Key Points
Question
What is the association of patient frailty with postoperative complications and failure to rescue after low-risk and high-risk inpatient surgery?
Findings
In this cohort study of the American College of Surgeons National Surgical Quality Improvement Program, there was a dose-response association between increasing patient frailty, the number of postoperative complications, and failure to rescue. These associations were apparent after low-risk and high-risk inpatient surgical procedures.
Meaning
Patient frailty should be considered an important component of the preoperative assessment because it may help identify patients who might benefit from perioperative interventions designed to enhance physiologic reserve and could provide a framework for shared decision making on initiating surgical care.
This cohort study assesses the association of frailty with failure to rescue in patients undergoing inpatient surgery.
Abstract
Importance
Failure to rescue (FTR), or death after a potentially preventable complication, is a nationally endorsed, publicly reported quality measure. However, little is known about the impact of frailty on FTR, in particular after low-risk surgical procedures.
Objective
To assess the association of frailty with FTR in patients undergoing inpatient surgery.
Design, Setting, and Participants
This study assessed a cohort of 984 550 patients undergoing inpatient general, vascular, thoracic, cardiac, and orthopedic surgery in the National Surgical Quality Improvement Program between January 1, 2005, and December 31, 2012. Frailty was assessed using the Risk Analysis Index (RAI), and patients were stratified into 5 groups (RAI score, ≤10, 11-20, 21-30, 31-40, and >40). Procedures were categorized as low mortality risk (≤1%) or high mortality risk (>1%). The association between RAI scores, the number of postoperative complications (0, 1, 2, or 3 or more), and FTR was evaluated using hierarchical modeling.
Main Outcomes and Measures
The number of postoperative complications and inpatient FTR.
Results
A total of 984 550 patients were included, with a mean (SD) age of 58.2 (17.1) years; women were 549 281 (55.8%) of the cohort. For patients with RAI scores of 10 or less, major complication rates after low-risk surgery were 3.2%; rates of those with RAI scores of 11 to 20, 21 to 30, 31 to 40, and more than 40 were 8.6%, 13.5%, 23.8%, and 36.4%, respectively. After high-risk surgery, these rates were 13.5% for those with scores of 10 or less, 23.7% for those with scores of 11 to 20, 31.1% for those with scores of 21 to 30, 42.5% for those with scores of 31 to 40, and 54.4% for those with scores of more than 40. Stratifying by the number of complications, significant increases in FTR were observed across RAI categories after both low-risk and high-risk procedures. After a low-risk procedure, odds of FTR after 1 major complication for patients with RAI scores of 11 to 20 increased 5-fold over those with RAI scores of 10 or less (odds ratio [OR], 5.3; 95% CI, 3.9-7.1). Odds ratios were 8.1 (95% CI, 5.6-11.7) for patients with RAI scores of 21 to 30; 22.3 (95% CI, 13.9-35.6) for patients with scores of 31 to 40; and 43.9 (95% CI, 19-101.1) for patients with scores of more than 40. For patients undergoing a high-risk procedure, the corresponding ORs were likewise consistently elevated (RAI score 11-20: OR, 2.5; 95% CI, 2.3-2.7; vs RAI score 21-30: 5.1; 95% CI, 4.6-5.5; vs RAI score 31-40: 8.9; 95% CI, 8.1-9.9; vs RAI score >40: 18.4; 95% CI, 15.7-21.4).
Conclusions and Relevance
Frailty has a dose-response association with complications and FTR, which is apparent after low-risk and high-risk inpatient surgery. Systematic assessment of frailty in preoperative patients may help refine estimates of surgical risk that could identify patients who might benefit from perioperative interventions designed to enhance physiologic reserve and potentially mitigate aspects of procedural risk, and would provide a framework for shared decision-making regarding the value of a given surgical procedure.
Introduction
The prevalence of frailty is estimated at 10% to 20% of the United States population older than 65 years and as many as 40% in those older than 80 years.1 (The population older than 65 years currently accounts for 15% of the total population and is projected to rise to 21% by 2030.1) Frailty is a state of vulnerability to adverse health outcomes because of stressors that result in disability, dependency, falls, need for long-term care, and mortality.2 Although frailty can be defined in many ways, an international panel of experts who convened in 2011 agreed that it is a multidimensional construct of 6 domains (physical performance, gait speed, mobility, nutritional status, mental health, and cognition) that together can indicate increased risk of death, disability, and institutionalization.3 While frailty increases with age, not all elderly patients are necessarily frail, and not all young patients are necessarily robust. As such, frailty can be conceptualized as a measure of physiologic reserve, which is defined as the critical threshold at which external stressors overwhelm the ability of the human body to adapt, resulting in decompensation.4
Although frailty is widely reported in the medical literature, its role in adverse postoperative outcomes has been established only recently.5,6,7 Failure to Rescue (FTR), defined as death after a serious, potentially preventable complication, is a nationally endorsed and publicly reported quality measure.8,9 However, the underlying mechanism by which this phenomenon occurs remains poorly understood. Frail patients may be particularly vulnerable to FTR because their diminished physiological reserve may contribute to occurrence of perioperative complications and reduce their ability to recover when such adverse events happen.
Although recently published data have demonstrated an association between frailty and FTR in patients undergoing trauma and vascular surgery, important but unanswered questions remain.10,11 Although frailty is associated with postoperative morbidity, the degree to which it is associated with patients’ risk of developing multiple complications (which have been shown to have a dose-response effect on FTR) is unclear.12 In addition, few studies have evaluated whether frailty impacts postoperative outcomes after lower-risk operations. To explore these 2 issues, we applied the Risk Analysis Index (RAI), a recently validated tool13 for screening large surgical populations, to data from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). We hypothesized that frailty was not only associated with the occurrence of multiple complications and FTR, but that this association was apparent after both high-risk and low-risk inpatient surgery.
Methods
Data
Data from ACS-NSQIP were used to conduct a retrospective cohort study of patients undergoing inpatient general, vascular, thoracic, cardiac, and orthopedic operations between January 1, 2005, to December 31, 2012. The database contains prospectively collected clinical and surgical information for all major inpatient and outpatient surgical procedures performed at more than 600 participating hospitals. This study was granted exempt status by the Roswell Park Cancer Institute institutional review board per institutional guidelines because of the deidentified nature of the data.
Patients
The cohort was restricted to patients who underwent an operation from January 1, 2005, to December 31, 2012, because the variables necessary to calculate the RAI score were only available in ACS-NSQIP in these years. In subsequent years, several of the necessary data points were omitted from abstraction. We excluded patients who underwent outpatient procedures because they had a very low mortality rate (0.09%). Within this data set, 2 395 937 patients underwent an inpatient surgical procedure for 1 of the included specialties. Any patient with an incomplete set of the data needed to calculate the RAI score was excluded (n = 1 403 218). Patients younger than 18 years were also excluded (n = 1687). The final analytic data set included 984 550 patients.
Variables
The RAI score for each patient was calculated. The RAI was adapted from the Minimum Data Set (MDS) Mortality Risk Index–Revised (MMRI-R).14,15 The MMRI-R consists of demographic and clinical variables in addition to assessing activities of daily living. The RAI was validated prospectively in the outpatient surgery clinics at the Veterans Affairs Nebraska–Western Iowa Health Care System and retrospectively using variables from ACS-NSQIP and the Veterans Affairs Surgical Quality Improvement Program.13 The RAI score predicts postoperative mortality and morbidity with as good or better predictive ability than other existing measures of frailty, such as the MMRI-R and the modified Frailty Index (mFI).16
The variables used to calculate the RAI score of each patient were age, sex, diagnosis of cancer (a composite of disseminated cancer, preoperative chemotherapy, and preoperative radiation therapy scores), weight loss, renal failure, congestive heart failure, shortness of breath, chronic care facility status, presence of cognitive deterioration, and functional status. Patients were then stratified into 5 groups per their RAI score (≤10 points, 11-20 points, 21-30 points, 31-40 points, and >40 points), in which larger scores indicated poorer overall health status. Days from operation to each complication were used to separately identify complications that occurred during the postoperative inpatient stay and those that occurred after discharge. Major complications included deep infection, organ-space infection, acute renal failure, postoperative bleeding requiring transfusion, myocardial infarction, pneumonia, pulmonary embolism, stroke, unplanned intubation, prolonged mechanical ventilation, or septic shock.17 The number of postoperative complications were calculated and then categorized as 0, 1, 2, and 3 or more.12 A procedure-specific risk categorization was also created. Current Procedural Terminology codes were used to identify individual procedures, and procedure-specific 30-day mortality rates were calculated. Procedures were then categorized as low-risk (30-day mortality rate, ≤1%) or high-risk (30-day mortality rate, >1%).17 A list of the 10 most common high-risk and low-risk procedures in the data set are provided in eAppendix 1 in the Supplement.
Statistical Analysis
Standard descriptive statistics were used to evaluate the distribution of variables across RAI categories. The Cochrane-Armitage test was used to evaluate for statistically significant trends in complication and FTR rates across RAI categories. We chose to use the Agency for Healthcare Research and Quality FTR definition for our primary outcome (inpatient FTR, defined as an inpatient death after an inpatient complication).18 However, the National Quality Forum also endorses a quality indicator using 30-day FTR, not just inpatient mortality.8 As such, we also chose to conduct a sensitivity analysis using this alternative definition for FTR. Additional sensitivity analyses were conducted among patients who only underwent elective (rather than emergent) surgical procedures. The results of all analyses were similar.
To address the fact that procedures across different specialties could have similar risk profiles, 2-level, hierarchical regression models (that clustered patients within specialties) were used. The association between the number of complications and RAI was evaluated using multinomial regression. The association between RAI score and FTR rate (or, more simply, mortality for those who had no complications) was evaluated using logistic regression. Because our main variable of interest, RAI score, is a composite variable combining many of the covariates we would have normally selected for inclusion in a multivariable model, no covariates were included to avoid collinearity issues. The exception was the logistic model evaluating FTR, which included an interaction between RAI score and the number of complications. All model assumptions were verified graphically using diagnostic plots as appropriate. A mediation analysis was performed to evaluate how RAI score was associated with FTR per the number of complications .19,20 To formally test how RAI scores mediated the number of complications, we applied the method of Baron and Kenny,21 which establishes partial or full mediation when (1) separate models demonstrate a significant association between the independent variable and the mediating variable and between the mediating variable and the dependent variable and (2) the association between the independent and dependent variable is significantly reduced after a mediating variable is added to the model.21
All analyses were completed in SAS version 9.4 (SAS Institute, Inc). A P value of .05 was used to denote statistical significance. Analyses were performed from February 2017 to July 2017.
Results
A total of 984 550 patients were included in our analysis. The mean (SD) age of the overall cohort was 58.2 (17.1) years; women constituted 549 281 (55.8%) of the cohort. A total of 715 443 patients (72.7%) underwent general surgery, 159 432 (16.2%) had vascular surgery, 11 727 (1.2%) had thoracic surgery, 12 310 (1.3%) had cardiac surgery, and 85 638 (8.7%) had orthopedic surgery.
Table 1 summarizes the demographic, clinical, morbidity, and mortality characteristics of the 5 RAI groups. Of the full study cohort, 131 775 (13.4%) had any complication, with 102 270 patients (10.4%) having a major complication. A total of 59 684 of 811 043 patients (7.4%) with RAI scores of 10 or less experienced major complications, as did 21 457 of 108 635 patients (19.8%) with RAI scores of 11 to 20, 13 505 of 47 304 patients (28.5%) with RAI scores of 21 to 30, 5897 of 14 348 patients (41.1%) with RAI scores of 31 to 40, and 1727 of 3220 patients (53.6%) with RAI scores of more than 40. The rates of any complication varied significantly between groups (RAI score ≤10: 79 880 of 811 043 [9.8%]; RAI scores 11-20: 26 917 of 108 635 [24.8%]; RAI scores 21-30: 16 320 of 47 304 [34.5%]; RAI scores 31-40: 6761 of 14 348 [47.1%]; and RAI scores ≥40: 1897 of 3220 [58.9%]; P < .001 across all groups).
Table 1. Demographic and Clinical Variables Stratified by Risk Analysis Index Score.
| Characteristic | RAI Score, No. (%) | ||||
|---|---|---|---|---|---|
| ≤10 | 11-20 | 21-30 | 31-40 | >40 | |
| Total | 811 043 (82.4) | 108 635 (11.0) | 47 304 (4.8) | 14 348 (1.5) | 3220 (0.3) |
| Age, mean/median (SD) | 56.3/58.0 (16.7) | 67.4/70.0 (16.4) | 66.4/67.0 (15.1) | 67.4/69.0 (14.8) | 66.6/68.0 (14.3) |
| Age, y | |||||
| ≤ 55 | 368 515 (45.4) | 25 986 (23.9) | 11 034 (23.3) | 3007 (21.0) | 665 (20.7) |
| 56-65 | 177 826 (21.9) | 19 811 (18.2) | 10 603 (22.4) | 3061 (21.3) | 763 (23.7) |
| 66-75 | 154 235 (19.0) | 22 667 (20.9) | 10 784 (22.8) | 3387 (23.6) | 821 (25.5) |
| >75 | 110 467 (13.6) | 40 171 (37.0) | 14 883 (31.5) | 4893 (34.1) | 971 (30.2) |
| Sex | |||||
| Male | 334 015 (41.2) | 55 707 (51.3) | 32 865 (69.5) | 10 248 (71.4) | 2434 (75.6) |
| Female | 477 028 (58.8) | 52 928 (48.7) | 14 439 (30.5) | 4100 (28.6) | 786 (24.4) |
| Race/Ethnicity | |||||
| White | 609 279 (83.7) | 80 816 (81.6) | 35 129 (81.6) | 10 268 (78.5) | 2242 (76.2) |
| Black | 80 617 (11.1) | 13 686 (13.8) | 5997 (13.9) | 2250 (17.2) | 560 (19.0) |
| Asian | 18 961 (2.6) | 2418 (2.4) | 1057 (2.5 | 295 (2.3 | 75 (2.6 |
| Hispanic | 12 980 (1.8) | 1443 (1.5) | 565 (1.3) | 187 (1.4) | 43 (1.5) |
| Native American | 5968 (0.8) | 716 (0.7) | 303 (0.7) | 86 (0.7) | 21 (0.7) |
| BMI, mean (SD) | 30.9 (12.9) | 27.3 (7.6) | 27.4 (7.4) | 26.9 (7.9) | 29.9 (7.3) |
| ASA level | |||||
| 1 | 54 355 (6.7) | 944 (0.9) | 217 (0.5) | 23 (0.2) | 1 (0.0) |
| 2 | 339 649 (41.9) | 19 036 (17.5) | 6386 (13.5) | 813 (5.7) | 54 (1.7) |
| 3 | 363 771 (44.9) | 62 554 (57.7) | 24 448 (51.8) | 5790 (40.4) | 919 (28.6) |
| 4/5 | 52 184 (6.4) | 25 947 (23.9) | 16 188 (34.3) | 7699 (53.7) | 2242 (69.7) |
| Current smoker (within 1 y) | 173 032 (21.3) | 22 297 (20.5) | 9473 (20.0) | 3067 (21.4) | 784 (24.3) |
| Functional status | |||||
| Independent | 802 653 (99.0) | 69 876 (64.3) | 20 687 (43.7) | 2976 (20.7) | 61 (1.9) |
| Partial dependent | 8390 (1.0) | 36 182 (33.3) | 15 181 (32.1) | 3016 (21.0) | 656 (20.4) |
| Total dependent | 0 | 2577 (2.4) | 11 436 (24.2) | 8356 (58.2) | 2503 (77.7) |
| Diabetes | |||||
| None | 673 992 (83.1) | 81 622 (75.1) | 34 796 (73.6) | 9975 (69.5) | 2204 (68.4) |
| Noninsulin | 36 114 (4.5) | 4944 (4.6) | 1901 (4.0) | 462 (3.2) | 105 (3.3) |
| Oral medication | 49 266 (6.1) | 7662 (7.1) | 3285 (6.9) | 1051 (7.3) | 237 (7.4) |
| Insulin | 51 671 (6.4) | 14 407 (13.3) | 7322 (15.5) | 2860 (19.9) | 674 (20.9) |
| Congestive heart failure | 2331 (0.3) | 6117 (5.6) | 3519 (7.4) | 2034 (14.2) | 830 (25.8) |
| Dyspnea | |||||
| None | 720 144 (88.8) | 86 148 (79.3) | 37 110 (78.5) | 8618 (60.1) | 1160 (36.0) |
| Moderate exertion | 88 591 (10.9 | 15 763 (14.5) | 5829 (12.3) | 1534 (10.7) | 259 (8.0) |
| At rest | 2308 (0.3) | 6724 (6.2) | 4365 (9.2) | 4196 (29.2) | 1801 (55.9) |
| Chronic obstructive pulmonary disease | 41 824 (5.2) | 12 577 (11.6) | 5826 (12.3) | 2432 (17.0) | 692 (21.5) |
| Preoperative pneumonia | 2103 (0.3) | 1973 (1.8) | 2164 (4.6) | 1652 (11.5) | 668 (20.7) |
| Preoperative ventilator | 1698 (0.2) | 2155 (2.0) | 4874 (10.3) | 3603 (25.1) | 1250 (38.8) |
| Preoperative coma | 59 (0.0) | 44 (0.0) | 269 (0.6) | 340 (2.4) | 139 (4.3) |
| Impaired sensorium | 2093 (0.3) | 2165 (2.0) | 2993 (6.3) | 2529 (17.6) | 1100 (34.2) |
| Transient ischemic attack | 27 841 (3.4) | 6358 (5.9) | 2252 (4.8) | 725 (5.1) | 185 (5.7) |
| Stroke | |||||
| None | 776 006 (95.7) | 97 270 (89.5) | 41 137 (87.0) | 11 411 (79.5) | 2540 (78.9) |
| Without deficit | 17 466 (2.2) | 5219 (4.8) | 2224 (4.7) | 677 (4.7) | 152 (4.7) |
| With deficit | 17 493 (2.2) | 6138 (5.7) | 3938 (8.3) | 2260 (15.8) | 528 (16.4) |
| Ascites | 6086 (0.8) | 3030 (2.8) | 2500 (5.3) | 1464 (10.2) | 570 (17.7) |
| Bleeding disorder | 50 312 (6.2) | 16 138 (14.9) | 8331 (17.6) | 3436 (23.9) | 1002 (31.1) |
| Disseminated cancer | 0 | 10 397 (9.6) | 12 777 (27.0) | 3416 (23.8) | 1247 (38.7) |
| Chemotherapy | 0 | 7587 (7.0) | 7047 (14.9) | 1783 (12.4) | 644 (20.0) |
| Radiotherapy | 0 | 3474 (3.2) | 5588 (11.8) | 1279 (8.9) | 318 (9.9) |
| Steroids | 27 641 (3.4) | 7021 (6.5) | 3345 (7.1) | 1353 (9.4) | 466 (14.5) |
| Transfusion | 4762 (0.6 | 2985 (2.7 | 2375 (5.0 | 1173 (8.2 | 383 (11.9) |
| Weight loss | 139 (0.0) | 19 795 (18.2) | 5039 (10.7) | 4241 (29.6) | 1186 (36.8) |
| Open/infected wound | 32 324 (4.0) | 16 166 (14.9) | 9951 (21.0) | 4358 (30.4) | 1129 (35.1) |
| Dialysis | 5121 (0.6) | 10 408 (9.6) | 5194 (11.0) | 2155 (15.0) | 749 (23.3) |
| Preoperative renal failure | 1162 (0.1) | 3093 (2.8) | 2055 (4.3) | 1321 (9.2) | 666 (20.7) |
| Hypertension requiring medications | 407 460 (50.2) | 71 080 (65.4) | 29 665 (62.7) | 9434 (65.8) | 2124 (66.0) |
| Type of operation | |||||
| General | 599 503 (73.9) | 70 003 (64.4) | 33 325 (70.4) | 10 183 (71.0) | 2429 (75.4) |
| Vascular | 120 128 (14.8) | 26 096 (24.0) | 9557 (20.2) | 3056 (21.3) | 595 (18.5) |
| Thoracic | 8179 (1.0) | 1939 (1.8) | 1197 (2.5) | 325 (2.3) | 87 (2.7) |
| Cardiac | 9301 (1.1) | 2203 (2.0) | 572 (1.2) | 200 (1.4) | 34 (1.1) |
| Orthopedic | 73 932 (9.1) | 8394 (7.7) | 2653 (5.6) | 584 (4.1) | 75 (2.3) |
| Length of stay, d, Mean/Median (SD) | 4.4/3 (6.1) | 8.5/6 (10.7) | 11.3/7 (13.9) | 14/9 (16.9) | 15.4/10 (17.8) |
| Emergency operation | 133 137 (16.4) | 22 071 (20.3) | 12 523 (26.5) | 5737 (40.0) | 1733 (53.8) |
| Procedural mortality risk | |||||
| <1% (low) | 483 286 (59.6) | 28 352 (26.1) | 6820 (14.4) | 1103 (7.7) | 140 (4.3) |
| >1% (high) | 327 757 (40.4) | 80 283 (73.9) | 40 484 (85.6) | 13 245 (92.3) | 3080 (95.7) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
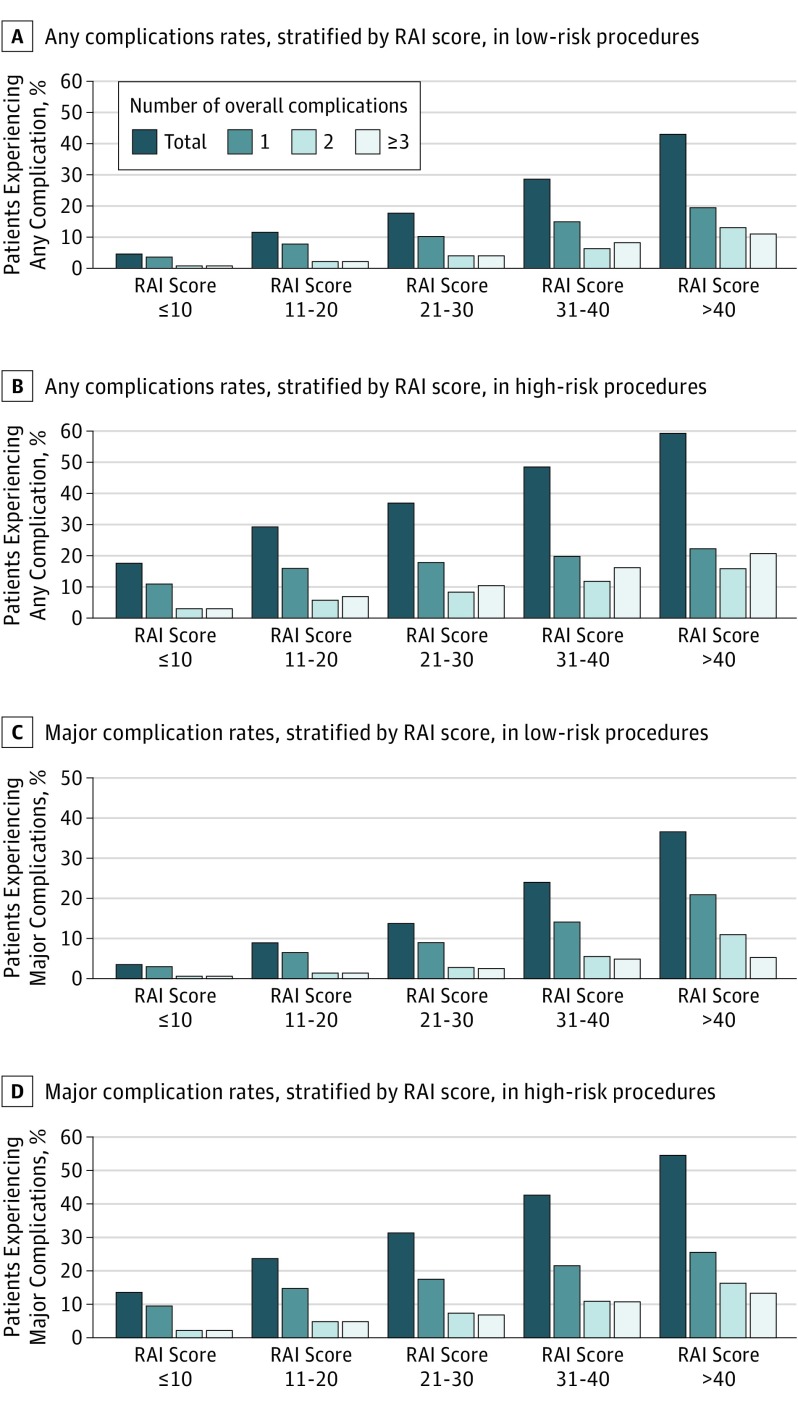
For those who underwent low-risk surgery, rates of major complication rates varied more than 10-fold (RAI scores ≤10: 15 551 of 483 286 [3.2%]; RAI scores 11-20: 2445 of 28 352 [8.6%]; RAI scores 21-30: 920 of 6820 [13.5%]; RAI scores 31-40: 262 of 1103 [23.8%]; RAI scores ≥40: 51 of 140 [36.4%]; P < .001 across all groups). For patients undergoing high-risk surgery, major complications occurred in 44 133 of 327 757 patients (13.5%) with RAI scores of 10 or less, 19 012 of 80 283 patients (23.7%) with RAI scores between 11 and 20, 12 585 of 40 484 patients (31.1%) with RAI scores between 21 and 30, 5635 of 13 245 patients (42.5%) with RAI scores between 31 and 40, and 1676 of 3080 patients (54.4%) with scores of more than 40 (P < .001 across all groups).
This trend was also observed for any complications. Individuals undergoing low-risk surgery with high RAI scores experienced rates of any complication that were much higher than individuals with lower RAI scores (RAI scores ≤10: 21 184 of 483 286 [4.4%]; RAI scores 11-20: 3176 of 28 352 [11.2%]; RAI scores 21-30: 1194 of 6820 [17.5%]; RAI scores 31-40: 314 of 1103 [28.5%]; RAI scores ≥40: 60 of 140 [42.9%]; P < .001 across all groups). A similar pattern was seen in those patients undergoing high-risk surgery (RAI scores ≤10: 58 696 of 327 757 [17.9%]; RAI scores 11-20: 23 741 of 80 283 [29.6%]; RAI scores 21-30: 15 126 of 40 484 [37.4%]; RAI scores 31-40: 6447 of 13 245 [48.7%]; and RAI scores ≥40: 1837 of 3080 [59.6%]; P < .001 across all groups). Figure 1 shows the number of major and any complication across RAI groups, stratified by procedural risk.
Figure 1. Complication Rates, Stratified by Procedural Risk and RAI Score.
RAI, Risk Analysis Index. Procedural risk was defined as low if mortality was less than 1% and high if mortality was more than 1%.
Across RAI scores, there were monotonic increases in the proportion of patients who had a specific number of major complications and any complications, regardless of level of procedural risk. For instance, the rate of any 1 complication in patients who underwent a low-risk procedure (RAI scores ≤10: 13 008 of 483 286 [2.7%]; RAI scores 11 to 20: 1785 of 28 352 [6.3%]; RAI scores 21-30: 595 of 6820 [8.7%]; RAI scores 31-40: 154 of 1103 [14.0%]; RAI scores ≥40: 29 of 140 [20.7%]) was uniformly lower in each score group than the rate of any 1 complication in patients in the same score group who underwent a high-risk procedure (RAI scores ≤10: 30 832 of 327 757 [9.4%]; RAI scores 11-20: 11 772 of 80 283 [14.7%]; RAI scores 21-30: 7026 of 40 484 [17.4%]; RAI scores 31-40: 2810 of 13 245 [21.2%]; RAI scores ≥40: 779 of 3080 [25.3%]).
Regardless of procedural risk level, increasing RAI score was also associated with an incremental increase in the likelihood of a given number of complications relative to patients who had no complications (Table 2). For instance, relative to patients who had no complications, the relative risk ratios (RR) of developing 1 major complication after a low-risk procedure were 2.2 (95% CI, 1.6-2.9) among patients with RAI scores of 11-20, 3.2 (95% CI, 2.3-4.5) among those with RAI scores of 21 to 30, 5.4 (95% CI, 3.7-7.8) among those with RAI scores of 31 to 40, and 9.4 (95% CI, 5.4-16.5) among those who scored more than 40.
Table 2. Association Between RAI Score and Number of Complications Stratified by Procedural Risk.
| RAI Scorea,b | Relative Risk (95% CI) | |
|---|---|---|
| Major Complication | Any Complication | |
| Low-risk, 1 complication | ||
| 11-20 | 2.19 (1.60-2.98) | 2.12 (1.57-2.85) |
| 21-30 | 3.23 (2.32-4.50) | 2.88 (2.10-3.96) |
| 31-40 | 5.35 (3.65-7.84) | 4.32 (2.98-6.24) |
| >40 | 9.44 (5.42-16.45) | 6.47 (3.73-11.20) |
| Low-risk, 2 complications | ||
| 11-20 | 4.18 (3.71-4.72) | 3.09 (2.81-3.40) |
| 21-30 | 9.23 (7.86-10.84) | 6.32 (5.53-7.21) |
| 31-40 | 19.66 (15.00-25.76) | 10.52 (8.16-13.56) |
| >40 | 42.95 (25.05-73.64) | 24.59 (14.96-40.41) |
| Low-risk, ≥3 complications | ||
| 11-20 | 4.42 (2.79-7.01) | 4.33 (2.78-6.72) |
| 21-30 | 8.32 (5.20-13.32) | 8.74 (5.62-13.58) |
| 31-40 | 17.82 (10.88-29.16) | 20.67 (13.02-32.84) |
| >40 | 19.46 (8.45-44.77) | 26.68 (13.87-51.31) |
| High-risk, 1 complication | ||
| 11-20 | 1.40 (0.96-2.06) | 1.36 (1.00-1.86) |
| 21-30 | 1.62 (1.10-2.38) | 1.46 (1.06-1.99) |
| 31-40 | 1.88 (1.27-2.77) | 1.60 (1.16-2.20) |
| >40 | 2.31 (1.52-3.52) | 1.95 (1.38-2.77) |
| High-risk, 2 complications | ||
| 11-20 | 2.27 (2.18-2.37) | 1.93 (1.87-2.00) |
| 21-30 | 3.63 (3.47-3.80) | 2.76 (2.65-2.87) |
| 31-40 | 5.81 (5.48-6.17) | 4.02 (3.80-4.25) |
| >40 | 8.99 (8.14-9.93) | 5.50 (4.99-6.07) |
| High-risk, ≥3 complications | ||
| 11-20 | 2.06 (1.66-2.56) | 2.09 (1.74-2.50) |
| 21-30 | 3.46 (2.77-4.31) | 3.60 (3.00-4.34) |
| 31-40 | 5.25 (4.14-6.65) | 5.34 (4.38-6.52) |
| >40 | 6.23 (4.72-8.23) | 6.98 (5.53-8.80) |
Abbreviation: RAI, Risk Analysis Index.
All comparisons are vs the group with RAI scores of 10 or less.
Low-risk procedures were defines as having a postoperative mortality rate of less than 1%, and high-risk procedures were defined as having a postoperative mortality rate of 1% or higher.
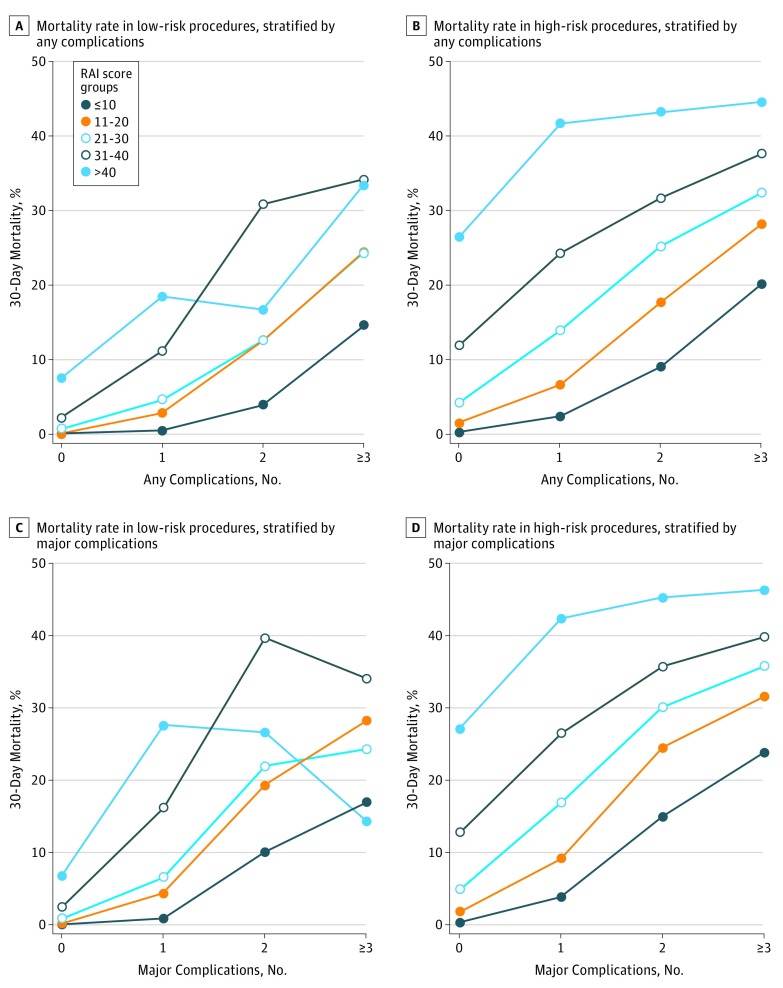
Figure 2 presents FTR as a function of RAI score. For high-risk procedures, there were significant increases in FTR rates across RAI categories, stratified by the number of complications. For patients undergoing a high-risk procedure who experienced 1 major complication, FTR occurred in 1187 of 30 832 patients with RAI scores of 10 or less (3.8%; reference); 1076 of 11 772 patients (9.1%) with RAI scores of 11 to 20 (OR, 2.5; 95% CI, 2.3-2.7); 1184 of 7026 patients (16.9%) with RAI scores of 21 to 30 (OR, 5.1; 95% CI, 4.6-5.5); 744 of 2810 patients (26.5%) with RAI scores of 31 to 40 (OR, 9.0; 95% CI, 8.1-10.0); and 330 of 779 patients (42.4%) with RAI scores of more than 40 (OR, 18.4; 95% CI, 15.7-21.4). Rates of FTR increased in patients who had high-risk procedures followed by 2 major complications (RAI score ≤10: 1013 of 6787 [14.9%]; vs RAI score 11-20: 884 of 3610 [24.5%]; OR, 1.8; 95% CI, 1.7-2.0; vs RAI score 21-30: 853 of 2835 [30.1%]; OR, 2.4; 95% CI, 2.2-2.7; vs RAI score 31-40: 515 of 1438 [35.8%]; OR, 3.2; 95% CI, 2.8-3.6; vs RAI score ≥40: 223 of 493 [45.2%]; OR, 4.7; 95% CI, 3.9-5.7; P < .001) and 3 or more major complications (RAI score ≤10: 1552 of 6514 [23.8%]; vs RAI score 11-20: 1146 of 3630 [31.6%]; OR, 1.5; 95% CI, 1.3-1.6; vs RAI score 21-30: 975 of 2724 [35.8%]; OR, 1.8; 95% CI, 1.6-2.0; RAI score 31-40: 552 of 1387 [39.8%]; OR, 2.1; 95% CI, 1.9-2.4; RAI score ≥40: 187 of 404 [46.3%]; OR, 2.7; 95% CI, 2.2-3.4; P < .001 for all comparisons; Table 3). The findings were similar for any complications.
Figure 2. Mortality Rates Associated With Major or Overall Complications, Stratified By Procedural Risk and RAI Score.
RAI, Risk Analysis Index. Procedural risk was defined as low if mortality was less than 1% and high if mortality was more than 1%.
Table 3. Association Between RAI Score and 30-day In-Hospital Mortality, Stratified by Number of Complications and Procedural Risk.
| RAI Scorea,b | Odds Ratiosc (95% CI) | |
|---|---|---|
| Major Complications | Any Complication | |
| Low-risk, 0 complications | ||
| 11-20 | 8.42 (6.10-11.63) | 8.31 (5.51-12.53) |
| 21-30 | 35.15 (25.26-48.91) | 43.04 (29.05-63.75) |
| 31-40 | 98.20 (60.80-158.62) | 139.37 (81.83-237.37) |
| >40 | 291.41 (124.76-680.66) | 513.74 (216.83-1217.23) |
| Low-risk, 1 complication | ||
| 11-20 | 5.26 (3.92-7.06) | 6.00 (4.27-8.42) |
| 21-30 | 8.08 (5.56-11.74) | 10.19 (6.70-15.49) |
| 31-40 | 22.31 (13.99-35.59) | 26.09 (15.23-44.69) |
| >40 | 43.86 (19.03-101.13) | 47.11 (17.40-127.55) |
| Low-risk, 2 complications | ||
| 11-20 | 2.15 (1.55-2.98) | 3.62 (2.62-5.01) |
| 21-30 | 2.52 (1.69-3.77) | 3.57 (2.34-5.44) |
| 31-40 | 5.89 (3.38-10.28) | 11.04 (6.31-19.33) |
| >40 | 3.26 (1.02-10.39) | 4.97 (1.42-17.42) |
| Low-risk, ≥3 complications | ||
| 11-20 | 1.93 (1.46-2.57) | 1.90 (1.51-2.39) |
| 21-30 | 1.58 (1.06-2.35) | 1.87 (1.38-2.55) |
| 31-40 | 2.53 (1.38-4.63) | 3.03 (1.92-4.78) |
| >40 | 0.82 (0.10-6.83) | 2.93 (0.99-8.63) |
| High-risk, 0 complications | ||
| 11-20 | 5.24 (4.81-5.71) | 5.83 (5.27-6.44) |
| 21-30 | 14.43 (13.28-15.68) | 16.67 (15.14-18.35) |
| 31-40 | 41.38 (37.75-45.35) | 51.14 (46.07-56.77) |
| >40 | 105.68 (92.52-120.73) | 137.40 (118.72-159.03) |
| High-risk, 1 complication | ||
| 11-20 | 2.51 (2.31-2.74) | 2.88 (2.62-3.17) |
| 21-30 | 5.06 (4.65-5.51) | 6.53 (5.94-7.17) |
| 31-40 | 8.99 (8.12-9.96) | 13.01 (11.65-14.54) |
| >40 | 18.35 (15.74-21.40) | 28.99 (24.59-34.19) |
| High-risk, 2 complications | ||
| 11-20 | 1.85 (1.67-2.04) | 2.20 (1.99-2.43) |
| 21-30 | 2.45 (2.21-2.72) | 3.43 (3.10-3.80) |
| 31-40 | 3.18 (2.80-3.61) | 4.71 (4.16-5.34) |
| >40 | 4.71 (3.89-5.69) | 7.75 (6.41-9.36) |
| High-risk, ≥3 complications | ||
| 11-20 | 1.47 (1.35-1.61) | 1.56 (1.45-1.68) |
| 21-30 | 1.78 (1.62-1.96) | 1.90 (1.76-2.06) |
| 31-40 | 2.11 (1.87-2.39) | 2.39 (2.17-2.64) |
| >40 | 2.75 (2.25-3.38) | 3.19 (2.71-3.75) |
Abbreviation: RAI, Risk Analysis Index.
All comparisons are vs the group with RAI scores of 10 or less.
Low-risk procedures were defines as having a postoperative mortality rate of less than 1%, and high-risk procedures were defined as having a postoperative mortality rate of 1% or higher.
Odds ratios are for 30-day in-hospital mortality.
This same trend was observed after low-risk procedures, although absolute event rates were lower. Increasing RAI scores were associated with a higher odds of FTR (or perioperative mortality for those who had no complications). The odds of FTR after 1 major complication for patients undergoing a low risk procedure increased as RAI scores rose (RAI score ≤10: 112 of 13 008 [0.9%]; RAI score 11-20: 78 of 1785 [4.4%]; OR, 5.3; 95% CI, 3.9-7.1; vs RAI score 21-30: 39 of 595 [6.6%]; OR, 8.1; 95% CI, 5.6-11.7; vs RAI score 31-40: 25 of 154 [16.2%]; OR, 22.3; 95% CI, 13.9-35.6; vs RAI score ≥40: 8 of 29 [27.6%]; OR, 43.9; 95% CI, 19-101.1; P < .001). Notably, the relative impact of increasing RAI score was attenuated as the number of complications increased after low-risk and high-risk procedures, suggesting that the number of complications mediates the effect of RAI score on FTR.
Having established the association between RAI and mortality and RAI and complications, we confirmed the association between complications and mortality with separate models, satisfying the assumptions of mediation analysis (eAppendix 2 in the Supplement). The magnitude of the direct effect of RAI on mortality (β = .143 and .097 for low-risk and high-risk cohorts) was reduced when complications were added to the model (β = .088 and .076 for low-risk and high-risk cohorts, respectively, with major complications, and β = .083 and .076 for low-risk and high-risk cohorts, respectively, with any complication). These differences between the direct and mediated effects of RAI score on mortality were significant (Sobel test of mediation, P < .001 in all models where RAI scores were treated as a continuous variable with integer increments), suggesting that the effect of RAI on mortality is partially mediated through postoperative complications in both low-risk and high-risk cohorts.
Discussion
Surgery induces substantial physiologic stress in even healthy patients. Recent studies suggest the early postoperative period is a time of particularly elevated risk for frail patients.10,11,22 There are 2 different conceptualizations of frailty: phenotypic frailty is conceptualized as a clinical syndrome driven by age associated with biologic changes, and deficit accumulation frailty is thought to be driven by the accumulation of medical, functional, and social deficits.23 The RAI score is a simple, clinically relevant tool that uses deficit accumulation to estimate the degree of phenotypic frailty in a given preoperative patient. Although this analysis used a form of the RAI score calculated from NSQIP variables, a survey version of the RAI score can be calculated prospectively in approximately 90 seconds to guide real-time decisions.13
The impact of frailty in surgical patients in relation to FTR is currently not well-characterized. In this regard, our study supports several novel and important conclusions. First, our data suggest there is a dose-response association between increasing frailty and both the number of postoperative complications and FTR. Second, these associations are observed after both low-mortality and high-mortality risk surgical procedures. Finally, the association between increasing frailty and FTR is partially mediated by the occurrence of complications (eg, frailty leads to complications that in turn lead to FTR). However, because the association between frailty and FTR persisted in our mediation analysis, this suggests frailty itself (and not simply the occurrence of complications in frail patients) has a clear impact on adverse postoperative outcomes.
Consistent with previously published work, our findings indicate that frailty is associated with incremental increases in the occurrence of postoperative complications.6,16,24,25,26 The fact that this has been a consistent finding across studies that used a variety of different models for measuring frailty not only emphasizes its importance as a perioperative risk factor, but also suggests a causal association. Furthermore, our findings suggest frailty is not only associated with an increasing number of postoperative complications, but that it also increases the risk of perioperative adverse events even after low-risk surgical procedures. This emphasizes the importance of frailty assessment for perioperative counseling before for procedures considered minor or low-risk by health care professionals providing surgery and anesthesia.
Failure to rescue was first described by Silber et al27 in the early 1990s. It has since been adopted as a quality indicator by both AHRQ and was endorsed by the National Quality Forum in 2010.8,18 Since then, significant interest has developed in understanding what factors influence FTR in postoperative patients.28 Several macrosystem hospital factors have been associated with FTR, including hospital size, occupancy, teaching status, hospital technology, nurse-to-patient ratio, and presence of more than 20 intensive care unit beds.29,30,31,32,33,34 Microsystem factors such as intensive care unit staffing, physician coverage, rapid response teams, attitudes of clinical staff with regards to a culture of safety, and specific behaviors have also been identified.35 While many of these health care structural factors are relatively immutable, frailty is believed to be a potentially modifiable patient-level risk factor. For example, in a recent randomized clinical trial evaluating a trimodal prehabilitation program (consisting of moderate-intensity physical exercise, nutritional counseling, protein supplementation, and anxiety reduction strategies), more than 80% of patients in the prehabilitation arm returned to preoperative functional capacity within 8 weeks, while only 40% of those in the control group regained their baseline level of functioning.36
Quality initiatives directed at identification and prehabilitation of frail patients are intended to inform shared decision making and mitigate perioperative surgical risk. This is particularly relevant when focusing on FTR, because it has been proposed that the inability to arrest a sequence of complications may underlie its occurrence.37 This is also significant given that higher FTR rates are seen with multiple complications and sequential complications.12 Because our findings demonstrate frailty is not only associated with the occurrence of complications, but also the number of complications, greater focus, and broader implementation of such efforts may help hospitals and health care professionals to identify at-risk patients and apply preoperative interventions to improve the patient’s physiologic reserve, decrease their perioperative risk, or improve early detection and intervention on postoperative complications. Efforts can also be developed to identify those patients who, after learning of their frailty-associated risks, may opt for a less invasive surgery or a nonsurgical option. This is particularly important given the fact that existing FTR methodology cannot disambiguate rescued patients who return to baseline function and those who experience permanent and dramatic disability that some have called rescue to failure.38
Thoroughly assessing of a level of preoperative frailty in a given patient and using this information to direct conversations around the goals of surgical care in the context of the attendant risks and desired outcome could provide valuable information in operative planning or could influence choices regarding the indication for a planned procedure. In this regard, 1 notable finding from our work was the impact of frailty after low-risk surgical procedures. Before the introduction of risk calculators and scoring systems to predict perioperative outcomes, surgical decisions were often based on the gut feeling of the operating surgeon. However, clinicians often do a poor job of estimating perioperative risk.39,40,41 The widespread development and implementation of objective risk prediction tools reflects the shift from traditional approaches of risk estimation and have become an integral decision support tool in the preoperative setting.
Limitations
There are several limitations to our study. The ACS-NSQIP was not specifically designed to assess frailty. However, we used a recently validated tool developed for use with this data set to estimate the degree of preoperative frailty. The ACS-NSQIP Participant User File does not provide a hospital identifier that would have allowed for adjustment of patients clustering within hospitals, and the data do not include information regarding clinician-level or hospital-level factors that could potentially impact FTR. Also, although the ACS-NSQIP captures data on the occurrence of several commonly occurring complications, this list is neither exhaustive nor inclusive of procedure-specific complications. Finally, there is no established RAI threshold as yet that can be used to clearly distinguish a patient as frail, although it is widely recognized that frailty exists on a continuum rather than a category, be it categorical or ordinal.
Conclusions
Preoperative patient frailty appears to be an important risk factor for postoperative complications and FTR, even after low-risk surgical procedures. Frailty assessment should be integrated into quality improvement efforts and preoperative informed consent conversations. Future work should focus on establishing clear definitions of frailty using the various measurement tools that would then enable clinicians to quickly identify patients who might either benefit from strategies directed at preoperative optimization or a more thorough discussion of the attendant risks of a given procedure and/or the goals of surgical care. In the meantime, our data suggest the risk-benefit ratio of performing even minor elective procedures needs to be carefully considered in individuals who are considered frail.
eAppendix 1. 10 most common low risk procedures and 10 most common high risk procedures
eAppendix 2. Mediation analysis
References
- 1.Colby SL, Ortman JM; United States Census Bureau Projections of the size and composition of the US population 2014 to 2060: population estimates and projections: current population reports. https://census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed February 15, 2018.
- 2.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):-. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Mañas L, Féart C, Mann G, et al. ; FOD-CC group (Appendix 1) . Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelaiditi E, Cesari M, Canevelli M, et al. ; IANA/IAGG . Cognitive frailty: rational and definition from an (IANA/IAGG) international consensus group. J Nutr Health Aging. 2013;17(9):726-734. [DOI] [PubMed] [Google Scholar]
- 5.Makary MA, Segev DL, Pronovost PJ, et al. . Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901-908. [DOI] [PubMed] [Google Scholar]
- 6.Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC Jr, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206(4):544-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Quality Forum Failure to rescue 30-day mortality (risk adjusted). http://www.qualityforum.org/QPS/0353. Published 2017. Accessed February 16, 2017.
- 9.Medicare Hospital Compare Surgical complications—recalibrated patient safety indicators. https://www.medicare.gov/hospitalcompare/Data/Serious-Complications.html. Published March 2017. Accessed April 3, 2017.
- 10.Joseph B, Phelan H, Hassan A, et al. . The impact of frailty on failure-to-rescue in geriatric trauma patients: A prospective study. J Trauma Acute Care Surg. 2016;81(6):1150-1155. [DOI] [PubMed] [Google Scholar]
- 11.Arya S, Kim SI, Duwayri Y, et al. . Frailty increases the risk of 30-day mortality, morbidity, and failure to rescue after elective abdominal aortic aneurysm repair independent of age and comorbidities. J Vasc Surg. 2015;61(2):324-331. [DOI] [PubMed] [Google Scholar]
- 12.Massarweh NN, Anaya DA, Kougias P, Bakaeen FG, Awad SS, Berger DH. Variation and impact of multiple complications on failure to rescue after inpatient surgery. Ann Surg. 2017;266(1):59-65. [DOI] [PubMed] [Google Scholar]
- 13.Hall DE, Arya S, Schmid KK, et al. . Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porock D, Oliver DP, Zweig S, et al. . Predicting death in the nursing home: development and validation of the 6-month Minimum Data Set mortality risk index. J Gerontol A Biol Sci Med Sci. 2005;60(4):491-498. [DOI] [PubMed] [Google Scholar]
- 15.Porock D, Parker-Oliver D, Petroski GF, Rantz M. The MDS mortality risk index: The evolution of a method for predicting 6-month mortality in nursing home residents. BMC Res Notes. 2010;3:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res. 2013;183(1):104-110. [DOI] [PubMed] [Google Scholar]
- 17.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361(14):1368-1375. [DOI] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality Patient safety network: patient safety primer failure to rescue. https://psnet.ahrq.gov/primers/primer/38/failure-to-rescue. Accessed March 10, 2017.
- 19.Liu SH, Ulbricht CM, Chrysanthopoulou SA, Lapane KL. Implementation and reporting of causal mediation analysis in 2015: a systematic review in epidemiological studies. BMC Res Notes. 2016;9:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mascha EJ, Dalton JE, Kurz A, Saager L. Statistical grand rounds: understanding the mechanism: mediation analysis in randomized and nonrandomized studies. Anesth Analg. 2013;117(4):980-994. [DOI] [PubMed] [Google Scholar]
- 21.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173-1182. [DOI] [PubMed] [Google Scholar]
- 22.McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg. 2016;151(6):538-545. [DOI] [PubMed] [Google Scholar]
- 23.Robinson TN, Walston JD, Brummel NE, et al. . Frailty for surgeons: review of a national institute on aging conference on frailty for specialists. J Am Coll Surg. 2015;221(6):1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg. 2011;253(6):1223-1229. [DOI] [PubMed] [Google Scholar]
- 25.Hall DE, Arya S, Schmid KK, et al. . Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg. 2017;152(3):233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009;48(1):78-83. [DOI] [PubMed] [Google Scholar]
- 27.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. 1992;30(7):615-629. [DOI] [PubMed] [Google Scholar]
- 28.Johnston MJ, Arora S, King D, et al. . A systematic review to identify the factors that affect failure to rescue and escalation of care in surgery. Surgery. 2015;157(4):752-763. [DOI] [PubMed] [Google Scholar]
- 29.Sheetz KH, Dimick JB, Ghaferi AA. Impact of hospital characteristics on failure to rescue following major surgery. Ann Surg. 2016;263(4):692-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henneman D, Snijders HS, Fiocco M, et al. . Hospital variation in failure to rescue after colorectal cancer surgery: results of the Dutch Surgical Colorectal Audit. Ann Surg Oncol. 2013;20(7):2117-2123. [DOI] [PubMed] [Google Scholar]
- 31.Ghaferi AA, Osborne NH, Birkmeyer JD, Dimick JB. Hospital characteristics associated with failure to rescue from complications after pancreatectomy. J Am Coll Surg. 2010;211(3):325-330. [DOI] [PubMed] [Google Scholar]
- 32.Wakeam E, Asafu-Adjei D, Ashley SW, Cooper Z, Weissman JS. The association of intensivists with failure-to-rescue rates in outlier hospitals: results of a national survey of intensive care unit organizational characteristics. J Crit Care. 2014;29(6):930-935. [DOI] [PubMed] [Google Scholar]
- 33.Wakeam E, Hevelone ND, Maine R, et al. . Failure to rescue in safety-net hospitals: availability of hospital resources and differences in performance. JAMA Surg. 2014;149(3):229-235. [DOI] [PubMed] [Google Scholar]
- 34.McIsaac DI, Wijeysundera DN, Huang A, Bryson GL, van Walraven C. Association of the hospital volume of frail surgical patients cared for with outcomes after elective, major noncardiac surgery: a retrospective population-based cohort study. Anesthesiology. 2017;126(4):602-613. [DOI] [PubMed] [Google Scholar]
- 35.Ghaferi AA, Dimick JB. Importance of teamwork, communication and culture on failure-to-rescue in the elderly. Br J Surg. 2016;103(2):e47-e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillis C, Li C, Lee L, et al. . Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937-947. [DOI] [PubMed] [Google Scholar]
- 37.Wakeam E, Hyder JA, Lipsitz SR, et al. . Hospital-level variation in secondary complications after surgery. Ann Surg. 2016;263(3):493-501. [DOI] [PubMed] [Google Scholar]
- 38.Wakeam E, Hyder JA. Raising the bar for failure to rescue: critical appraisal of current measurement and strategies to catalyze improvement. JAMA Surg. 2015;150(11):1023-1024. [DOI] [PubMed] [Google Scholar]
- 39.Pettigrew RA, Burns HJ, Carter DC. Evaluating surgical risk: the importance of technical factors in determining outcome. Br J Surg. 1987;74(9):791-794. [DOI] [PubMed] [Google Scholar]
- 40.Pettigrew RA, Hill GL. Indicators of surgical risk and clinical judgement. Br J Surg. 1986;73(1):47-51. [DOI] [PubMed] [Google Scholar]
- 41.Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis. 2009;24(5):569-576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. 10 most common low risk procedures and 10 most common high risk procedures
eAppendix 2. Mediation analysis