Key Points
Question
How is systolic blood pressure associated with outcomes in patients with heart failure with preserved ejection fraction?
Findings
In a propensity score–matched observational study of hospitalized patients with HF and ejection fraction 50% or greater in the national Medicare-linked OPTIMIZE-HF registry, a discharge systolic blood pressure level of less than 120 mm Hg was associated with a significantly higher risk of 30-day, 1-year, and long-term all-cause mortality.
Meaning
A systolic blood pressure level of less than 120 mm Hg identifies patients with heart failure with preserved ejection fraction at higher risk for short- and long-term mortality and emphasizes the need for future prospective studies to evaluate optimal systolic blood pressure treatment goals in this patient population.
Abstract
Importance
Lower systolic blood pressure (SBP) levels are associated with poor outcomes in patients with heart failure. Less is known about this association in heart failure with preserved ejection fraction (HFpEF).
Objective
To determine the associations of SBP levels with mortality and other outcomes in HFpEF.
Design, Setting, and Participants
A propensity score–matched observational study of the Medicare-linked Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry included 25 354 patients who were discharged alive; 8873 (35.0%) had an ejection fraction of at least 50%, and of these, 3915 (44.1%) had stable SBP levels (≤20 mm Hg admission to discharge variation). Data were collected from 259 hospitals in 48 states between March 1, 2003, and December 31, 2004. Data were analyzed from March 1, 2003, to December 31, 2008.
Exposure
Discharge SBP levels less than 120 mm Hg. A total of 1076 of 3915 (27.5%) had SBP levels less than 120 mm Hg, of whom 901 (83.7%) were matched by propensity scores with 901 patients with SBP levels of 120 mm Hg or greater who were balanced on 58 baseline characteristics.
Main Outcomes and Measures
Thirty-day, 1-year, and overall all-cause mortality and heart failure readmission through December 31, 2008.
Results
The 1802 matched patients had a mean (SD) age of 79 (10) years; 1147 (63.7%) were women, and 134 (7.4%) were African American. Thirty-day all-cause mortality occurred in 91 (10%) and 45 (5%) of matched patients with discharge SBP of less than 120 mm Hg vs 120 mm Hg or greater, respectively (hazard ratio [HR], 2.07; 95% CI, 1.45-2.95; P < .001). Systolic blood pressure level less than 120 mm Hg was also associated with a higher risk of mortality at 1 year (39% vs 31%; HR, 1.36; 95% CI, 1.16-1.59; P < .001) and during a median follow-up of 2.1 (overall 6) years (HR, 1.17; 95% CI, 1.05-1.30; P = .005). Systolic blood pressure level less than 120 mm Hg was associated with a higher risk of heart failure readmission at 30 days (HR, 1.47; 95% CI, 1.08-2.01; P = .02) but not at 1 or 6 years. Hazard ratios for the combined end point of heart failure readmission or all-cause mortality associated with SBP level less than 120 mm at 30 days, 1 year, and overall were 1.71 (95% CI, 1.34-2.18; P < .001), 1.21 (95% CI, 1.07-1.38; P = .004), and 1.12 (95% CI, 1.01-1.24; P = .03), respectively.
Conclusions and Relevance
Among hospitalized patients with HFpEF, an SBP level less than 120 mm Hg is significantly associated with poor outcomes. Future studies need to prospectively evaluate optimal SBP treatment goals in patients with HFpEF.
This observational study examines the mortality of patients with systolic blood pressure and other outcomes in heart failure with preserved ejection fraction.
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is common, and patients with HFpEF have similar poor outcomes as those with HF with reduced ejection fraction (EF).1,2 The American College of Cardiology/American Heart Association/Heart Failure Society of America guideline for HF recommends that systolic blood pressure (SBP) should be controlled in patients with HFpEF,3 and its 2017 update4 makes a new recommendation for an optimal SBP target level of less than 130 mm Hg for patients with HFpEF and persistent hypertension. However, an optimal SBP target level is less clear for patients with HFpEF. Hypertension is a major risk factor for incident HF, and treatment of hypertension, particularly to an SBP target level of less than 120 mm Hg, has been demonstrated to substantially lower the risk of incident HF.5,6,7,8 However, once patients develop HF, a lower SBP level may have a paradoxical association with a higher risk of cardiovascular morbidity and mortality.9,10,11 Less is known about this association in patients with HFpEF, the examination of which is the objective of this study.
Methods
Data Source and Study Population
The current analyses are based on data from the Medicare-linked Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry, the details of which have been published before.12,13,14 Briefly, medical records of 48 612 HF hospitalizations from 259 hospitals in 48 states were abstracted using a web-based information system. These patients were hospitalized between March 1, 2003, and December 31, 2004, and had an International Classification of Diseases, Ninth Revision, Clinical Modification code for HF as a principal discharge diagnosis. Data on demographics characteristics, medical history, symptoms, signs, admission and discharge medications, inpatient procedures, and short-term outcomes were collected.12,15 Long-term outcomes data were obtained by linking data from the OPTIMIZE-HF registry to the Medicare data up to December 31, 2008, using a probabilistic linking approach, which identified 26 376 unique patients. A total of 25 354 were discharged alive,13,15 of whom 8873 (35.0%) had HFpEF defined as EF of 50% or greater.3
The original OPTIMIZE-HF protocol was approved by the local or central institutional review boards. The current study is based on a deidentified copy of the Medicare-linked OPTIMZE-HF data approved by the Centers for Medicare and Medicaid Services and by the local institutional review board and Research and Development Committee. Written informed consent was not required owing to the retrospective nature of the study.
Assembly of a Cohort With Stable SBP
Data on SBP levels were collected from patients in the supine position from times closest to admission and discharge, and automated electronic data checks were used to prevent outlying SBP values.9 We excluded 85 patients who had SBP levels greater than 300 mm Hg or less than 60 mm Hg. We restricted our analysis to 3915 patients (44.1%) with stable inpatient SBP levels, defined as an admission to discharge variation of 20 mm Hg or less (median, –4; range, –20 to 20). Of the 3915 patients with stable SBP levels, 1076 patients (27.5%) had a discharge SBP level of less than 120 mm Hg (eFigure 1 in the Supplement). We chose the SBP cutoff of 120 because SBP level less than 120 mm Hg has been shown to be associated with poor outcomes in HF.9,10,11
Of the 4873 patients (54.9%) with a variable inpatient SBP level (admission to discharge variation >20 mm Hg), 4077 patients (83.7%) had a mean (median; range) decrease of –47 mm Hg (–41 mm Hg; –178 to –21 mm Hg), and 796 patients (14.5%) had a mean (median; range) increase of 35 mm Hg (32 mm Hg; 21 to 102 mm Hg). We chose to restrict our primary analysis to patients with stable inpatient SBP levels to minimize bias due to measurement errors and acute inpatient events affecting SBP. This also allowed us to avoid potential exposure misclassification. For example, a patient with a high admission SBP level (eg, 180 mm Hg) and an in-hospital decrease of 80 mm Hg would be misclassified in the lower (<120 mm Hg) discharge SBP group.
Assembly of a Balanced Cohort
To attenuate bias due to imbalances in baseline characteristics and to enhance our ability to draw inferences about association, we used propensity score matching to assemble a cohort in which patients with SBP levels less than 120 mm Hg vs 120 mm Hg or greater would be expected to be balanced on all measured baseline characteristics.16,17,18,19 We estimated propensity scores for discharge SBP level less than 120 mm Hg for each of the 3915 patients with a stable SBP using a nonparsimonious multivariable logistic regression model, in which SBP level less than 120 mm Hg was used as the dependent variable and the 58 baseline variables were used as covariates (eFigure 2 in the Supplement). We included diastolic blood pressure (DBP) in the model to attenuate any independent association of DBP level with outcomes. However, we also repeated the model after excluding DBP.
The propensity score for SBP level less than 120 mm Hg for a patient is that patient’s probability of having an SBP level less than 120 mm Hg given the 58 baseline characteristics used in the propensity score model (57 for the model without DBP). We then used a matching algorithm, described previously,20 to match 901 of 1076 patients (83.7%) with an SBP level of less than 120 mm Hg with 901 patients with an SBP level of 120 mm Hg or greater who had similar propensity scores, thus assembling a matched cohort of 1802 patients (eFigure 1A in the Supplement). Between-group postmatch balance was assessed by estimating absolute standardized differences for each of the 58 baseline characteristics and presented as a Love plot (eFigure 2 in the Supplement).21 An absolute standardized difference of 0% indicates no residual bias, and values less than 10% indicate inconsequential residual bias.
Assembly of Sensitivity Cohorts
To ensure that the findings of our primary cohort were not sensitive to methodological approaches described earlier, we assembled 4 separate sensitivity cohorts. First, we repeated the above process using 4873 patients (54.9%) without a stable SBP level, defined as an admission to discharge variation of at least 20 mm Hg. Of these, 4077 patients (83.7%) had a decrease in SBP level from admission to discharge and a median discharge SBP level of 122 mm Hg. Overall, 1736 patients (42.6%) had a SBP level less than 120 mm Hg, of which 1116 (64.3%) could be matched by propensity scores, thus assembling a matched cohort of 2232 patients (eFigure 1B in the Supplement). The 2232 matched patients had a median (range) decrease in SBP from admission to discharge of –40 mm Hg (–21 to –165 mm Hg) and were balanced on 58 baseline characteristics. We excluded the 796 patients with a variable increase in SBP because they would be expected to be characteristically (only 6% had and SBP level <120 mm Hg) and prognostically different from those with a decrease in SBP level.
We then assembled 2 other sensitivity cohorts using all patients regardless of admission to discharge SBP variations: 4582 matched patients with admission SBP level less than 120 mm Hg vs 120 mm Hg or greater (eFigure 1C in the Supplement) and 2958 matched patients with discharge SBP level less than 120 vs 120 mm Hg or greater (eFigure 1D in the Supplement). Finally, because of the recent guideline recommendations of a target SBP level of less than 130 mm Hg in patients with HFpEF and persistent hypertension,22,23 we assembled another sensitivity cohort in which lower SBP level was defined as less than 130 mm Hg using the prematching data of 3915 patients of our primary analysis. We matched 1114 of 1785 patients (62.4%) with an SBP level less than 130 mm Hg, thus assembling a propensity score–matched cohort of 2228 patients with discharge SBP levels less than 130 mm Hg vs 130 mm Hg or greater that were balanced on 58 baseline characteristics.
Outcomes Data
Our primary outcomes included all-cause mortality and HF readmission at 30 days, 1 year, and during overall median follow-up of 2.1 (overall 6) years up to December 31, 2008. Secondary outcomes included all-cause readmission and the 2 combined end points of all-cause readmission or all-cause mortality and HF readmission or all-cause mortality. All outcomes data were collected from Medicare 100% MedPAR File and 100% Beneficiary Summary File.13
Statistical Analyses
Between-group baseline characteristics were compared using Pearson χ2 and Wilcoxon rank-sum tests, as appropriate. All outcome analyses were conducted using matched data. Kaplan-Meier survival analysis was used to compare cumulative risk of all-cause mortality by discharge SBP level less than 120 vs 120 mm Hg or greater, censoring patients at readmission or study end, whichever came first. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals for outcomes associated with SBP level less than 120 mm Hg using SBP level of 120 mm Hg or greater as reference. For the readmission model, patients were censored at death or study end, whichever came first. To assess for nonlinearity, we fitted restricted cubic spline models with 4 knots at SBP levels of 110 mm Hg, 120 mm Hg (reference), 140 mm Hg, and 150 mm Hg using both matched data and prematched data, adjusting for propensity scores. Formal sensitivity analyses were conducted to quantify the degree of hidden bias that could potentially explain any significant associations. Subgroup analyses were conducted to assess homogeneity of the association between discharge SBP less than 120 mm Hg and overall all-cause mortality in clinically relevant subgroups of patients in the primary matched cohort. All statistical analyses were conducted using SPSS statistical software version 24 (IBM SPSS) and SAS statistical software version 9.4 (SAS Institute Inc).
Results
Baseline Characteristics
The 1802 matched patients had a mean (SD) age of 79 (10) years and a mean (SD) EF of 59% (7%). A total of 1147 (63.7%) were women and 134 (7.4%) were African American. The mean (median; range) SBP level was 121 (120; 75-193) mm Hg, with only 13 matched patients having an SBP level less than 90 mm Hg (2 had <80 mm Hg). Before matching, patients with a discharge SBP level less than 120 mm Hg had a lower prevalence of hypertension and diabetes and a higher prevalence of atrial fibrillation, and fewer of these patients were taking angiotensin converting enzyme (ACE) inhibitors and β-blockers (Table 1). These and other measured baseline characteristics were balanced after matching; the absolute standardized differences for all 58 baseline characteristics were less than 10% (Table 1; eFigure 2 in the Supplement).
Table 1. Baseline Characteristics by Discharge Systolic Blood Pressure (SBP) Level in Patients With Heart Failure With Left Ventricular Ejection Fraction of 50% or Greater.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Before Propensity Score Matching (n = 3915) | After Propensity Score Matching (n = 1802) | |||||
| Discharge SBP Level | P Value | Discharge SBP Level | P Value | |||
| <120 mm Hg (n = 1076) | ≥120 mm Hg (n = 2839) | <120 mm Hg (n = 901) | ≥120 mm Hg (n = 901) | |||
| Age, mean (SD), y | 79 (11) | 78 (10) | .27 | 79 (11) | 79 (10) | .19 |
| Women | 670 (62) | 1879 (66) | .02 | 576 (64) | 571 (63) | .81 |
| African American | 85 (8) | 342 (12) | <.001 | 72 (8) | 62 (7) | .37 |
| Left ventricular ejection fraction | 59 (7) | 59 (7) | .66 | 59 (7) | 59 (7) | .82 |
| Medical history | ||||||
| No known prior heart failure | 121 (11) | 376 (13) | .09 | 101 (11) | 105 (12) | .77 |
| Hypertension | 670 (62) | 2234 (79) | <.001 | 591 (66) | 599 (67) | .69 |
| Coronary artery disease | 485 (45) | 1267 (45) | .80 | 409 (45) | 409 (45) | >.99 |
| Acute myocardial infarction | 172 (16) | 428 (15) | .48 | 146 (16) | 150 (17) | .80 |
| Coronary revascularization | 237 (22) | 664 (23) | .37 | 208 (23) | 200 (22) | .65 |
| Diabetes | 364 (34) | 1282 (45) | <.001 | 333 (37) | 325 (36) | .70 |
| Cerebrovascular disease | 182 (17) | 520 (18) | .31 | 151 (17) | 159 (18) | .62 |
| Peripheral vascular disease | 131 (12) | 409 (14) | .07 | 110 (12) | 112 (12) | .89 |
| Atrial fibrillation | 484 (45) | 1016 (36) | <.001 | 381 (42) | 391 (43) | .63 |
| Chronic obstructive pulmonary disease | 370 (34) | 893 (32) | .08 | 303 (34) | 315 (35) | .55 |
| Admission clinical findings | ||||||
| Dyspnea at rest | 450 (42) | 1222 (43) | .49 | 372 (41) | 383 (43) | .60 |
| Dyspnea on exertion | 667 (62) | 1774 (63) | .77 | 557 (62) | 569 (63) | .60 |
| Orthopnea | 248 (23) | 738 (26) | .06 | 201 (22) | 218 (24) | .34 |
| Paroxysmal nocturnal dyspnea | 115 (11) | 397 (14) | .006 | 96 (11) | 97 (11) | .94 |
| Chest pain | 197 (18) | 625 (22) | .01 | 168 (19) | 169 (19) | .95 |
| Jugular venous pressure elevation | 296 (28) | 721 (25) | .18 | 239 (27) | 232 (26) | .71 |
| Peripheral edema | 725 (67) | 1939 (68) | .58 | 599 (67) | 591 (66) | .69 |
| SBP, mm Hga | 113 (12) | 143 (19) | <.001 | 114 (12) | 137 (16) | <.001 |
| Discharge clinical findings | ||||||
| Heart rate, bpm | 76 (14) | 75 (19) | .01 | 76 (14) | 75 (14) | .08 |
| SBP, mm Hga | 108 (8) | 141 (16) | <.001 | 109 (8) | 135 (13) | <.001 |
| Diastolic blood pressure, mm Hg | 60 (9) | 69 (12) | <.001 | 62 (8) | 61 (9) | .61 |
| Serum creatinine, mean (SD), mg/dL | 1.53 (0.9) | 1.67 (1.3) | .001 | 1.53 (0.9) | 1.55 (1.2) | .75 |
| Discharge medications | ||||||
| ACE inhibitors or ARBs | 503 (47) | 1616 (57) | <.001 | 437 (49) | 447 (50) | .64 |
| β- blockers | 573 (53) | 1611 (57) | .049 | 489 (54) | 480 (53) | .67 |
| Metoprolol | 298 (28) | 877 (31) | .05 | 251 (28) | 258 (29) | .71 |
| Carvedilol | 125 (12) | 278 (10) | .09 | 112 (12) | 90 (10) | .10 |
| Aldosterone antagonists | 108 (10) | 202 (7) | .003 | 76 (8) | 82 (9) | .62 |
| Digoxin | 266 (25) | 526 (19) | <.001 | 206 (23) | 195 (22) | .53 |
| Loop diuretics | 893 (83) | 2266 (80) | .03 | 739 (82) | 742 (82) | .85 |
| Nitrates | 206 (19) | 714 (25) | <.001 | 179 (20) | 184 (20) | .77 |
| Amlodipine | 57 (5) | 347 (12) | <.001 | 54 (6) | 45 (5) | .35 |
| Antiarrhythmic drugs | 132 (12) | 296 (10) | <.001 | 113 (13) | 110 (12) | .83 |
| Warfarin | 348 (32) | 722 (25) | <.001 | 275 (31) | 279 (31) | .84 |
| Aspirin | 450 (42) | 1237 (44) | .32 | 380 (42) | 388 (43) | .97 |
| Statins | 299 (28) | 925 (33) | .004 | 268 (30) | 265 (29) | .88 |
| Hospital length of stay, d | 6 (5) | 6 (5) | <.001 | 6 (5) | 6 (5) | .64 |
| Hospital characteristics | ||||||
| Bed size, No. | 390 (236) | 394 (240) | .56 | 389 (234) | 390 (239) | .86 |
| Academic center | 462 (43) | 1195 (42) | .71 | 475 (42) | 390 (43) | .48 |
| Transplant center | 169 (16) | 394 (14) | .15 | 141 (16) | 132 (15) | .55 |
| Interventional center | 845 (79) | 2166 (76) | .14 | 699 (78) | 697 (77) | .91 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
SI conversion factor: To convert serum creatinine to micromoles per liter, multiply by 88.4.
Systolic blood pressure is the exposure variable and would not be expected to be balanced in the matched cohort; presented for descriptive purposes only.
Outcomes in Patients With HF, EF 50% or Greater, and Stable SBP
Thirty-day all-cause mortality occurred in 91 (10%) and 45 (5%) of matched patients (n = 1802) with a discharge SBP level less than 120 vs 120 mm Hg or greater, respectively (HR, 2.07; 95% CI, 1.45-2.95; P < .001) ( Table 2). The association between an SBP level less than 120 mm Hg and higher risk of all-cause mortality persisted both at 1 year (39% vs 31%; HR, 1.36; 95% CI, 1.16-1.59; P < .001) (Table 2) and 6 years (75% vs 71%; HR, 1.17; 95% CI, 1.05-1.30; P = .005) (Table 2; Figure 1). Findings from our restricted cubic spline analysis demonstrate that there was no evidence of a nonlinear association between SBP and all-cause mortality (P > .20) (Figure 2). Findings from our subgroup analyses demonstrate that the association between SBP levels less than 120 mm Hg and overall all-cause mortality in our matched cohort was homogenous across various clinically relevant subgroups of patients, except those with a glomerular filtration rate less than 45 mL/min/1.73 m2 and those who received a discharge prescription for ACE inhibitors (Figure 3).
Table 2. Outcomes by Discharge Systolic Blood Pressure (SBP) Level in 1802 Propensity Score–Matched Patients With Heart Failure With Ejection Fraction of 50% or Greater.
| Outcome | Events, No. (%) | SBP Level <120 mm Hg, Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Discharge SBP Level <120 mm Hg (n = 901) | Discharge SBP Level ≥120 mm Hg (n = 901) | |||
| 30 d | ||||
| All-cause mortality | 91 (10) | 45 (5) | 2.07 (1.45-2.95) | .001 |
| All-cause readmission | 238 (26) | 198 (22) | 1.24 (1.03-1.50) | .02 |
| Heart failure readmission | 95 (11) | 67 (7) | 1.47 (1.08-2.01) | .02 |
| All-cause readmission or all-cause mortality | 290 (32) | 222 (25) | 1.35 (1.13-1.61) | .001 |
| Heart failure readmission or all-cause mortality | 172 (19) | 104 (12) | 1.71 (1.34-2.18) | .001 |
| 1 y | ||||
| All-cause mortality | 349 (39) | 278 (31) | 1.36 (1.16-1.59) | .001 |
| All-cause readmission | 588 (65) | 578 (64) | 1.13 (1.01-1.26) | .04 |
| Heart failure readmission | 251 (28) | 246 (27) | 1.11 (0.93-1.33) | .23 |
| All-cause readmission or all-cause mortality | 683 (76) | 639 (71) | 1.18 (1.06-1.32) | .002 |
| Heart failure readmission or all-cause mortality | 485 (54) | 437 (49) | 1.21 (1.07-1.38) | .004 |
| Overall | ||||
| All-cause mortality | 671 (75) | 640 (71) | 1.17 (1.05-1.30) | .01 |
| All-cause readmission | 759 (84) | 786 (87) | 1.09 (0.99-1.21) | .09 |
| Heart failure readmission | 376 (42) | 390 (43) | 1.06 (0.92-1.22) | .41 |
| All-cause readmission or all-cause mortality | 870 (97) | 873 (97) | 1.12 (1.02-1.23) | .02 |
| Heart failure readmission or all-cause mortality | 762 (85) | 750 (83) | 1.12 (1.01-1.24) | .03 |
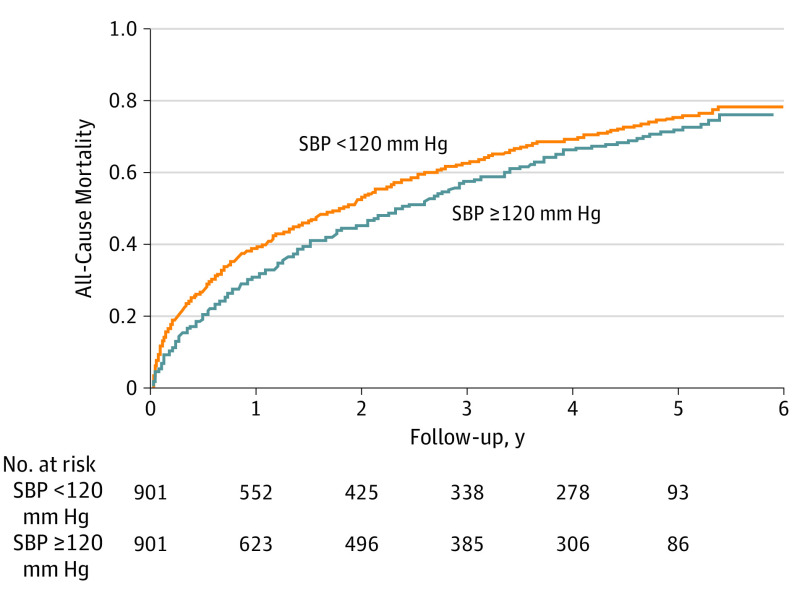
Figure 1. Kaplan-Meier Plots for All-Cause Mortality by Systolic Blood Pressure (SBP) Level.
Kaplan-Meier plot for all-cause mortality in 901 pairs of propensity score–matched patients with heart failure and left ventricular ejection fraction of 50% or greater, by SBP level less than 120 vs 120 mm Hg or greater. Hazard ratios for all-cause mortality at 1 month, 1 year, and overall were 2.07 (95% CI, 1.45-2.95; P < .001), 1.36 (95% CI, 1.16-1.59; P < .001), and 1.17 (95% CI, 1.05-1.30; P = .005), respectively.
Figure 2. Restricted Cubic Spline Plots for All-Cause Mortality by Systolic Blood Pressure.
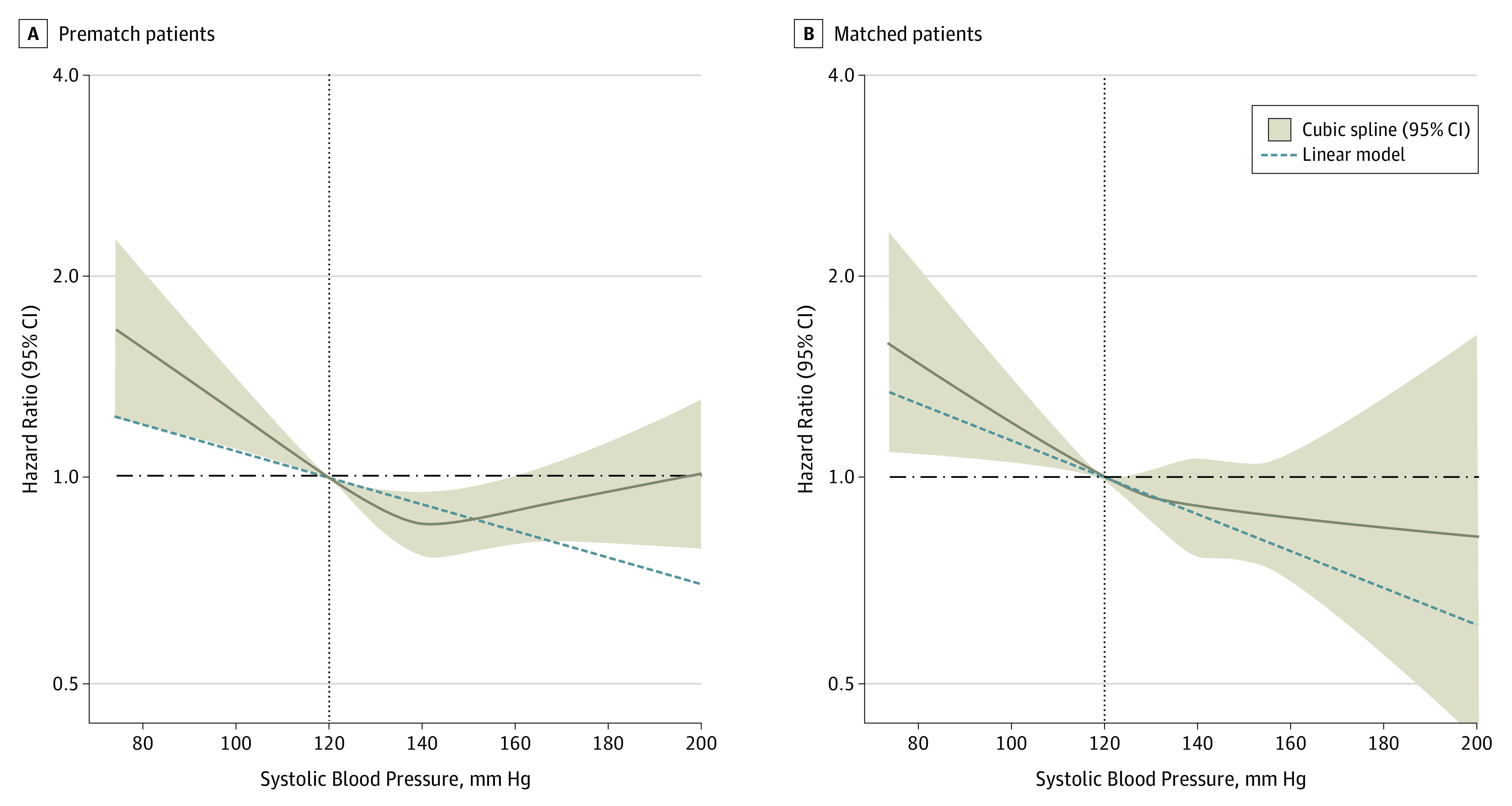
Hazard ratios and 95% confidence intervals for all-cause mortality by discharge systolic blood pressure level in 3915 patients with heart failure with preserved ejection fraction of 50% or greater according to restricted cubic spline regression models using 4 knots at blood pressures of 110 mm Hg, 120 mm Hg (reference), 140 mm Hg, and 150 mm Hg. Solid black lines indicate hazard ratios, and shaded areas indicate 95% CI. Plots on the left panel (A) are based on 3906 prematch patients (9 prematch patients had systolic blood pressure levels >200 mm Hg and were excluded), adjusting for propensity scores, and those on the right panel (B) are based on 1802 matched patients (none had systolic blood pressure levels >200 mm Hg) balanced on 58 baseline characteristics. Spline curves were truncated at a systolic blood pressure level of 200 mm Hg.
Figure 3. Forest Plots for Subgroup Analyses of Mortality by Systolic Blood Pressure (SBP) Level.
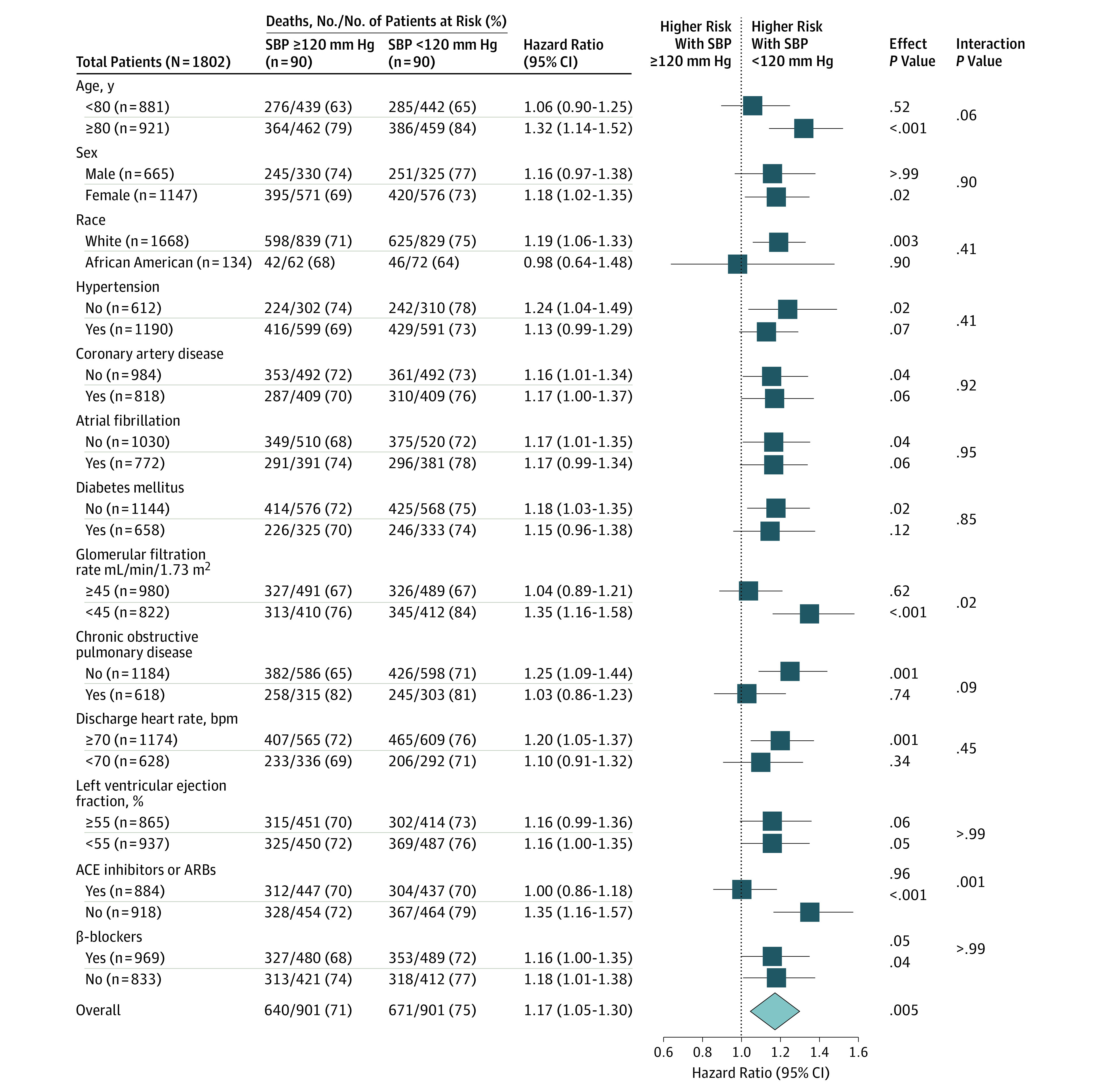
Forest plots displaying hazard ratios and 95% confidence intervals for all-cause mortality in subgroups of propensity score–matched patients with heart failure and a left ventricular ejection fraction of 50% or greater by discharge SBP level less than 120 vs 120 mm Hg or greater. ACE indicates angiotensin-converting enzyme; ARB, angiotensin II receptor blocker.
Of the 901 matched pairs, 125 pairs (13.9%) clearly had a shorter 30-day survival rate than their matched counterparts, and 85 of 125 pairs (68%) of those patients belonged to the lower SBP group (sign score test P < .001). A hidden covariate that is a near-perfect probability of 30-day all-cause mortality and not strongly associated with any of the 58 variables used in our propensity score model could potentially explain this association if it would also increase the odds of having an SBP level less than 120 mm Hg by about 56%. Patients with SBP levels less than 120 mm Hg also had shorter 1-year and overall survival than their matched counterparts, and these associations were also rather insensitive (sign score test P < .001) to bias by a hidden confounder. Associations of SBP level less than 120 mm Hg with other outcomes are displayed in Table 2.
When we excluded DBP from the logistic regression model for propensity score, we were able to assemble a matched balanced cohort of 2104 patients who had a mean (SD) age of 79 (11) years and a mean (SD) EF of 59% (7%); 1321 (62.8%) were women, and 160 (7.6%) were African American. Matched patients in the 2 SBP groups were balanced on all 57 baseline characteristics. Mean (SD) for DBP patients in the SBP level less than 120 vs 120 mm Hg or greater groups were 60 and 69 mm Hg, respectively, which is similar to the prematch DBP level values (Table 1). Respective SBP values were 108 and 138 mm Hg. Among the 2104 matched patients with imbalanced discharge DBP, a discharge SBP less than 120 mm Hg was associated with a higher risk of all-cause mortality at 30 days (113 [10.7%] vs 45 [4.3%]; HR, 2.59; 95% CI, 1.83-3.66; P < .001), 1 year (422 [40.1%] vs 332 [31.6%]; HR, 1.39; 95% CI, 1.20-1.60; P < .001), and 6 years (790 [75.1%] vs 737 [70.1%]; HR, 1.22; 95% CI, 1.10-1.35; P < .001). Systolic blood pressure level less than 120 mm Hg was also significantly associated with a higher risk of the combined end point of HF readmission or all-cause mortality at all 3 times.
Outcomes in Patients With HF, EF of 50% or Greater, and Variable SBP
The 2232 matched patients with an EF of 50% or greater and a variable SBP level (decrease of >20 mm Hg from admission to discharge) had a mean (SD) age of 79 (10) years and a mean (SD) EF of 59% (7%); 1573 (70.5%) were women, and 237 (10.6%) were African American. The 2 SBP groups were balanced on all 58 baseline characteristics. Before matching, 1285 (74%) of the patients in the lower SBP group had a history of hypertension (vs 670 [62%] in the stable SBP cohort) (Table 1). Overall, all-cause mortality occurred in 771 (69%) and 750 (67.2%) of patients with a discharge SBP level less than 120 vs 120 mm Hg or greater, respectively (HR, 1.10; 95% CI, 0.99-1.22; P = .07) (eTable in the Supplement). Associations with other outcomes are displayed in the eTable in the Supplement.
Outcomes in Patients With HF and EF 50% or Greater, Regardless of SBP Stability
The 4582 matched patients with an EF of 50% or greater regardless of SBP stability had a mean (SD) age of 79 (10) years and a mean (SD) EF of 59% (7%), and 3074 (67.1%) were women and 410 (8.9%) were African American. These patients were balanced on all 58 baseline characteristics. All-cause mortality over 6 years of follow-up occurred in 1637 (72%) and 1595 (69.6%) of patients with a discharge SBP level less than 120 vs 120 mm Hg or greater, respectively (HR, 1.09; 95% CI, 1.02-1.17; P = .01) (eTable in the Supplement). Associations with other outcomes are displayed in the eTable in the Supplement.
Association With Admission SBP Less Than 120 mm Hg
The 2958 matched patients with an EF of 50% or greater had a mean (SD) age of 79 (10) years, a mean (SD) EF of 59% (7%) and 1850 (62.5%) were women and 203 (6.9%) were African American. These patients were balanced on all 58 baseline characteristics. Six-year all-cause mortality occurred in 1128 (76.3%) and 1041 (70.4%) of patients with an admission SBP level less than 120 vs 120 mm Hg or greater, respectively (HR, 1.28; 95% CI, 1.18-1.40; P < .001). Associations with other outcomes are displayed in eTable in the Supplement.
Association With Discharge SBP Less Than 130 mm Hg
The 2228 matched patients had a mean (SD) age of 79 (10) years, a mean (SD) EF of 59% (7%); 1437 (64.5%) were women, and 181 (8.1%) were African American. The 2 SBP groups were balanced on all 58 baseline characteristics. Thirty-day all-cause mortality occurred in 79 (7.1%) and 54 (4.8%) of matched patients with a discharge SBP level less than 130 vs 130 mm Hg or greater, respectively (HR, 1.47; 95% CI, 1.04-2.08; P = .03). A discharge SBP level less than 130 mm Hg was also associated with a higher risk of all-cause mortality at 1 year (375 [33.7%] vs 318 [28.5%]; HR, 1.24; 95% CI, 1.07-1.44; P = .005) and 6 years (803 [72.1%] vs 778 [69.8%]; HR, 1.11; 95% CI, 1.01-1.22; P = .045). Systolic blood pressure level less than 130 mm Hg was also significantly associated with a higher risk of the combined end point of HF readmission or all-cause mortality at all 3 times.
Discussion
Findings from the current study demonstrate that among hospitalized patients with HFpEF, a discharge SBP level of less than 120 mm Hg was associated with a significantly higher risk of 30-day, 1-year, and overall all-cause mortality and that this association was essentially unchanged when lower SBP was defined as SBP levels less than 130 mm Hg. An SBP level less than 120 mm Hg was also associated with a significantly higher risk of the combined end points of HF readmission or mortality at all 3 times. These findings, taken together with those from multiple sensitivity cohorts, provide evidence of a consistent association between a lower SBP level and poor outcomes in patients with HFpEF.
Higher mortality associated with a lower SBP level in patients with HFpEF may reflect differences in cause, pathophysiology, and stage of disease. Hypertension often precedes the development of clinical HF and is the most common cause of morbidity in patients with HF.5,6,24 However, fewer (prematched) patients in the SBP level less than 120 mm Hg group in our study (Table 1) had a history of hypertension, suggesting that HFpEF in these patients may not have been associated with hypertension. A lower SBP level in patients with HFpEF may be due to SBP-lowering drugs; however, fewer patients in the lower SBP group were receiving these drugs. It is possible that a lower SBP level in HFpEF may also reflect a more advanced disease state and lower cardiac output. A higher pulse pressure has been shown to be associated with a higher risk of death in HFpEF (EF ≥ 50%).25 However, that is unlikely to explain our findings because patients in the higher SBP group had a lower mortality despite a higher pulse pressure.
A higher proportion of prematched patients with a lower SBP level were receiving loop diuretics, which may have contributed to the lower SBP level, greater reflex neurohormonal activation, and poor outcomes.16 While our propensity score matching achieved substantial between-group balance in all measured confounders, imbalances in their severity may remain and persist during follow-up. Furthermore, imbalances in unmeasured confounders may also in part explain the observed poor outcomes in the lower SBP group. Nearly half of the matched patients with lower SBP levels were receiving ACE inhibitors and β-blockers, which could have further lowered SBP levels during follow-up. Hypotension has been shown to be associated with sudden cardiac death.26,27,28 Thus, a higher incidence of sudden cardiac death in the lower SBP group may also in part explain the lack of association with readmission in that group.
The association between SBP level less than 120 mm Hg and HF readmission was modest and variable. If a higher proportion of patients in the lower SBP group had more advanced HF, then pump failure could in part explain the higher HF readmission in that group.29 However, lack of consistency of this association across various cohorts and timeframes suggest this association may not be intrinsic in nature. For example, among patients with stable SBP levels, a discharge SBP level less than 120 mm Hg had a significant association with 30-day HF readmission but not with 1-year and overall HF readmissions. In contrast, among those with variable SBP levels, a discharge SBP level less than 120 mm Hg had a significant association with HF readmission overall but not during shorter follow-up. Finally, an admission SBP level less than 120 mm Hg had a significant association with all outcomes but not with HF readmission.
Several prior studies have examined the association of a lower SBP level with outcomes in hospitalized older adults with HF.9,30,31,32,33 Most of these studies were small, and only 1 was dedicated to HFpEF.33 In contrast, our study is distinguished by a larger sample size, the use of a cohort with stable admission to discharge SBP, the use of an EF cutoff of 50% or greater to define HFpEF, the use of propensity score matching to assemble a balanced cohort, the use of subgroup analyses to demonstrate homogeneity, the use of multiple sensitivity analyses, and the use of formal sensitivity analyses to assess bias by a potential unmeasured confounder.
National guidelines for HF and hypertension recommend that SBP levels should be controlled in patients with HFpEF in general and to less than 130 mm Hg in patients with HF and persistent hypertension.22,23 These recommendations are extrapolated from populations without HF because direct evidence from patients with HFpEF is limited. The ACE inhibitors and angiotensin II receptor blockers have been shown to modestly lower SBP levels in patients with HFpEF, but this did not translate into better outcomes.34,35 Findings from observational studies suggest that a lower SBP level may be associated with poor outcomes in patients with HF.9,10,11 This poorly understood phenomenon of reverse epidemiology is not unique to SBP and has also been described for other traditional HF risk factors.36,37,38 Findings from the current study provide evidence that a lower SBP level is a marker of underlying pathophysiologic processes that is associated with poor outcomes in patients with HFpEF, an observation that may help design future trials testing new therapies in HFpEF.39 Future prospective randomized clinical trials also need to examine the effect of various SBP target levels on outcomes in patients with HFpEF.
Limitations
There are several limitations to our study. Because of the observational nature of our study, bias due to unmeasured confounders is possible. Findings from our sensitivity analyses suggest that the harmful association of SBP level less than 120 mm Hg and all-cause mortality is rather insensitive to a hidden bias. We had no data on postdischarge SBP level, and SBP crossover during follow-up may result in regression dilution and potential underestimation of the true association.40 Our database on hospitalized patients with HFpEF may not be generalizable to ambulatory patients with HFpEF because determinants of blood pressure as well as measurement of blood pressure in these 2 settings are different.
Conclusions
In hospitalized older patients with HFpEF, an SBP level less than 120 mm Hg is independently associated with a higher risk of all-cause mortality and the combined end points of readmissions or mortality. Future studies need to prospectively evaluate optimal SBP treatment goals in patients with HFpEF.
eTable. Outcomes by Systolic Blood Pressure (SBP) in Propensity Score–Matched Patients with Heart Failure With Ejection Fraction ≥50% in the Main Cohort and 3 Sensitivity Cohorts
eFigure 1. Flow Chart Displaying Assembly of Propensity Score Matched Cohorts of Patients With Heart Failure and Left Ventricular Ejection Fraction ≥50%, by Systolic Blood Pressure (SBP) <120 vs ≥120 mm Hg
eFigure 2. Love Plot Displaying Absolute Standardized Differences Comparing 58 Baseline Characteristics Between Patients With Heart Failure And Left Ventricular Ejection Fraction ≥50% and Systolic Blood Pressure Level <120 vs ≥120 mm Hg Before and After Propensity Score Matching
References
- 1.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288(17):2144-2150. [DOI] [PubMed] [Google Scholar]
- 2.Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194-202. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137-e161. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275(20):1557-1562. [PubMed] [Google Scholar]
- 6.Kostis JB, Davis BR, Cutler J, et al. ; SHEP Cooperative Research Group . Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA. 1997;278(3):212-216. [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, et al. ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. [DOI] [PubMed] [Google Scholar]
- 8.Williamson JD, Supiano MA, Applegate WB, et al. ; SPRINT Research Group . Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315(24):2673-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gheorghiade M, Abraham WT, Albert NM, et al. ; OPTIMIZE-HF Investigators and Coordinators . Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296(18):2217-2226. [DOI] [PubMed] [Google Scholar]
- 10.Desai RV, Banach M, Ahmed MI, et al. Impact of baseline systolic blood pressure on long-term outcomes in patients with advanced chronic systolic heart failure (insights from the BEST trial). Am J Cardiol. 2010;106(2):221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banach M, Bhatia V, Feller MA, et al. Relation of baseline systolic blood pressure and long-term outcomes in ambulatory patients with chronic mild to moderate heart failure. Am J Cardiol. 2011;107(8):1208-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148(1):43-51. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Kilgore ML, Arora T, et al. Design and rationale of studies of neurohormonal blockade and outcomes in diastolic heart failure using OPTIMIZE-HF registry linked to Medicare data. Int J Cardiol. 2013;166(1):230-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam PH, Dooley DJ, Deedwania P, et al. Heart rate and outcomes in hospitalized patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;70(15):1861-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonarow GC, Stough WG, Abraham WT, et al. ; OPTIMIZE-HF Investigators and Hospitals . Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J Am Coll Cardiol. 2007;50(8):768-777. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27(12):1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. Primer on statistical interpretation or methods report card on propensity-score matching in the cardiology literature from 2004 to 2006: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1(1):62-67. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 19.Rubin DB. Using propensity score to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001; 2(3-4):169-188. doi: 10.1023/A:1020363010465 [DOI] [Google Scholar]
- 20.Ahmed MI, White M, Ekundayo OJ, et al. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J. 2009;30(16):2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahle C, Adamopoulos C, Ekundayo OJ, Mujib M, Aronow WS, Ahmed A. A propensity-matched study of outcomes of chronic heart failure (HF) in younger and older adults. Arch Gerontol Geriatr. 2009;49(1):165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. [DOI] [PubMed] [Google Scholar]
- 23.Whelton P, Carey R, Aronow W, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. 2017;2017. [DOI] [PubMed] [Google Scholar]
- 24.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360(12):1179-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laskey WK, Wu J, Schulte PJ, et al. Association of arterial pulse pressure with long-term clinical outcomes in patients with heart failure. JACC Heart Fail. 2016;4(1):42-49. [DOI] [PubMed] [Google Scholar]
- 26.Greene HL. Sudden arrhythmic cardiac death: mechanisms, resuscitation and classification: the Seattle perspective. Am J Cardiol. 1990;65(4):4B-12B. [DOI] [PubMed] [Google Scholar]
- 27.Davies DW, Kadar D, Steward DJ, Munro IR. A sudden death associated with the use of sodium nitroprusside for induction of hypotension during anaesthesia. Can Anaesth Soc J. 1975;22(5):547-552. [DOI] [PubMed] [Google Scholar]
- 28.Franciosa JA, Heckel R. Significance of hypotension preceding fatal ventricular tachyarrhythmias in post-coronary obstruction sudden death. Am Heart J. 1981;101(4):421-427. [DOI] [PubMed] [Google Scholar]
- 29.Zile MR, Gaasch WH, Anand IS, et al. ; I-Preserve Investigators . Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121(12):1393-1405. [DOI] [PubMed] [Google Scholar]
- 30.Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ; ADHERE Scientific Advisory Committee, Study Group, and Investigators . Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580. [DOI] [PubMed] [Google Scholar]
- 31.Núñez J, Núñez E, Fonarow GC, et al. Differential prognostic effect of systolic blood pressure on mortality according to left-ventricular function in patients with acute heart failure. Eur J Heart Fail. 2010;12(1):38-44. [DOI] [PubMed] [Google Scholar]
- 32.Vidán MT, Bueno H, Wang Y, et al. The relationship between systolic blood pressure on admission and mortality in older patients with heart failure. Eur J Heart Fail. 2010;12(2):148-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buiciuc O, Rusinaru D, Lévy F, et al. Low systolic blood pressure at admission predicts long-term mortality in heart failure with preserved ejection fraction. J Card Fail. 2011;17(11):907-915. [DOI] [PubMed] [Google Scholar]
- 34.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J; PEP-CHF Investigators . The Perindopril In Elderly People With Chronic Heart Failure (PEP-CHF) Study. Eur Heart J. 2006;27(19):2338-2345. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Pfeffer MA, Swedberg K, et al. ; CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362(9386):777-781. [DOI] [PubMed] [Google Scholar]
- 36.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43(8):1439-1444. [DOI] [PubMed] [Google Scholar]
- 37.Khalid U, Ather S, Bavishi C, et al. Pre-morbid body mass index and mortality after incident heart failure: the ARIC study. J Am Coll Cardiol. 2014;64(25):2743-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonarow GC, Abraham WT, Albert NM, et al. A smoker’s paradox in patients hospitalized for heart failure: findings from OPTIMIZE-HF. Eur Heart J. 2008;29(16):1983-1991. [DOI] [PubMed] [Google Scholar]
- 39.Butler J, Fonarow GC, Zile MR, et al. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail. 2014;2(2):97-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150(4):341-353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Outcomes by Systolic Blood Pressure (SBP) in Propensity Score–Matched Patients with Heart Failure With Ejection Fraction ≥50% in the Main Cohort and 3 Sensitivity Cohorts
eFigure 1. Flow Chart Displaying Assembly of Propensity Score Matched Cohorts of Patients With Heart Failure and Left Ventricular Ejection Fraction ≥50%, by Systolic Blood Pressure (SBP) <120 vs ≥120 mm Hg
eFigure 2. Love Plot Displaying Absolute Standardized Differences Comparing 58 Baseline Characteristics Between Patients With Heart Failure And Left Ventricular Ejection Fraction ≥50% and Systolic Blood Pressure Level <120 vs ≥120 mm Hg Before and After Propensity Score Matching