Key Points
Question
How common is albuminuria, and does its presence predict prognosis in adults with congenital heart disease?
Findings
In this cohort study of 612 adult outpatients with congenital heart disease, albuminuria was present in 106 (17.3%) and was more prevalent among patients with cyanosis, Fontan circulation, systemic right ventricle, worse functional class, or greater disease complexity. Albuminuria was associated with increased risk for adverse outcomes in patients with biventricular circulation but not those with Fontan circulation.
Meaning
Albuminuria is prevalent and may provide an additional tool to identify increased risk in adults with biventricular congenital heart disease.
This study assesses the prevalence, risk factors, and prognostic implications of albuminuria in adults with congenital heart disease.
Abstract
Importance
Albuminuria is associated with adverse outcomes in diverse groups of patients, but the importance of albuminuria in the emerging population of increasingly complex adults with congenital heart disease (ACHD) remains unknown.
Objective
To assess the prevalence, risk factors, and prognostic implications of albuminuria in ACHD.
Design, Setting, and Participants
This prospective study assessed a cohort of ambulatory patients aged 18 years and older who were examined at an ACHD referral center and enrolled in the Boston ACHD Biobank between May 17, 2012, to August 5, 2016. Albuminuria was defined as an urine albumin-to-creatinine (ACR) ratio of 30 mg/g or more.
Main Outcomes and Measures
Death or nonelective cardiovascular hospitalization, defined as overnight admission for heart failure, arrhythmia, thromboembolic events, cerebral hemorrhage, and/or disease-specific events.
Results
We measured the ACR of 612 adult patients with CHD (mean [SD] age, 38.6 [13.4] years; 308 [50.3%] women). Albuminuria was present in 106 people (17.3%) and was associated with older age (patients with ACR <30 mg/g: mean [SD]: 37.5 [13.2] years; vs patients with ACR ≥30 mg/g: 43.8 [13.1] years; P < .001), presence of diabetes mellitus (ACR <30 mg/g: 13 of 506 [2.6%]; vs ≥30 mg/g: 11 of 106 [10.4%]; P < .001), lower estimated glomerular filtration rate (ACR <30 mg/g: median [interquartile range (IQR)]: 103.3 [90.0-116.4] mL/min/1.73 m2; ACR ≥30 mg/g: 99.1 [78.8-108.7] mL/min/1.73 m2; P = .002), and cyanosis (ACR <30 mg/g: 23 of 506 [5.1%]; vs ACR ≥30 mg/g: 21 of 106 [22.6%]; P < .001). After a mean (SD) follow-up time of 270 (288) days, 17 patients (2.5%) died, while 68 (11.1%) either died or experienced overnight inpatient admission. Albuminuria predicted outcome, with 30 of 106 patients with albuminuria (28.3%) affected vs 38 of 506 patients without albuminuria (7.5%; hazard ratio [HR], 3.0; 95% CI, 1.9-4.9; P < .001). Albuminuria was also associated with increased mortality (11 of 106 [10.4%]; vs 6 of 506 [1.2%] in patients with and without albuminuria, respectively; HR, 6.4; 95% CI, 2.4-17.3; P < .001). Albuminuria was associated with the outcomes only in patients with a biventricular circulation (HR, 4.5; 95% CI, 2.5-8.0) and not those with single-ventricle circulation (HR, 1.0; 95% CI, 0.4-2.8; P = 0.01 compared with biventricular circulation group). Among 133 patients (21.7%) in NYHA functional class 2, albuminuria was strongly associated with death or nonelective hospitalization.
Conclusions and Relevance
Albuminuria is common and is associated with increased risk for adverse outcome in patients with ACHD with biventricular circulation. Albuminuria appears especially useful in stratifying risk in patients categorized as NYHA functional class 2.
Introduction
There is a growing population of adults with congenital heart disease (ACHD) of increasingly complexity and a corresponding rise in health care utilization. This disease category encompasses a wide diversity of diagnoses with a broad range of overall prognosis and specific outcome risks. Biomarkers that better stratify risk for patients across the ACHD spectrum would have great clinical value.
Albuminuria is present in approximately 5% to 8% of the general population, with a higher prevalence in patients with diabetes mellitus (DM) or hypertension. Normally, little albumin is excreted in the urine because of charge-selective and size-selective properties of the glomerular capillary wall and tubular reabsorption. Albuminuria indicates increased glomerular permeability that arises from endothelial dysfunction, generalized vasculopathy, or tubular dysfunction. The presence of albuminuria is associated with increased risk for adverse renal and cardiovascular outcomes in the general population and in patients with a broad array of diseases.
The association between cyanotic congenital heart disease (CHD) and albuminuria has been known for more than a century. However, to our knowledge, few studies have explored this phenomenon in other subtypes of CHD in the contemporary era and in which most patients undergo surgical or catheter-based repair. In this study, we investigated the prevalence, correlates, and prognostic implications of albuminuria in adults with CHD, hypothesizing that those who have albuminuria on a single spot urine measurement would be at increased risk for adverse outcomes.
Methods
Study Sample
We prospectively enrolled outpatients with CHD who were 18 years or older between 2012 and 2016 at Boston Children’s Hospital and Brigham and Women’s Hospital. We excluded 82 patients who had an albumin-to-creatinine ratio (ACR) of 30 mg/g or more after they had performed an exercise test on the same day, because strenuous exertion can lead to transient albuminuria.
The study protocol was approved by the Boston Children’s Hospital institutional review board, and informed consent was obtained from all participants. This study was conducted under a reliance agreement with Brigham and Women’s Hospital to cede direct oversight to Boston Children’s Hospital.
Collected Variables and Definitions
Biospecimens were collected as part of the Boston Adult Congenital Heart Disease Biobank. Details of the protocol have been published. Briefly, clinical data were collected from tests completed within 2 years of biospecimen collection, including patient demographics, vital signs, cardiac diagnosis, medications, medical comorbidities, and New York Heart Association functional classification (NYHA FC). Patients were classified into groups by primary diagnosis, pathophysiology, and complexity, as detailed in the eMethods in the Supplement. For a subset of patients who had cardiopulmonary exercise testing within 2 years of enrollment, we collected and analyzed peak oxygen consumption and ventilatory efficiency data.
Albuminuria
Random spot urine was collected during a clinic visit from spontaneous clean-catch void. Urine underwent centrifugation at 4000 rpm for 5 minutes at 4°C. Creatinine and albumin were measured on fresh samples in a Clinical Laboratory Improvement Amendments–certified laboratory. We defined albuminuria per existing guidelines: (1) an ACR of less than 30 mg/g is normal to mildly increased albuminuria, (2) an ACR of 30 to 300 mg/g is moderately increased albuminuria, and (3) an ACR of more than 300 mg/g is severely increased albuminuria. For simplicity, the term albuminuria is used in this article to indicate an ACR or 30 mg/g or greater.
Outcomes
The primary combined outcome was death from any cause or nonelective cardiovascular hospitalization. The latter was defined as an overnight inpatient admission for heart failure, arrhythmia or symptoms of arrhythmia, ischemic or thromboembolic events, cerebral hemorrhage, or disease-specific events (eg, hyperviscosity symptoms in Eisenmenger syndrome). Secondary analyses were performed for the outcome of all-cause mortality.
Data Analysis
Owing to its right-skewed distribution, ACR was analyzed as a natural log-transformed continuous variable and as a categorical variable. Continuous variables are presented as mean (SD) if normally distributed or median (interquartile range [IQR]) if not normally distributed. Either a 2-sided unpaired t test or Wilcoxon rank-sum test, as appropriate for distribution, was used to compare continuous variables between groups, and either the χ2 test or Fisher exact was used to compare proportions for categorical variables. The log-rank test was used to perform univariate survival analysis per albuminuria severity. Time to event was defined from date of biospecimen collection to date of first clinical event, with censoring of event-free individuals at the most recent date when event status was known. For multivariable Cox regression analyses, we used forward selection with a P value of less than .05 for entry, considering for inclusion any variable associated with log ACR at a P value of .10 in univariate analysis; a maximum of 1 covariate was included per 10 events. We applied the Harrell method to estimate of the concordance statistic (C-statistic) for this model, first without ACR and then after adding ACR as both a categorical variable and a log-transformed continuous variable. In addition, we analyzed the association by adjusting for a loaded model that itself adjusted a priori for covariates considered likely to be important: age, diagnosis severity, cyanosis, DM, estimated glomerular filtration rate (eGFR), and NYHA FC category.
Based on a prior analysis, we anticipated the possibility that albuminuria would have a different meaning in those with single-ventricle Fontan (SVF) circulation compared with those who have biventricular CHD physiology; therefore, we planned a priori to report the outcomes analysis stratifying by SVF status. We explored all 2-way interactions, confirming a statistically significant interaction between SVF status and the association between ACR and outcomes; there were no other significant 2-way interactions.
Log ACR was also analyzed as a continuous variable using restricted cubic splines to assess for a nonlinear association with outcomes. A 2-sided P value less than .05 was considered statistically significant. Analyses were performed with SAS version 9.4 (SAS Institute Inc), R version 3.4.1 (R Foundation for Statistical Computing), and GraphPad Prism (GraphPad Software, Inc).
Results
A total of 753 patients were enrolled in the Boston Adult Congenital Heart biobank during the study period. Urine was not collected from 59 individuals; another 82 individuals were excluded because urine was collected after an exercise test. A total of 612 patients were included in the analysis (eFigure 1 in the Supplement).
Albuminuria in ACHD
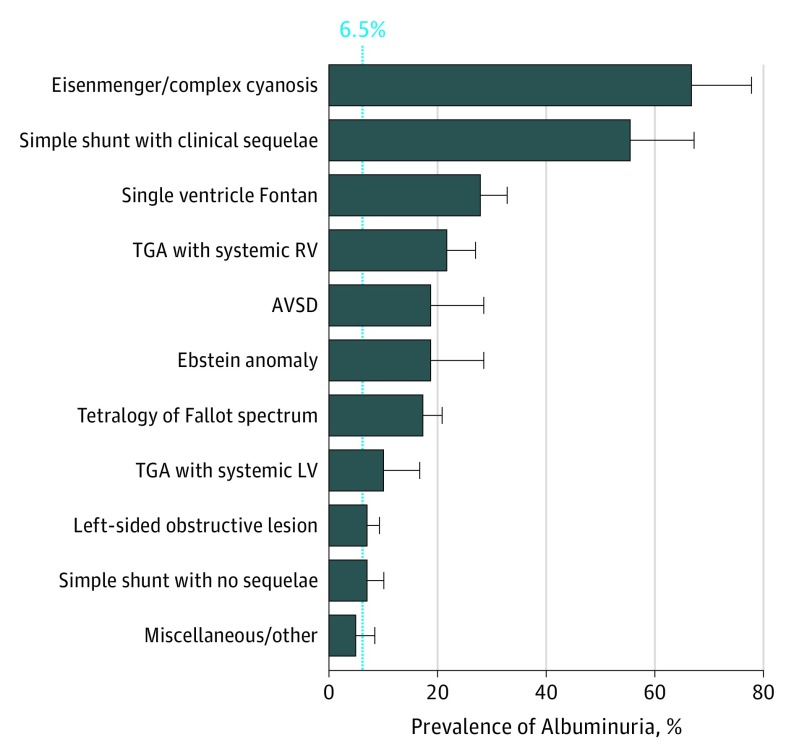
The most common CHD diagnosis groups were left-sided obstructive lesions (n = 141 [23.0%]), tetralogy of Fallot spectrum diagnoses (n = 126 [20.6%]), SVF (n = 86 [14.1%]), and repaired simple shunts (n = 89 [14.5%], of whom 18 [2.9%] had sequelae and 71 [11.6%] did not). Albuminuria was present in 106 patients (17.3%), moderately increased ACR (30-300 mg/g) in 90 patients (14.7%), and severely increased ACR (>300 mg/g) in 16 patients (2.6%). Patients with Eisenmenger syndrome or complex cyanotic CHD (12 of 18 [66.7%]), simple shunts with clinical sequelae (10 of 18 [55.6%]), SVF (24 of 86 [27.9%]), and transposition of the great arteries with a systemic right ventricle (13 of 60 [21.7%]) all had at least 3-fold higher prevalence of albuminuria compared with the prevalence in the general population (approximately 5% to 8%). However, the prevalence of albuminuria was similar to the general population in patients with simple shunts without clinical sequelae (5 of 71 [7.0%]) and left-sided obstructive lesions (10 of 141 [7.1%]; Figure 1).
Figure 1. Prevalence of Albuminuria in Adults by Type of Congenital Heart Disease.
Frequency of albuminuria (defined as an albumin-to-creatinine ratio ≥30 mg/g) according to underlying congenital heart disease diagnosis. The solid line represents the approximate general population prevalence (6.5%). Subgroups included 18 patients with Eisenmenger syndrome/complex cyanotic, 18 with a simple shunt with clinical sequelae, 86 with single ventricle Fontan circulation, 60 with TGA with systemic RV, 16 with AVSD, 16 with Ebstein anomaly, 126 with tetralogy of Fallot, 20 with TGA with systemic left ventricle, 141 with left ventricular obstructive lesions, 71 with a simple shunt with no sequelae, and 40 with miscellaneous other disorders. AVSD indicates atrioventricular septal defect; LV, left ventricle; RV, right ventricle; TGA, transposition of the great arteries.
Factors Associated With Albuminuria
Albuminuria was associated with older age (patients with ACR<30 mg/g: mean [SD] age, 37.5 [13.2] years vs patients with ACR ≥30 mg/g: 43.8 [13.1] years; P < .001), presence of DM (patients with ACR <30 mg/g: 13 of 506 [2.6%]; vs patients with ACR ≥30 mg/g: 11 of 106 [10.4%]; P < .001), lower eGFR (patients with ACR <30 mg/g: median [IQR], 103.3 [90.0-116.4] mL/min/1.73 mg/m2; patients with ACR ≥30 mg/g: 99.1 [78.8-108.7] mL/min/1.73 mg/m2; P = .002), and cyanosis (patients with ACR <30 mg/g: 23 of 506 [5.1%]; vs patients with ACR ≥30 mg/g: 21 of 106 [22.6%]; P < .001).
Albuminuria was also associated with higher (worse) NYHA FC category and greater disease complexity. Most patients with ACR of less than 30 were NYHA FC category 1 (392 of 506 [77.8%]), while the same was true for just over half of patients with albuminuria (58 of 106 [55.2%]). Patients with ACR levels of less than 30 mg/g experienced albuminuria at varying rates per their NYHA FC category (NYHA FC 1: 392 of 506 [77.8%]; vs NYHA FC 2: 97 of 506 [19.3%]; vs NYHA FC 3/4: 15 of 506 [3.0%]). Albuminuria prevalence also varied in patients with ACR levels of 30 mg/g or more per this classification (NYHA FC 1: 58 of 106 [55.2%]; vs NYHA FC 2: 36 of 106 [34.3%]; vs NYHA FC 3/4: 11 of 106 [10.5%]; P < .001 across all comparisons in both subgroups). Albuminuria varied with disease complexity patients with ACR levels of less than 30 mg/g (mild: 106 of 506 [20.6%]; vs moderate: 237 of 506 [46.8%]; vs severe: 63 of 506 [12.4%]) and in patients with ACR of 30 mg/g or more (mild: 17 of 106 [16.0%]; vs moderate: 32 of 106 [30.2%]; vs severe: 57 of 106 [53.7%]; P < .001 across all comparisons in both subgroups).
There was no statistically significant association between albuminuria and hypertension, BMI, or tobacco use (Table 1). The high prevalence of albuminuria was not attributable to the presence of hypertension or DM, both of which had relatively low prevalence (83 of 612 [13.6%] and 24 of 612 [3.9%], respectively).
Table 1. Demographic and Clinical Characteristics of 612 Adults With Congenital Heart Disease, Categorized by Presence of Albuminuria.
| Variable | No. (%) | P Value | |
|---|---|---|---|
| ACR <30 mg/g (n = 506) | ACR ≥30 mg/g (n = 106) | ||
| Age, mean (SD), y | 37.5 (13.2) | 43.8 (13.1) | <.001 |
| White race | 373 (73.7) | 78 (73.6) | >.99 |
| Men | 252 (49.8) | 52 (49.1) | .88 |
| BMI, mean (SD)a | 27.2 (9.8) | 26.5 (6.4) | .23 |
| BMI >30a | 131 (25.8) | 25 (23.6) | .57 |
| Hypertension | 72 (14.2) | 11 (10.4) | .29 |
| Diabetes mellitus | 13 (2.6) | 11 (10.4) | <.001 |
| Current tobacco use | 21 (4.2) | 6 (5.7) | .49 |
| Chronic kidney diseaseb | 12 (2.4) | 13 (12.3) | <.001 |
| Coronary artery disease | 9 (1.8) | 1 (0.9) | .53 |
| Pulmonary hypertension | 12 (2.4) | 14 (13.2) | <.001 |
| Liver cirrhosis | 7 (1.4) | 7 (6.6) | .01 |
| Obstructive sleep apnea | 36 (7.1) | 18 (17.0) | .001 |
| Stroke | 20 (4.0) | 9 (8.5) | .05 |
| Hyperlipidemia | 65 (6.1) | 15 (14.1) | .71 |
| Cyanosisa,c | 23 (5.1) | 21 (22.6) | <.001 |
| Mechanical valve | 24 (4.7) | 6 (5.7) | .70 |
| Single-ventricle Fontan circulation | 62 (12.2) | 24 (22.6) | <.001 |
| NYHA functional classa | |||
| 1 | 392 (77.8) | 58 (55.2) | <.001 |
| 2 | 97 (19.3) | 36 (34.3) | |
| 3/4 | 15 (3.0) | 11 (10.5) | |
| Disease complexity | |||
| Mild | 106 (20.9) | 17 (16.0) | <.001 |
| Moderate | 237 (46.8) | 32 (30.2) | |
| Severe | 63 (12.4) | 57 (53.7) | |
| Medications | |||
| Aspirin | 147 (29.1) | 44 (41.5) | .04 |
| ACE inhibitor or ARB | 151 (29.8) | 33 (31.1) | .79 |
| β-Blocker | 144 (28.5) | 46 (43.4) | .003 |
| Potassium-sparing diuretic | 38 (7.5) | 18 (17.0) | .002 |
| Loop diuretic | 72 (14.2) | 49 (46.2) | <.001 |
| Statin | 51 (10.1) | 17 (16.0) | .08 |
| Warfarin | 76 (15.0) | 39 (36.8) | <.001 |
| Digoxin | 37 (7.3) | 23 (21.7) | <.001 |
| Laboratory testsa | |||
| Hemoglobin, mean (SD), g/dL | 14.2 (1.6) | 14.7 (2.7) | .09 |
| Serum creatinine, median (IQR), mg/dL | 0.8 (0.7-1.0) | 0.8 (0.7-1.0) | .36 |
| eGFR, median (IQR), mL/min/1.73 m2 | 103.3 (90.0-116.4) | 99.1 (78.8-108.7) | .002 |
| C-reactive protein, median (IQR), mg/L | 1.2 (0.4-2.6) | 2.0 (0.9-4.8) | <.001 |
| Serum albumin, mean (SD), g/dL | 4.6 (0.4) | 4.4 (0.4) | .007 |
| Serum sodium, mean (SD), mmol/L | 140 (2.3) | 140 (2.8) | .29 |
Abbreviations: ACE, angiotensin converting enzyme; ACR, albumin-to-creatinine ratio; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; IQR, interquartile range; NYHA, New York Heart Association.
Data were missing from 3 patients for NYHA functional class, 6 patients for BMI, 28 patients for blood laboratory tests, and 67 patients for cyanosis status (oxygen saturation).
Chronic kidney disease was defined as eGFR less than 60 mL/min/1.73 m2.
Cyanosis was defined as a resting oxygen saturation of less than 92%.
Albuminuria was more frequently observed in patients who were taking diuretics, β-blockers, digoxin, and warfarin. Patients with ACR levels of less than 30 mg/g and patients with ACR levels of 30 mg/g or more differed significantly with respect to use of loop diuretics (72 of 506 [14.2%]; vs 49 of 106 [46.2%], respectively; P < .001). Similar results were seen with rates of β-blocker use (patients with ACR <30 mg/g: 144 of 506 [28.5%]; vs patients with ACR ≥30 mg/g: 46 of 106 [43.4%]; P = .003); rates of digoxin use (patients with ACR<30 mg/g: 37 of 506 [7.3 %]; vs patients with ACR ≥30 mg/g: 23 of 106 [21.7%]; P < .001); and rates of warfarin use (patients with ACR <30 mg/g: 76 of 506 [15.0%]; vs patients with ACR ≥30 mg/g: 39 of 106 [36.8%]; P < .001). However, albuminuria was not associated with the use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker medications (Table 1).
Survival Analysis
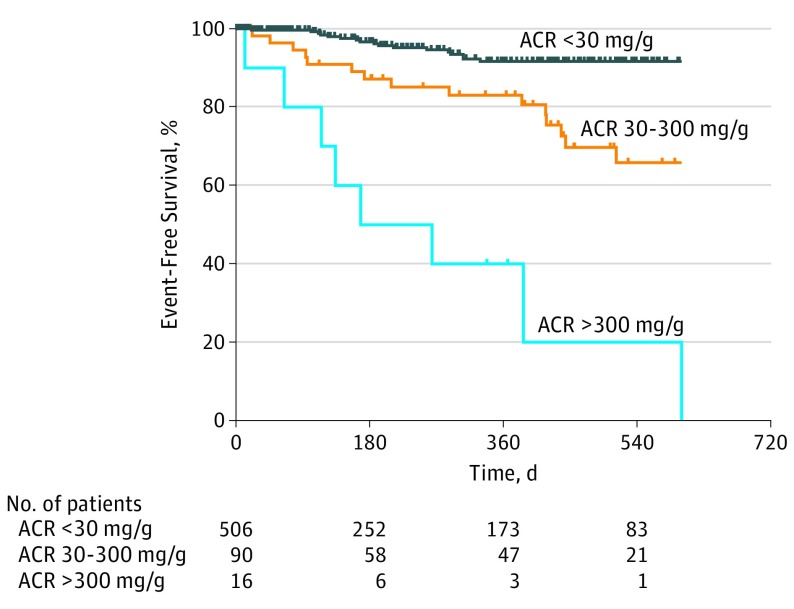
After a mean (SD) follow-up time of 270 (288) days (median, 183.5 days; range, 0-1183 days), there were 17 deaths (2.5%) and 68 patients (11.1%) experienced the primary outcome, which was either death or nonelective cardiovascular hospitalization. On survival analysis by degree of albuminuria, we found a stepwise increase in the risk for sustaining the combined primary outcome (death and nonelective hospitalization for cardiovascular causes) with increasing degree of albuminuria, from normal or mildly increased to moderately increased to severely increased albuminuria (Figure 2).
Figure 2. Survival Time Free From Death or Nonelective Cardiovascular Hospitalization, Categorized by Degree of Albuminuria.
Kaplan-Meier survival plots for adults with congenital heart disease classified by degree of albuminuria: normal or mildly increased (ACR <30 mg/g; n = 506; vs moderately increased (ACR ≥30-300 mg/g; n = 90; P < .001 compared with normal/mild albuminuria); vs severely increased, (ACR >300 mg/g; n = 16; P < .001 in comparison to moderately increased albuminuria). ACR indicates albumin-to-creatinine ratio.
Among those with any degree of albuminuria (ACR ≥30 mg/g), the primary outcome occurred in 30 of 106 patients (28.3%); in contrast, only 38 of 506 people without albuminuria (7.5%) experienced the same (Table 2). Albuminuria was a significant predictor of combined outcome (univariate hazard ratio [HR], 3.0; 95% CI, 1.9-4.9; P < .001; multivariable HR, 2.4; 95% CI, 1.4-4.1; P = .002, after adjusting for cyanosis and NYHA FC category based on automated forward selection). The C-statistic calculated via the base model with cyanosis and NYHA FC category was 0.72; this increased to 0.75 with the addition of dichotomous albuminuria and to 0.76 with the addition of continuous log ACR data.
Table 2. Hazard Ratios for the Combined Outcome and All-Cause Mortality.
| Outcome | All Patients | Biventricular Circulation | Fontan Circulation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No (%), ACR ≥30 vs <30 mg/g | HR (95% CI) | P Value | No (%), ACR ≥30 vs <30 mg/g | HR (95% CI) | P Value | No (%), ACR ≥30 vs <30 mg/g | HR (95% CI) | P Value | |
| Combined outcomea | |||||||||
| ACR ≥30 mg/g, univariate | 30/106 (28.3) vs 38/506 (7.5) |
3.0 (1.9-4.9) |
<.001 | 25/82 (30.5) vs 21/444 (4.7) |
4.5 (2.5-8.0) |
<.001 | 5/24 (20.8) vs 17/62 (27.4) |
1.0 (0.4-2.8) |
.95 |
| ACR ≥30 mg/g, adjusted model 1b | NA | 2.4 (1.4-4.1) |
.002 | NA | 2.6 (1.3-5.4) |
.01 | NA | 0.9 (0.3-2.9) |
.89 |
| ACR ≥30 mg/g, adjusted model 2c | NA | 2.2 (1.3-4.0) |
.01 | NA | 2.6 (1.2-5.6) |
.01 | NA | 0.9 (0.3-3.0) |
.85 |
| Mortalityd | |||||||||
| ACR ≥30 mg/g | 11/106 (10.4) vs 6/506 (1.2) |
6.4 (2.4-17.3) |
<.001 | 10/82 (12.2) vs 1/444 (0.2) |
37.9 (4.8-297) |
<.001 | 1/24 (4.2) vs 5/62 (8.1) |
0.6 (0.1-5.4) |
.67 |
Abbreviations: ACR, albumin-to-creatinine ratio; HR, hazard ratio; NA, not applicable.
The combined outcome was mortality or nonelective cardiovascular hospitalization; the combined outcome and mortality alone were each associated with albuminuria in the overall cohort.
Model 1 was based on automated forward selection and was adjusted for New York Heart Association functional class and cyanosis.
Model 2 was adjusted for age, diabetes mellitus, New York Heart Association functional class, cyanosis, congenital heart disease severity, and estimated glomerular filtration rate.
Given the low absolute number of deaths (n = 17), multivariable adjustment was not performed for mortality alone.
Since the selected multivariable model did not include several clinically relevant variables, we repeated the analysis adjusting for age, cyanosis, DM, NHYA FC level, disease complexity, and eGFR with similar results (HR, 2.2; 95% CI, 1.3-4.0; P = .01). Of particular note was the association between degree of albuminuria and HRs for the combined outcome was consistent at all levels of eGFR (eFigure 2 in the Supplement).
Albuminuria was also predictive of mortality. Death occurred in 11 of 106 patients with albuminuria (10.4%) compared with only 6 of 506 patients who did not have albuminuria (1.2%; HR, 6.4; 95% CI, 2.4-17.3; P < .001; Table 2).
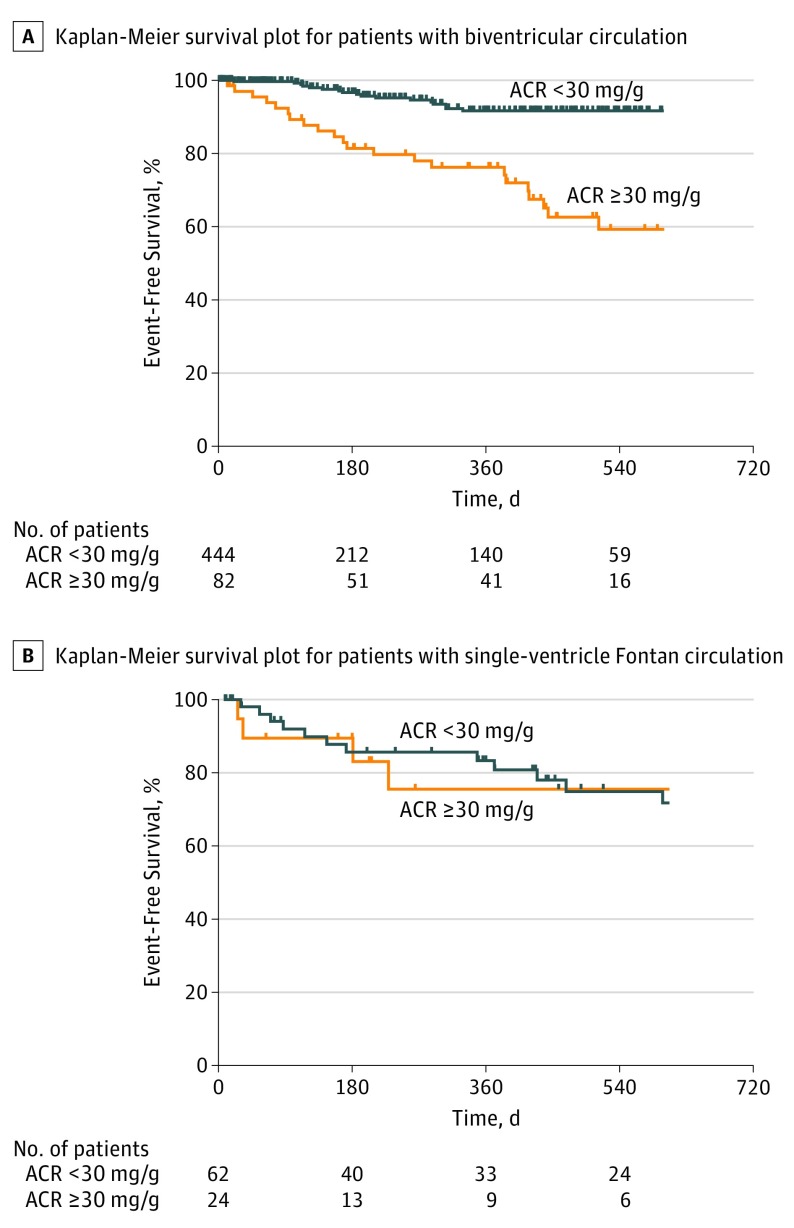
There was a significant 2-way interaction between the type of circulation and the association of albuminuria with the combined outcome. Albuminuria was associated with adverse outcomes only in patients with a biventricular circulation, but not those with a SVF (biventricular circulation: n = 526; HR, 4.5; 95% CI, 2.5-8.0; vs SVF circulation: n = 86; HR, 1.0; 95% CI, 0.4-2.8; P = .01 for 2-way interaction; Figure 3; Table 2). This was also true for mortality alone (biventricular circulation: HR, 37.9; 95% CI, 4.8-297; vs SVF circulation: HR, 0.6; 95% CI, 0.1-4.5; P = .01 for 2-way interaction; Table 2). There was a continual increase in risk associated with increasing ACR among patients with a biventricular circulation, even within the normal range (<30 mg/g), to a plateau of approximately 300 mg/g (eFigure 3 in the Supplement).
Figure 3. Survival Time Free from Death or Nonelective Cardiovascular Hospitalization, Stratified by Type of Circulation.
A, Kaplan-Meier survival plot for adult congenital heart disease patients by albuminuria status, for patients with biventricular circulation; an ACR level of 30 mg/g or greater was a significant predictor of the combined outcome (hazard ratio, 4.5; 95% CI, 2.5-8.0; P < .001 by log-rank test). B, For patients with single-ventricle Fontan circulation, an ACR level of 30 mg/g or greater was not a significant predictor of the combined outcome (hazard ratio, 1.0; 95% CI, 0.4-2.8; P = .95 by log-rank test). ACR indicates albumin-to-creatinine ratio.
After stratifying the survival analysis by baseline NYHA FC, event-free survival was poor regardless of albuminuria status among patients with markedly limited functional status (NYHA FC 3 or 4); likewise, prognosis was good for patients at the NYHA FC 1 category whether or not they had albuminuria. However, among the 133 patients with NYHA FC 2 symptoms, albuminuria was strongly predictive of death or nonelective hospitalization (eFigure 4 in the Supplement).
Exercise Testing
A subset of patients (n = 404; 66.0%) underwent cardiopulmonary exercise testing within 2 years of enrollment. Albuminuria was associated with lower peak oxygen consumption (or VO2; mean [SD], 18.5 [7.6] mL/kg/min vs 24.2 [8.0] mL/kg/min; P < .001; 58.6% vs 74.1% predicted; P < .001), and less efficient ventilation (minute ventilation/carbon dioxide production [VE/VCO2] slope, 31.4 [8.8] vs 27.6 [5.6]; P < .001).
As with the survival analysis, there was a 2-way interaction by SVF circulation status (P = .02). Albuminuria was associated with lower VO2 in patients with biventricular CHD (mean [SD], 17.8 [8.2] mL/kg/min vs 24.7 [8.0] mL/kg/min; P < .001), but not with SVF circulation (mean [SD], 20.5 [5.7] mL/kg/min vs 21.1 [6.5] mL/kg/min; P = .73; eTable in the Supplement).
Discussion
This study suggests that approximately 1 in every 6 adults with CHD have albuminuria, which is notably higher than the general population prevalence of 5% to 8%. Adult patients with CHD who have albuminuria are at increased risk for mortality and nonelective cardiovascular hospitalization, particularly those who have a biventricular circulation. As in other populations, higher levels of ACR, even below the cut-off level of 30 mg/g, appeared to be associated with increased risk. Despite a correlation between albuminuria and markers of disease severity and prognosis, the prognostic value of albuminuria was not accounted for by known markers of ACHD prognosis, including NYHA FC category, disease complexity, and renal function. Albuminuria provided particular prognostic insight among patients with mild to moderate functional limitation, those classified as NYHA FC 2.
The prognostic implication of low eGFR in patients with ACHD has been described, and the association between cyanosis and renal function has been detailed. In patients with cyanotic CHD, functional abnormalities (eg, decreased renal plasma flow and GFR, impaired uric acid secretion, and proteinuria) and pathological abnormalities (eg, mesangial hypercellularity, glomerular capillary congestion, and segmental sclerosis) have both been reported. However, little has been published about the prevalence and prognostic value of albuminuria across the wider spectrum of patients with ACHD. In a study of 14 patients with cyanosis and 22 patients without cyanosis with CHD, 13 patients (35.7%) had moderately increased albuminuria, and 8 patients (21%) had proteinuria (defined as urine protein >1 g/24 hours). Notably, albuminuria was only present among the patients with cyanosis. The current study supports that the frequency of albuminuria parallels the general prevalence among certain groups of patients, including those with left-sided obstructive lesions, repaired simple shunts without clinical sequelae, and transposition with a systemic left ventricle. Conversely, the prevalence of albuminuria is elevated among patients with ongoing cyanosis and others, such as those with SVF, simple shunts with clinical sequelae, and transposition with a systemic right ventricle.
There are likely several distinct reasons that adults with CHD are prone to albuminuria. One is cyanosis and erythrocytosis, as outlined above. Hemodynamic abnormalities, specifically limited perfusion and renal congestion, may be another. Several studies have shown a high prevalence of albuminuria in adults with acquired congestive heart failure with a similar prevalence among those with reduced and preserved ejection fractions. In patients with acquired heart failure, albuminuria is similarly associated with increased risk for death and nonfatal heart failure hospitalizations. The ACHD diagnoses with the highest burden of albuminuria also tend to be those most strongly associated with heart failure and high systemic venous pressure. Further, medications commonly used to treat heart failure (eg, diuretics, β-blockers, and digoxin) were more frequently prescribed to patients with albuminuria. Interestingly, there was no difference in angiotensin-converting enzyme inhibitor or receptor blocker use between groups with or without albuminuria. While the current analysis is unable to delve deeply into the reason for this discrepancy, presumably this could reflect a protective effect of these medications on albuminuria.
Many variables associated with albuminuria in ACHD tended to mirror findings in the general population and those with heart failure: older age, DM, lower eGFR, and worse NYHA FC status. Conversely, hypertension, tobacco use, and elevated BMI were not associated with albuminuria in this study sample. Most importantly, only a small subset of patients in this study had DM (24 [3.9%]) or hypertension (83 [13.6%]), and these diagnoses did not account for the high prevalence of albuminuria observed.
As with studies in other patient populations, albuminuria was associated with adverse outcomes in the overall group of patients with ACHD. In fact, it is challenging to identify an adequately powered study in any population that does not support an association between albuminuria and adverse outcomes. This makes it especially notable that while we found albuminuria to be strongly predictive of outcomes in patients with biventricular CHD, there was no such association with adverse outcome among patients with SVF circulation. This is not readily explained by low statistical power: the prevalence of albuminuria in the group with SVF was high, and we were able to demonstrate a statistically significant interaction between underlying circulation and the association between albuminuria and outcome. Further, an equivalent pattern was observed with continuous measures of exercise performance (ie, in peak VO2 and VE/VCO2 slope), which are themselves associated with outcomes in adults with CHD. Albuminuria was associated with markedly lower peak VO2 and higher VE/VCO2 slope in patients with a biventricular circulation; there was no such association among those with a SVF circulation. We believe this suggests either a distinct mechanism of albuminuria or that albuminuria reflects competing mechanisms in SVF patients. For example, it may be that exercise is associated with more prominent albuminuria in the SVF circulation because of striking increases in systemic venous pressure; this could be associated with some adverse effect among patients who engage in regular aerobic conditioning but the positive effects of such training may counterbalance any negative impact.
These results demonstrate the high prevalence of albuminuria in adults with CHD overall, with a variable prevalence by CHD diagnosis. Albuminuria identifies a cohort of patients with more severe illness, and the predictive value of albuminuria appears to be independent of its association with known markers of risk. Albuminuria was especially useful in predicting outcomes in patients with NYHA FC 2 symptoms, a group at intermediate risk who would therefore especially benefit from further risk stratification. Urine ACR is an inexpensive, noninvasive, simple test that can be performed during routine clinical visits; with further validation, the presence of albuminuria could be used to identify a higher risk group among patients who may appear to be stable. The mechanism and implications of albuminuria in patients with SVF require further research, including potential interactions with other end organ (eg, liver) dysfunction.
Limitations
The population under study, adults with CHD, is heterogeneous. We classified patients based on 1 commonly applied approach to defining primary pathophysiologic subtype. This classification scheme was not developed with renal pathophysiology in mind, and patients within a single diagnostic group may not share the same renal effects (eg, coarctation and aortic stenosis were grouped under left-sided obstructive lesion). This is also a single-center study, and the distribution of CHD diagnoses and historical management may not be universally generalizable. The diversity of diagnoses also constrains the use of certain variables. For example, we chose not to present data on ventricular ejection fraction because of the inherent difficulties of this measure in patients with CHD and the variability between diagnoses. Our definitions of comorbidities, including DM and hypertension, depended on a documented clinical diagnosis from the medical record.
Urine ACR is a spot measurement of urine albumin excretion and may be affected by factors such as fever or active urinary tract infection; however, these are unlikely to be common in a substantial proportion of stable outpatients. Much like the diagnosis of systemic hypertension, documentation of albuminuria in practice requires repeated measurement. While this is a limitation, the lack of such confirmation would be expected to cause nondifferential misclassification and thereby bias the results toward the null. One reason that confirmation with repeat testing is required is that strenuous exertion can also cause benign transient albuminuria; we therefore excluded patients with albuminuria who underwent an exercise stress test prior to urine collection. The resulting prevalence estimates may, therefore, underestimate the actual prevalence of albuminuria.
Conclusions
Albuminuria is common in adults with CHD, with the highest prevalence among patients with SVF, cyanotic CHD, and transposition of the great arteries with a systemic right ventricle. Albuminuria predicts adverse clinical outcomes in adults with biventricular CHD but not in those with SVF. The mechanisms and implications of albuminuria in patients with SVF merit further study. Albuminuria appears to have especially powerful prospective use in defining risk in patients with NYHA FC 2 status.
eMethods. Supplemental Methods.
eFigure 1. Patient Enrollment and Inclusion in the Analysis.
eFigure 2. Hazard Ratios for the Combined Outcome by Estimated Glomerular Filtration Rate and Degree of Albuminuria.
eFigure 3. Risk of Adverse Outcomes for Adults with Biventricular Congenital Heart Disease, n=526, by Urinary Albumin-to-Creatinine Ratio (ACR) Value.
eFigure 4. Kaplan-Meier Plots of Albuminuria as a Predictor of Adverse Outcomes, stratified by New York Heart Association Functional Class.
eTable. Exercise Test Results by Albuminuria Status, Stratified by Type of Circulation.
References
- 1.Gilboa SM, Devine OJ, Kucik JE, et al. . Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134(2):101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Leary JM, Siddiqi OK, de Ferranti S, Landzberg MJ, Opotowsky AR. The changing demographics of congenital heart disease hospitalizations in the United States, 1998 through 2010. JAMA. 2013;309(10):984-986. [DOI] [PubMed] [Google Scholar]
- 3.van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ. The changing epidemiology of congenital heart disease. Nat Rev Cardiol. 2011;8(1):50-60. [DOI] [PubMed] [Google Scholar]
- 4.Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54(5):460-467. [DOI] [PubMed] [Google Scholar]
- 5.Jones CA, Francis ME, Eberhardt MS, et al. . Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39(3):445-459. [DOI] [PubMed] [Google Scholar]
- 6.Hillege HL, Janssen WM, Bak AA, et al. ; Prevend Study Group . Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249(6):519-526. [DOI] [PubMed] [Google Scholar]
- 7.Coresh J, Selvin E, Stevens LA, et al. . Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038-2047. [DOI] [PubMed] [Google Scholar]
- 8.Seliger SL, Salimi S, Pierre V, Giffuni J, Katzel L, Parsa A. Microvascular endothelial dysfunction is associated with albuminuria and CKD in older adults. BMC Nephrol. 2016;17(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo LM, Bakris GL, Comper WD. Renal handling of albumin: a critical review of basic concepts and perspective. Am J Kidney Dis. 2002;39(5):899-919. [DOI] [PubMed] [Google Scholar]
- 10.Ochodnicky P, Henning RH, van Dokkum RP, de Zeeuw D. Microalbuminuria and endothelial dysfunction: emerging targets for primary prevention of end-organ damage. J Cardiovasc Pharmacol. 2006;47(suppl 2):S151-S162. [DOI] [PubMed] [Google Scholar]
- 11.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310(6):356-360. [DOI] [PubMed] [Google Scholar]
- 12.Keane WF, Brenner BM, de Zeeuw D, et al. ; RENAAL Study Investigators . The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63(4):1499-1507. [DOI] [PubMed] [Google Scholar]
- 13.Wachtell K, Ibsen H, Olsen MH, et al. . Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139(11):901-906. [DOI] [PubMed] [Google Scholar]
- 14.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. . Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110(1):32-35. [DOI] [PubMed] [Google Scholar]
- 15.Jackson CE, Solomon SD, Gerstein HC, et al. ; CHARM Investigators and Committees . Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374(9689):543-550. [DOI] [PubMed] [Google Scholar]
- 16.Niizeki T, Takeishi Y, Sasaki T, et al. . Usefulness of albuminuria as a prognostic indicator in patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2013;111(8):1180-1186. [DOI] [PubMed] [Google Scholar]
- 17.Astor BC, Hallan SI, Miller ER III, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167(10):1226-1234. [DOI] [PubMed] [Google Scholar]
- 18.Yan L, Ma J, Guo X, et al. . Urinary albumin excretion and prevalence of microalbuminuria in a general Chinese population: a cross-sectional study. BMC Nephrol. 2014;15:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerstein HC, Mann JF, Yi Q, et al. ; HOPE Study Investigators . Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421-426. [DOI] [PubMed] [Google Scholar]
- 20.Scirica BM, Mosenzon O, Bhatt DL, et al. . Cardiovascular outcomes according to urinary albumin and kidney disease in patients with type 2 diabetes at high cardiovascular risk: observations from the SAVOR-TIMI 53 trial [published online December 6, 2017]. JAMA Cardiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber FP. Congenital heart disease, with extreme secondary polycythaemia and orthostatic albuminuria. Proc R Soc Med. 1909; 2(Clin Sect): 23-24. [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-Quintana E, Rodríguez-González F, Fábregas-Brouard M, Nieto-Lago V. Serum and 24-hour urine analysis in adult cyanotic and noncyanotic congenital heart disease patients. Congenit Heart Dis. 2009;4(3):147-152. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-Quintana E, Rodríguez-González F. Medium-term follow-up of renal function in hypoxaemic congenital heart disease patients. Cardiol Young. 2016;26(6):1137-1143. [DOI] [PubMed] [Google Scholar]
- 24.Bellinghieri G, Savica V, Santoro D. Renal alterations during exercise. J Ren Nutr. 2008;18(1):158-164. [DOI] [PubMed] [Google Scholar]
- 25.Opotowsky AR, Loukas B, Ellervik C, et al. . Design and implementation of a prospective adult congenital heart disease biobank. World J Pediatr Congenit Heart Surg. 2016;7(6):734-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Criteria Committee of the New York Heart Association Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed Boston: Little, Brown; 1994. [Google Scholar]
- 27.Levin A, Stevens P, Bilous R, et al. . Kidney disease: improving global outcomes (KDIGO) CKD work group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):e150. [Google Scholar]
- 28.Harrell FE Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3(2):143-152. [DOI] [PubMed] [Google Scholar]
- 29.Opotowsky AR, Baraona FR, Mc Causland FR, et al. . Estimated glomerular filtration rate and urine biomarkers in patients with single-ventricle Fontan circulation. Heart. 2017;103(6):434-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka F, Komi R, Makita S, et al. ; Iwate-Kenco Study Group . Low-grade albuminuria and incidence of cardiovascular disease and all-cause mortality in nondiabetic and normotensive individuals. J Hypertens. 2016;34(3):506-512. [DOI] [PubMed] [Google Scholar]
- 31.Dimopoulos K, Diller GP, Koltsida E, et al. . Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117(18):2320-2328. [DOI] [PubMed] [Google Scholar]
- 32.Passwell J, Orda S, Modan M, Shem-Tov A, Aladjem A, Boichis H. Abnormal renal functions in cyanotic congential heart disease. Arch Dis Child. 1976;51(10):803-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burke JR, Glasgow EF, McCredie DA, Powell HR. Nephropathy in cyanotic congenital heart disease. Clin Nephrol. 1977;7(1):38-42. [PubMed] [Google Scholar]
- 34.Dittrich S, Kurschat K, Dähnert I, Vogel M, Müller C, Lange PE. Cyanotic nephropathy and use of non-ionic contrast agents during cardiac catherization in patients with cyanotic congenital heart disease. Cardiol Young. 2000;10(1):8-14. [DOI] [PubMed] [Google Scholar]
- 35.Flanagan MF, Hourihan M, Keane JF. Incidence of renal dysfunction in adults with cyanotic congenital heart disease. Am J Cardiol. 1991;68(4):403-406. [DOI] [PubMed] [Google Scholar]
- 36.Perloff JK, Latta H, Barsotti P. Pathogenesis of the glomerular abnormality in cyanotic congenital heart disease. Am J Cardiol. 2000;86(11):1198-1204. [DOI] [PubMed] [Google Scholar]
- 37.Ross EA, Perloff JK, Danovitch GM, Child JS, Canobbio MM. Renal function and urate metabolism in late survivors with cyanotic congenital heart disease. Circulation. 1986;73(3):396-400. [DOI] [PubMed] [Google Scholar]
- 38.Burlet A, Drukker A, Guignard JP. Renal function in cyanotic congenital heart disease. Nephron. 1999;81(3):296-300. [DOI] [PubMed] [Google Scholar]
- 39.Scott HW Jr, Elliott SR II. Renal hemodynamics in congenital cyanotic heart disease. Bull Johns Hopkins Hosp. 1950;86(1):58-71. [PubMed] [Google Scholar]
- 40.Watanabe H, Iino K, Ito H. Subclinical microalbuminuria as a predictor of heart failure prognosis. Circ J. 2014;78(12):2838-2839. [DOI] [PubMed] [Google Scholar]
- 41.Diller GP, Dimopoulos K, Broberg CS, et al. . Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J. 2006;27(14):1737-1742. [DOI] [PubMed] [Google Scholar]
- 42.Ohuchi H, Negishi J, Noritake K, et al. . Prognostic value of exercise variables in 335 patients after the Fontan operation: a 23-year single-center experience of cardiopulmonary exercise testing. Congenit Heart Dis. 2015;10(2):105-116. [DOI] [PubMed] [Google Scholar]
- 43.Radojevic J, Inuzuka R, Alonso-Gonzalez R, et al. . Peak oxygen uptake correlates with disease severity and predicts outcome in adult patients with Ebstein’s anomaly of the tricuspid valve. Int J Cardiol. 2013;163(3):305-308. [DOI] [PubMed] [Google Scholar]
- 44.Dimopoulos K, Okonko DO, Diller GP, et al. . Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation. 2006;113(24):2796-2802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods.
eFigure 1. Patient Enrollment and Inclusion in the Analysis.
eFigure 2. Hazard Ratios for the Combined Outcome by Estimated Glomerular Filtration Rate and Degree of Albuminuria.
eFigure 3. Risk of Adverse Outcomes for Adults with Biventricular Congenital Heart Disease, n=526, by Urinary Albumin-to-Creatinine Ratio (ACR) Value.
eFigure 4. Kaplan-Meier Plots of Albuminuria as a Predictor of Adverse Outcomes, stratified by New York Heart Association Functional Class.
eTable. Exercise Test Results by Albuminuria Status, Stratified by Type of Circulation.