Key Points
Question
Are women with schizophrenia at a higher risk for breast cancer?
Findings
In this meta-analysis of 12 cohort studies in which conventional methods of meta-analysis had been used, schizophrenia in women was associated with an increased breast cancer incidence compared with the general population. However, substantial between-study variance is present that is reflected by the wide prediction interval.
Meaning
The results suggest that the incidence of breast cancer in women with schizophrenia is higher than that of the general female population; however, significant heterogeneity exists among the included studies, and it is possible that a future study will show a decreased breast cancer risk in women with schizophrenia compared with the general population.
Abstract
Importance
Patients with schizophrenia are considered to have many risk factors for the development of cancer. However, the incidence of breast cancer in women with schizophrenia compared with the general population remains uncertain.
Objective
To perform an updated meta-analysis to evaluate the association between schizophrenia and the risk of breast cancer.
Data Sources
A systematic search of the PubMed and EMBASE databases was conducted using the search terms schizophrenia, schizophrenic, psychosis, combined with breast and cancer, tumor, neoplasm, or carcinoma. The final literature search was performed on August 15, 2017.
Study Selection
Cohort studies reporting the standardized incidence ratio (SIR) for the risk of breast cancer in women with schizophrenia compared with the general population.
Data Extraction and Synthesis
The meta-analysis adhered to Meta-analysis of Observational Studies in Epidemiology and the Cochrane Handbook for Systematic Reviews of Interventions. Data extraction was performed independently. A random-effects model was used to pool the results, and a recently proposed prediction interval was calculated to describe the heterogeneity.
Main Outcomes and Measures
The SIR for the risk of breast cancer in women with schizophrenia compared with the general population or those without schizophrenia.
Results
Twelve cohorts were included in this meta-analysis. The results of the meta-analysis showed that schizophrenia was associated with a significantly increased risk of breast cancer incidence in women (SIR, 1.32; 95% CI, 1.15-1.51; P < .001), with significant heterogeneity (P < .001; I2 = 90%). Substantial between-study variance was also suggested by the wide prediction interval (0.82-2.11), which indicated that it is possible that a future study will show a decreased breast cancer risk in women with schizophrenia compared with the general population. The subgroup analysis results showed that the association was not significantly affected by whether breast cancer cases were excluded at baseline or the sample size of the included studies.
Conclusions and Relevance
The incidence of breast cancer in women with schizophrenia is higher than that of the general female population. However, significant heterogeneity exists among the included studies. Women with schizophrenia deserve intensive prevention and treatment of breast cancer.
This meta-analysis examines the incidence of breast cancer in women with schizophrenia.
Introduction
The status of physical health in patients with schizophrenia has become an important topic in health care management research.1 Because patients with schizophrenia may be prone to have an unhealthy lifestyle, they are vulnerable to many chronic diseases, such as metabolic disorders,2,3 diabetes,4,5 and cardiovascular diseases.6,7 However, the risk of cancer in patients with schizophrenia remains uncertain.8 Although the health of patients with schizophrenia is complicated by many risk factors for the development of cancer, including smoking,9,10 alcohol and substance abuse,11 obesity,12,13 and lack of exercise,14,15 epidemiologic studies have shown inconsistent results. In a previous meta-analysis of cohort studies,16 the overall risk of cancer was not statistically significantly increased in patients with schizophrenia compared with the general population without schizophrenia. The results of subsequent analyses have shown that the association between schizophrenia and cancer risk may not be explained only by the risk factors related to an unhealthy lifestyle; many other factors, including genetic mechanisms, may be involved in the interactions between schizophrenia and cancer pathogenesis.17 Because schizophrenia has been associated with lowered risks of many types of cancer, including colorectal cancer, malignant melanoma, and prostate cancer,16 it has been hypothesized that the genetic factors involved in the pathogenesis of schizophrenia may be protective against cancer.18,19,20 However, the association between schizophrenia and breast cancer remains uncertain. The results of subgroup analyses from a previous meta-analysis have shown an association between schizophrenia and an increased risk of breast cancer compared with the general female population.16 A subsequent systematic review challenged these results by including more recent studies, but the results were mixed.21 Many published cohort studies were not included in the previous meta-analysis,22,23,24,25,26 and the results of some previously included cohort studies have been updated.27,28,29 Another meta-analysis30 used a conventional method that evaluates the heterogeneity among studies with the I2 statistic and combines the results with the random-effects model. However, this method was challenged by recent findings that I2 may not be an appropriate index of heterogeneity, and instead, a prediction interval (PI) that describes the heterogeneity in a random-effects meta-analysis should be reported to quantify the heterogeneity.31 Therefore, we thought it important to perform an updated meta-analysis regarding the association between schizophrenia and the risk of breast cancer with the reporting of the PI. The results of this study may lead to better prevention and early treatment of breast cancer in women with schizophrenia.
Methods
This systematic review and meta-analysis was designed and performed in accordance with the Meta-analysis of Observational Studies in Epidemiology32 and the Cochrane Handbook for Systematic Reviews of Interventions guidelines.33 The study was approved by Tianjin Medical University.
Literature Search
We systematically searched the PubMed and EMBASE databases with the terms schizophrenia, schizophrenic, and psychosis, combined with breast and cancer, tumor, neoplasm, or carcinoma. The search was limited to studies in humans that were published in English. We also manually screened the reference lists of original and review articles. The final literature search was performed on August 15, 2017.
Inclusion and Exclusion Criteria
Studies that fulfilled the following criteria were included: (1) published as full-length articles in English, (2) designed as cohort studies (prospective or retrospective, regardless of the sample size and follow-up duration), (3) included adult women (age ≥18 years), (4) schizophrenia identified as exposure at baseline, (5) the general population without a diagnosis of schizophrenia was used as a control, (6) documented incidence of breast cancer on follow-up, and (7) reported the adjusted standardized incidence ratios (SIRs), at least adjusted for age, and their corresponding 95% CIs for breast cancer incidence in women with schizophrenia compared with controls. The diagnosis of schizophrenia and the confirmation of breast cancer cases were consistent with the criteria applied in the original articles. If studies with overlapping participants were encountered, the reports with the larger sample size were included in the present meta-analysis. Abstracts, letters to the editor, reviews, and investigations with designs other than a cohort study were excluded from the present analysis. Studies reporting breast cancer–related mortality rather than incidence were also excluded because the mortality outcome may be affected by many factors other than breast cancer incidence, such as comorbidities and treatments.
Data Extraction and Quality Evaluation
We performed the literature search, data extraction, and quality assessment independently, according to the predefined inclusion criteria. Discrepancies were resolved by consensus. The following data regarding the characteristics of the studies were extracted: name of first author, year of publication, country where the study was conducted, sample size, source of the study population, years of follow-up, number of breast cancer cases, strategies for confirmation of breast cancer cases, and whether cancer incidence before the diagnosis of schizophrenia was excluded. The Newcastle-Ottawa Scale34 was used to evaluate the quality of the included studies. This scale ranges from 1 (lowest quality) to 9 (highest quality) stars and judges each study regarding the aspects of study group selection, comparability of the groups, and ascertainment of the outcome of interest.
Statistical Analysis
Data of SIRs and the lower and upper limits of their 95% CIs were extracted to calculate log SIRs and their corresponding SEs. The logarithmically transformed SIRs and their corresponding SEs were used to stabilize the variance and normalize the distribution. The Cochran Q test and the I2 statistic were used to evaluate the heterogeneity among the included cohort studies.30 A random-effects model was used for the meta-analysis of the SIR data because this model is considered to produce a more generalized result by considering heterogeneity between studies.33 A PI was calculated based on the methods provided by Borenstein et al31 indicating the range of a true SIR of a future study in 95% of all populations. Sensitivity analyses by omitting 1 study at a time were performed to evaluate the robustness of the results.35 Because a previous systematic review has indicated that studies with more than 100 breast cancer cases tend to show an evident association between schizophrenia and the risk of breast cancer,21 we performed stratified analyses according to whether the number of breast cancer cases in the included studies was over 100. Subgroup analysis was performed according to whether the cases of breast cancer that occurred before the diagnosis of schizophrenia were excluded in each study. Potential publication bias was assessed by funnel plots with the Egger regression asymmetry test.36 RevMan, version 5.1 (Cochrane Collaboration) and Stata software, version 12.0 (StataCorp) were used for the statistical analyses.
Results
Literature Search Results
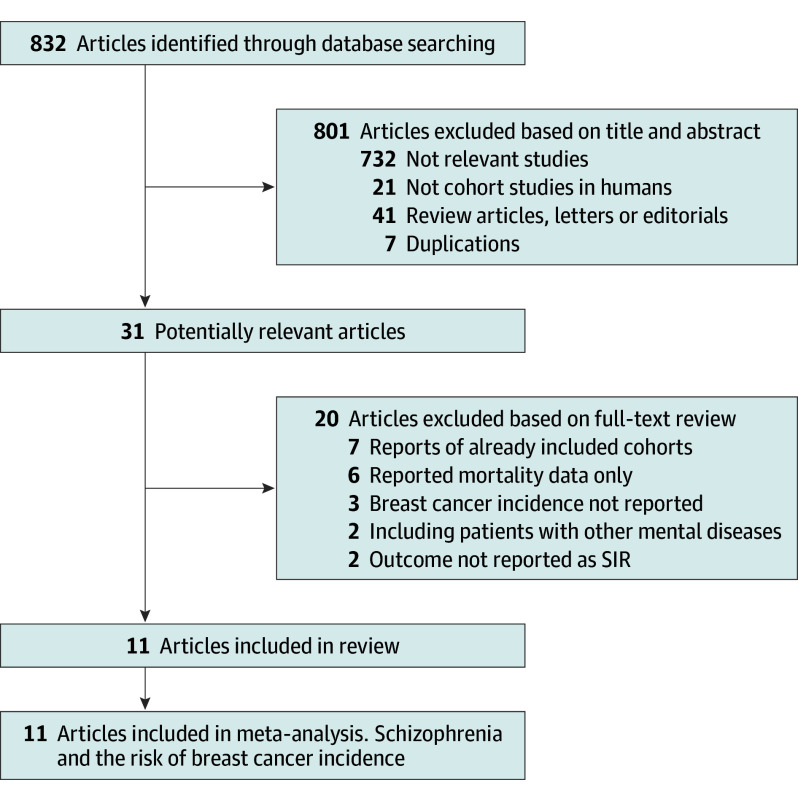
The literature searching process and study identification are summarized in Figure 1. In brief, 832 records were identified after initial database searching and exclusion of the duplicate records. Further screening of titles and abstracts excluded 801 records, mainly because they were irrelevant to the aim of the study. For the 31 records that underwent full-text review, 20 were excluded because 2 of them included patients with mental diseases other than schizophrenia, 7 were reports of already included cohorts, 3 did not report the incidence of breast cancer, 6 only reported data regarding breast cancer mortality, and the other 2 did not provide outcome data as SIRs.
Figure 1. Flowchart of the Literature Search.
SIR indicates standardized incidence ratio.
Study Characteristics and Quality Evaluation
Overall, 11 database-derived retrospective cohort studies8,22,23,24,25,26,37,38,39,40,41 were included. Because 1 study36 included data from 2 different cohorts, 12 cohorts were included in our meta-analysis. The baseline characteristics of the included cohorts are reported in the eTable in the Supplement. Overall, the studies were published between 1992 and 2016 and included participants from Europe,23,25,38,39,40 the United States,22,37 and Asia.8,24,26,37,41 Five cohorts included hospitalized patients with schizophrenia,23,26,38,39,40 while the others did not specify the source of the patients. The number of women with schizophrenia included in each study varied from 1388 to 46 447, and the number of the breast cancer cases ranged from 42 to 1034. Six studies8,23,24,25,26,39 excluded the breast cancer cases that occurred before the diagnosis of schizophrenia; the other studies did not specify. The qualities of the included studies were generally good, with Newcastle-Ottawa Scale values between 6 and 8.
Schizophrenia and the Incidence of Breast Cancer
The meta-analysis results of the 12 cohorts showed that schizophrenia was associated with a significantly increased risk of breast cancer incidence in women (SIR, 1.32; 95% CI, 1.15-1.51; P < .001) (Figure 2), with significant heterogeneity (P < .001; I2 = 90%). The existence of substantial between-study variance is reflected by the wide PI (0.82-2.11). Accordingly, it is possible that a future study will show a decreased breast cancer risk in women with schizophrenia compared with the general population. The findings of sensitivity by omitting 1 study at a time did not significantly change the results, with the SIR varying between 1.29 and 1.39 (all P < .01).
Figure 2. Incidence of Breast Cancer in Women With Schizophrenia.
Pooled results of 12 cohorts indicated that schizophrenia was associated with a significantly increased risk of breast cancer incidence in women. IV indicates inverse variance; SIR, standardized incidence ratio.
Results of Subgroup Analyses
The results of subgroup analyses showed that the association between schizophrenia and an increased breast cancer incidence was significant in studies in which breast cancer occurred before the diagnosis of schizophrenia were excluded (SIR, 1.35; 95% CI, 1.20-1.53; P < .001; I2 = 86%) (Figure 3) and in studies with more than 100 breast cancer cases (SIR, 1.32; 95% CI, 1.18-1.48; P < .001; I2 = 86%) (Figure 4), but the association between schizophrenia and breast cancer incidence was not significant in studies that did not specify the exclusion of breast cancer cases that occurred before the diagnosis of schizophrenia (SIR, 1.38; 95% CI, 0.89-2.14; P = .15; I2 = 91%) (Figure 3) or in studies with fewer than 100 breast cancer cases (SIR, 1.50; 95% CI, 0.78-2.87; P = .23; I2 = 93%) (Figure 4). However, the differences between subgroups were not statistically significant (P = .92 and P = .71 for subgroup interaction).
Figure 3. Subgroup Analyses According to Whether the Breast Cancer Cases That Occurred Before the Diagnosis of Schizophrenia Were Excluded.
Subgroup analysis results showed that the association between schizophrenia and increased risk of breast cancer incidence in women was not significantly affected by whether breast cancer cases were excluded at baseline. IV indicates inverse variance; SIR, standardized incidence ratio.
Figure 4. Subgroup Analyses According to the Number of Breast Cancer Cases in Each Study.
Subgroup analysis results showed that the association between schizophrenia and increased risk of breast cancer incidence in women was not significantly affected by the sample size of the included studies. IV indicates inverse variance; SIR, standardized incidence ratio.
Publication Bias
The funnel plot for the association between schizophrenia and breast cancer incidence was symmetric on visual inspection (eFigure 1 in the Supplement). The Egger regression test also did not indicate a potential publication bias (P = .65).
Discussion
In this study, by pooling the results of all available cohort studies with the conventional method of meta-analysis, we found that women with schizophrenia are at a higher risk for the incidence of breast cancer compared with the general population. These results were not significantly affected by whether only breast cancer cases after the diagnosis of schizophrenia were considered or the sample size of the studies. However, substantial between-study variance is present, which is reflected by the wide PI (0.82-2.11). Accordingly, it is possible that a future study will show a decreased breast cancer risk in women with schizophrenia compared with the general population. Because breast cancer is the most common cancer in women, affecting 1 in 9 women during their lifetime,42 our findings highlight that intensive prevention and treatment against breast cancer are warranted for women with schizophrenia.
The results of this study have important clinical implications. First, we found that schizophrenia in women was associated with an increased breast cancer incidence compared with the general population, which is contrary to the previous hypothesis that schizophrenia may be protective against cancer. These results, together with our recent meta-analysis results showing no association with lung cancer risk but a reduced hepatic cancer risk in schizophrenia (data available on request), indicated that the association between schizophrenia and cancer risk may be complicated and depend on the cancer site. Second, because the current status of breast cancer prevention and treatment options are less optimal in women with schizophrenia,43 our results highlight that women with schizophrenia deserve focused care for breast cancer screening and treatment. These gaps were also reflected by the results of a recent study showing that patients with schizophrenia are at a significantly increased risk of cancer mortality compared with the general population or individuals without schizophrenia,44 although the incidence of cancer in these patients may not necessarily differ from that of the general population. For the early prevention of breast cancer, an initial evaluation is needed to stratify the risk of breast cancer in women with schizophrenia. Subsequently, antipsychotics that may increase the prolactin level and produce a higher breast cancer risk should be avoided in high-risk women. Regular screening, including imaging or biomarker tests, should be performed. If an early diagnosis of breast cancer is made in women with schizophrenia, collaborations with oncologists are needed for clinical psychiatrists to make an optimal treatment recommendation. In addition, every effort should be made to improve the patients’ adherence during the prevention, treatment, and follow-up.
An important strength of our study is that we calculated the PI to describe the heterogeneity in a random-effects meta-analysis. We found substantial between-study variance, which is reflected by the wide PI (0.82-2.11). Accordingly, it is possible that a future study will show a decreased breast cancer risk in women with schizophrenia compared with the general population. Within the context of significant heterogeneity among the previously published cohort studies focusing on the association between schizophrenia and breast cancer risk, whether performing an epidemiologic study is sufficient to answer the question is of concern. Instead, large database research, such as deep neural network analysis centered on a genetic or other biological association between schizophrenia and breast cancer risk, may be optimal. These studies are warranted.
As for the potential mechanisms underlying the association between an increased breast cancer risk in women with schizophrenia, many factors may be involved (eFigure 2 in the Supplement). First, many clinical conditions that are commonly seen in patients with schizophrenia may also be risk factors for the development of breast cancer, such as obesity, nulliparity, and breastfeeding.45 As breast cancer may be a hormone-dependent cancer, a significant positive association between plasma prolactin levels and the risk of breast cancer, has been observed46; in addition, increased prolactin levels have been documented in women with schizophrenia, particularly for those receiving certain antipsychotics.47 Finally, schizophrenia and breast cancer may share some other pathophysiologic factors during their development, including pathways involved in angiogenesis48 and cell cycle regulation.49 However, further experimental studies are needed to determine the exact mechanisms underlying the association between schizophrenia and breast cancer incidence.
Limitations
Our study has some limitations that should be considered when interpreting the results. First, as inherent in other meta-analyses of observational studies, we could not exclude the possibility that some residual factors may confound the association between schizophrenia and breast cancer, such as dietary factors and the use of antipsychotics. As a common shortcoming of meta-analyses of observational studies, the results of this study could not indicate a causative association between schizophrenia and breast cancer in women. In addition, significant heterogeneity remained in our meta-analysis, and the source of the heterogeneity could not be fully analyzed because we did not have access to individual patient data of the included cohorts. Also, we did not include studies found in other databases, not written in English, or published as a conference abstract. We acknowledge this as a limitation. However, including literature reports from PubMed, EMBASE, and the Cochrane Library published only in English should have covered the majority of the cases. Including conference abstracts that did not undergo peer review may potentially cause other bias. Finally, factors such as age at onset of breast cancer, treatment, or cancer subtypes may substantially affect the association between schizophrenia and breast cancer incidence. These factors were generally not reported in the original studies; therefore, they could not be analyzed accordingly in the meta-analysis. Future studies are needed to determine the association between schizophrenia and the different pathologic subtypes of breast cancer as well as whether the association may be affected by the woman’s age at breast cancer onset, antipsychotic medications used, and the cancer subtype.
Conclusions
The results of this meta-analysis demonstrated that women with schizophrenia are at a higher risk for the incidence of breast cancer, compared with the general female population, although substantial between-study variance is present. Intensive screening and treatment for breast cancer in women with schizophrenia are clinically important.
eTable. Characteristics of the Included Studies
eFigure 1. Funnel Plot for the Meta-analysis of the Incidence of Breast Cancer in Women With Schizophrenia
eFigure 2. Proposed Mechanisms Underlying the Association Between Schizophrenia and Breast Cancer Risk
References
- 1.Oud MJ, Meyboom-de Jong B. Somatic diseases in patients with schizophrenia in general practice: their prevalence and health care. BMC Fam Pract. 2009;10:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai D, Lang Y, Feng Y, et al. Early onset of cardiometabolic risk factor profiles in drug naïve adolescents and young adults with first-episode schizophrenia. Schizophr Res. 2017;190:60-62. [DOI] [PubMed] [Google Scholar]
- 3.Zhai D, Cui T, Xu Y, et al. Cardiometabolic risk in first-episode schizophrenia (FES) patients with the earliest stages of both illness and antipsychotic treatment. Schizophr Res. 2017;179:41-49. [DOI] [PubMed] [Google Scholar]
- 4.Morris A. Diabetes: linking diabetes and schizophrenia. Nat Rev Endocrinol. 2017;13(3):126. [DOI] [PubMed] [Google Scholar]
- 5.Vancampfort D, Correll CU, Galling B, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15(2):166-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Fan YL, Tang ZY, Cheng XS. Schizophrenia and risk of stroke: a meta-analysis of cohort studies. Int J Cardiol. 2014;173(3):588-590. [DOI] [PubMed] [Google Scholar]
- 7.Fan Z, Wu Y, Shen J, Ji T, Zhan R. Schizophrenia and the risk of cardiovascular diseases: a meta-analysis of thirteen cohort studies. J Psychiatr Res. 2013;47(11):1549-1556. [DOI] [PubMed] [Google Scholar]
- 8.Grinshpoon A, Barchana M, Ponizovsky A, et al. Cancer in schizophrenia: is the risk higher or lower? Schizophr Res. 2005;73(2-3):333-341. [DOI] [PubMed] [Google Scholar]
- 9.Miyauchi M, Kishida I, Suda A, et al. Long term effects of smoking cessation in hospitalized schizophrenia patients. BMC Psychiatry. 2017;17(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cather C, Pachas GN, Cieslak KM, Evins AE. Achieving smoking cessation in individuals with schizophrenia: special considerations. CNS Drugs. 2017;31(6):471-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang CK. Impact of additive alcohol and substance use disorders on the mortality of people with schizophrenia and mood disorders. Evid Based Ment Health. 2016;19(2):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Du X, Zhang Y, et al. The prevalence, risk factors and clinical correlates of obesity in Chinese patients with schizophrenia. Psychiatry Res. 2017;251:131-136. [DOI] [PubMed] [Google Scholar]
- 13.Sugai T, Suzuki Y, Yamazaki M, et al. High prevalence of obesity, hypertension, hyperlipidemia, and diabetes mellitus in Japanese outpatients with schizophrenia: a nationwide survey. PLoS One. 2016;11(11):e0166429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stubbs B, Chen LJ, Chung MS, Ku PW. Physical activity ameliorates the association between sedentary behavior and cardiometabolic risk among inpatients with schizophrenia: a comparison versus controls using accelerometry. Compr Psychiatry. 2017;74:144-150. [DOI] [PubMed] [Google Scholar]
- 15.Vancampfort D, Stubbs B, Probst M, et al. Physical activity as a vital sign in patients with schizophrenia: evidence and clinical recommendations. Schizophr Res. 2016;170(2-3):336-340. [DOI] [PubMed] [Google Scholar]
- 16.Catts VS, Catts SV, O’Toole BI, Frost AD. Cancer incidence in patients with schizophrenia and their first-degree relatives−a meta-analysis. Acta Psychiatr Scand. 2008;117(5):323-336. [DOI] [PubMed] [Google Scholar]
- 17.Fond G, Macgregor A, Attal J, et al. Antipsychotic drugs: pro-cancer or anti-cancer? a systematic review. Med Hypotheses. 2012;79(1):38-42. [DOI] [PubMed] [Google Scholar]
- 18.Bushe CJ, Hodgson R. Schizophrenia and cancer: in 2010 do we understand the connection? Can J Psychiatry. 2010;55(12):761-767. [DOI] [PubMed] [Google Scholar]
- 19.Hodgson R, Wildgust HJ, Bushe CJ. Cancer and schizophrenia: is there a paradox? J Psychopharmacol. 2010;24(4)(suppl):51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jablensky A, Lawrence D. Schizophrenia and cancer: is there a need to invoke a protective gene? Arch Gen Psychiatry. 2001;58(6):579-580. [DOI] [PubMed] [Google Scholar]
- 21.Bushe CJ, Bradley AJ, Wildgust HJ, Hodgson RE. Schizophrenia and breast cancer incidence: a systematic review of clinical studies. Schizophr Res. 2009;114(1-3):6-16. [DOI] [PubMed] [Google Scholar]
- 22.McGinty EE, Zhang Y, Guallar E, et al. Cancer incidence in a sample of Maryland residents with serious mental illness. Psychiatr Serv. 2012;63(7):714-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji J, Sundquist K, Ning Y, Kendler KS, Sundquist J, Chen X. Incidence of cancer in patients with schizophrenia and their first-degree relatives: a population-based study in Sweden. Schizophr Bull. 2013;39(3):527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin CY, Lane HY, Chen TT, Wu YH, Wu CY, Wu VY. Inverse association between cancer risks and age in schizophrenic patients: a 12-year nationwide cohort study. Cancer Sci. 2013;104(3):383-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osborn DP, Limburg H, Walters K, et al. Relative incidence of common cancers in people with severe mental illness: cohort study in the United Kingdom THIN primary care database. Schizophr Res. 2013;143(1):44-49. [DOI] [PubMed] [Google Scholar]
- 26.Chen LY, Hung YN, Chen YY, et al. Cancer incidence in young and middle-aged people with schizophrenia: nationwide cohort study in Taiwan, 2000-2010 [published online November 21, 2016]. Epidemiol Psychiatr Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mortensen PB. The incidence of cancer in schizophrenic patients. J Epidemiol Community Health. 1989;43(1):43-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortensen PB. The occurrence of cancer in first admitted schizophrenic patients. Schizophr Res. 1994;12(3):185-194. [DOI] [PubMed] [Google Scholar]
- 29.Barak Y, Achiron A, Mandel M, Mirecki I, Aizenberg D. Reduced cancer incidence among patients with schizophrenia. Cancer. 2005;104(12):2817-2821. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. [DOI] [PubMed] [Google Scholar]
- 31.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8(1):5-18. [DOI] [PubMed] [Google Scholar]
- 32.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 33.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Version 5.1.0. The Cochrane Collaboration. http://training.cochrane.org/handbook. Updated June 2017. Accessed January 18, 2018.
- 34.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2010, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Updated 2014. Accessed October 15, 2017.
- 35.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulbinat W, Dupont A, Jablensky A, et al. Cancer incidence of schizophrenic patients: results of record linkage studies in three countries. Br J Psychiatry Suppl. 1992;18(18):75-83. [PubMed] [Google Scholar]
- 38.Lichtermann D, Ekelund J, Pukkala E, Tanskanen A, Lönnqvist J. Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry. 2001;58(6):573-578. [DOI] [PubMed] [Google Scholar]
- 39.Dalton SO, Mellemkjaer L, Thomassen L, Mortensen PB, Johansen C. Risk for cancer in a cohort of patients hospitalized for schizophrenia in Denmark, 1969-1993. Schizophr Res. 2005;75(2-3):315-324. [DOI] [PubMed] [Google Scholar]
- 40.Goldacre MJ, Kurina LM, Wotton CJ, Yeates D, Seagroat V. Schizophrenia and cancer: an epidemiological study. Br J Psychiatry. 2005;187:334-338. [DOI] [PubMed] [Google Scholar]
- 41.Barak Y, Levy T, Achiron A, Aizenberg D. Breast cancer in women suffering from serious mental illness. Schizophr Res. 2008;102(1-3):249-253. [DOI] [PubMed] [Google Scholar]
- 42.Warner E. Clinical practice: breast-cancer screening. N Engl J Med. 2011;365(11):1025-1032. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal A, Pandurangi A, Smith W. Disparities in breast and cervical cancer screening in women with mental illness: a systematic literature review. Am J Prev Med. 2013;44(4):392-398. [DOI] [PubMed] [Google Scholar]
- 44.Zhuo C, Tao R, Jiang R, Lin X, Shao M. Cancer mortality in patients with schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2017;211(1):7-13. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Sun X, Miller E, et al. BMI, reproductive factors, and breast cancer molecular subtypes: a case-control study and meta-analysis. J Epidemiol. 2017;27(4):143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M, Wu X, Chai F, Zhang Y, Jiang J. Plasma prolactin and breast cancer risk: a meta-analysis. Sci Rep. 2016;6:25998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Hert M, Peuskens J, Sabbe T, et al. Relationship between prolactin, breast cancer risk, and antipsychotics in patients with schizophrenia: a critical review. Acta Psychiatr Scand. 2016;133(1):5-22. [DOI] [PubMed] [Google Scholar]
- 48.Lopes R, Soares R, Figueiredo-Braga M, Coelho R. Schizophrenia and cancer: is angiogenesis a missed link? Life Sci. 2014;97(2):91-95. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, He G, He L, McGrath J. Do shared mechanisms underlying cell cycle regulation and synaptic plasticity underlie the reduced incidence of cancer in schizophrenia? Schizophr Res. 2011;130(1-3):282-284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Characteristics of the Included Studies
eFigure 1. Funnel Plot for the Meta-analysis of the Incidence of Breast Cancer in Women With Schizophrenia
eFigure 2. Proposed Mechanisms Underlying the Association Between Schizophrenia and Breast Cancer Risk