Key Points
Question
What are the cognitive outcomes of children born extremely or very preterm since 1990, and what perinatal and demographic factors predict outcome?
Findings
This meta-analysis of 71 studies (7752 extremely or very preterm and 5155 full-term children) showed a large (0.86 standard deviation) difference in intelligence between extremely or very preterm children and controls, which was stable in children born between 1990 and 2008. Bronchopulmonary dysplasia explained 65% of the variance in intelligence across studies.
Meaning
Despite advancing perinatal care, cognitive outcomes of children born extremely or very preterm did not improve between 1990 and 2008; preventive strategies to reduce the incidence of bronchopulmonary dysplasia may be crucial to improve outcomes after extremely or very preterm birth.
Abstract
Importance
Despite apparent progress in perinatal care, children born extremely or very preterm (EP/VP) remain at high risk for cognitive deficits. Insight into factors contributing to cognitive outcome is key to improve outcomes after EP/VP birth.
Objective
To examine the cognitive abilities of children of EP/VP birth (EP/VP children) and the role of perinatal and demographic risk factors.
Data Sources
PubMed, Web of Science, and PsycINFO were searched without language restriction (last search March 2, 2017). Key search terms included preterm, low birth weight, and intelligence.
Study Selection
Peer-reviewed studies reporting intelligence scores of EP/VP children (<32 weeks of gestation) and full-term controls at age 5 years or older, born in the antenatal corticosteroids and surfactant era, were included. A total of 268 studies met selection criteria, of which 71 covered unique cohorts.
Data Extraction and Synthesis
MOOSE guidelines were followed. Data were independently extracted by 2 researchers. Standardized mean differences in intelligence per study were pooled using random-effects meta-analysis. Heterogeneity in effect size across studies was studied using multivariate, random-effects meta-regression analysis.
Main Outcomes and Measures
Primary outcome was intelligence. Covariates included gestational age, birth weight, birth year, age at assessment, sex, race/ethnicity, socioeconomic status, small for gestational age, intraventricular hemorrhage, periventricular leukomalacia, bronchopulmonary dysplasia (BPD), necrotizing enterocolitis, sepsis, and postnatal corticosteroid use.
Results
The 71 included studies comprised 7752 EP/VP children and 5155 controls. Median gestational age was 28.5 weeks (interquartile range [IQR], 2.4 weeks) and the mean age at assessment ranged from 5.0 to 20.1 years. The median proportion of males was 50.0% (IQR, 8.7%). Preterm children had a 0.86-SD lower IQ compared with controls (95% CI, −0.94 to −0.78, P < .001). Results were heterogeneous across studies (I2 = 74.13; P < .001). This heterogeneity could not be explained by birth year of the cohort. Multivariate meta-regression analysis with backward elimination revealed that BPD explained 65% of the variance in intelligence across studies, with each percent increase in BPD rate across studies associated with a 0.01-SD decrease in IQ (0.15 IQ points) (P < .001).
Conclusions and Relevance
Extremely or very preterm children born in the antenatal corticosteroids and surfactant era show large deficits in intelligence. No improvement in cognitive outcome was observed between 1990 and 2008. These findings emphasize that improving outcomes after EP/VP birth remains a major challenge. Bronchopulmonary dysplasia was found to be a crucial factor for cognitive outcome. Lowering the high incidence of BPD may be key to improving long-term outcomes after EP/VP birth.
This meta-analysis of 71 studies examines the cognitive outcomes of 7752 children born extremely or very preterm.
Introduction
Rising preterm birth rates together with improved survival identify a growing number of children surviving preterm birth. However, despite apparent progress in perinatal care, long-term morbidity rates have not decreased. Extremely or very preterm (EP/VP) birth (<32 weeks of gestation) is associated with higher risks for motor, learning, and behavior problems, and particularly, cognitive impairment. Among the most immature infants (<28 weeks of gestation), impairment rates in 1 or more of these neurodevelopmental domains are reported to be as high as 70%. An important and frequently used measure of outcome is intelligence, as it provides a broad index of cognitive functioning and is substantially correlated with important life outcomes, such as physical and mental health, academic achievement, socioeconomic success, and life chances. Earlier meta-analyses showed that preterm birth is associated with lower intelligence, with intelligence level being proportionally related to the degree of immaturity. The compromised intelligence of children of EP/VP birth (EP/VP children) poses a significant burden on individuals, families, and society, and provides challenges for education and health care professionals.
During 24 to 40 weeks of gestation, multiple complex events critical for brain development take place, including axonal and subplate growth, differentiation of premyelinating oligodendrocytes, and proliferation and migration of γ-aminobutyric acid–ergic neurons. These processes are highly vulnerable to pathogenic factors, such as hemorrhagic events, hypoxic-ischemic events, and inflammation, and may be further exacerbated by environmental factors, such as stress exposure during neonatal intensive care unit stay and malnutrition. Neuropathology associated with EP/VP birth includes both direct brain injury, of which white matter lesions are most common, and secondary neuronal abnormalities affecting gray matter. These disturbances in brain development are reflected in reduced overall brain volume in childhood and adolescence, with reductions being evident in both gray and white matter as well as alterations in white matter microstructure. White matter abnormalities in particular have been associated with neurocognitive impairments.
Insight into factors contributing to alterations in brain development and subsequent cognitive impairment in EP/VP children is of interest because such knowledge is key to improving outcomes of these children. Neonatal morbidities, such as intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), bronchopulmonary dysplasia (BPD), and infectious diseases (eg, sepsis, necrotizing enterocolitis) have been associated with brain abnormalities and increased risks for cognitive impairment in preterm children. Moreover, certain treatments, such as postnatal corticosteroid use, and demographic factors (eg, sex, socioeconomic status) may play a role. However, the relative contribution of each of the possible risk factors is not known. This lack of knowledge hampers clear conclusions to be drawn about factors that are key in enhancing the outcomes of EP/VP children and thus might be the target of interventions. Furthermore, knowledge of risk factors for cognitive impairment would benefit clinical decision making and parental counseling in the neonatal period and contribute to the identification of high-risk infants for close monitoring and early intervention. Therefore, a meta-analysis and meta-regression were conducted to examine the intelligence of EP/VP children and the role of perinatal and demographic risk factors.
Methods
Study Selection
This meta-analysis was conducted according to MOOSE guidelines. Inclusion criteria for studies were (1) the sample consisted of infants born EP/VP (<32 weeks’ gestational age [GA]) and/or with extremely low (<1000 g) or very low (<1500 g) birth weight; (2) a term control group was included; (3) infants were born in the antenatal corticosteroids and surfactant era (ie, 1990 or later) or participated in studies with earlier antenatal corticosteroid and surfactant therapy availability; (4) age at assessment of at least 5 years; (5) intelligence was assessed in both groups using standardized, validated tests; and (6) the study was published in a peer-reviewed journal.
PubMed, Web of Science, and PsycINFO (last search March 2, 2017) were searched without language restriction using combinations of the following search terms: prematur*, preterm, low birth weight, elbw, vlbw, intelligence, intellect*, IQ, cognit*, mental, abilit*, perform*, function*, disabilit*, impair*, disorder*, and retard*. In case of overlapping cohorts, selection was based on the following criteria (in order of importance): (1) the study with the longest follow-up interval (ie, oldest age at assessment), (2) the study with the largest sample size, and (3) the study reporting the highest number of perinatal variables.
Outcomes and Covariates
Intelligence scores (IQ) and information on perinatal factors and demographics were extracted from the articles. For studies not reporting information on all pertinent variables, authors were contacted to provide additional data. Various intelligence tests, including full-scale, verbal, and performance measures, were used across studies (eTable in the Supplement). For studies reporting verbal and performance IQ but no full-scale IQ, the mean of the verbal and performance scores was computed to provide a full-scale estimate. A categorical moderator variable was included in the analyses to assess the type of test as a source of heterogeneity in outcomes across studies. Information on GA, birth weight, birth year, age at assessment, sex, race/ethnicity, socioeconomic status, small for GA status, IVH grade I/II, IVH grade III/IV, PVL, BPD, necrotizing enterocolitis, sepsis, and postnatal corticosteroid use was extracted from the articles and/or provided by authors. Definitions used, if described, varied across studies and are reported in the eTable in the Supplement.
Study Quality
The Newcastle-Ottawa Scale for cohort studies was used to assess study quality. All included studies were independently rated by 2 of us (E.S.T., J.F.d.K.) (eTable in the Supplement) on aspects of participant selection, group comparability, and outcome assessment. Two aspects of the scale (“demonstration that outcome of interest was not present at the start of study” and “was follow-up long enough for outcomes to occur?”) were not applicable and therefore omitted. This revision resulted in a 7-point rating scale, with higher scores representing better study quality.
Statistical Analysis
Analyses were performed using Comprehensive Meta-Analysis, version 3.0 (Biostat). The standardized mean difference in IQ between EP/VP and full-term children was used as effect size. The mean difference of each study was weighted by the inverse of its variance. If studies reported data for independent subgroups, a combined effect across subgroups was computed. Random-effects meta-analysis was performed to calculate summary estimates. Dispersion in effect sizes was quantified using I2. Publication bias was evaluated using funnel plots and the Egger test.
Random-effects meta-regression analysis quantified the association of demographic and perinatal factors with study-level effects. Studies were weighted by the inverse of the sum of the within- and between-study variance. Analyses were performed on complete cases. To evaluate whether complete-case analysis introduced bias, the correlation between IQ and the amount of missing values for each covariate was computed. Univariate preselection of covariates was applied to reduce the number of covariates. Covariates were selected for further modeling using an α level of .05. Subsequently, preselected covariates were included in a backward multiple meta-regression analysis with an α level of .05 as cutoff point for removal.
Results
eFigure 1 in the Supplement shows the study selection process. Screening based on title and abstract indicated 1865 possibly relevant articles. Full-text examination revealed 268 eligible studies, of which 71 covered unique cohorts. These 71 studies comprised 7752 EP/VP children and 5155 controls. Median GA and birth weight of means in the studies were 28.5 weeks (interquartile range [IQR], 2.4 weeks) and 1103.6 g (IQR, 265.0 g), respectively. Mean age at assessment varied from 5.0 to 20.1 years. The median proportion of males was 50.0% (IQR, 8.7%). The eTable in the Supplement provides details of all included studies with accompanying references (eReferences in the Supplement). Median incidence rates were highest for sepsis (28.9%) and BPD (27.0%), and lowest for IVH grade III/IV (3.1%) and PVL (3.6%).
Intelligence
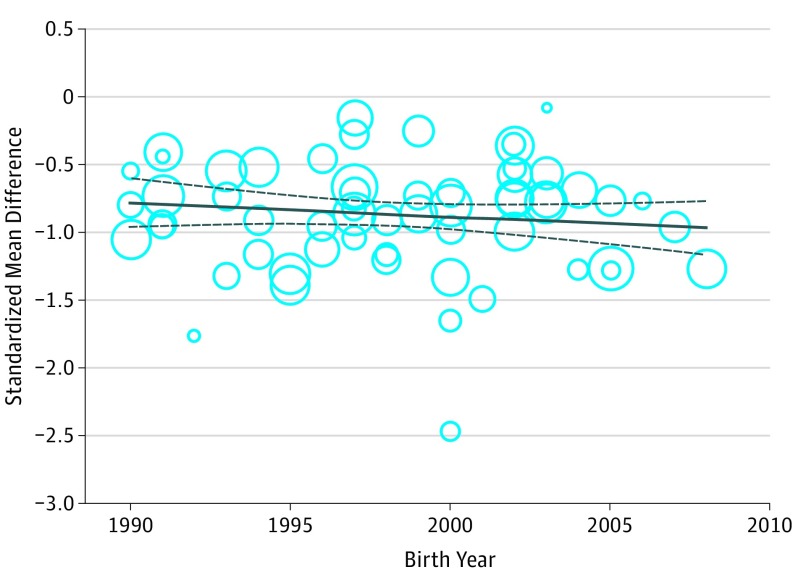
The IQs of EP/VP children were 0.86 SDs lower compared with those of full-term children (95% CI, −0.94 to −0.78, P < .001), corresponding to a difference of 12.9 IQ points. This difference represents a large effect. A forest plot is provided in eFigure 2 in the Supplement. Results across studies were heterogeneous (I2 = 74.13, P < .001). This heterogeneity could not be explained by type of intelligence test (95% CI, −0.37 to 0.05; P = .13), study quality (95% CI, −0.09 to 0.03; P = .36), or age at assessment (95% CI, −0.02 to 0.03; P = .85). Furthermore, as depicted in Figure 1, the standardized mean difference in IQ across studies did not vary as a function of birth year of the cohort—neither unadjusted (95% CI, −0.02 to 0.02; P = .77) nor adjusted for GA (95% CI, −0.03 to 0.01; P = .30). Sensitivity analyses were performed to explore whether this finding was affected by study quality or region of origin. Analysis including only high-quality studies (Newcastle-Ottawa Scale score >5) showed similar results (95% CI, −0.06 to 0.02; P = .25). The same was true for analysis including only cohorts from Western countries (95% CI, −0.01 to 0.03; P = .58). Differentiation between studies originating from North America (95% CI, −0.05 to 0.04; P = .71) and Europe (95% CI, −0.01 to 0.04; P = .22) did not change the results. Further differentiation between regions was not possible due to the small numbers of studies from other regions.
Figure 1. Association Between Birth Year and the Standardized Mean Difference in IQ Between Extremely or Very Preterm Born Children and Full-term Controls.
Meta-regression analysis was adjusted for gestational age. Plotting characters are proportional to the study weight.
Demographic and Perinatal Risk Factors
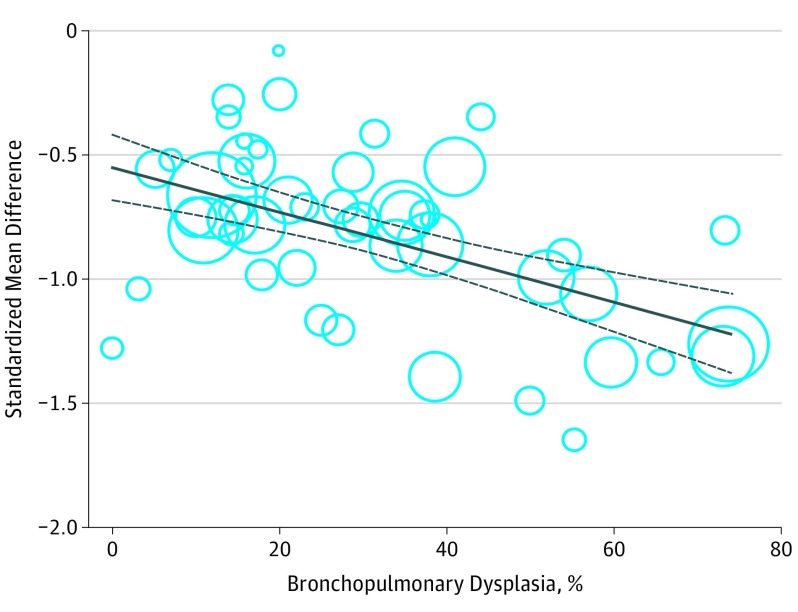
Weak, nonsignificant correlations (r = 0.03-0.23) were observed between IQ and the amount of missing values in covariates. Relevant covariates were preselected using univariate meta-regression analyses. Results of all univariate analyses are presented in the Table. Gestational age, birth weight, IVH grade I/II, PVL, BPD, and postnatal corticosteroid use significantly explained heterogeneity in effect size across studies and were therefore considered for further modeling. Small for GA, IVH grade III/IV, necrotizing enterocolitis, sepsis, sex, race/ethnicity, and maternal educational level were nonsignificant covariates. Backward multiple meta-regression analysis including all studies with complete data for aforementioned covariates (k = 24) revealed that heterogeneity in effect size across studies was significantly explained by GA (95% CI, 0.04 to 0.17; P = .002) and BPD (95% CI, −0.01 to −0.001; P = .02). This model was retested including all studies with complete data for both GA and BPD (k = 49). Gestational age was a nonsignificant covariate (95% CI, −0.007 to 0.10; P = .09) when BPD was included in the model and inclusion of GA did not affect the coefficient of BPD, implying that the association between BPD and the outcome was not confounded by GA. Furthermore, the variance seemed not to be inflated due to multicollinearity (variance inflation factor = 1.93). The final model included only BPD (95% CI, −0.01 to −0.006; P < .001), resulting in a total explained variance of 65% (95% CI, 32% to 98%; P<.001). Each percent increase in BPD rate across studies was associated with a 0.01-SD decrease in IQ (0.15 IQ points) in EP/VP children compared with controls. Given these results, a BPD rate of 0% is associated with a difference in IQ of 0.55 SDs between EP/VP children and controls, whereas a BPD rate of 100% is associated with a 1.55-SD difference. At the individual level, this finding suggests that a diagnosis of BPD is associated with a 1-SD decrease in IQ (15 IQ points) compared with the absence of BPD. Figure 2 shows the regression plot of BPD on the standardized mean difference in IQ across studies. Postnatal corticosteroid use was found to explain 33% of the variance in the univariate analysis. Postnatal corticosteroid use and BPD were significantly correlated (r = −0.57). However, adding BPD to the regression model (k = 30) rendered the result for postnatal corticosteroid use nonsignificant (95% CI, −0.005 to 0.004; P = .75). No evidence for multicollinearity was found (variance inflation factor = 1.49). The confounding effect of BPD definition could not be assessed because the definition used was unknown for a number of studies. Alternatively, birth year was used as a proxy for BPD definition since the definition utilized is likely related to birth year of the cohort. Controlling for birth year did not alter the results.
Table. Results of Univariate Meta-Regression Analyses of the Association Between Perinatal and Demographic Covariates and Intelligence Scores.
| Covariate | κ Value | Change in IQ Points per Unit Change in Covariate (95% CI)a | R2 Value |
|---|---|---|---|
| GA, wk | 70 | 1.26 (0.52 to 2.00)b | 23.94 |
| BW, g | 68 | 0.02 (0.003 to 0.02)c | 19.84 |
| SGA, % | 50 | −0.04 (−0.15 to 0.07) | 0.79 |
| IVH grade I/II, % | 50 | −0.17 (−0.31 to −0.03)d | 13.07 |
| IVH grade III/IV, % | 54 | −0.26 (−0.54 to 0.03) | 9.15 |
| PVL, % | 46 | −0.26 (−0.48 to −0.04)d | 13.08 |
| BPD, % | 49 | −0.15 (−0.19 to −0.09)b | 64.99 |
| NEC, % | 41 | 0.06 (−0.13 to 0.24) | 0.26 |
| Sepsis, % | 38 | −0.05 (−0.12 to 0.03) | 1.36 |
| Postnatal corticosteroid use, % | 31 | −0.09 (−0.17 to −0.01)d | 32.68 |
| Sex, % male | 66 | 0.10 (−0.05 to 0.26) | 1.62 |
| Race/ethnicity, % nonwhite | 33 | −0.001 (−0.11 to 0.09) | 0.17 |
| Maternal education <12 y, % | 15 | −0.03 (−0.20 to 0.07) | 0 |
Abbreviations: BPD, bronchopulmonary dysplasia; BW, birth weight; GA, gestational age; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; PVL, periventricular leukomalacia; SGA, small for gestational age.
These values indicate the change in IQ points for each unit change in the covariate. For example, a 1-week change in GA is associated with a 1.26-point change in IQ. A negative value indicates a negative association (ie, a decrease in the covariate is associated with an increase in IQ and vice versa). For example, a decrease of 1% in the rate of BPD is associated with an increase in IQ of 0.15 points.
P < .001.
P < .01.
P < .05.
Figure 2. Association Between Incidence of Bronchopulmonary Dysplasia and the Standardized Mean Difference in IQ Between Extremely or Very Preterm Born Children and Full-term Controls.
Plotting characters are proportional to the study weight.
Publication Bias
Inspection of the funnel plot (eFigure 3 in the Supplement) did not suggest publication bias. This finding was confirmed by the nonsignificant Egger test (t = 0.78, P = .44).
Discussion
This meta-analysis and meta-regression aimed to clarify the role of perinatal and demographic factors for long-term cognitive outcome of EP/VP children. Based on 71 studies including 7752 EP/VP children and 5155 controls, our results demonstrated a large difference (0.86 SD) in intelligence between EP/VP children and their full-term peers, which corresponds to approximately 13 points in IQ. This difference was stable over age (5-20 years) and birth year (1990-2008). Second, considering a wide range of perinatal and demographic risk factors, we showed a strong association between BPD and long-term cognitive outcome in EP/VP children.
A difference in intelligence of almost 1 SD is likely to have important consequences for academic achievement and socioeconomic outcomes. Preterm birth is associated with poor academic outcome, lower incomes, and increased social security dependency.Assuming a normal distribution of IQ, a lowering of almost 1 SD suggests that approximately 16% of the EP/VP children have an IQ that is 2 SDs below the population mean compared with 2.5% in the total population, which is indicative of intellectual disability according to the DSM-5.
In 2002, Bhutta and colleagues showed the detrimental effect of preterm birth on intelligence based on cohorts of children born before 1990. Based on cohorts of children born in the antenatal corticosteroid and surfactant era and having almost 5 times more studies at our disposal, we found a similarly large effect. Our results are also comparable to the findings of Kerr-Wilson et al, based on 27 studies including preterm children born between 1975 and 2000. Apart from the lack of improvement in cognitive outcomes shown by the present meta-analysis in comparison with the meta-analysis of Bhutta and colleagues, our study presents no evidence of improvement of cognitive outcome between 1990 and 2008, even when differences in mean GA over time were taken into consideration. These findings suggest that, regarding long-term cognitive outcomes, no significant progress has been made since the early 1990s. Changes in neonatal practice, for example, the largely decreased use of postnatal corticosteroids, may have resulted in some improvement in cerebral palsy and neurosensory disability rates. However, other studies showed no improvement in these outcomes over time. Overall, improvement of neurodevelopmental outcomes, including severe cognitive and neurosensory disabilities, seems to be only marginal. This finding emphasizes the need to identify strategies to improve long-term cognitive outcomes of EP/VP children.
Neonatal morbidities have been associated with brain abnormalities and cognitive impairment in preterm children. Moreover, these complications are modifiable and thus factors for further study and intervention. Several large cohort studies reported neonatal morbidity rates between 1990 and 2010. Studies from Israel, the Netherlands, Switzerland, the United States, England, and Canada reported decreased mortality rates for each week of gestation, while overall morbidity rates remained unchanged. One study from France showed reductions in overall morbidity. For the specific complications, results were mixed. Incidence rates of severe IVH, PVL, and necrotizing enterocolitis remained either stable or decreased. Moreover, as shown in the present meta-analysis, rates of these complications in follow-up samples are relatively low (3.1% [severe IVH], 3.6% [PVL], and 4.6% [necrotizing enterocolitis]). This is different for BPD. Aforementioned cohort studies showed stable or increased incidence rates for BPD over the past decades, and the median incidence rate based on studies included in this meta-analysis was as high as 27%. We showed that BPD plays a key role in long-term cognitive outcomes in EP/VP children.
Previous studies showed a positive association between BPD and brain abnormalities in preterm infants. The mechanisms underlying the association between BPD, brain development, and subsequent cognitive outcome remain to be further elucidated. One factor implicated in the pathogenesis of both BPD and brain injury in preterm infants is oxidative stress, which is defined as an imbalance between the generation of free radicals and antioxidant defense capacity. Because of exposure to high oxygen concentrations, infection and inflammation, reduced antioxidant defense, and free iron release, EP/VP children are at high risk for oxidative stress. Immaturity of organs, hypoxia-ischemia, hyperoxia, inadequate nutrition, and mechanical ventilation further increase the risk for free radical–induced injury. Animal studies have shown that hyperoxia results in both lung and brain injury, and the severity of lung injury was positively related to the severity of brain injury. Regardless of whether it is causal, the association between BPD and brain abnormalities may depend on common pathogenic mechanisms induced by shared and unshared risk factors. Moreover, postnatal corticosteroids were widely used in the past to treat BPD, while later evidence showed substantial adverse consequences for neurodevelopment. Results of the present meta-regression analysis do not suggest that the strong association between BPD and intelligence can be mainly attributed to postnatal corticosteroid use. However, given the well-established negative consequences for brain development and the interrelatedness of BPD and postnatal corticosteroid use, their influence on neurodevelopment cannot be seen in isolation. Future studies on the mechanisms underlying the association between BPD and neurodevelopment should take the postnatal use of corticosteroids into account. Furthermore, the neurodevelopmental safety of corticosteroid therapies other than dexamethasone—the most common kind of corticosteroid used in the present studies—should be explored. For example, recent evidence suggests that hydrocortisone may affect neurocognitive development to a lesser extent than dexamethasone.
The rates of BPD varied across studies and were associated with the immaturity of the infants included. It remains to be evaluated in future studies whether differences in clinical practice may also explain part of this variation. To our knowledge, no single prevention or intervention strategy was proven to have a considerable influence on the incidence of BPD. For example, lowering the initial oxygen concentration during resuscitation to 30% had no benefits with respect to the risk for BPD and oxidative stress compared with 65% oxygen. The fact that multiple factors seem to be involved in the development of BPD and neonatal brain injury advocates a multifactorial approach for prevention and treatment. Potential strategies may include an optimal ventilation strategy and oxygen concentration, anti-inflammatory agents, antioxidant therapy, and adequate nutritional support. Finally, recent evidence points at the potential of stem cell–based therapies as a highly promising treatment for neonatal lung and brain injury.
Limitations
One limitation of the present meta-analysis is the number of missing details for demographic and perinatal risk factors. Despite our effort to contact all authors for additional data and the subsequent effort of many authors to provide these data, the proportion of missing data for covariates was relatively high. Although the correlation between the proportion of missing data and the outcome of studies was weak, missing data may still form a source of bias as meta-regression analyses could be performed only using a subsample of available studies. The number of studies in our analyses was nevertheless large and we found no evidence for publication bias or bias related to study quality. Another limitation relates to the differences in definitions of morbidities across studies, subsequently affecting incidence rates. In addition, a number of studies did not report which definition was used. This lack of information hampered direct assessment of the influence of varying definitions on our results. Differences in definitions may arise as a function of time (eg, the 28-day definition of BPD may be mainly used in older cohorts). However, controlling for birth year did not alter our results. Studies used various measures of socioeconomic status, which limited the possibility to properly assess its role. In contrast to the present findings, previous studies showed the importance of socioeconomic factors for cognitive outcomes of preterm children. This association may even be underestimated since it is recognized that children from lower socioeconomic backgrounds are more frequently lost to follow-up. Meta-regression uses aggregated instead of individual patient data, which results in a loss of information and may explain the absence of this association in our study. While reducing neonatal complications or counteracting their effects is important to improve long-term outcomes of preterm children, socioeconomic factors should not escape our attention.
Conclusions
In this meta-analysis, robust evidence was found for large deficits in intelligence in children born EP/VP from 1990 to 2008. Despite advancing neonatal health care, the results show no indication of improvements in long-term cognitive outcome during this period. Bronchopulmonary dysplasia was found to be a crucial factor for long-term cognitive outcome and the incidence rate remains high. This finding suggests that reducing the incidence of BPD in EP/VP children would be beneficial.
eTable. Study Characteristics, IQ of EP/VP Born and Full-Term Born Children, and Demographic and Perinatal Characteristics of the EP/VP Born Sample
eFigure 1. PRISMA Flowchart of the Study Selection Procedure
eFigure 2. Forest Plot of Standardized Mean Differences (SMD) in IQ Between Extremely/Very Preterm Children and Full-Term Controls
eFigure 3. Funnel Plot of the Standardized Mean Difference in IQ for Each Study Plotted Against Its Standard Error
eReferences
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162-2172. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RM, Luby AM, Scanlon JW, Kellogg RJ. Effect of surfactant on morbidity, mortality, and resource use in newborn infants weighing 500 to 1500 g. N Engl J Med. 1994;330(21):1476-1480. [DOI] [PubMed] [Google Scholar]
- 3.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115(4):997-1003. [DOI] [PubMed] [Google Scholar]
- 4.Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717-728. [DOI] [PubMed] [Google Scholar]
- 5.de Kieviet JF, Piek JP, Aarnoudse-Moens CS, Oosterlaan J. Motor development in very preterm and very low-birth-weight children from birth to adolescence: a meta-analysis. JAMA. 2009;302(20):2235-2242. [DOI] [PubMed] [Google Scholar]
- 6.Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32(1):51-58. [DOI] [PubMed] [Google Scholar]
- 7.Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol. 2008;21(2):123-128. [DOI] [PubMed] [Google Scholar]
- 8.Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ; Victorian Infant Collaborative Study Group . School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013;131(4):e1053-e1061. [DOI] [PubMed] [Google Scholar]
- 9.Koenen KC, Moffitt TE, Roberts AL, et al. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry. 2009;166(1):50-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottfredson LS. Intelligence: is it the epidemiologists’ elusive “fundamental cause” of social class inequalities in health? J Pers Soc Psychol. 2004;86(1):174-199. [DOI] [PubMed] [Google Scholar]
- 11.Petrill SA, Wilkerson B. Intelligence and achievement: a behavioral genetic perspective. Educ Psychol Rev. 2000;12(2):185-199. [Google Scholar]
- 12.Strenze T. Intelligence and socioeconomic success: a meta-analytic review of longitudinal research. Intelligence. 2007;35(5):401-426. [Google Scholar]
- 13.Gottfredson LS. Why g matters: the complexity of everyday life. Intelligence. 1997;24(1):79-132. [Google Scholar]
- 14.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728-737. [DOI] [PubMed] [Google Scholar]
- 15.Kerr-Wilson CO, Mackay DF, Smith GC, Pell JP. Meta-analysis of the association between preterm delivery and intelligence. J Public Health (Oxf). 2012;34(2):209-216. [DOI] [PubMed] [Google Scholar]
- 16.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70(4):541-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keunen K, van Elburg RM, van Bel F, Benders MJ. Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr Res. 2015;77(1-2):148-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 2012;54(4):313-323. [DOI] [PubMed] [Google Scholar]
- 20.Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121(2):306-316. [DOI] [PubMed] [Google Scholar]
- 21.Skranes J, Vangberg TR, Kulseng S, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130(pt 3):654-666. [DOI] [PubMed] [Google Scholar]
- 22.Soria-Pastor S, Gimenez M, Narberhaus A, et al. Patterns of cerebral white matter damage and cognitive impairment in adolescents born very preterm. Int J Dev Neurosci. 2008;26(7):647-654. [DOI] [PubMed] [Google Scholar]
- 23.Short EJ, Klein NK, Lewis BA, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112(5):e359-e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M. Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr. 2006;149(2):169-173. [DOI] [PubMed] [Google Scholar]
- 25.Zubiaurre-Elorza L, Soria-Pastor S, Junqué C, et al. Thalamic changes in a preterm sample with periventricular leukomalacia: correlation with white-matter integrity and cognitive outcome at school age. Pediatr Res. 2012;71(4, pt 1):354-360. [DOI] [PubMed] [Google Scholar]
- 26.Stoll BJ, Hansen NI, Adams-Chapman I, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357-2365. [DOI] [PubMed] [Google Scholar]
- 27.Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92(3):F193-F198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284(15):1939-1947. [DOI] [PubMed] [Google Scholar]
- 29.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153(2):170-175, 175.e1. [DOI] [PubMed] [Google Scholar]
- 30.Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr. 2001;1(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson S, Fawke J, Hennessy E, et al. Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics. 2009;124(2):e249-e257. [DOI] [PubMed] [Google Scholar]
- 32.Wong HS, Edwards P. Nature or nurture: a systematic review of the effect of socio-economic status on the developmental and cognitive outcomes of children born preterm. Matern Child Health J. 2013;17(9):1689-1700. [DOI] [PubMed] [Google Scholar]
- 33.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 34.Borenstein M, Hedges LV, Higgins J, Rothstein HR. Independent Subgroups Within a Study: Introduction to Meta-analysis. Chichester, UK: John Wiley & Sons, Ltd; 2009:217-224. [Google Scholar]
- 35.Joseph RM, O’Shea TM, Allred EN, et al. ; ELGAN Study Investigators . Neurocognitive and academic outcomes at age 10 years of extremely preterm newborns. Pediatrics. 2016;137(4):e20154343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basten M, Jaekel J, Johnson S, Gilmore C, Wolke D. Preterm birth and adult wealth: mathematics skills count. Psychol Sci. 2015;26(10):1608-1619. [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 38.Wilson-Costello D, Friedman H, Minich N, et al. Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000-2002. Pediatrics. 2007;119(1):37-45. [DOI] [PubMed] [Google Scholar]
- 39.Cheong JLY, Anderson PJ, Burnett AC, et al. ; Victorian Infant Collaborative Study Group . Changing neurodevelopment at 8 years in children born extremely preterm since the 1990s. Pediatrics. 2017;139(6):e20164086. [DOI] [PubMed] [Google Scholar]
- 40.Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grisaru-Granovsky S, Reichman B, Lerner-Geva L, et al. ; Israel Neonatal Network . Population-based trends in mortality and neonatal morbidities among singleton, very preterm, very low birth weight infants over 16 years. Early Hum Dev. 2014;90(12):821-827. [DOI] [PubMed] [Google Scholar]
- 42.Groenendaal F, Termote JU, van der Heide-Jalving M, van Haastert IC, de Vries LS. Complications affecting preterm neonates from 1991 to 2006: what have we gained? Acta Paediatr. 2010;99(3):354-358. [DOI] [PubMed] [Google Scholar]
- 43.Chen F, Bajwa NM, Rimensberger PC, Posfay-Barbe KM, Pfister RE; Swiss Neonatal Network . Thirteen-year mortality and morbidity in preterm infants in Switzerland. Arch Dis Child Fetal Neonatal Ed. 2016;101(5):F377-F383. [DOI] [PubMed] [Google Scholar]
- 44.Fanaroff AA, Stoll BJ, Wright LL, et al. ; NICHD Neonatal Research Network . Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147.e1-147.e8. [DOI] [PubMed] [Google Scholar]
- 45.Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ. 2012;345:e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah PS, Sankaran K, Aziz K, et al. ; Canadian Neonatal Network . Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J Perinatol. 2012;32(2):132-138. [DOI] [PubMed] [Google Scholar]
- 47.Ancel P-Y, Goffinet F, Kuhn P, et al. ; EPIPAGE-2 Writing Group . Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015;169(3):230-238. [DOI] [PubMed] [Google Scholar]
- 48.Neubauer V, Junker D, Griesmaier E, Schocke M, Kiechl-Kohlendorfer U. Bronchopulmonary dysplasia is associated with delayed structural brain maturation in preterm infants. Neonatology. 2015;107(3):179-184. [DOI] [PubMed] [Google Scholar]
- 49.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130(pt 3):667-677. [DOI] [PubMed] [Google Scholar]
- 50.Perrone S, Tataranno LM, Stazzoni G, Ramenghi L, Buonocore G. Brain susceptibility to oxidative stress in the perinatal period. J Matern Fetal Neonatal Med. 2015;28(suppl 1):2291-2295. [DOI] [PubMed] [Google Scholar]
- 51.Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA. Reprint of “the developing oligodendrocyte: key cellular target in brain injury in the premature infant.” Int J Dev Neurosci. 2011;29(6):565-582. [DOI] [PubMed] [Google Scholar]
- 52.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003;8(1):39-49. [DOI] [PubMed] [Google Scholar]
- 53.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82(2):291-295. [DOI] [PubMed] [Google Scholar]
- 54.Perrone S, Tataranno ML, Buonocore G. Oxidative stress and bronchopulmonary dysplasia. J Clin Neonatol. 2012;1(3):109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pham H, Vottier G, Pansiot J, et al. Inhaled NO prevents hyperoxia-induced white matter damage in neonatal rats. Exp Neurol. 2014;252:114-123. [DOI] [PubMed] [Google Scholar]
- 56.Poon AWH, Ma EXH, Vadivel A, et al. Impact of bronchopulmonary dysplasia on brain and retina. Biol Open. 2016;5(4):475-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baud O, Trousson C, Biran V, Leroy E, Mohamed D, Alberti C; PREMILOC Trial Group . Association between early low-dose hydrocortisone therapy in extremely preterm neonates and neurodevelopmental outcomes at 2 years of age. JAMA. 2017;317(13):1329-1337. [DOI] [PubMed] [Google Scholar]
- 58.Ali Z, Schmidt P, Dodd J, Jeppesen DL. Bronchopulmonary dysplasia: a review. Arch Gynecol Obstet. 2013;288(2):325-333. [DOI] [PubMed] [Google Scholar]
- 59.Boronat N, Aguar M, Rook D, et al. Survival and neurodevelopmental outcomes of preterms resuscitated with different oxygen fractions. Pediatrics. 2016;138(6):e20161405. [DOI] [PubMed] [Google Scholar]
- 60.Cools F, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2015;(3):CD000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saugstad OD, Aune D, Aguar M, Kapadia V, Finer N, Vento M. Systematic review and meta-analysis of optimal initial fraction of oxygen levels in the delivery room at ≤32 weeks. Acta Paediatr. 2014;103(7):744-751. [DOI] [PubMed] [Google Scholar]
- 62.Wright CJ, Kirpalani H. Targeting inflammation to prevent bronchopulmonary dysplasia: can new insights be translated into therapies? Pediatrics. 2011;128(1):111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JW, Davis JM. Future applications of antioxidants in premature infants. Curr Opin Pediatr. 2011;23(2):161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biniwale MA, Ehrenkranz RA. The role of nutrition in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 2006;30(4):200-208. [DOI] [PubMed] [Google Scholar]
- 65.Mitsialis SA, Kourembanas S. Stem cell–based therapies for the newborn lung and brain: possibilities and challenges. Semin Perinatol. 2016;40(3):138-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howe LD, Tilling K, Galobardes B, Lawlor DA. Loss to follow-up in cohort studies: bias in estimates of socioeconomic inequalities. Epidemiology. 2013;24(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Study Characteristics, IQ of EP/VP Born and Full-Term Born Children, and Demographic and Perinatal Characteristics of the EP/VP Born Sample
eFigure 1. PRISMA Flowchart of the Study Selection Procedure
eFigure 2. Forest Plot of Standardized Mean Differences (SMD) in IQ Between Extremely/Very Preterm Children and Full-Term Controls
eFigure 3. Funnel Plot of the Standardized Mean Difference in IQ for Each Study Plotted Against Its Standard Error
eReferences