00 (0.99-1.01) | .88 | 1.02 (1.01-1.04) | <.001 |
| Cardiac index, L/min/m2 | 0.95 (0.90-1.01) | .10 | 1.04 (0.98-1.09) | .19 | 0.78 (0.72-0.85) | <.001 |
| PA pulse pressure, mm Hg | 1.02 (1.01-1.02) | <.001 | 1.00 (1.00-1.01) | .12 | 1.01 (1.01-1.02) | <.001 |
| PA compliance, mL/mm Hgc | 0.77d (0.73-0.82) | <.001 | 1.00 (0.97-1.04) | .94 | 0.87 (0.83-0.92) | <.001 |
| Arterial elastance in the right ventricle, mm Hg/mLc | 2.81e (2.17-3.63) | <.001 | 1.00 (0.92-1.10) | .94 | 1.29 (1.17-1.42) | <.001 |
| TPG, mm Hg | 1.02 (1.01-1.02) | <.001 | 1.00 (1.00-1.01) | .41 | 1.01 (1.01-1.02) | <.001 |
| PVR, mm Hg/L/minc | 1.06f (1.04-1.08) | <.001 | 1.01 (0.99-1.03) | .40 | 1.06 (1.03-1.08) | <.001 |
| DPG, mm Hg | 1.02 (1.01-1.02) | <.001 | 1.00 (0.99-1.01) | .67 | 1.01 (1.00-1.02) | .03 |
Abbreviations: DPG, diastolic pulmonary gradient; HR, hazard ratio; mPAP, mean pulmonary artery pressure; PA, pulmonary artery; PAWP, pulmonary artery wedge pressure; PH-HFpEF, pulmonary hypertension associated with heart failure with preserved ejection fraction; PVR, pulmonary vascular resistance; TPG, transpulmonary gradient.
International Classification of Diseases, Ninth Revision, codes 410 to 429.
P < .001 for interaction with follow-up time.
For variables with proportional hazards violations, spline models were created (B-splines with 1 knot) to address the violation of assumptions, as noted for PA compliance, arterial elastance in the right ventricle, and PVR.
For PA compliance of 5 or less including 1897 (94%) of observations. Hazard ratio for PA compliance or more than 5 was 1.08 (P = .11) per unit increase.
For arterial elastance in the right ventricle of 1 or less including 1684 (69%) of observations. Hazard ratio for arterial elastance of more than 1 was 1.09 (P = .12) per unit increase.
For PVR of more than 1 including 1935 (91%) of observations. Hazard ratio for PVR of 1 or less was 1.33 (P = .3) per unit increase.
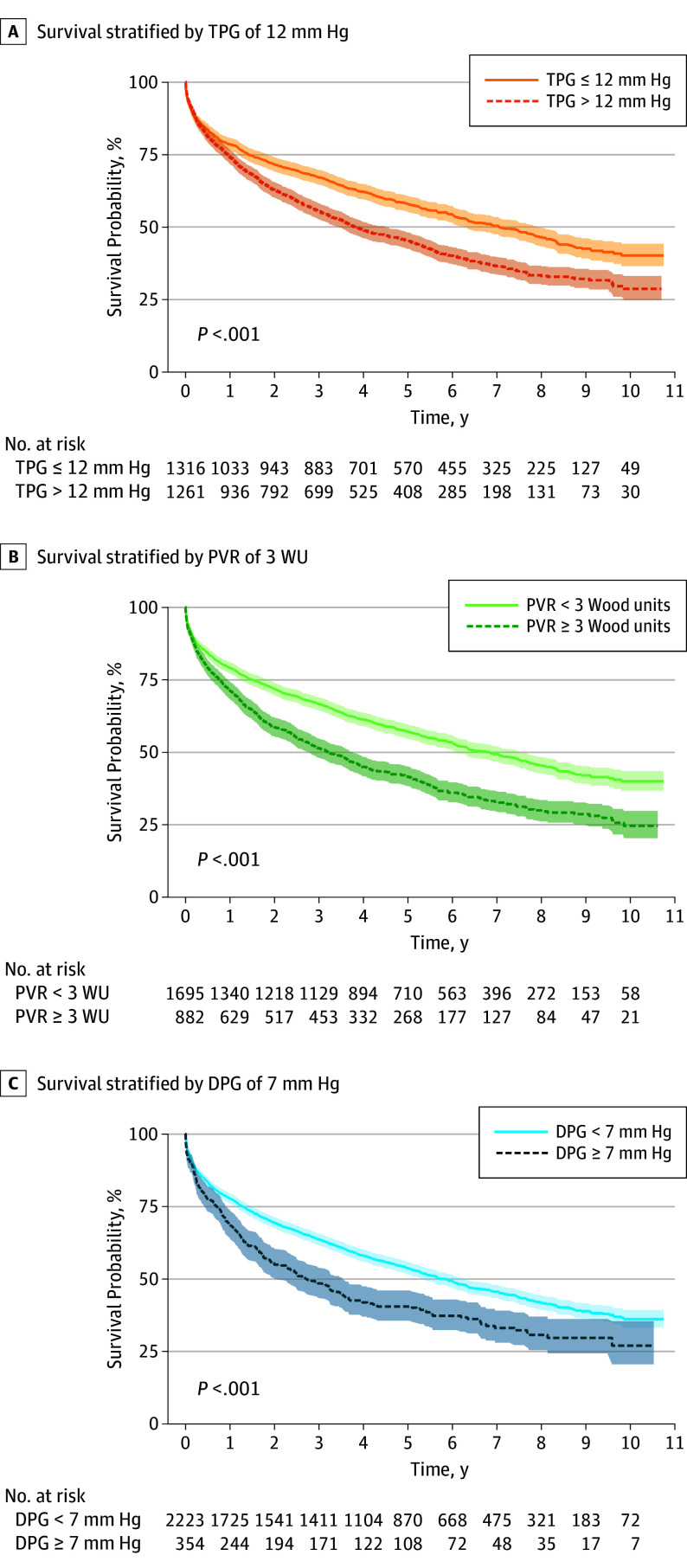
Survival was significantly worse for participants with TPG of 12 mm Hg or more (Figure 3A), as well as participants with PVR of 3 Woods units or more (Figure 3B) and in participants with DPG of 7 mm Hg or more (Figure 3C). Detailed survival tables stratified by TPG, PVR, or DPG are presented in eTables 5 to 7 in the Supplement.
Figure 3. Elevated TPG, PVR, and DPG Are Associated With Increased Mortality in the PH-HFpEF Cohort.
Transpulmonary gradient (TPG) (A), pulmonary vascular resistance (PVR) (B), and diastolic pulmonary gradient (DPG) (C) are associated with increased mortality. WU indicates Wood units.
Association Between Hemodynamic Markers of Precapillary Involvement and Hospitalizations in PH-HFpEF
The median (IQR) time between index right heart catheterization and cardiac hospitalization was 171 (28-931) days, and there were 1591 cardiac hospitalizations. Significantly more cardiac hospitalizations occurred in participants who were older, had a high right atrial pressure, high left atrial size, high mPAP, high PA pulse pressure, high PVR, high arterial elastance in the RV, low LVEF, low cardiac index, or a low PA compliance (Table 2). When modeled as continuous variables, age, right atrial pressure, mPAP, PA pulse pressure, PA compliance, TPG, and PVR were associated with cardiac hospitalizations (Table 2). Using the clinically defined cut-off values, a PVR of 3 Woods units or more had a high risk for cardiac hospitalizations (HR, 1.47; 95% CI, 1.28-1.69), as did TPG of more than 12 mm Hg (HR, 1.37; 95% CI, 1.20-1.57). Diastolic pulmonary gradient as a continuous variable did significantly associate with cardiac hospitalizations (HR, 1.01; 95% CI, 1.00-1.02) in the PH-HFpEF cohort but not as the clinically defined cut-off value of 7 mm Hg or more.
Time to cardiac hospitalization was significantly worse in participants with a TPG of more than 12 mm Hg (eFigure 4A in the Supplement), as well as participants with a PVR of 3 Woods units or more (eFigure 4B in the Supplement) and in participants with a DPG of 7 mm Hg or more (eFigure 4C in the Supplement). Detailed tables for cardiac hospitalization stratified by TPG, PVR, or DPG are presented in supplemental eTables 8 to 10 in the Supplement.
Discussion
We report on the clinical outcomes of hemodynamically defined PH-HFpEF in a large single-center database. There are multiple important findings. First, we confirm that PH-LHD is common in participants undergoing a right heart catheterization (46%) of which 56% is PH-HFpEF. Survival in PH-LHD, and more specifically PH-HFpEF, is quite poor, similar in severity to precapillary PH but notably with more cardiac hospitalizations. Additionally, hemodynamic markers of precapillary involvement in PH-HFpEF predict mortality and cardiac hospitalizations. We also present detailed outcomes for mortality and hospitalizations in eTables 1, 2, and 5 to 10 in the Supplement, which may help inform future clinical trials.
It is generally accepted that the presence of PH-LHD is associated with worse outcomes.31,32 Even mild increases in PA pressure within the accepted normal range are associated with poor outcomes.33 Therefore, PH-HFpEF is a continuum of disease. However, although PH is defined by invasive hemodynamic measurements, most reports have focused on estimates of PH from echocardiography given the difficulty in amassing a large enough data set to effectively evaluate outcomes. By leveraging information technology in a large clinical data repository, it is possible to critically evaluate prevalence and outcomes of hemodynamically defined PH. Our findings confirm that the presence of PH by hemodynamic criteria (mean PA pressure, ≥25 mm Hg) in left heart disease, and more specifically HFpEF, is associated with poor outcomes similar to patients with precapillary PH.34,35 We confirm several recent smaller studies of mortality rates in PH-HFpEF (19.3% at 1 year and 43.5% at 5 years).34,35,36,37 Further, we document a tremendous burden on the health care system in terms of hospitalizations in PH-HFpEF. However, if the population is further stratified by hemodynamic definitions suggested for the definition of combined precapillary and postcapillary PH (PVR, ≥3 and DPG, ≥7),30 then the prevalence of precapillary PH in HFpEF in this cohort is much more limited (354 of 2587 [14%] with mPAP, ≥25 mm Hg). This is similar to other recent reports and may be informative to future trial design to best target new therapies.4,17,38
Beyond diagnosis, there is conflicting data in the literature regarding prognosis associated with hemodynamic definitions of PH-HFpEF. We found multiple hemodynamic criteria (TPG, >12 mm Hg; PVR, ≥3 Woods units; DPG, ≥7 mm Hg) predicted mortality and cardiac hospitalizations. This is similar to Gerges et al,5 who found increased mortality and pulmonary vascular medial hypertrophy in patients with PH-LHD with a TPG of more than 12 mm Hg and a DPG of 7 mm Hg or more compared with those with a DPG of less than 7 mm Hg.5 However, several other groups have found that DPG is not predictive of mortality in PH-LHD.21,39 Disparity in the association of DPG with outcomes may be associated with sources of error in the retrospectively recorded pressures, such as errors in the PAWP (underwedging or overwedging, excessive v-waves), errors in diastolic PA pressure due to high frequency noise, as well as excessive respiratory variation and/or inadequate calibration, which may result in negative values and limit clinical utility.21,40 We performed a systematic review of 200 randomly selected participants for a nested case study of DPG in which we found that catheter whip (with underestimation of PA diastolic pressure) was the most common cause of a negative DPG and that systematic overread revealed a more than 4 mm Hg bias in DPG (the retrospective data set was underestimated by an average of 4 mm Hg). These findings suggest that DPG must be assessed carefully and may be restricted to a research setting.
The limitations of interventional trials to date for PH-HFpEF point out the heterogeneity of disease and need for rigorous phenotyping to find effective therapies.41,42,43 To date and to our knowledge, there are no approved therapies for postcapillary PH or combined precapillary and postcapillary PH. Specifically, there is a lack of evidence for the use of current pulmonary arterial hypertension therapies.30 The data we provide, including detailed survival and hospitalization in eTables 1, 2, and 5 to 10 in the Supplement may be informative for future randomized clinical trial design, albeit recognizing the potential referral bias of more advanced disease in this cohort referred for invasive hemodynamic assessment. Although invasively measured cardiopulmonary hemodynamics may be more difficult for clinical trial enrollment and carry more risk, PH can only be diagnosed invasively and therefore must be included in trials targeting this disease. Further, our data on poor outcomes in these patients provide evidence for a reasonable risk-benefit profile for such an approach.
Limitations
This study has several limitations. Although this was a large study, it is from a single-center experience, which may limit generalizability. The design of the study, based on analysis of patients referred for measurement of invasive hemodynamics, while allowing for a large study of such data, necessarily comes with a referral bias toward patients with potentially more advanced disease and therefore may not be representative of the overall HFpEF population. The administrative data on hospitalizations was limited to the UPMC network; therefore, hospitalizations may have been missed if occurring at a non-UPMC facility, which would result in an underestimation of hospitalization outcomes. The size of this study precluded hemodynamic review of all raw data from right heart catheterization, which may result in errors, as noted in the nested case-control study of DPG in this report. The size of the study also limited the ability to include detailed information on the indication for referral for right heart catheterization, medications, and more detailed echocardiographic indices of LV filling, diastology, and hypertrophy. It was not possible to exclude other potential causes of PH such as chronic pulmonary embolism. To obtain an LVEF in as many participants as possible in this large retrospective data set, we used advanced search methods of the clinical data repository including echocardiography (67.3%) and nuclear imaging (26.8%), which yielded an LVEF in 94.1% of the whole cohort. While including both echocardiography and nuclear imaging limited missing data, the different techniques may induce variability.
Conclusions
In a large cohort referred for invasive hemodynamic assessment, PH-HFpEF was very common. Transpulmonary gradient, PVR, and DPG are all predictors of mortality and cardiac hospitalizations. These findings may be useful for future clinical trial design.
eAppendix 1. Methods
eAppendix 2. Results
eTable 1. Table of the Survival Probability and the Number of Participants at Risk
eTable 2. Freedom from Cardiac Hospitalization and the Number of Participants at Risk
eTable 3. Overlap of Hemodynamic Markers Quantifying Precapillary Involvement in PH-LHD and PH-HFpEF
eTable 4. Characteristics of the Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction (PH-HFpEF) Cohort Stratified by Clinically Defined Hemodynamic Cutoffs
eTable 5. Survival Probability and the Number of PH-HFpEF Participants at Risk Stratified by Transpulmonary Gradient
eTable 6. Survival Probability and the Number of PH-HFpEF Participants at Risk Stratified by Pulmonary Vascular Resistance
eTable 7. Survival Probability and the Number of PH-HFpEF Participants at Risk Stratified by Diastolic Pulmonary Gradient
eTable 8. Freedom From Cardiac Hospitalization and the Number of PH-HFpEF Individuals at Risk Stratified by Transpulmonary Gradient
eTable 9. Freedom From Cardiac Hospitalization and the Number of PH-HFpEF Individuals at Risk Stratified by Pulmonary Vascular Resistance
eTable 10. Freedom From Cardiac Hospitalization and the Number of PH-HFpEF Individuals at Risk Stratified by Diastolic Pulmonary Gradient
eFigure 1. Development of the Clinical Data Repository Enhanced Right Heart Catheterization Database (CDR-enhanced RHC Database) and Association of PH Groups With Mortality
eFigure 2. Descriptive Information on the Extracted Data in the CDR-Enhanced RHC Database
eFigure 3. Prevalence of precapillary Pulmonary Vascular Disease in PH-LHD and PH-HFpEF Based on Hemodynamic Definitions
eFigure 4. Elevated TPG, PVR, and DPG are Associated With Increased Cardiac Hospitalizations in the PH-HFpEF Cohort
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics: 2013 update: a report from the American Heart Association. Circulation. 2013;127(1):143-152. [DOI] [PubMed] [Google Scholar]
- 2.Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194-202. [DOI] [PubMed] [Google Scholar]
- 3.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296(18):2209-2216. [DOI] [PubMed] [Google Scholar]
- 4.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134(1):73-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143(3):758-766. [DOI] [PubMed] [Google Scholar]
- 6.Guazzi M. Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail. 2014;7(2):367-377. [DOI] [PubMed] [Google Scholar]
- 7.Bursi F, McNallan SM, Redfield MM, et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59(3):222-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37(1):183-188. [DOI] [PubMed] [Google Scholar]
- 9.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salamon JN, Kelesidis I, Msaouel P, et al. Outcomes in World Health Organization group II pulmonary hypertension: mortality and readmission trends with systolic and preserved ejection fraction-induced pulmonary hypertension. J Card Fail. 2014;20(7):467-475. [DOI] [PubMed] [Google Scholar]
- 11.Kjaergaard J, Akkan D, Iversen KK, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol. 2007;99(8):1146-1150. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed SF, Hussain I, AbouEzzeddine OF, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study [published correction appears in Circulation. 2015;131:e424]. Circulation. 2014;130(25):2310-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vachiéry JL, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25)(suppl):D100-D108. [DOI] [PubMed] [Google Scholar]
- 14.Lai YC, Tabima DM, Dube JJ, et al. SIRT3-AMP-Activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation. 2016;133(8):717-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgado JF, Conde E, Sánchez V, et al. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail. 2005;7(6):1011-1016. [DOI] [PubMed] [Google Scholar]
- 16.Vanderpool RR, Pinsky MR, Naeije R, et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. 2015;101(1):37-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerges M, Gerges C, Pistritto AM, et al. Pulmonary hypertension in heart failure. epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med. 2015;192(10):1234-1246. [DOI] [PubMed] [Google Scholar]
- 18.Maron BA. Hemodynamics should be the primary approach to diagnosing, following, and managing pulmonary arterial hypertension. Can J Cardiol. 2015;31(4):515-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164-172. [DOI] [PubMed] [Google Scholar]
- 20.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2):156-163. [DOI] [PubMed] [Google Scholar]
- 21.Tampakakis E, Leary PJ, Selby VN, et al. The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. JACC Heart Fail. 2015;3(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25)(suppl):D42-D50. [DOI] [PubMed] [Google Scholar]
- 23.Fang JC, DeMarco T, Givertz MM, et al. World Health Organization pulmonary hypertension group 2: pulmonary hypertension due to left heart disease in the adult: a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2012;31(9):913-933. [DOI] [PubMed] [Google Scholar]
- 24.Thenappan T, Shah SJ, Gomberg-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4(3):257-265. [DOI] [PubMed] [Google Scholar]
- 25.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail. 2013;1(4):290-299. [DOI] [PubMed] [Google Scholar]
- 26.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108(8):609-613. [DOI] [PubMed] [Google Scholar]
- 27.Yount RJ, Vries JK, Councill CD. The medical archival system: an information retrieval system based on distributed parallel processing. Inf Process Manage. 1991;27(4):379-389. doi: 10.1016/0306-4573(91)90091-Y [DOI] [Google Scholar]
- 28.McMurray JJ, Adamopoulos S, Anker SD, et al. ; Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology; ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology: developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):803-869. [DOI] [PubMed] [Google Scholar]
- 29.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. [DOI] [PubMed] [Google Scholar]
- 30.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119. [DOI] [PubMed] [Google Scholar]
- 31.Guazzi M, Gomberg-Maitland M, Arena R. Pulmonary hypertension in heart failure with preserved ejection fraction. J Heart Lung Transplant. 2015;34(3):273-281. [DOI] [PubMed] [Google Scholar]
- 32.Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiéry JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37(12):942-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assad TR, Hemnes AR, Larkin EK, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol. 2016;68(23):2525-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opitz CF, Hoeper MM, Gibbs JS, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol. 2016;68(4):368-378. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal R, Shah SJ, Foreman AJ, et al. Risk assessment in pulmonary hypertension associated with heart failure and preserved ejection fraction. J Heart Lung Transplant. 2012;31(5):467-477. [DOI] [PubMed] [Google Scholar]
- 37.Hoeper MM, Meyer K, Rademacher J, Fuge J, Welte T, Olsson KM. Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4(6):441-449. [DOI] [PubMed] [Google Scholar]
- 38.Assad TR, Brittain EL, Wells QS, et al. Hemodynamic evidence of vascular remodeling in combined post- and precapillary pulmonary hypertension. Pulm Circ. 2016;6(3):313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail. 2015;3(6):467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagy AI, Venkateshvaran A, Merkely B, Lund LH, Manouras A. Determinants and prognostic implications of the negative diastolic pulmonary pressure gradient in patients with pulmonary hypertension due to left heart disease. Eur J Heart Fail. 2017;19(1):88-97. [DOI] [PubMed] [Google Scholar]
- 41.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124(2):164-174. [DOI] [PubMed] [Google Scholar]
- 42.Redfield MM, Chen HH, Borlaug BA, et al. ; RELAX Trial . Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah AM, Shah SJ, Anand IS, et al. ; TOPCAT Investigators . Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014;7(1):104-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Methods
eAppendix 2. Results
eTable 1. Table of the Survival Probability and the Number of Participants at Risk
eTable 2. Freedom from Cardiac Hospitalization and the Number of Participants at Risk
eTable 3. Overlap of Hemodynamic Markers Quantifying Precapillary Involvement in PH-LHD and PH-HFpEF
eTable 4. Characteristics of the Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction (PH-HFpEF) Cohort Stratified by Clinically Defined Hemodynamic Cutoffs
eTable 5. Survival Probability and the Number of PH-HFpEF Participants at Risk Stratified by Transpulmonary Gradient
eTable 6. Survival Probability and the Number of PH-HFpEF Participants at Risk Stratified by Pulmonary Vascular Resistance
eTable 7. Survival Probability and the Number of PH-HFpEF Participants at Risk Stratified by Diastolic Pulmonary Gradient
eTable 8. Freedom From Cardiac Hospitalization and the Number of PH-HFpEF Individuals at Risk Stratified by Transpulmonary Gradient
eTable 9. Freedom From Cardiac Hospitalization and the Number of PH-HFpEF Individuals at Risk Stratified by Pulmonary Vascular Resistance
eTable 10. Freedom From Cardiac Hospitalization and the Number of PH-HFpEF Individuals at Risk Stratified by Diastolic Pulmonary Gradient
eFigure 1. Development of the Clinical Data Repository Enhanced Right Heart Catheterization Database (CDR-enhanced RHC Database) and Association of PH Groups With Mortality
eFigure 2. Descriptive Information on the Extracted Data in the CDR-Enhanced RHC Database
eFigure 3. Prevalence of precapillary Pulmonary Vascular Disease in PH-LHD and PH-HFpEF Based on Hemodynamic Definitions
eFigure 4. Elevated TPG, PVR, and DPG are Associated With Increased Cardiac Hospitalizations in the PH-HFpEF Cohort