Key Points
Question
What factors are associated with the development of secondary attention-deficit/hyperactivity disorder in children 5 to 10 years after traumatic brain injury?
Findings
In this cohort study that included 187 children, severe traumatic brain injury and lower levels of maternal educational level were significantly associated with increased risk for secondary attention-deficit/hyperactivity disorder. Family dysfunction was significantly associated with increased risk in patients with traumatic brain injury but not in patients with orthopedic injury.
Meaning
Injury and environmental factors were associated with risk of secondary attention-deficit/hyperactivity disorder, with new onset up to 6.8 years after injury, highlighting the importance of identifying risk and promoting long-term follow-up of patients with high risk for secondary attention-deficit/hyperactivity disorder.
Abstract
Importance
After traumatic brain injury (TBI), children often experience impairment when faced with tasks and situations of increasing complexity. Studies have failed to consider the potential for attention problems to develop many years after TBI or factors that may predict the development of secondary attention-deficit/hyperactivity disorder (SADHD). Understanding these patterns will aid in timely identification of clinically significant problems and appropriate initiation of treatment with the hope of limiting additional functional impairment.
Objective
To examine the development of SADHD during the 5 to 10 years after TBI and individual (sex, age at injury, and injury characteristics) and environmental (socioeconomic status and family functioning) factors that may be associated with SADHD.
Design, Setting, and Participants
Concurrent cohort/prospective study of children aged 3 to 7 years hospitalized overnight for TBI or orthopedic injury (OI; used as control group) who were screened at 3 tertiary care children’s hospitals and 1 general hospital in Ohio from January 2003 to June 2008. Parents completed assessments at baseline (0-3 months), 6 months, 12 months, 18 months, 3.4 years, and 6.8 years after injury. A total of 187 children and adolescents were included in the analyses: 81 in the TBI group and 106 in the OI group.
Main Outcomes and Measures
Diagnosis of SADHD was the primary outcome. Assessments were all completed by parents. Secondary ADHD was defined as an elevated T score on the DSM-Oriented Attention-Deficit/Hyperactivity Problems Scale of the parent-reported Child Behavior Checklist, report of an ADHD diagnosis, and/or current treatment with stimulant medication not present at the baseline assessment. The Family Assessment Device–Global Functioning measurement was used to assess family functioning; scores ranged from 1 to 4, with greater scores indicating poorer family functioning.
Results
The analyzed sample included 187 children with no preinjury ADHD. Mean (SD) age was 5.1 (1.1) years; 108 (57.8%) were male, and 50 (26.7%) were of nonwhite race/ethnicity. Of the 187 children, 48 (25.7%) met our definition of SADHD. Severe TBI (hazard ratio [HR], 3.62; 95% CI, 1.59-8.26) was associated with SADHD compared with the OI group. Higher levels of maternal education (HR, 0.33; 95% CI, 0.17-0.62) were associated with a lower risk of SADHD. Family dysfunction was associated with increased risk of SADHD within the TBI group (HR, 4.24; 95% CI, 1.91-9.43), with minimal association within the OI group (HR, 1.32; 95% CI, 0.36-4.91).
Conclusions and Relevance
Early childhood TBI was associated with increased risk for SADHD. This finding supports the need for postinjury monitoring for attention problems. Consideration of factors that may interact with injury characteristics, such as family functioning, will be important in planning clinical follow-up of children with TBI.
This cohort study compares the development of secondary attention-deficit/hyperactivity disorder in children who were previously hospitalized with traumatic brain injury vs children previously hospitalized with orthopedic injury.
Introduction
Traumatic brain injury (TBI) is a leading cause of hospitalization in children and adolescents, with more than 1 million children, adolescents, and young adults visiting emergency departments for TBI in the United States annually.1 Sustaining TBI in early childhood is associated with increased risk for development of psychiatric disorders; rates of new psychiatric disorders among patients with TBI are as high as 49% compared with 13% in control samples of children with orthopedic injury (OI).2,3 Attention-deficit/hyperactivity disorder (ADHD), defined by developmentally inappropriate and impairing levels of inattention and/or hyperactivity/impulsivity in multiple settings,4 is the most common psychiatric disorder among children with a history of TBI, with a prevalence of approximately 20%.5,6,7 Among typically developing school-aged children, the prevalence of ADHD is estimated at 8%.8 Onset of ADHD symptoms after an injury such as TBI and/or OI is referred to as secondary ADHD (SADHD). Patients who develop SADHD within the first year after injury demonstrate greater functional impairment than those who do not develop SADHD,9,10 highlighting the importance of understanding factors associated with the development of SADHD.11
Risk factors for development of SADHD after pediatric TBI are not well understood. The effect of injury severity is unclear and varies depending on the severity of TBI examined.6,7,10,12,13,14,15,16,17,18 Although some studies report that injury severity does not predict the development of SADHD,9,16 others report a dose-response effect, with severe injuries predictive of greater risk for SADHD than less severe injuries.10,13,14,15,16,17,18 Karver et al12 noted that children who sustained a severe TBI at a younger age were more likely to develop ADHD symptoms than those injured at older ages.
In addition to injury characteristics, environmental factors have been considered. Poor preinjury family functioning and lower socioeconomic status have been linked to an increased risk for SADHD.7,9,13,16 When injury severity, family psychiatric history, socioeconomic status, and family functioning were examined simultaneously, only family functioning was significantly associated with ADHD symptoms, with poor family functioning associated with more severe ADHD symptoms.15
Most studies examining the development of SADHD were limited to the initial 2 years after injury and did not consider the potential for SADHD to develop many years after injury.7,9,10,13,15,16,17 However, children with a history of TBI might experience new types of impairment, even many years after injury, when faced with tasks and situations of increasing complexity. Early adolescence is a time of great change in brain development and behavioral functioning.19 In addition, parents and teachers place increasing expectations on children for independently organizing and completing schoolwork, navigating multiple classrooms, and managing the expectations of multiple teachers. These physical and social changes, coupled with evidence that early injuries may disrupt the acquisition of developmentally appropriate skills,20 suggest that children who sustain a TBI in early childhood may demonstrate an emergence or worsening of ADHD symptoms during this developmental period. Finally, little is known about how injury characteristics and environmental factors interact over time to predict the development of SADHD. The present study aimed to fill these gaps by examining the development of SADHD during the 5 to 10 years after injury in children with TBI relative to a control group of children with OI. We further evaluated individual (age at injury, injury severity) and environmental predictors (maternal educational level and family functioning) of SADHD.
Methods
Participants and Study Design
A concurrent cohort/prospective research design was used. Consecutive admissions of children between the ages of 3 and 7 years hospitalized overnight for TBI or OI were screened at 3 tertiary care children’s hospitals and 1 general hospital. Patients were enrolled from January 2003 to June 2008, data collection for the final follow-up occurred from December 2009 through April 2015, and statistical analyses for the present paper were conducted from September 2016 through December 2017. Children with OI served as the comparison group to control for preinjury child and family factors, including child attention problems that increase the likelihood of sustaining an injury requiring hospitalization.21 Additional eligibility criteria included accidental cause of injury, no preinjury neurologic problems or developmental delays, and English as the primary language. Children were not excluded if they had a history of attention problems. Institutional review boards of participating institutions, Cincinnati Children’s Hospital Medical Center, Nationwide Children’s Hospital, Rainbow Babies and Children’s Hospital, and MetroHealth Medical Center, approved all procedures, and written informed consent or assent was obtained from all participants. Assent was collected in congruence with individual institutional review board requirements or guidelines. At Cincinnati Children’s Hospital Medical Center, verbal assent was obtained for all children younger than 11 years, and written assent was collected for all children 11 years of age and older; at Nationwide Children’s Hospital, assent was obtained from patients between the ages of 9 and 18 years; and at Rainbow Babies and MetroHealth, written assent was obtained from children 7 years and older, and verbal assent was obtained from children younger than 7 years.
Assessments were completed at baseline (mean [SD] of 1.3 [6.6] months after injury), 6, 12, and 18 months after injury, with longer-term follow-up visits a mean (SD) of 3.4 (0.84) years and 6.8 (1.05) years after injury. Children were invited for the final assessment when they transitioned to middle school, with the expectation that deficits in executive function and attention would emerge at this time. Severity of TBI was characterized using the lowest postresuscitation Glasgow Coma Scale (GCS) score. Severe TBI was defined as a GCS score of 8 or lower. Moderate TBI was defined as a GCS score of 9 to 12, and complicated mild TBI was defined as a GCS score of 13 to 15 with abnormal findings on brain imaging. The OI group included children who sustained a bone fracture (excluding skull fracture) and did not exhibit alterations in consciousness or other signs or symptoms of brain injury.
Measures
At each visit, parents completed a demographic questionnaire that asked about basic demographic information (including maternal educational level) as well as behavioral and mental health diagnoses and treatment for behavioral or emotional concerns. Specifically, parent report of diagnosis of ADHD (before or after injury) and whether the child was currently taking stimulant medication were obtained. The developmentally appropriate version of the Child Behavior Checklist (CBCL),22 CBCL 1.5 to 5 years or CBCL 6 to 18 years, was collected at each visit. The CBCL is a parent-report measure of child emotional and behavioral problems with high test-retest reliability and criterion validity that has been shown to be sensitive to behavioral problems after TBI.23,24 The DSM-Oriented Attention-Deficit/Hyperactivity Problems Scale T score was used to identify patients with elevated levels of attention problems, with higher scores indicative of greater behavioral concerns. The Family Assessment Device–Global Functioning25 is a parent self-report measure assessing family functioning with established reliability and validity. Scores range from 1 to 4, with higher scores indicating poorer functioning. Consistent with previous work, a raw score greater than 2.17 was used to define clinically significant levels of family dysfunction,26 with binary classification (presence/absence) of dysfunction used in the analyses.
Defining ADHD
In the current study, ADHD was defined as T scores higher than 65 on the DSM-Oriented Attention-Deficit/Hyperactivity Problems scale on the CBCL, parent-reported history of ADHD diagnosis, or prescribed stimulant medication. Participants who met these ADHD criteria at the baseline assessment (when parents were asked to report behavior, ADHD diagnosis, and treatment before injury) were considered to have primary/preinjury ADHD. To focus on the development of new-onset attention problems, those with primary ADHD were excluded from analyses. Children who did not meet the criteria for primary ADHD but met the criteria for ADHD at any subsequent assessment visit were considered to have secondary ADHD (SADHD). Clinically significant elevations in CBCL ratings of attention problems are robustly associated with diagnoses made via structured diagnostic interview, highlighting the clinical utility of this approach.9
Statistical Analyses
The primary outcome measure was the development of SADHD. Interval-censored Cox proportional hazards regression modeling was used to model the time from injury to development of SADHD among the injury groups and the moderating effects of family functioning. Two separate models were completed. The first was a main-effects model examining differences in the hazard of SADHD development by injury severity (OI, complicated mild TBI, moderate TBI, and severe TBI) and demographic and environmental factors (age at injury, sex, maternal educational level, and family functioning). Because family functioning has been one of the most consistent environmental factors shown to influence recovery7,9,15 and moderate the effects of injury,27,28 a second model was run to explore the injury group by family functioning interaction on SADHD development. This model included injury group (TBI vs OI), demographic/environmental factors (age at injury, sex, maternal educational level, and family functioning), and family functioning by injury group interaction. Site of enrollment was controlled for in both models.
The threshold for statistical significance was set at 2-sided P < .05, and 95% CIs for hazard ratios (HRs) were calculated. To better understand factors associated with development of SADHD, we tested the proportional hazards assumption for the Cox proportional hazards regression models by examining the Pearson product moment correlation between the Schoenfeld residual and the rank of survival time for cases that progressed to SADHD. All statistics analyses were completed using SAS software (version 9.4; SAS Institute Inc).
Results
Two hundred twenty-one participants were originally enrolled in the study based on the inclusion criteria reviewed above. To focus on the consequences of more severe injuries, 15 children with uncomplicated mild TBI (GCS score >13 with no clinically significant neuroimaging findings) were excluded from analyses. An additional 16 children (1 with severe TBI, 1 with moderate TBI, 3 with complicated mild TBI, and 11 with OI) with primary ADHD were excluded, and 3 children were excluded because they were missing parent rating scales at the baseline assessment. The analyzed sample included 187 children. Of these, the mean (SD) age at injury was 5.1 (1.1) years, 108 (57.8%) were male, and 50 (26.7%) were of nonwhite race/ethnicity (Table 1). One hundred sixty-seven of the 187 (89.3%) of the sample analyzed were retained at the 6-month visit, 151 (80.7%) at the 12-month visit, 149 (79.7%) at the 18-month visit, 134 (71.7%) at the 3.4-year visit, and 120 (64.2%) at the final time point (6.8-year visit). Retention rates did not differ across injury groups at any visit. One hundred (53.5%) of the participants completed all 6 visits, and children who completed all visits did not differ from those who did not complete the long-term follow-up on any of the demographic variables (eTable in the Supplement).
Table 1. Demographic Characteristics of All Injury Groups at Baseline Visit.
| Characteristic | OI (n = 106) | Complicated Mild TBI (n = 40) | Moderate TBI (n = 20) | Severe TBI (n = 21) | Total Sample (N = 187) | P Value |
|---|---|---|---|---|---|---|
| Age at injury, mean (SD), y | 5.13 (1.07) | 5.07 (1.19) | 5.16 (1.18) | 4.92 (0.96) | 5.10 (1.09) | .88 |
| Time since injury, mean (SD), ya | 0.10 (0.04) | 0.12 (0.06) | 0.13 (0.08) | 0.14 (0.07) | 0.11 (0.06) | .003b |
| Males, No. (%) | 60 (56.6) | 23 (57.5) | 11 (55.0) | 14 (66.7) | 108 (57.8) | .85 |
| Nonwhite race/ethnicity, No. (%) | 23 (21.7) | 10 (25.0) | 10 (50.0) | 7 (33.3) | 50 (26.7) | .06 |
| FAD-GF score, mean (SD) | 1.49 (0.46) | 1.55 (0.40) | 1.57 (0.38) | 1.67 (0.52) | 1.53 (0.45) | .40 |
| High FAD-GF score, No. (%)c | 8 (7.5) | 5 (12.5) | 2 (10.0) | 5 (23.8) | 20 (10.7) | .07 |
| Maternal educational level, No. (%)d | 102 (96.2) | 37 (92.5) | 14 (70.0) | 16 (76.2) | 169 (90.4) | .003e |
| Single parent, No. (%) | 23 (21.7) | 14 (35.0) | 11 (55.0) | 11 (52.4) | 59 (31.6) | .003f |
Abbreviations: FAD-GF, Family Assessment Device–Global Functioning25; OI, orthopedic injury; TBI, traumatic brain injury.
Time from injury to completion of baseline visit in years.
OI < complicated mild TBI, OI < moderate TBI, and OI < severe TBI.
Scores of 2.17 or higher are considered high FAD-GF scores.
Mothers with at least a high school diploma.
OI > severe TBI, OI > moderate TBI, and complicated mild TBI > moderate TBI.
OI < severe TBI, OI < moderate TBI.
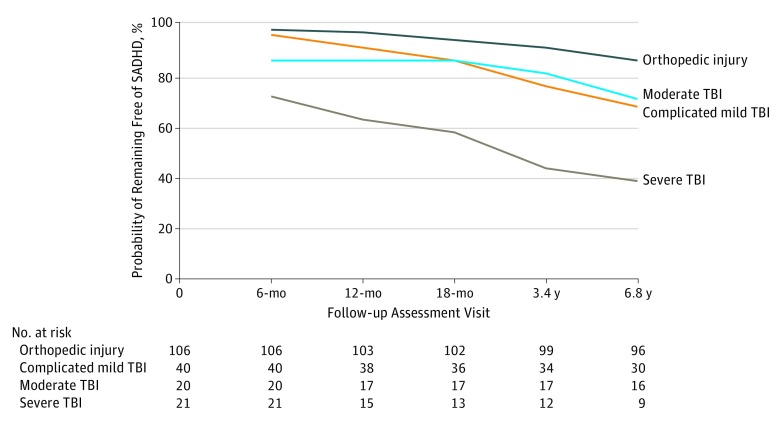
Of the 187 children with no preinjury ADHD, 48 children (25.7%) met our definition of SADHD. These included 13 children with severe TBI (incidence rate, 0.19 event per person-year), 6 with moderate TBI (incidence rate, 0.06 event per person-year), 13 complicated mild TBI (incidence rate, 0.06 event per person-year), and 16 with OI (incidence rate, 0.03 event per person-year). Children met criteria for SADHD for the following reasons: 26, CBCL elevation only; 6, reported diagnosis only; 4, ADHD medication only; 4 CBCL elevation and ADHD medication; 5, reported diagnosis and ADHD medication; and 3, CBCL elevation and reported diagnosis and ADHD medication. None of the children had CBCL elevation and reported diagnosis. The SADHD criteria did not differ across injury groups. The number of children at risk at the start of each visit as well as new-onset cases for each injury group at each visit are presented in the Figure. The probability of not developing SADHD (cumulative survival rate) for each group over time is presented in the Figure. Of note, 8 of the 13 children (61.5%) with severe TBI who developed SADHD did so within the first year after their injury, whereas 3 of 6 children (50.0%) with moderate TBI and 7 of 13 (53.8%) with complicated mild TBI who developed SADHD did so later than the first year after injury.
Figure. Cumulative Survival Rate for Each Injury Group Across Study Period.
Number at risk is calculated as the baseline sample minus the number of participants previously diagnosed with secondary attention-deficit/hyperactivity disorder (SADHD). TBI indicates traumatic brain injury.
Results of the main-effects model showed that injury groups differed significantly in the hazard for the development of SADHD (Table 2). Specifically, children with severe TBI had significantly increased risk for the development of SADHD compared with the OI group (HR, 3.62; 95% CI, 1.59-8.26). The complicated mild TBI and moderate TBI group did not differ significantly from the OI group. Lower maternal educational level (HR, 0.33; 95% CI, 0.17-0.62) and greater family dysfunction (HR, 3.22; 95% CI, 1.60-6.45) were also significantly associated with risk for SADHD (Table 2).
Table 2. Association of Injury Severity and Demographic and Environmental Factors With Secondary Attention Problemsa.
| Factor | Hazard Ratio (95% CI) |
|---|---|
| Injury groupb | |
| Complicated mild TBI vs OI | 1.67 (0.77-3.58) |
| Moderate TBI vs OI | 1.73 (0.66-4.68) |
| Severe TBI vs OI | 3.62 (1.59-8.26) |
| Age at injury, per 1-y increase in age | 1.17 (0.90-1.53) |
| Sex, male vs female | 1.93 (0.99-3.74) |
| Maternal educational level ≥HS vs <HS | 0.33 (0.17-0.62) |
| FAD-GF score, per 1.0-U increase | 3.22 (1.60-6.45) |
| Site of enrollment | |
| Cincinnati vs Cleveland | 1.17 (0.42-3.22) |
| Cincinnati vs Columbus | 1.28 (0.68-2.50) |
| Cleveland vs Columbus | 1.09 (0.37-3.20) |
Abbreviations: FAD-GF, Family Assessment Device–Global Functioning25; HS, high school diploma; OI, orthopedic injury; TBI, traumatic brain injury.
Data were generated from a Cox proportional hazards regression model.
The OI group was used as the reference group.
Results from the interaction-effects model revealed that male sex (HR, 1.97; 95% CI, 1.03-3.77) and lower levels of maternal education (HR, 0.34; 95% CI, 0.18-0.66) were associated with increased risk for SADHD (Table 3). Although the TBI by family dysfunction interaction was not statistically significant, family dysfunction was associated with increased risk of SADHD within the TBI group (HR, 4.24; 95% CI, 1.91-9.43) and had a minimal, but not significant, association with risk of ADHD in the OI group (HR, 1.32; 95% CI, 0.36-4.91).
Table 3. Role of Injury and Demographic and Environmental Factors in Risk for Developing SADHDa.
| Factor | Hazard Ratio (95% CI) |
|---|---|
| Age at injury, per 1-y increase in age | 1.18 (0.90-1.54) |
| Sex, male vs female | 1.97 (1.03-3.77) |
| Maternal educational level ≥HS vs <HS | 0.34 (0.18-0.66) |
| Site of enrollment | |
| Cincinnati vs Cleveland | 1.20 (0.44-3.36) |
| Cincinnati vs Columbus | 1.09 (0.57-2.10) |
| Cleveland vs Columbus | 0.91 (0.31-2.64) |
| FAD-GF score by injury, per 1.0-U increaseb | |
| OI group | 1.32 (0.36-4.91) |
| TBI group | 4.24 (1.91-9.43) |
Abbreviations: FAD-GF, Family Assessment Device–General Functioning25; HS, high school diploma; OI, orthopedic injury; SADHD, secondary attention-deficit/hyperactivity disorder; TBI, traumatic brain injury.
Data were generated from a Cox proportional hazards regression model.
Two-sided P = .13 for comparison of the TBI group vs the OI group.
Discussion
As expected, injury type and severity were associated with increased risk of SADHD. Specifically, 61.9% of children with severe TBI (13 of 21) developed SADHD, whereas 15.1% of children with OI (16 of 106) developed SADHD by the start of middle school (approximately 7 years after injury). Overall, across the spectrum of TBI severity, the risk for SADHD was increased, with strongest associations with severe TBI. Although the risk of SADHD developing after complicated mild TBI and moderate TBI did not meet the threshold for statistical significance, all TBI severity groups demonstrated almost twice the risk for SADHD compared with children with OI, with risks as much as 4 times higher for the severe TBI group compared with the OI group. Although most children with severe TBI who developed SADHD did so within the first 18 months after injury, a portion of those with complicated mild and moderate TBI demonstrated new onset of SADHD at the final 2 assessments, highlighting the importance of continued monitoring even years after TBI. Maternal educational level and family factors influenced the risk for developing SADHD. Most notably, poor family functioning increased the risk among those with TBI, and minimal association with family dysfunction was noted within the OI sample. Because children with high levels of family dysfunction may be at particular risk for SADHD, follow-up of this subgroup is critical.
This study fills a gap in the literature on development of SADHD after pediatric brain injury by following up children injured in early childhood for a mean of 7 years after injury. The findings confirm previous research documenting an increased risk for SADHD after childhood TBI and support the need for postinjury monitoring to ensure timely identification and management of attention problems. Although children with severe TBI were at highest risk for SADHD compared with children with OI, those with less severe TBI had about twice the risk of developing attention problems throughout development than their peers without brain injury. Taken together, findings suggest that physicians and other clinicians should continue to be vigilant in monitoring attention problems in patients with a history of brain injury, even if it has been a number of years since the injury, the injury was moderate in nature, or the patient experienced a predominantly positive recovery. As observed by Max and colleagues,15 when environmental variables were included in the model along with the interaction of injury group and family functioning, TBI was no longer associated with an increased risk for SADHD compared with OI, although poor family functioning was associated with increased risk for SADHD among the children with TBI. Injury characteristics may increase risks for family dysfunction more immediately after injury, and such dysfunction may either mediate or exacerbate the adverse effects of TBI. In view of these findings, consideration of factors that may interact with injury characteristics, such as family functioning, will be important in planning clinical follow-up. The present findings support the pattern of greater environmental effects after TBI that has been reported in other cohorts28,29,30,31,32,33,34 and highlight the idea that family environment continues to play a role in recovery even years after injury.
Limitations
Results of this study should be considered in the context of its limitations. All reports (ADHD symptoms, history of diagnosis and prescribed medication, and family functioning) were based on parent report and were thus susceptible to reporting bias. In addition, ADHD (primary ADHD and SADHD) was identified based on parent report with no information regarding symptoms or impairment in other settings, such as school. Furthermore, the cutoff for SADHD was determined by CBCL scores in the borderline-clinical range. Future studies examining this topic would benefit from using a structured diagnostic interview and collection of teacher ratings to better document symptoms across settings. Similarly, the use of the CBCL DSM-Oriented Attention-Deficit/Hyperactivity Problems Scale does not allow for the examination of symptoms by domain (eg, hyperactivity, inattention). The use of structured interviews, symptom rating scales, or both would permit a more fine-grained analysis of the association of risk factors across ADHD symptom domains. Although we controlled for new-onset ADHD in children in the OI group after injury, this group had a greater proportion of preinjury ADHD, suggesting that this group may have greater behavioral or attentional difficulties than their noninjured peers. Inclusion of noninjured, typically developing controls would allow for comparison of ADHD development with the general population. Furthermore, this study focused on children hospitalized for more severe TBI; thus, the findings may not have implications for the development of SADHD in children with mild TBI. Future larger studies would benefit from inclusion of a wider range of injury severity and assessment of environmental, genetic, and other injury-related factors to better understand which individuals are at risk for development of attention problems and to advance the development of individualized assessment and management approaches. In addition to understanding the mechanisms that may underlie the development of SADHD, exploring the association of SADHD with functional outcomes (eg, executive functioning, psychosocial adjustment, or family/caregiver stress) will be crucial in understanding the specific needs of this population.
Conclusions
Despite these limitations, findings from this study have important clinical implications. Children with a history of TBI, even those with less severe injuries, have an increased risk for the development of new-onset attention problems even many years after injury. Notably, the treatment of attention problems after TBI in childhood is hindered by a paucity of relevant evidence and inconsistent findings.35 Although treatments were not the focus of this study, a critical need exists to evaluate the effectiveness of interventions for attention problems after pediatric TBI, including medication and cognitive training. A better understanding of how a range of factors predicts the development of attention problems after TBI would inform delivery of interventions in the most timely and effective manner. Findings about the association of family functioning with the development of attention problems after TBI also support previous research highlighting the importance of allocating resources to the injured child’s family throughout recovery.18,27,28,29,31,32,34,36 Assessing family functioning, identifying families at risk, and developing programs to promote healthy family functioning to foster positive outcomes should be integrated into clinical practice when working with patients and families with a history of TBI.
eTable. Baseline Demographic Information for Those Who Completed All Six Study Visits and Those Who Did Not Complete All Six Study Visits
References
- 1.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Max JE, Schachar RJ, Ornstein TJ. Preinjury and secondary attention-deficit/hyperactivity disorder in pediatric traumatic brian injury forensic cases In: Sheman EMS, Brooks BL, eds. Pediatric Forensic Neuropsychology. New York, NY: Oxford University Press; 2012:258-274. [Google Scholar]
- 3.Emery CA, Barlow KM, Brooks BL, et al. A systematic review of psychiatric, psychological, and behavioural outcomes following mild traumatic brain injury in children and adolescents. Can J Psychiatry. 2016;61(5):259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 5.Max JE, Wilde EA, Bigler ED, et al. Psychiatric disorders after pediatric traumatic brain injury: a prospective, longitudinal, controlled study. J Neuropsychiatry Clin Neurosci. 2012;24(4):427-436. [DOI] [PubMed] [Google Scholar]
- 6.Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34-46.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Max JE, Schachar RJ, Levin HS, et al. Predictors of secondary attention-deficit/hyperactivity disorder in children and adolescents 6 to 24 months after traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2005;44(10):1041-1049. [DOI] [PubMed] [Google Scholar]
- 8.Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857-864. [DOI] [PubMed] [Google Scholar]
- 9.Gerring JP, Brady KD, Chen A, et al. Premorbid prevalence of ADHD and development of secondary ADHD after closed head injury. J Am Acad Child Adolesc Psychiatry. 1998;37(6):647-654. [DOI] [PubMed] [Google Scholar]
- 10.Slomine BS, Salorio CF, Grados MA, Vasa RA, Christensen JR, Gerring JP. Differences in attention, executive functioning, and memory in children with and without ADHD after severe traumatic brain injury. J Int Neuropsychol Soc. 2005;11(5):645-653. [DOI] [PubMed] [Google Scholar]
- 11.Yeates KO, Armstrong K, Janusz J, et al. Long-term attention problems in children with traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2005;44(6):574-584. [DOI] [PubMed] [Google Scholar]
- 12.Karver CL, Wade SL, Cassedy A, et al. Age at injury and long-term behavior problems after traumatic brain injury in young children. Rehabil Psychol. 2012;57(3):256-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Max JE, Lansing AE, Koele SL, et al. Attention deficit hyperactivity disorder in children and adolescents following traumatic brain injury. Dev Neuropsychol. 2004;25(1-2):159-177. [DOI] [PubMed] [Google Scholar]
- 14.Yang LY, Huang CC, Chiu WT, Huang LT, Lo WC, Wang JY. Association of traumatic brain injury in childhood and attention-deficit/hyperactivity disorder: a population-based study. Pediatr Res. 2016;80(3):356-362. [DOI] [PubMed] [Google Scholar]
- 15.Max JE, Arndt S, Castillo CS, et al. Attention-deficit hyperactivity symptomatology after traumatic brain injury: a prospective study. J Am Acad Child Adolesc Psychiatry. 1998;37(8):841-847. [DOI] [PubMed] [Google Scholar]
- 16.Max JE, Schachar RJ, Levin HS, et al. Predictors of attention-deficit/hyperactivity disorder within 6 months after pediatric traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2005;44(10):1032-1040. [DOI] [PubMed] [Google Scholar]
- 17.Wassenberg R, Max JE, Lindgren SD, Schatz A. Sustained attention in children and adolescents after traumatic brain injury: relation to severity of injury, adaptive functioning, ADHD and social background. Brain Inj. 2004;18(8):751-764. [DOI] [PubMed] [Google Scholar]
- 18.Anderson V, Godfrey C, Rosenfeld JV, Catroppa C. Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics. 2012;129(2):e254-e261. [DOI] [PubMed] [Google Scholar]
- 19.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47(3-4):296-312. [DOI] [PubMed] [Google Scholar]
- 20.Ewing-Cobbs L, Barnes M, Fletcher JM, Levin HS, Swank PR, Song J. Modeling of longitudinal academic achievement scores after pediatric traumatic brain injury. Dev Neuropsychol. 2004;25(1-2):107-133. [DOI] [PubMed] [Google Scholar]
- 21.DiScala C, Lescohier I, Barthel M, Li G. Injuries to children with attention deficit hyperactivity disorder. Pediatrics. 1998;102(6):1415-1421. [DOI] [PubMed] [Google Scholar]
- 22.Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms and Profiles. Burlington: Research Center for Children Youth and Families, University of Vermont; 2001. [Google Scholar]
- 23.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529-1540. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz L, Taylor HG, Drotar D, Yeates KO, Wade SL, Stancin T. Long-term behavior problems following pediatric traumatic brain injury: prevalence, predictors, and correlates. J Pediatr Psychol. 2003;28(4):251-263. [DOI] [PubMed] [Google Scholar]
- 25.Epstein NB, Baldwin LM, Bishop DS. The McMaster Family Assessment Device. J Marital Fam Ther. 1983;9(2):171-180. doi: 10.1111/j.1752-0606.1983.tb01497.x [DOI] [Google Scholar]
- 26.Wade SL, Taylor HG, Drotar D, Stancin T, Yeates KO. Family burden and adaptation during the initial year after traumatic brain injury in children. Pediatrics. 1998;102(1, pt 1):110-116. [DOI] [PubMed] [Google Scholar]
- 27.Wade SL, Cassedy A, Walz NC, Taylor HG, Stancin T, Yeates KO. The relationship of parental warm responsiveness and negativity to emerging behavior problems following traumatic brain injury in young children. Dev Psychol. 2011;47(1):119-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeates KO, Taylor HG, Walz NC, Stancin T, Wade SL. The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology. 2010;24(3):345-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurowski BG, Taylor HG, Yeates KO, Walz NC, Stancin T, Wade SL. Caregiver ratings of long-term executive dysfunction and attention problems after early childhood traumatic brain injury: family functioning is important. PM R. 2011;3(9):836-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wade SL, Zhang N, Yeates KO, Stancin T, Taylor HG. Social environmental moderators of long-term functional outcomes of early childhood brain injury. JAMA Pediatr. 2016;170(4):343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Burant C. Bidirectional child-family influences on outcomes of traumatic brain injury in children. J Int Neuropsychol Soc. 2001;7(6):755-767. [DOI] [PubMed] [Google Scholar]
- 32.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology. 2002;16(1):15-27. [DOI] [PubMed] [Google Scholar]
- 33.Yeates KO, Taylor HG, Drotar D, et al. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. J Int Neuropsychol Soc. 1997;3(6):617-630. [PubMed] [Google Scholar]
- 34.Wade SL, Taylor HG, Drotar D, Stancin T, Yeates KO, Minich NM. Parent-adolescent interactions after traumatic brain injury: their relationship to family adaptation and adolescent adjustment. J Head Trauma Rehabil. 2003;18(2):164-176. [DOI] [PubMed] [Google Scholar]
- 35.Backeljauw B, Kurowski BG. Interventions for attention problems after pediatric traumatic brain injury: what is the evidence? PM R. 2014;6(9):814-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman LA, Wade SL, Walz NC, Taylor HG, Stancin T, Yeates KO. Clinically significant behavior problems during the initial 18 months following early childhood traumatic brain injury. Rehabil Psychol. 2010;55(1):48-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Baseline Demographic Information for Those Who Completed All Six Study Visits and Those Who Did Not Complete All Six Study Visits