Key Points
Question
Do patients who develop postoperative atrial fibrillation after coronary artery bypass graft surgery carry a similar long-term risk of thromboembolism as patients with nonsurgical, nonvalvular atrial fibrillation?
Findings
In this cohort study that compared 2108 patients with postoperative atrial fibrillation with 8432 matched patients with nonvalvular atrial fibrillation, postoperative atrial fibrillation was associated with a significantly lower risk of thromboembolism.
Meaning
These data appear not to support the notion that new-onset postoperative atrial fibrillation should be regarded as equivalent to primary nonvalvular atrial fibrillation in terms of long-term thromboembolic risk.
This cohort study examines long-term risk of thromboembolism in patients with new-onset postoperative atrial fibrillation after first-time isolated coronary artery bypass graft surgery compared with patients with nonvalvular atrial fibrillation.
Abstract
Importance
New-onset postoperative atrial fibrillation (POAF) is a common complication of coronary artery bypass graft (CABG) surgery. However, the long-term risk of thromboembolism in patients who develop POAF after CABG surgery remains unknown. In addition, information on stroke prophylaxis in this setting is lacking.
Objective
To examine stroke prophylaxis and the long-term risk of thromboembolism in patients with new-onset POAF after first-time isolated CABG surgery compared with patients with nonsurgical, nonvalvular atrial fibrillation (NVAF).
Design, Setting, and Participants
This cohort study used data from a clinical cardiac surgery database and Danish nationwide registries to identify patients undergoing first-time isolated CABG surgery who developed new-onset POAF from January 1, 2000, through June 30, 2015. These patients were matched by age, sex, CHA2DS2-VASc score, and year of diagnosis to patients with nonsurgical NVAF in a 1 to 4 ratio. Data analysis was completed from February 2017 to January 2018.
Main Outcomes and Measures
The proportion of patients initiating oral anticoagulation therapy within 30 days and the rates of thromboembolism.
Results
A total of 2108 patients who developed POAF after CABG surgery were matched with 8432 patients with NVAF. In the full population of 10 540 patients, the median (interquartile range) age was 69.2 (63.7-74.7) years; 8675 patients (82.3%) were men. Oral anticoagulation therapy was initiated within 30 days postdischarge in 175 patients with POAF (8.4%) and 3549 patients with NVAF (42.9%). The risk of thromboembolism was lower in the POAF group than in the NVAF group (18.3 vs 29.7 events per 1000 person-years; adjusted hazard ratio [HR], 0.67; 95% CI, 0.55-0.81; P < .001). Anticoagulation therapy during follow-up was associated with a lower risk of thromboembolic events in both patients with POAF (adjusted HR, 0.55; 95% CI, 0.32-0.95; P = .03) and NVAF (adjusted HR, 0.59; 95% CI, 0.51-0.68; P < .001) compared with patients who did not receive any anticoagulation therapy. Further, the risk of thromboembolism was not significantly higher in patients with POAF compared with those who did not develop POAF after CABG surgery (adjusted HR, 1.11; 95% CI, 0.94-1.32; P < .24).
Conclusions and Relevance
New-onset POAF in patients who had undergone CABG surgery was associated with a lower long-term thromboembolic risk than that of patients who had NVAF. These data do not support the notion that new-onset POAF should be regarded as equivalent to primary NVAF in terms of long-term thromboembolic risk.
Introduction
New-onset postoperative atrial fibrillation (POAF) is a frequent complication of coronary artery bypass graft (CABG) surgery, with a reported incidence between 11% and 40%.1,2,3,4 Although new-onset POAF has traditionally been thought to be transient and benign, there is mounting evidence that these patients face a greater risk of perioperative complications, including stroke, prolonged hospital stay, in-hospital mortality, and long-term mortality compared with patients who remain in sinus rhythm post-CABG surgery.5,6,7,8,9,10,11
Further, it is well-known that nonvalvular atrial fibrillation (NVAF) is associated with an increased risk of stroke and systemic embolism,12,13 and that oral anticoagulation (OAC) therapy, whether with vitamin K or non–vitamin K antagonist OACs, considerably reduces this risk.14,15 However, data on stroke prophylaxis in new-onset POAF after CABG are lacking, and international guidelines on the management of patients with atrial fibrillation (AF) do not provide clear recommendations on this issue.16,17 More importantly, longitudinal data on the long-term risk of thromboembolic events in these patients are sparse and conflicting.18,19,20,21,22 To address these gaps in knowledge, we conducted a retrospective cohort study in Denmark to examine stroke prophylaxis and the long-term risk of thromboembolism in patients with new-onset POAF after first-time isolated CABG surgery compared with a matched cohort of patients with NVAF.
Methods
Data Sources
All residents in Denmark are assigned a unique and permanent civil registration number that allows accurate linkage of nationwide administrative and clinical registries at an individual level over time. We used data from a prospectively collected cardiac surgery database composed of detailed clinical and procedural information on all patients undergoing cardiac surgery at the Copenhagen University Hospital, Rigshospitalet, from January 1, 2000, to June 30, 2015. These data were linked to several nationwide administrative registries. The Danish National Patient Registry contains information on all hospital admissions and outpatient contacts, coded according to the International Classification of Diseases, Eighth Revision [ICD-8] and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10], as well as all surgical procedures, coded according to the Nordic Medico-Statistical Committee Classification of Surgical Procedures23; the Danish National Prescription Registry holds detailed information on dispensing date, strength, and quantity on all filled drug prescriptions in Denmark24; and the Danish Civil Registration System contains information on birth date, sex, and vital status (a dataset recording whether an individual is alive and residing in Denmark; has emigrated from Denmark; has unknown residential status, a condition termed disappeared; or has died, as well as dates on which such status began).25
Ethical Approval
The Danish Data Protection Agency approved this study. In Denmark, register-based studies in which individuals cannot be identified do not require ethical approval or informed consent procedures, and therefore none were done.
Study Population
We identified all Danish residents above 18 years of age undergoing first-time isolated CABG surgery (ie, no concomitant valve or other cardiac surgical procedure) at the Copenhagen University Hospital, Rigshospitalet, between January 1, 2000, and June 30, 2015. Patients were referred from East Denmark, which has a population of 2.6 million people. Patients were included in the study if they met all of the inclusion criteria: (1) they had no history of AF (ie, those who did not have a primary or secondary hospital discharge diagnosis of AF at any time prior to hospitalization for CABG surgery) but developed POAF during hospitalization (defined as an AF rhythm requiring either medical therapy or cardioversion); (2) they had no prescription for OAC medications in the 6 months prior to the CABG surgery; (3) they had no history of deep venous thrombosis or pulmonary embolism, and (4) they remained alive at discharge. In addition, we identified a nationwide cohort of patients diagnosed as having nonsurgical NVAF during hospitalization or in an outpatient clinic who were not prescribed OAC medications for at least 6 months prior to diagnosis, had not undergone any cardiac surgery prior to diagnosis, and did not have a history of deep venous thrombosis or pulmonary embolism. Patients with POAF were matched by age (up to 1 year difference in age), sex, CHA2DS2-VASc-score, and year of index date to patients with NVAF in a 1 to 4 ratio using risk-set matching. The index date was defined as the discharge date for patients with POAF or NVAF who were diagnosed with AF during hospitalization. For patients with NVAF who were diagnosed in an outpatient clinic, the index date was defined as the date of diagnosis.
Comorbidity and Pharmacotherapy at Baseline
Comorbidity was obtained using hospital discharge diagnoses any time prior to baseline, which was defined as the date of hospitalization for CABG in patients with POAF or the date of first diagnosis for AF in patients with NVAF (eTable 1 in the Supplement for ICD-8 and ICD-10 codes). Patients with diabetes and hypertension were identified using filled drug prescriptions, as has been done previously.26,27 Alcohol abuse was defined from associated prescription fulfillment and ICD-10 diagnosis codes. Pharmacotherapy at baseline was defined as filled prescriptions within 180 days prior to baseline (eTable 2 in the Supplement presents a list of the relevant Anatomical Therapeutic Chemical Classification System codes). The estimated risk of stroke (CHADS2 and CHA2DS2-VASc-scores) and bleeding (HAS-BLED score) was calculated as described previously.28,29
Postdischarge Anticoagulation Therapy
Oral anticoagulation therapy (whether a vitamin K or a non–vitamin K antagonist) was assessed continuously for each individual during the follow-up period using filled prescriptions, taking administered dosages and packing size into account, as described previously.30,31 Exposure to OAC therapy was defined as the point at which patients had medication available, and discontinuation was defined as the point at which patients had had no more medication available for at least 30 days. Thus, patients were allowed to change exposure status during the follow-up period per their filled OAC prescriptions.
Outcomes
The primary outcome was thromboembolism (a composite of ischemic stroke, transient cerebral ischemia, and thrombosis or embolism in peripheral arteries). Secondary outcomes were all-cause mortality and recurrent AF hospitalization defined as a new hospital admission with AF as the primary diagnosis. (The diagnoses of AF and ischemic stroke in the Danish National Patient Registry have been validated with a positive predictive value of 92.6% and 97%, respectively.32,33) Patients were followed from the index date until occurrence of the outcome of interest, emigration out of Denmark, the passage of 10 years after index date, or the end of the study (December 31, 2015), whichever came first.
Statistical Analysis
Descriptive data were reported as frequencies and percentages or median with interquartile range (IQR), as appropriate. Differences in baseline characteristics were tested by applying the χ2 test for categorical variables and the Mann-Whitney test for continuous variables. Crude incidence rates were calculated as number of events per 1000 person-years. Survival curves according to groups were constructed by the Kaplan-Meier method, and differences between groups were assessed using the log-rank test. The cumulative incidence of thromboembolism and recurrent hospitalization for AF per group was estimated using the Aalen-Johansen estimator, while taking into account the competing risk of death. Differences between groups were assessed using Gray test. Cox proportional hazard regression models conditional on matching (ie, comparing cases with their matched controls) were used to estimate hazard ratios (HRs) with 95% CIs, adjusted for comorbidities (Table), concomitant pharmacotherapy (Table), and OAC therapy as a time-dependent covariate. Patients with NVAF served as the reference in all models. The proportional hazards assumption was tested and found to be valid. Clinical relevant interactions, including age, sex, several comorbidities, and OAC treatment during the follow-up period, were tested for and found insignificant, unless otherwise stated. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Inc). A 2-sided P value of less than .05 was considered statistically significant. Data analysis occurred from February 2017 to January 2018.
Table. Baseline Characteristics of Study Population.
| Characteristic | No. (%) | ||
|---|---|---|---|
| NVAF (n = 8432) |
POAF (n = 2108) |
P Value | |
| Demographics | |||
| Age, median (interquartile range), y | 69.2 (63.7-74.7) | 69.2 (63.7-74.7) | NA |
| Men | 6940 (82.3) | 1735 (82.3) | NA |
| Comorbidities | |||
| Ischemic heart disease | 3478 (41.3) | 2108 (100) | <.001 |
| Heart failure | 1459 (17.3) | 351 (16.7) | .48 |
| Thromboembolism | 2204 (26.1) | 214 (10.2) | <.001 |
| Peripheral vascular disease | 668 (7.9) | 129 (6.1) | .005 |
| Hypertension | 5258 (62.4) | 1056 (50.1) | <.001 |
| Coagulopathy | 43 (0.5) | 8 (0.4) | .44 |
| Bleeding | 1089 (12.9) | 214 (10.2) | <.001 |
| Diabetes | 1913 (22.7) | 357 (16.9) | <.001 |
| Malignancy | 1210 (14.4) | 192 (9.1) | <.001 |
| Chronic renal disease | 571 (6.8) | 90 (4.3) | <.001 |
| Chronic obstructive pulmonary disease | 1106 (13.1) | 129 (6.1) | <.001 |
| Liver disease | 269 (3.2) | 31 (1.5) | <.001 |
| Alcohol abuse | 653 (7.7) | 71 (3.4) | <.001 |
| Concomitant medical treatment | |||
| Statins | 3232 (38.3) | 1316 (62.4) | <.001 |
| Aspirin | 4192 (49.7) | 1290 (61.2) | <.001 |
| Adenosine diphosphate-receptor inhibitors | 681 (8.1) | 227 (10.8) | <.001 |
| Dipyridamole | 581 (6.9) | 60 (2.9) | <.001 |
| Nonsteroidal anti-inflammatory drugs | 1779 (21.1) | 367 (17.4) | <.001 |
| Risk scores, mean (SD) | |||
| CHA2DS2-VASca | 3.1 (1.4) | 3.1 (1.4) | NA |
| CHADS2b | 1.8 (1.1) | 1.3 (1.2) | <.001 |
| HAS-BLEDc | 2.5 (1.1) | 2.2 (1.1) | <.001 |
Abbreviations: NA, not applicable; NVAF, nonvalvular atrial fibrillation; POAF, postoperative atrial fibrillation.
The CHA2DS2-VASc score is composed of points for congestive heart failure; hypertension; age of 75 years or older (2 points); diabetes; history of stroke, transient ischemic attack, or systemic thromboembolism (2 points); vascular disease; age of 65 to 74 years, and female sex.
The CHADS2 score is composed of points for congestive heart failure; hypertension; age of 75 years or older; diabetes; history of stroke, transient ischemic attack, or systemic thromboembolism (2 points).
The HAS-BLED score is composed of points for hypertension; abnormal renal or liver function; history of stroke; history of bleeding; labile international normalized ratio; age older than 65 years; and drug consumption of antiplatelet agents, nonsteroidal anti-inflammatory drugs, or alcohol abuse. In this study, international normalized ratio scores were left out due to missing data.
Results
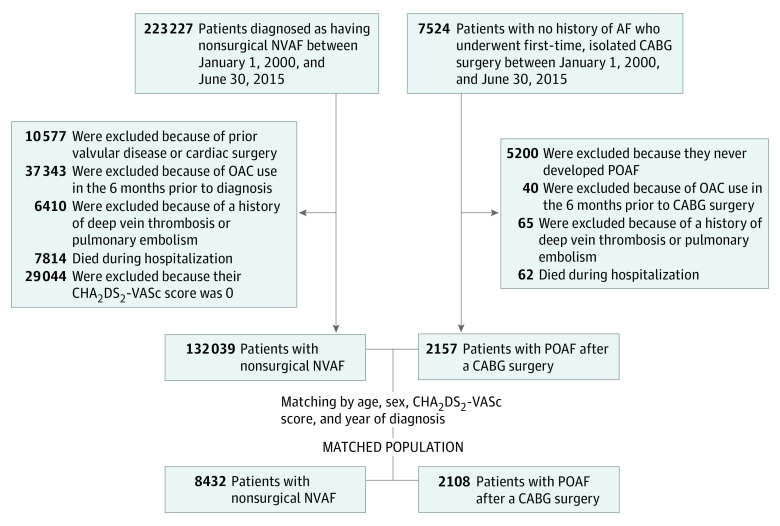
A flowchart of the study population selection process is presented in Figure 1. From January 1, 2000, to June 30, 2015, 7524 patients with no history of AF underwent isolated CABG surgery; of these, 2324 patients (30.9%) developed POAF during hospitalization. After exclusion criteria were applied, 2108 patients with POAF were matched with 8432 patients with NVAF. Baseline characteristics per group are summarized in the Table. The median age of the study population was 69.2 years (IQR, 63.7-74.7 years) and 8674 (82.3%) were men. The mean (SD) CHA2DS2-VASc34 score in the population was 3.1 (1.4) points on a scale of 0 to 9. The NVAF group was characterized by greater prevalence of cardiovascular and noncardiovascular comorbidities compared with the POAF group.
Figure 1. Flowchart of the Study Population Selection Process.
AF indicates atrial fibrillation; CABG, coronary artery bypass grafting; CHA2DS2-VASc, details in De Jong34; DVT, deep vein thrombosis; NVAF, nonvalvular atrial fibrillation; OAC, oral anticoagulation; PE, pulmonary embolism; POAF, postoperative atrial fibrillation.
Postdischarge Anticoagulation Therapy
In the POAF group, 175 patients (8.4%) initiated OAC therapy (of whom 146 patients [83.4%] received warfarin) within 30 days after the index date. The proportion of patients with POAF who received antiplatelet therapy within 30 days after discharge was 138 (78.9%) and 1527 (79.7%) of those receiving and not receiving concomitant OAC therapy, respectively. Correspondingly, 3549 patients (42.9%) initiated OAC therapy (of whom 2771 [78.4%] received warfarin) in the NVAF group within 30 days after the index date. The proportion of patients receiving OAC therapy within 30 days after the index date per CHA2DS2-VASc score and year of surgery or diagnosis in the POAF and NVAF group are displayed in eTable 3 and eFigure 1 in the Supplement; during the study period, the proportion of patients initiating OAC therapy within 30 days of the index date increased in the NVAF group, but not in the POAF group, with a steep increase after 2010.
Among patients with POAF who initiated OAC therapy within 30 days after the index date and were alive (n = 174), 172 (98.9%) were observed to be continuing this treatment at 1 month, 96 (55.8%) at 3 months, 66 (39.1%) at 6 months, and 39 (24.8%) at 1 year.
Thromboembolism
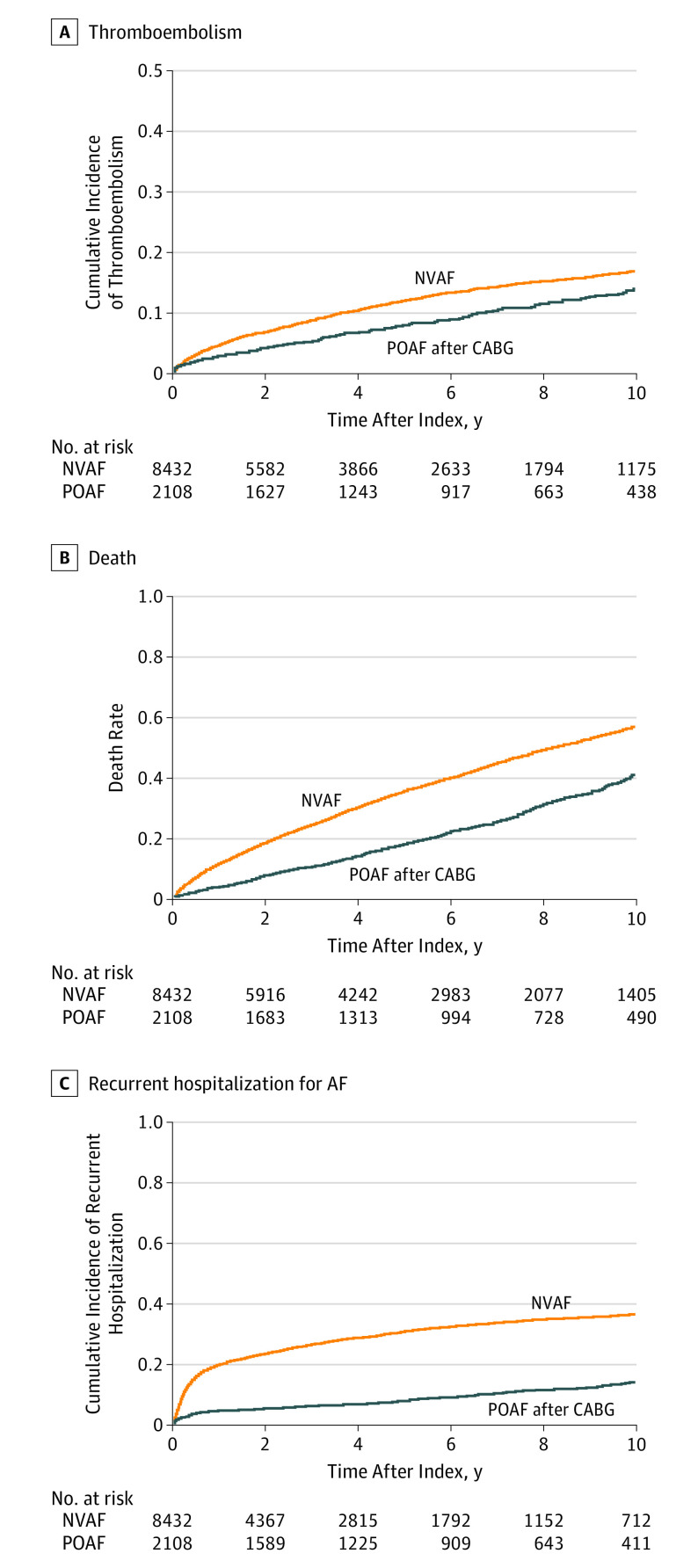
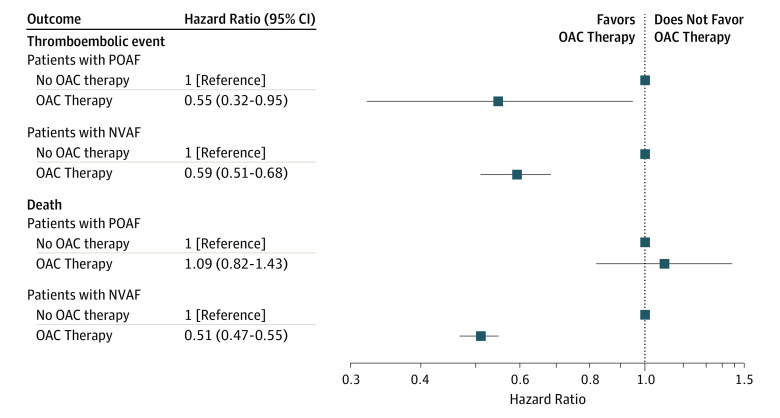
The median follow-up time from the index date until occurrence of a thromboembolic event, death, emigration, or end of the study period was 5.1 years (IQR, 2.2-9.2 years) for patients with POAF and 3.5 years (IQR, 1.4-7.1 years) for patients with NVAF. The crude incidence rates of thromboembolism were 18.3 (95% CI, 16.0-20.9) events per 1000 person-years for patients with POAF and 29.7 (95% CI, 28.0-31.5) events per 1000 person-years for patients with NVAF. The cumulative incidences of thromboembolism according to groups are illustrated in Figure 2A. In multivariable Cox proportional hazard analysis, POAF was associated with a significantly lower risk of thromboembolism compared with NVAF (adjusted HR, 0.67; 95% CI, 0.55-0.81; P < .001). No interaction between OAC treatment and groups was found during the follow-up period (P = .81 for interaction). The use of OAC therapy during the follow-up period was associated with a significantly lower risk of thromboembolic events in both patients with POAF (adjusted HR, 0.55; 95% CI, 0.32-0.95; P = .03) and NVAF (adjusted HR, 0.59; 95% CI, 0.51-0.68; P < .001) compared with patients who had no OAC therapy (Figure 3).
Figure 2. Long-term Outcomes in Patients Who Develop POAF After CABG Surgery and Patients With NVAF.
A, Cumulative incidence of thromboembolism, a composite of ischemic stroke, transient cerebral ischemia, and thrombosis or embolism in peripheral arteries (P < .001 by Grays test). B, Kaplan-Meier curves for all-cause mortality (P < .001 by log-rank test). C, Cumulative incidence of recurrent hospitalization for AF (P < .001 by the Gray test). AF indicates atrial fibrillation; CABG, coronary artery bypass graft; NVAF, nonvalvular atrial fibrillation; POAF, postoperative atrial fibrillation.
Figure 3. Adjusted Hazard Ratios of Thromboembolism and All-Cause Mortality in Patients Developing POAF After CABG Surgery and Patients With NVAF During Follow-up per OAC Therapy.
CABG indicates coronary artery bypass graft; NVAF, nonvalvular atrial fibrillation; OAC, oral anticoagulation; POAF, postoperative atrial fibrillation.
All-Cause Mortality and Recurrent Hospitalization for Atrial Fibrillation
The crude incidence rates of all-cause mortality were 46.9 (95% CI, 43.2-50.9) events per 1000 person-years for patients with POAF and 88.0 (95% CI, 85.1-90.9) events per 1000 person-years for patients with NVAF. Figure 2B depicts Kaplan-Meier curves for death in patients with POAF and NVAF. In multivariable Cox proportional hazard analysis, POAF was associated with a significantly lower risk of all-cause mortality compared with NVAF (adjusted HR, 0.55; 95% CI, 0.49-0.61; P < .001). The use of OAC therapy during the follow-up period was associated with a significantly lower risk of all-cause mortality in patients with NVAF (adjusted HR, 0.51; 95% CI, 0.47-0.55; P < .001) but not in patients with POAF (adjusted HR, 1.09; 95% CI, 0.82-1.43; P = .56) compared with patients who had no OAC therapy (Figure 3).
The crude incidence rates of recurrent hospitalization for AF were 19.9 (95% CI, 17.5-22.7) events per 1000 person-years for patients with POAF and 96.3 (95% CI, 92.7-100.0) events per 1000 person-years for patients with NVAF. The cumulative incidences of recurrent hospitalization for AF in patients with POAF and NVAF are illustrated in Figure 2C. In multivariable Cox proportional hazard analysis, POAF was associated with a significantly lower risk of recurrent hospitalization for AF than NVAF (adjusted HR, 0.29; 95% CI, 0.25-0.34; P < .001).
Sensitivity Analysis
To test the robustness of our findings, we compared the risk of hospitalization for orthopedic fractures in patients with POAF and NVAF. This falsification end point analysis was performed to evaluate the presence of potential unmeasured confounding in the adjusted analyses. The risk of orthopedic fractures was similar among groups (adjusted HR, 0.83; 95% CI, 0.63-1.08; P = .16). Further, we excluded patients with a history of thromboembolism (after matching, 7372 patients with NVAF and 1843 patients with POAF were included in this analysis), and we found a similar association as the main analysis for the primary outcome of thromboembolism (adjusted HR, 0.71; 95% CI, 0.57-0.89; P = .003). We also excluded patients with NVAF who had been diagnosed in an outpatient clinic; after matching, 8268 patients with NVAF and 2067 patients with POAF were included in this analysis. Restricting the analysis to patients diagnosed as having NVAF during hospitalization yielded similar results as the main analysis for the primary outcome of thromboembolism (adjusted HR, 0.62; 95% CI, 0.51-0.76; P < .001). In addition, we restricted the primary outcome of thromboembolism to ischemic stroke and found a similar association as the main analysis (adjusted HR, 0.73; 95% CI, 0.58-0.90; P = .004). Further, we matched patients with POAF by the components of the CHA2DS2-VASc-score (ie, heart failure, hypertension, age, diabetes, a history of thromboembolism, vascular disease, and sex) and year of diagnosis to patients with NVAF in a 1:2 ratio (n = 1904 and n = 3808, respectively). Baseline characteristics per groups and outcomes are displayed in eTable 4 and eFigure 2 in the Supplement, respectively. This analysis yielded similar results as the main analysis in terms of outcomes (ie, thromboembolism, all-cause mortality, and recurrent hospitalization for AF).
Finally, we compared the risk of outcomes in patients developing and not developing POAF after isolated CABG surgery (n = 2157 and n = 4964, respectively). Baseline characteristics per groups are displayed in eTable 5 in the Supplement. Patients with POAF after CABG surgery had a similar associated risk of thromboembolism (adjusted HR, 1.11; 95% CI, 0.94-1.32; P = .24) but a higher associated risk of all-cause mortality (adjusted HR, 1.32; 95% CI 1.18-1.47; P < .001) and rehospitalization for AF (adjusted HR, 2.27; 95% CI, 1.84-2.80; P < .001) compared with those who did not develop POAF after CABG surgery.
Discussion
This study examined the long-term risk of thromboembolism in patients with new-onset POAF after first-time isolated CABG surgery compared with a matched cohort of patients with NVAF and yielded several major findings. First, among patients with no history of AF who underwent CABG surgery, 30.9% developed POAF during hospitalization, of whom fewer than 1 in 10 (8.4%) received OAC therapy within 30 days after discharge. Second, POAF was associated with a significantly lower risk of thromboembolism compared with NVAF. Third, POAF was associated with a significantly lower risk of all-cause mortality and recurrent AF hospitalization than NVAF.
Although the association between new-onset POAF after cardiac surgery and short-term risk of stroke is well-established,6,7,8 few studies have evaluated the long-term risk of thromboembolic events in this setting. In a study using administrative data, Gialdini et al18 found that AF in conjunction with cardiac surgery was associated with an increased long-term risk of ischemic stroke. However, this study, as well as other studies18,19,21,22 examining the association between POAF and long-term stroke risk, did not account for postdischarge anticoagulation therapy because of the lack of data on medication use. To our knowledge, our study is the first to evaluate the long-term risk of thromboembolism in POAF compared with NVAF with data on postdischarge anticoagulation therapy. We found that new-onset POAF after CABG surgery was associated with a lower long-term risk of thromboembolic events compared with that of primary NVAF, despite a similar predicted risk of thromboembolism. The association remained true after adjusting for comorbidities, concomitant pharmacotherapy, and postdischarge anticoagulation medication. Thus, our data do not support the notion that new-onset POAF should be regarded as similar to primary NVAF in terms of long-term thromboembolic risk.
The lack of evidence for OAC therapy in patients who develop POAF after cardiac surgery is reflected in the most recent guidelines for the management of patients with AF. The 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines state that “it is reasonable to administer antithrombotic medication in patients who develop postoperative AF, as advised for nonsurgical patients,”16(p2089) and the 2016 European Society of Cardiology guidelines17 provide a similar recommendation on this issue. Thus, guidelines are unclear on whether POAF should be considered as NVAF in regards to prophylaxis for subsequent stroke and thromboembolism. Although it may seem reasonable to initiate OAC therapy if the risk of thromboembolism (as assessed by the CHADS228,29 or CHA2DS2-VASc score34) outweighs the risk of bleeding (as assessed by the HAS-BLED score34) in this setting, it is important to keep in mind that this recommendation is based on low-quality evidence and also that these risk stratification scores have not been validated in surgical patients. In our study, only 175 patients (8.4%) developing POAF were prescribed an anticoagulant within 30 days after discharge for CABG surgery, despite a high predicted stroke risk and a moderate predicted bleeding risk. Other studies evaluating OAC use in this setting have reported similar low rates of warfarin use at discharge (14%-24%).7,9,20,35 In this study, we found that OAC therapy was associated with a similar lower risk of thromboembolic events in the group with POAF and the group with NVAF, suggesting a similar effectiveness of anticoagulation for the prevention of stroke. Given that POAF is 1 of the most common complications of cardiac surgery, more studies specifically addressing the role of OAC therapy in patients with POAF are warranted to examine the efficacy, safety, timing, and duration of OAC therapy in this setting, preferably in a randomized clinical trial.
Although the primary outcome in this study was thromboembolism, mortality is of great importance. We found that POAF was associated with a lower risk of all-cause mortality than NVAF. This may be explained by a selection of patients chosen for CABG surgery and by the higher cardiovascular and noncardiovascular disease burden in the the NVAF cohort.
The impact of POAF on the development of late arrhythmia has been sparsely studied. In a retrospective study comprising 571 patients undergoing CABG surgery, Ahlsson et al22 found that 25% of patients who developed POAF were diagnosed with paroxysmal or persistent AF after discharge during a 5-year follow-up period compared with 3% of patients without POAF. In another study36 including 305 patients who had undergone CABG surgery, symptomatic episodes of AF requiring medical care were more common among patients developing POAF than those not developing POAF during a median follow-up of 2 years. Although these data suggest that POAF is associated with an increased risk of recurrent AF, to our knowledge, no study has investigated whether patients with new-onset POAF have a risk of recurrent AF similar to primary NVAF. We found that POAF after CABG surgery, compared with NVAF, was associated with a significantly lower risk of recurrent AF hospitalization for AF (as defined by a new hospital admission with AF as the primary diagnosis). This finding supports the notion that POAF is not to be regarded as equivalent to primary NVAF in terms of risk of hospitalization with AF.
Strengths
The main strength of this study is the completeness of data in a cohort of patients undergoing first-time isolated CABG surgery and a nationwide unselected cohort of patients with NVAF who were followed in a real-world setting. The Danish health care system, funded by taxes, provides free and equal access to health care for all residents regardless of socioeconomic or insurance status. In Denmark, all OAC therapies can be purchased only through prescription. Because of a system of partial reimbursement of drug expenses by the Danish health care system, pharmacies are required to register all fulfilled prescriptions, which ensures complete and accurate registration.
Limitations
The findings of this study should be viewed in the context of a number of limitations. The main limitations of this study are inherent to its observational design. Despite adjustment for potential confounders and a similar associated risk of the falsification end point among groups, the possibility of residual confounding in our analyses cannot be excluded. Further, no causal inference can be made from the analysis on the effectiveness of OAC therapy for the prevention of thromboembolism in the 2 cohorts, although these data suggest that OAC therapy is associated with a similar lower thromboembolic risk among groups. In this study, POAF was defined as a rhythm requiring either medical therapy or cardioversion, and thus short episodes of POAF might have been missed. Similarly, we were unable to determine the duration of POAF and did not have any information on discharge rhythm. In addition, data on important clinical parameters such as plasma creatinine levels, international normalized ratio of prothrombin time, body mass index (calculated as weight in kilograms divided by height in meters squared), and smoking habits, as well as electrocardiograms, were unavailable. Finally, we did not have data on the type of AF in patients with NVAF.
Conclusions
Developing new-onset POAF after CABG surgery was associated with a lower long-term thromboembolic risk compared with NVAF. Further, patients with POAF did not have significantly higher associated risk of thromboembolism compared with those who did not develop POAF after CABG surgery. These data do not support the notion that POAF should be regarded as equivalent to primary NVAF in terms of long-term thromboembolic risk.
eTable 1 ICD-8 and ICD-10 codes
eTable 2 ATC codes
eTable 3 OAC initiation within 30 days after the index date according to CHA2DS2-VASc in patients developing POAF following CABG and NVAF
eTable 4 Baseline characteristics of patients developing POAF following CABG and NVAF
eTable 5 Baseline characteristics of patients developing and not developing POAF following CABG
eFigure 1 OAC initiation within 30 days after the index date according to year of surgery or diagnosis in patients developing POAF following CABG and NVAF
eFigure 2 Long-term outcomes in patients developing POAF following CABG and NVAF
References
- 1.Walkey AJ, Benjamin EJ, Lubitz SA. New-onset atrial fibrillation during hospitalization. J Am Coll Cardiol. 2014;64(22):2432-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathew JP, Fontes ML, Tudor IC, et al. ; Investigators of the Ischemia Research and Education Foundation; Multicenter Study of Perioperative Ischemia Research Group . A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291(14):1720-1729. [DOI] [PubMed] [Google Scholar]
- 3.Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135(12):1061-1073. [DOI] [PubMed] [Google Scholar]
- 4.Ommen SR, Odell JA, Stanton MS. Atrial arrhythmias after cardiothoracic surgery. N Engl J Med. 1997;336(20):1429-1434. [DOI] [PubMed] [Google Scholar]
- 5.Kaw R, Hernandez AV, Masood I, Gillinov AM, Saliba W, Blackstone EH. Short- and long-term mortality associated with new-onset atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2011;141(5):1305-1312. [DOI] [PubMed] [Google Scholar]
- 6.Saxena A, Dinh DT, Smith JA, Shardey GC, Reid CM, Newcomb AE. Usefulness of postoperative atrial fibrillation as an independent predictor for worse early and late outcomes after isolated coronary artery bypass grafting (multicenter Australian study of 19,497 patients). Am J Cardiol. 2012;109(2):219-225. [DOI] [PubMed] [Google Scholar]
- 7.Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43(5):742-748. [DOI] [PubMed] [Google Scholar]
- 8.Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226(4):501-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Chami MF, Kilgo P, Thourani V, et al. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55(13):1370-1376. [DOI] [PubMed] [Google Scholar]
- 10.Phan K, Ha HS, Phan S, Medi C, Thomas SP, Yan TD. New-onset atrial fibrillation following coronary bypass surgery predicts long-term mortality: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2015;48(6):817-824. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg BA, Zhao Y, He X, et al. Management of postoperative atrial fibrillation and subsequent outcomes in contemporary patients undergoing cardiac surgery: insights from the Society of Thoracic Surgeons CAPS-Care Atrial Fibrillation Registry. Clin Cardiol. 2014;37(1):7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313(19):1950-1962. [DOI] [PubMed] [Google Scholar]
- 13.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983-988. [DOI] [PubMed] [Google Scholar]
- 14.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857-867. [DOI] [PubMed] [Google Scholar]
- 15.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. [DOI] [PubMed] [Google Scholar]
- 16.January CT, Wann LS, Alpert JS, et al. ; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071-2104. [DOI] [PubMed] [Google Scholar]
- 17.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50(5):e1-e88. [DOI] [PubMed] [Google Scholar]
- 18.Gialdini G, Nearing K, Bhave PD, et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014;312(6):616-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwich P, Buth KJ, Légaré JF. New onset postoperative atrial fibrillation is associated with a long-term risk for stroke and death following cardiac surgery. J Card Surg. 2013;28(1):8-13. [DOI] [PubMed] [Google Scholar]
- 20.Mariscalco G, Klersy C, Zanobini M, et al. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008;118(16):1612-1618. [DOI] [PubMed] [Google Scholar]
- 21.Rubin DA, Nieminski KE, Reed GE, Herman MV. Predictors, prevention, and long-term prognosis of atrial fibrillation after coronary artery bypass graft operations. J Thorac Cardiovasc Surg. 1987;94(3):331-335. [PubMed] [Google Scholar]
- 22.Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37(6):1353-1359. [DOI] [PubMed] [Google Scholar]
- 23.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39(7)(suppl):30-33. [DOI] [PubMed] [Google Scholar]
- 24.Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7)(suppl):38-41. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7)(suppl):22-25. [DOI] [PubMed] [Google Scholar]
- 26.Schramm TK, Gislason GH, Køber L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117(15):1945-1954. [DOI] [PubMed] [Google Scholar]
- 27.Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olesen JB, Sørensen R, Hansen ML, et al. Non-vitamin K antagonist oral anticoagulation agents in anticoagulant naïve atrial fibrillation patients: Danish nationwide descriptive data 2011-2013. Europace. 2015;17(2):187-193. [DOI] [PubMed] [Google Scholar]
- 29.Staerk L, Fosbøl EL, Gadsbøll K, et al. Non-vitamin K antagonist oral anticoagulation usage according to age among patients with atrial fibrillation: Temporal trends 2011-2015 in Denmark. Sci Rep. 2016;6:31477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gislason GH, Jacobsen S, Rasmussen JN, et al. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation. 2006;113(25):2906-2913. [DOI] [PubMed] [Google Scholar]
- 31.Schjerning Olsen AM, Gislason GH, McGettigan P, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA. 2015;313(8):805-814. [DOI] [PubMed] [Google Scholar]
- 32.Krarup LH, Boysen G, Janjua H, Prescott E, Truelsen T. Validity of stroke diagnoses in a National Register of Patients. Neuroepidemiology. 2007;28(3):150-154. [DOI] [PubMed] [Google Scholar]
- 33.Rix TA, Riahi S, Overvad K, Lundbye-Christensen S, Schmidt EB, Joensen AM. Validity of the diagnoses atrial fibrillation and atrial flutter in a Danish patient registry. Scand Cardiovasc J. 2012;46(3):149-153. [DOI] [PubMed] [Google Scholar]
- 34.De Jong J. CHA2DS2-VASc/HASBLED/EHRA atrial fibrillation risk score calculator. https://www.chadsvasc.org/. Accessed February 22, 2018.
- 35.Al-Khatib SM, Hafley G, Harrington RA, et al. Patterns of management of atrial fibrillation complicating coronary artery bypass grafting: Results from the Project of Ex-Vivo Vein Graft Engineering via Transfection IV (PREVENT-IV) Trial. Am Heart J. 2009;158(5):792-798. [DOI] [PubMed] [Google Scholar]
- 36.Antonelli D, Peres D, Freedberg NA, Feldman A, Rosenfeld T. Incidence of postdischarge symptomatic paroxysmal atrial fibrillation in patients who underwent coronary artery bypass graft: long-term follow-up. Pacing Clin Electrophysiol. 2004;27(3):365-367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1 ICD-8 and ICD-10 codes
eTable 2 ATC codes
eTable 3 OAC initiation within 30 days after the index date according to CHA2DS2-VASc in patients developing POAF following CABG and NVAF
eTable 4 Baseline characteristics of patients developing POAF following CABG and NVAF
eTable 5 Baseline characteristics of patients developing and not developing POAF following CABG
eFigure 1 OAC initiation within 30 days after the index date according to year of surgery or diagnosis in patients developing POAF following CABG and NVAF
eFigure 2 Long-term outcomes in patients developing POAF following CABG and NVAF