Key Points
Question
What are neural markers of resilience in adolescent females at risk for depression?
Findings
In this longitudinal study of 65 adolescent females, we examined functional connectivity in limbic, salience, and executive control networks. High-risk adolescent females who were resilient to depression had greater connectivity between regions in limbic and executive control networks than did high-risk adolescent females who developed depression and low-risk control adolescents; further, the strength of this connectivity was correlated with positive life events in the group of resilient adolescent females.
Meaning
Our findings highlight functional neuroimaging biomarkers of resilience to adolescent depression that may be candidate targets for the prevention and treatment of depression.
Abstract
Importance
Adolescence is a neurodevelopmental period during which experience-dependent plasticity in brain circuitry may confer vulnerability to depression as well as resilience to disorder. Little is known, however, about the neural mechanisms that underlie resilience during this critical period of brain development.
Objective
To examine neural functional connectivity correlates of resilience in adolescent females at high and low familial risk for depression who did and did not develop the disorder.
Design, Setting, and Participants
A longitudinal study was conducted at Stanford University from October 1, 2003, to January 31, 2017. Sixty-five female adolescents participated in the study: 20 at high risk in whom depression did not develop (resilient), 20 at high risk in whom depression developed (converted), and 25 at low risk with no history of psychopathology (control).
Main Outcomes and Measures
We compared functional connectivity between resilient and converted, and between resilient and control, adolescent females using voxelwise 2-sided t tests to examine neural markers of resilience to depression as the main outcomes of interest. Specifically, we assessed differences in connectivity of the limbic (amygdala seed), salience (anterior insula seed), and executive control (dorsolateral prefrontal cortex seed) networks, implicated in emotion regulation. We also examined the association between functional connectivity and life events.
Results
Of the 65 participants (mean [SD] age, 18.9 [2.5] years), adolescent females in the resilient group had greater connectivity between the amygdala and orbitofrontal cortex (z score = 0.23; P < .001) and between the dorsolateral prefrontal cortex and frontotemporal regions (z score = 0.24; P < .001) than did converted adolescent females. In adolescent females in the resilient group only, strength of amygdala-orbitofrontal cortex connectivity was correlated with positive life events (r18 = 0.48; P = .03). Resilient adolescent females had greater connectivity within frontal (z score = 0.07; P < .001) and limbic (z score = 0.21; P < .001) networks than did control individuals. Both high-risk groups had greater salience network connectivity: the converted group had greater intranetwork connectivity than did the resilient (z score = 0.13; P < .001) and control (z score = 0.10; P < .001) groups, and the adolescent females in the resilient group had greater salience network connectivity with the superior frontal gyrus than did the converted (z score = 0.24; P < .001) adolescent females.
Conclusions and Relevance
Resilient adolescent females have compensatory functional connectivity patterns in emotion regulatory networks that correlate with positive life events, suggesting that experience-dependent plasticity within these networks may confer resilience to depression. Further studies are warranted concerning connectivity-associated targets for promoting resilience in high-risk individuals.
This study examines neural functional connectivity correlates of resilience in adolescent females at high and low familial risk for depression who did and did not develop the disorder.
Introduction
An estimated 1 in 3 adolescent females in the United States is diagnosed as having major depressive disorder (MDD),1 the leading cause of disability and second leading cause of death during adolescence,2 with an estimated 17% lifetime prevalence.3 In contrast to the large body of research examining neural aspects of depression in adolescents,4,5 little is known about neurobiological factors that confer resilience to this disorder. The American Psychological Association defines resilience as the process of adapting well in the face of significant sources of stress and bouncing back from difficult life experiences.6 Debate is ongoing about the definition and determinants of resilience, which likely include biological, psychological, social, and cultural factors that help shape response to stressful experiences.7 Extending the focus of psychopathology-based models to incorporate strength- and competency-based approaches may facilitate the development of strategies designed to prevent and treat adolescent depression as well as promote resilience in high-risk youth.
Adolescence is marked by stress and increased risk for the onset of depression3; it is also a neurodevelopmental period with the potential for heightened learning, flexibility, and development of adaptive emotion regulation skills.8,9 Significant maturation of neural networks involved in emotion regulation10,11 and experience-dependent plasticity within brain networks12 occur over adolescence. Emotion dysregulation in adolescents has been associated with anomalies in the limbic network,13,14,15 the executive control network,16 and the salience network.17 Moreover, researchers have found increased plasticity within emotion regulation circuitry after treatment with antidepressants and psychotherapy, first-line interventions for adolescent depression.18,19,20 Specifically, abnormally decreased prefrontal activity, implicated in deficits in emotion regulation and cognitive control in depression,21,22 normalizes with successful treatment in individuals with depression.18,19,20 Researchers have also documented changes in connectivity between the amygdala (within the limbic network) and the anterior insula (within the salience network),19,23 as well as in executive control network connectivity, after psychotherapy. While these treatment-induced changes in connectivity are not necessarily markers of resilience, they are promising targets for putative networks implicated in neural mechanisms underlying resilience to developing adolescent depression.
The present study was designed to examine resilience in adolescent females at familial risk for depression. Using a seed-based resting-state functional magnetic resonance imaging (fMRI) approach, we compared functional connectivity of the limbic network, executive control network, and salience network in high-risk adolescent females in the resilient group with both high-risk adolescent females in whom depression developed (converted) and low-risk adolescent females with no history of psychopathology (control) group. Based on previous findings,24,25 we hypothesized that compared with their conversion and control peers, adolescent females in the resilient group will show adaptive compensatory changes in their brain networks, including greater connectivity between the amygdala and prefrontal regions implicated in emotion regulation, and greater overall executive control network connectivity. We also hypothesized that the converted group will show reduced executive control network and greater salience network connectivity compared with the resilient and control groups. Finally, given the modulatory roles of significant life events on brain development,26 we examined whether greater amygdala-frontal connectivity is associated with these significant life events.
Methods
Study Design
This longitudinal study of familial risk for depression recruited 190 female adolescents. Data were collected at Stanford University from October 1, 2003, to January 31, 2017. Participants were followed over the course of adolescence from age 9 through age 18 years, for a mean (SD) of 7.6 (2.4) years, and completed clinical and behavioral assessments at 18-month intervals. The neuroimaging data were acquired toward the end of the study, at 1 time (mean [SD] age 18.9 [2.5] years of participants when neuroimaging data were acquired). The study was approved by the institutional review board at Stanford University; written assent was obtained from all study participants and written consent was obtained from their parents.
Participants
At the time of study entry, participants were between 9 and 14 years of age and had no current or lifetime history of any Axis I disorder. Half of the participants had a mother who had recurrent MDD episodes during the daughter’s lifetime (high risk); the other half had mothers with no history of Axis I disorder (low risk). Approximately 6 years after entering the study, 92 of the 190 participants (43 high-risk adolescent females; 49 low-risk adolescent females) completed a resting-state functional magnetic resonance imaging (fMRI) scan. We report here on 65 of these 92 adolescent females: 20 high-risk females in whom MDD developed after entry to the study but who no longer had MDD by the time of the scan (converted); 20 high-risk females in whom MDD did not develop (resilient); and 25 low-risk females in whom no Axis I disorder developed (control). A more detailed description of sample selection is presented in eAppendix 1 in the Supplement.
Clinical and Behavioral Assessments
At each assessment at 18-month intervals, interviewers administered the Kiddie Schedule for Affective Disorders and Schizophrenia present and lifetime version (K-SADS-PL)27 to participants who were younger than 18 years and the Structured Clinical Interview for DSM (SCID)28 to participants who were older than 18 years. Receiving a diagnosis of MDD required that participants meet DSM-IV criteria with no history of manic, hypomanic, or mixed episodes.29
In addition to the K-SADS-PL and SCID administered at baseline and follow-up visits, participants completed the Children’s Depression Inventory (CDI)30 to assess depressive symptomatology and the Life Experiences Survey (LES)31 to assess positive and negative life events and the perceived meaning of these events. Participants reported on the presence, valence (positive or negative), and significance (4-point scale; 0 indicating no effect to 3 indicating great effect) of 50 life events (eg, moving to a new home, new relationship, special recognition at school, parent’s divorce). The summed significance score of each event created the cumulative negative or positive impact score.
Seed-Based Functional Connectivity Analyses
The acquisition of fMRI and parameters for preprocessing are described in eAppendix 2 in the Supplement. We conducted resting-state functional connectivity analyses using a seed-driven approach in Functional Connectivity SPM Toolbox (CONN).32 We selected a whole-brain seed-to-voxel analysis approach to test a priori hypotheses involving specific neural networks, and to facilitate comparisons with the extant resting-state fMRI literature on adolescent MDD.14,15,33,34 We generated whole-brain seed-to-voxel correlation maps by extracting the residual Blood Oxygenation Level Dependent (BOLD) signal time course from a priori regions of interest, including the amygdala (limbic network), anterior insula (salience network), and dorsolateral prefrontal cortex (executive control network) (Figure 1). Seed regions were 5 mm in diameter and created based on peak coordinates from the literature.35,36,37,38 We used methods that minimize the influence of motion and artifact (eAppendix 3 in the Supplement). We computed Pearson correlation coefficients between the time course of each seed and the time course of all other voxels in the brain. We converted correlation coefficients to z scores using Fisher transformation and used them in second-level general linear model analyses to examine group differences. We performed voxelwise 2-sided t tests to directly compare data from participants in the resilient group with data from participants in the conversion group and data from participants in the resilient group with data from participants in the control group as the main outcomes of interest. For all connectivity analyses, we set voxel-level thresholds to P < .001 and corrected for multiple comparisons using cluster-level false discovery rate (FDR) thresholding P < .05 based on gaussian field theory.39 We applied Bonferroni correction to the FDR-corrected cluster-level P values to correct for 6 a priori seeds (bilateral amygdala, anterior insula, and dorsolateral prefrontal cortex).
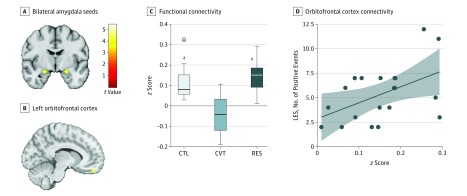
Figure 1. Limbic Network Connectivity.
A, Bilateral amygdala seeds (coordinates in Montreal Neurological Institute space: left: −22, −5, 17; right: 22, −5, 17). B, Region of the left OFC that had greater connectivity with the limbic network (right amygdala seed) in RES as compared with CVT group. Color bar represents t values from the between-group t test (RES>CVT). C, Box-and-whisker plots of amygdala to OFC functional connectivity (quantified as z score) for each group. The horizontal line in the middle of each box indicates the median, while the top and bottom borders mark the 75th and 25th percentiles, respectively. The whiskers above and below the box mark the 90th and 10th percentile. The points beyond the whiskers are outliers. D, Right amygdala to left OFC connectivity plotted against number of LES positive events within the RES group (r18 = 0.48, P = .03). The shaded area represents the 95% CI. CTL indicates control; CVT, converted; LES, Life Experiences Survey; OFC, orbitofrontal cortex; and RES, high-risk resilient.
aP < .001 compared with CVT.
Statistical Analyses
The analyses were performed in SPSS software, version 23 (SPSS Corp) and R package, version 3.3.1. Statistical significance was set at 2-sided P < .05 for behavioral and demographic data. We compared the 3 groups on demographic, clinical, and behavioral assessments using 1-way analysis of variance with post hoc Tukey tests, as appropriate, for continuous variables. We used the Wilcoxon signed rank test to compare the 3 groups on income and educational level, and χ2 tests to examine group differences in race/ethnicity and medication.
We first used Pearson correlations to examine the association between amygdala-orbitofrontal cortex (OFC) functional connectivity and LES scores (positive and negative, separately) within each high-risk group (resilient, converted). We then performed linear regression to test whether the high-risk groups differed in associations between LES scores on amygdala-OFC functional connectivity. We further tested that LES scores were not correlated with motion parameters.40
Results
Demographic and Clinical Information
In total, 65 female adolescents were followed in this longitudinal study, categorized into 3 groups: 20 at high risk in whom depression did not develop (resilient), 20 at high risk in whom depression developed (converted), and 25 at low risk with no history of psychopathology (control). Other demographic and clinical data are presented in Table 1. The 3 groups did not differ in age at the time of the scan or recruitment, length of time in the study, race/ethnicity, or household income. Although no participant experienced a psychiatric disorder prior to entering the study, participants in the high-risk groups had higher CDI scores at baseline than did the control group; however, the mean CDI scores at study entry were well below the diagnostic cutoff of a score of 13. On the day of the scan, the converted group had higher CDI scores and lower global assessment of functioning scores than did the resilient and control groups. Few of the participants (n = 4) were taking psychotropic medication at the time of the scan; the 3 groups did not differ on this variable. Three resilient participants had a lifetime history of alcohol and cannabis abuse (n = 1), specific phobia (n = 1), or binge eating disorder (n = 1); all participants were in full remission at the time of the scan except for the participant who met criteria for specific phobia whose score on the Beck Anxiety Inventory scale was 4, well within the normal range (0-9). Beck Anxiety Inventory scale scores range from 0 to 63. A total score of 0 to 7 is interpreted as a “minimal” level of anxiety; 8 to 15 as “mild”; 16 to 25 as “moderate,” and; 26 to 63 as “severe.” The resilient and converted groups did not differ significantly with respect to the total number of maternal episodes of depression (t19 = −1.14; P = .27).
Table 1. Demographic Information and Clinical Data.
| Variable | CTL (n = 25) | RES (n = 20) | CVT (n = 20) | Statistical Value | P Value |
|---|---|---|---|---|---|
| Demographic Information | |||||
| Age at scan, mean (SD), y | 18.99 (2.61) | 18.94 (2.62) | 18.75 (2.50) | F2,62 = 0.05 | .95 |
| Age at study entry, mean (SD) | 12.61 (1.81) | 13.18 (1.57) | 11.99 (1.37) | F2,62 = 2.71 | .07 |
| Years in study, mean (SD) | 6.38 (1.85) | 5.77 (1.86) | 6.76 (2.22) | F2,62 = 1.31 | .28 |
| Race/ethnicity, No. | χ28 = 4.53 | .81 | |||
| White | 15 | 13 | 12 | ||
| Black | 1 | 1 | 0 | ||
| Hispanic | 1 | 2 | 0 | ||
| Asian | 1 | 0 | 1 | ||
| Other/multiracial | 7 | 4 | 6 | ||
| Declined to state | 0 | 0 | 1 | ||
| Baseline annual household income, $, mean rank | 32.00 | 26.63 | 23.71 | χ22 = 2.84 | .24 |
| <10 000 | 0 | 1 | 0 | ||
| 10 000-25 000 | 0 | 1 | 2 | ||
| 25 001-50 000 | 0 | 2 | 5 | ||
| 50 001-75 000 | 4 | 2 | 1 | ||
| 75 001-100 000 | 4 | 5 | 2 | ||
| >100 000 | 10 | 8 | 7 | ||
| Declined to state | 7 | 1 | 3 | ||
| Baseline daughter WISC score, mean (SD) | 47.88 (7.53) | 49.70 (6.30) | 47.61 (6.23) | F2,61 = 0.56 | .57 |
| Baseline mother WAIS score, mean (SD) | 57.83 (7.00) | 63.11 (5.79) | 58.84 (7.88) | F2,61 = 3.29 | .04 |
| Baseline mother educational level, mean rank | 32.14 | 34.03 | 31.37 | χ22 = 0.23 | .89 |
| No high school diploma/GED | 0 | 0 | 0 | ||
| High school diploma/GED | 1 | 0 | 2 | ||
| Some college | 2 | 3 | 3 | ||
| 2-y College degree | 1 | 2 | 1 | ||
| 4-y College degree | 12 | 5 | 6 | ||
| Master’s degree | 7 | 8 | 2 | ||
| Doctorate | 2 | 2 | 5 | ||
| Decline to state | 0 | 0 | 1 | ||
| Clinical Data | |||||
| Baseline CDI score, mean (SD) | 0.88 (1.17) | 2.10 (1.62) | 2.55 (3.10) | F2,62 = 3.99 | .02 |
| Scan CDI score, mean (SD) | 0.16 (0.47) | 1.05 (1.54) | 2.80 (2.69) | F2,62 = 12.99 | <.001 |
| Baseline GAF score, mean (SD) | 88.52 (5.12) | 80.94 (7.80) | 79.56 (7.62) | F2,57 = 10.53 | <.001 |
| Scan GAF score, mean (SD) | 88.0 (8.44) | 84.95 (6.76) | 75.82 (12.54) | F2,58 = 9.07 | <.001 |
| Age of MDE onset, mean (SD), y | NA | NA | 15.50 (2.61) | NA | NA |
| Rate of MDE Recurrence, No. (%) | NA | NA | 8 (40) | NA | NA |
| Scan psychotropic medication, No. (%) | 0 (0) | 1 (5) | 3 (15) | χ22 = 4.58 | .10 |
| Antidepressant | 0 | 1 | 3 | ||
| Antipsychotic | 0 | 0 | 0 | ||
| Stimulant | 0 | 0 | 0 | ||
| Lifetime Clinical History | |||||
| Major depressive disorder | 0 | 0 | 20 | NA | NA |
| Alcohol use disorder | 0 | 1 | 2 | NA | NA |
| Cannabis use disorder | 0 | 1 | 1 | NA | NA |
| Social phobia | 0 | 0 | 4 | NA | NA |
| Specific phobia | 0 | 1 | 1 | NA | NA |
| Obsessive compulsive disorder | 0 | 0 | 0 | NA | NA |
| Posttraumatic stress disorder | 0 | 0 | 0 | NA | NA |
| Generalized anxiety disorder | 0 | 0 | 2 | NA | NA |
| Anorexia nervosa | 0 | 0 | 1 | NA | NA |
| Bulimia/binge eating disorder | 0 | 1 | 1 | NA | NA |
| Life Events Survey Scores | |||||
| Positive life events, mean (SD), No. | 5.88 (2.51) | 5.35 (2.83) | 5.42 (2.85) | F2,61 = 0.26 | .77 |
| Negative life events, mean (SD) | 2.88 (2.64) | 3.40 (2.58) | 4.68 (4.14) | F2,61 = 1.83 | .17 |
| Cumulative positive impact, mean (SD) | 9.44 (4.25) | 7.65 (4.23) | 8.63 (8.63) | F2,61 = 0.67 | .51 |
| Cumulative negative impact, mean (SD) | 4.88 (5.46) | 6.20 (5.35) | 8.55 (8.73) | F2,61 = 1.73 | .19 |
| fMRI Motion Parameters | |||||
| Censored No. of volumes, mean (SD) | 6.12 (5.46) | 4.75 (5.79) | 4.25 (3.35) | F2,62 = 0.85 | .43 |
| Frame-wise displacement, mean (SD), mm | 0.07 (0.04) | 0.09 (0.07) | 0.09 (0.09) | F2,62 = 0.95 | .39 |
| Frame-wise rotation, mean (SD), degrees | 0.002 (0.001) | 0.003 (0.003) | 0.002 (0.002) | F2,62 = 1.42 | .25 |
Abbreviations: CDI, Child Depression Inventory; CTL, control; CVT, converted; fMRI, functional magnetic resonance imaging; GAF, global assessment of functioning score; GED, General Educational Development; MDE, major depressive episode; NA, not applicable; RES, high-risk resilient; WAIS, Wechsler Adult Intelligence Scale; WISC, Wechsler Intelligence Scale for Children.
Life Events and Resting-State Functional Connectivity
Scores on the LES are summarized in Table 1. The 3 groups did not differ in number of negative or positive life events or in positive or negative impact scores at the time of the scan. Results of the analyses comparing resilient vs converted and results of comparisons between resilient and control are summarized in Table 2.
Table 2. Between-Group Connectivity Differences.
| Group Characteristic | Cluster Size, mm3 | MNI Coordinates | t Value | z Scores, Mean (SD) | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | RES | CVT | CTL | |||
| RES vs CVT | ||||||||
| Seed (comparison): left amygdala (RES>CVT)a | ||||||||
| Right superior frontal gyrus | 164 | 18 | 54 | 30 | t38 = 4.29 | 0.04 (0.14) | −0.15 (0.11) | 0.01 (0.13) |
| Left fusiform gyrus | 60 | −42 | −34 | −24 | t38 = 4.27 | 0.14 (0.14) | −0.06 (0.10) | −0.01 (0.11) |
| Seed (comparison): right amygdala (RES>CVT)a | ||||||||
| Left orbitofrontal cortex | 120 | −10 | 52 | −24 | t38 = 5.43 | 0.18 (0.08) | −0.05 (0.09) | 0.11 (0.08) |
| Left inferior temporal gyrus | 66 | −34 | −32 | −22 | t38 = 3.86 | 0.11 (0.12) | −0.05 (0.08) | 0.03 (0.13) |
| Right inferior temporal gyrus | 64 | 36 | −10 | −46 | t38 = 3.31 | 0.12 (0.06) | −0.04 (0.11) | 0.06 (0.12) |
| Seed (comparison): left anterior insula (CVT>RES)b | ||||||||
| Right inferior temporal gyrus | 173 | 54 | −32 | −18 | t38 = 4.87 | −0.18 (0.13) | 0.02 (0.11) | −0.08 (0.11) |
| Left inferior temporal gyrus | 100 | −62 | −20 | −24 | t38 = 5.17 | −0.24 (0.14) | −0.06 (0.12) | −0.08 (0.14) |
| Right MD thalamus | 73 | 18 | −30 | 0 | t38 = 2.92 | −0.20 (0.17) | 0.11 (0.07) | −0.06 (0.10) |
| Right anterior insula | 66 | 30 | 24 | 10 | t38 = 3.35 | 0.35 (0.13) | 0.54 (0.20) | 0.21 (0.16) |
| Seed (comparison): right anterior insula (RES>CVT)a | ||||||||
| Right superior frontal gyrus | 129 | 18 | 62 | 28 | t38 = 4.51 | 0.20 (0.17) | −0.04 (0.17) | 0.08 (0.18) |
| Seed (comparison): left dorsolateral prefrontal cortex (RES>CVT)a | ||||||||
| Left ventrolateral PFC (IFG) | 59 | −50 | 20 | 24 | t38 = 4.04 | 0.31 (0.16) | 0.07 (0.14) | 0.17 (0.11) |
| Seed (comparison): right dorsolateral prefrontal cortex (RES>CVT)a | ||||||||
| Left superior temporal gyrus | 185 | −48 | −22 | −2 | t38 = 5.48 | 0.08 (0.09) | −0.16 (0.12) | 0.05 (0.18) |
| RES vs CTL | ||||||||
| Seed (comparison): left amygdala (RES>CTL)c | ||||||||
| Right angular gyrus | 307 | 52 | −50 | 28 | t43 = 3.29 | 0.01 (0.13) | −0.08 (0.18) | −0.20 (0.13) |
| Seed (comparison): right amygdala (RES>CTL)c | ||||||||
| Left angular gyrus | 235 | −36 | −76 | 46 | t43 = 4.60 | 0.09 (0.16) | 0.00 (0.16) | −0.12 (0.15) |
| Seed (comparison): left anterior insula | ||||||||
| RES>CTL | ||||||||
| Right middle frontal gyrus | 146 | 44 | 36 | 46 | t43 = 4.06 | 0.11 (0.13) | 0.03 (0.12) | −0.04 (0.12) |
| Right anterior insula | 127 | 44 | 6 | 0 | t43 = 3.71 | 0.14 (0.08) | 0.18 (0.08) | 0.03 (0.05) |
| Left precentral gyrus | 95 | −34 | −12 | 38 | t43 = 4.75 | 0.17 (0.12) | 0.02 (0.15) | −0.04 (0.09) |
| CTL>RES | ||||||||
| Left superior frontal gyrus | 158 | −4 | 54 | 38 | t43 = 4.92 | −0.27 (0.16) | −0.17 (0.13) | 0.05 (0.09) |
| Seed (comparison): right anterior insula (CTL>RES)d | ||||||||
| Left middle temporal gyrus | 138 | −60 | −18 | −22 | t43 = 5.45 | −0.26 (0.17) | −0.18 (0.12) | −0.05 (0.14) |
| Right middle temporal gyrus | 115 | 56 | −12 | −22 | t43 = 4.33 | −0.27 (0.18) | −0.13 (0.12) | −0.07 (0.13) |
| Seed (comparison): right dorsolateral prefrontal cortex (RES>CTL)c | ||||||||
| Right middle frontal gyrus | 224 | 42 | 16 | 46 | t43 = 3.69 | 0.08 (0.17) | −0.03 (0.18) | 0.01 (0.17) |
Abbreviations: CTL, control; CVT, converted; IFG, inferior frontal gyrus; MD, medial dorsal; MNI, Montreal Neurological Institute; PFC, prefrontal cortex; RES, high-risk resilient.
No between-group connectivity differences were noted for CVT>RES.
No between-group connectivity differences were noted for RES>CVT.
No between-group connectivity differences were noted for CTL>RES.
No between-group connectivity differences were noted for RES>CTL.
Amygdala Seed Results
Compared with the converted group, adolescent females in the resilient group had greater connectivity between the left amygdala and right superior frontal gyrus (SFG) (z score = 0.19; P < .001), right amygdala and left OFC (z score = 0.23; P < .001), and bilateral inferior temporal gyri (z score = 0.16; P < .001). Compared with the control group, the resilient group had greater bilateral amygdala connectivity with the left and right angular gyri (z score = 0.09, P < .001). See Figure 1B and C for more details, and Table 2 for a complete list of our results.
Anterior Insula Seed Results
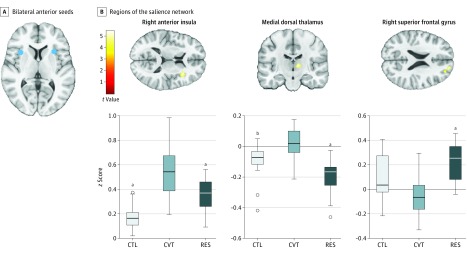
Compared with the control group, both high-risk groups had greater salience network connectivity: the converted group had greater intranetwork connectivity than did the resilient (z score = 0.13; P < .001) and control (z score = 0.10; P < .001) groups, and the adolescent females in the resilient group had greater salience network connectivity with the superior frontal gyrus than did the converted (z score = 0.24; P < .001) adolescent females. See Figure 2 for more details and Table 2 for a complete list of our results.
Figure 2. Salience Network Connectivity.
A, Bilateral anterior insula seeds (coordinates in Montreal Neurological Institute space: left: -30, 18, 5; right: 34, 16, 5). B, Regions of the salience network (anterior insula seeds) that exhibited greater connectivity within the CVT as compared with RES and CTL groups as well as greater salience network connectivity with the superior frontal gyrus in the RES group. Color bar represents t values from the between-group paired t tests. B, Box-and-whisker plots of functional connectivity quantified as z scores for CTL, CVT, and RES groups. The horizontal line in the middle of each box indicates the median, while the top and bottom borders mark the 75th and 25th percentiles, respectively. The whiskers above and below the box mark the 90th and 10th percentile. The points beyond the whiskers are outliers. CTL indicates control; CVT, converted; and RES, high-risk resilient.
aP < .001 compared with CVT.
bP < .01 compared with CVT.
Dorsolateral Prefrontal Cortex Seed Results
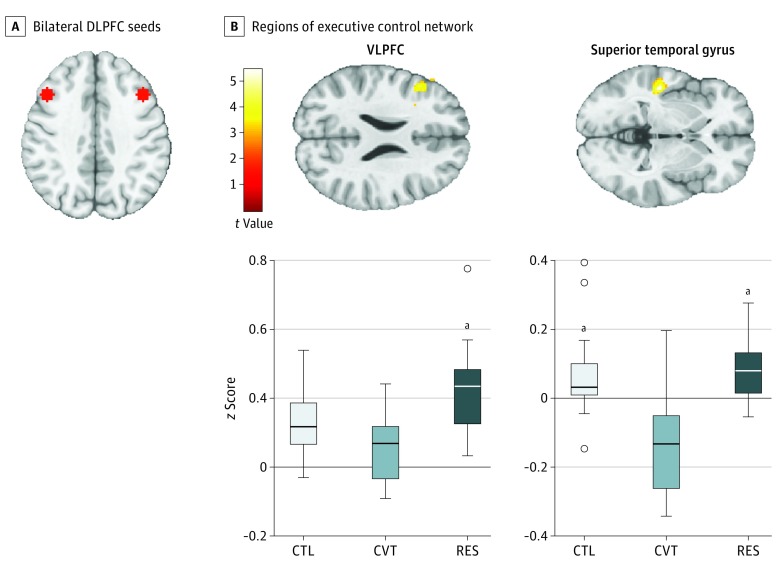
Compared with the converted group, adolescent females in the resilient group had greater left dorsolateral prefrontal cortex connectivity with the left inferior frontal gyrus (IFG) including the ventrolateral prefrontal cortex and with the left superior temporal gyrus (STG) (z score = 0.24; P < .001). Compared with the control group, adolescent females in the resilient group had greater right dorsolateral prefrontal cortex connectivity with the right MFG (z score = 0.07; P < .001). See Figure 3 for more details and Table 2 for a complete list of our results.
Figure 3. Executive Control Network Connectivity.
A, Bilateral DLPFC seeds (coordinates in Montreal Neurological Institute space: left: -43, 22, 34; right: 43, 22, 34). B, Regions of executive control network (DLPFC seeds) that exhibited greater connectivity within the RES compared with CVT and CTL groups. Color bar represents t values from the between-group paired t tests. B, Box-and-whisker plot of functional connectivity quantified as z scores for CTL, CVT, and RES groups. The horizontal line in the middle of each box indicates the median, while the top and bottom borders mark the 75th and 25th percentiles, respectively. The whiskers above and below the box mark the 90th and 10th percentile. The points beyond the whiskers are outliers. CTL indicates control; CVT, converted; DLPFC, dorsolateral prefrontal cortex; RES, high-risk resilient; and VLPFC, ventrolateral prefrontal cortex.
aP < .001 compared with CVT.
Differential Effects of Life Events on Amygdala-OFC Connectivity
No significant correlations between amygdala-OFC functional connectivity were found with negative events, meaning of negative events, or meaning of positive events within either high-risk group . We found a significant correlation between positive life events and connectivity between right amygdala and left OFC in the resilient (r18 = 0.48; P = .03) but not in the converted (r18 = −0.18; P = .47) groups. We compared these associations between the high-risk groups directly using linear regression, which yielded a significant interaction effect of group and positive life events on amygdala-OFC FC (t35 = 2.09; P = .04). The LES scores were not significantly correlated with any of the 18 motion parameters in the resilient group.
Supplemental Analyses
Given significant group differences in CDI and maternal Wechsler Adult Intelligence Scale scores, we included these measures as covariates (separately) to confirm that these group differences did not drive our findings (eAppendix 4 in the Supplement).
Discussion
Adolescence is a neurodevelopmental period during which experience-dependent plasticity in emotion regulatory neural circuitry may provide opportunities to enhance resilience. Previous research has focused on neural substrates of adolescent depression4,5; in this study, we sought to investigate neural markers of resilience to depression in adolescence. Thus, we compared profiles of functional connectivity between adolescent females in the resilient group and both converted and control adolescent females within 3 large-scale networks implicated both in adolescent depression and in emotion regulation: the limbic network, the salience network, and the executive control network. The resilient group had notable and potentially protective connectivity characteristics: compared with converted and control adolescent females, adolescent females in the resilient group exhibited greater connectivity between the amygdala and prefrontal cortex, with an association between amygdala-OFC connectivity and the experience of positive life events. Adolescent females in the resilient group also showed greater connectivity between regions of the executive control network than did their converted and control peers. Finally, both the converted and resilient high-risk adolescent females differed from control adolescent females in salience network connectivity: converted adolescent females showed greater intranetwork connectivity than did both resilient and control adolescent females, and adolescent females in the resilient group showed greater connectivity with frontal cortical regions than did converted adolescent females. These findings provide insights into brain circuitry that may be involved in resilience and, therefore, may inform more effective approaches to the prevention and treatment of adolescent-onset depression.
Several studies have found aberrant corticoamygdalar connectivity in adolescent females with depression compared with healthy controls.14,15 To our knowledge, the present study is the first to examine the connectivity of this circuit in high-risk adolescent females in the resilient group. The amygdala plays a central role in emotion processing, motivation, and learning.41 We found corticoamygdalar connectivity differences in adolescent females in the resilient group compared with their converted and control peers. The OFC (encompassed within the prefrontal cortex) undergoes extensive maturation during adolescence and is implicated in motivation, interpretation of affect, and emotion regulation.42 The OFC is also implicated in modulating amygdala function,43 with greater positive coupling associated with reduced depressive symptomatology over the course of adolescence.44,45 In this context, greater amygdala-OFC connectivity in the resilient group may serve a protective role in behavioral and emotion regulation that confers resilience to adolescent females at risk for depression.
We found a significant association between amygdala-OFC connectivity and positive life events in the adolescent females in the resilient group only. Although this finding must be replicated in future studies and we cannot establish directionality of this association, positive life experiences may strengthen amygdala-OFC connectivity or, conversely, greater amygdala-OFC connectivity may lead individuals to interpret life events in a more positive light. Although speculative, differences in the meaning and interpretation of stressful life events, particularly those that are interpreted as positive, may distinguish high-risk adolescent females who remain resilient from those who develop MDD. Experience-dependent plasticity may have a particularly strong association with brain network connectivity in adolescence.12 Consequently, the inevitable transitions and life experiences that occur during adolescence may provide an ideal opportunity to administer targeted preventions designed to strengthen adaptive coping and cognitive appraisal and interpretation.
Resilient adolescent females also showed strong connectivity between regions of the executive control network including the ventrolateral prefrontal cortex, dorsolateral prefrontal cortex, and temporal regions. This network is implicated in voluntary (ie, explicit) emotion regulation, including cognitive reappraisal and impulse control. Our findings suggest that high-risk adolescent females in the resilient group have greater “top-down” control over emotions and behavior than do high-risk adolescent females who develop depression. Researchers have posited that adaptive selection of goal-directed behavior to obtain a desired outcome is mediated by prefrontal cortex regions within this network46; this provides a possible mechanism and target through which therapeutic interventions aimed at strengthening these connections could increase resilience in high-risk populations. Greater plasticity within the executive control network following treatment with psychotherapy and selective serotonin reuptake inhibitors18,19,20 may be the basis of more adaptive interpretation of experiences and emotion regulation.21,22 Our findings suggest that this network serves a neuroprotective role in adolescence, or alternatively, that positive life experiences result in enriched executive control network development. Therefore, strengthening executive control network connectivity or providing opportunities for positive life experiences to strengthen connectivity within this network prior to the manifestation of symptoms may increase resilience to adolescent-onset depression in high-risk youth.
Both high-risk groups (resilient and converted) differed from controls in salience network connectivity, with the strongest salience network connectivity in the converted group. The salience network is implicated in self-awareness and in integrating internally and externally salient stimuli.47 Insula dysfunction is associated with a negative interpretation bias of emotions and life events16,48 and is posited to be responsible for misinterpreting salient information as negative.49 Altered insula responses have also been found when adolescent females with depression view negative stimuli.50 Our finding of greater salience network connectivity with frontal regions but less intranetwork connectivity in resilient than in converted adolescent females suggests that adolescent females in the resilient group experience negative processing biases of emotionally salient information but have compensatory connectivity in executive control network and frontolimbic regions to counter adverse effects of this processing.
Limitations
We note several limitations of this study. First, although we monitored this cohort of adolescent females longitudinally, we conducted a resting-state scan only at a single time. Thus, it is unclear whether the resting-state functional connectivity differences between resilient and converted that we found in this study were present prior to onset of depression or, alternatively, were a consequence of having developed depression. Examining baseline or serial resting-state scans to assess the trajectory of functional connectivity over the course of adolescence would also be informative to develop age-specific connectivity profiles reflecting resilience.51 Second, some of the participants we categorized as resilient may experience depression after age 18 years; because our study focused on resilience in high-risk adolescent females, we limited our analysis to adolescent-onset MDD. Third, only 3 participants with resilience had a past psychiatric diagnosis; thus, we could not reliably examine differences between resilient participants with and without a lifetime history of psychopathology. We were also unable to examine group differences in specific life events experienced. Future studies with larger sample sizes are needed to replicate our findings and to examine mechanisms through which intervening life events and their attributed meaning and significance may confer resilience to psychopathology in high-risk individuals. Finally, while all of the high-risk adolescent females had mothers who had experienced at least 2 episodes of MDD during their lifetime, we did not assess extended family history beyond maternal depression. We present additional study limitations and alternative interpretations in the Supplement (eAppendix 5 in the Supplement).
Conclusions
In this study, we investigated neural underpinnings of resilience in adolescent females at familial risk for depression and the influence of positive and negatively perceived life events. The characteristics of brain circuitry implicated in emotion regulation that we documented appear to be neural biomarkers of resilience. Our findings suggest an association of experience-dependent plasticity with brain networks, with a direct association between positive life events and amygdala-frontal connectivity in adolescent females in the resilient group. While further research is needed to examine the neural basis of resilience and whether stronger familial risk for depression is associated with higher risk of depression in adolescence, the present findings illuminate key functional connectivity patterns that may be targets for novel prevention and treatment approaches. By shifting the focus of future research to a resilience-based model of mental health, and by investigating characteristics of high-risk individuals who remain resilient, we may increase our understanding of how to optimize resilience to psychopathology.
eAppendix
eAppendix 1. Subjects
eAppendix 2. fMRI and Resting-State fMRI Data Preprocessing
eAppendix 3. Artifact and Motion Correction
eAppendix 4. No Main Effects of CDI or WAIS on Group Differences in Functional Connectivity
eAppendix 5. Additional Study Limitations, Alternative Explanations, and Future Directions
References
- 1.Breslau J, Gilman SE, Stein BD, Ruder T, Gmelin T, Miller E. Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Transl Psychiatry. 2017;7(5):e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heron M. Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65(1):1-96. [PubMed] [Google Scholar]
- 3.Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry. 2015;54(1):37-44.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller CH, Hamilton JP, Sacchet MD, Gotlib IH. Meta-analysis of functional neuroimaging of major depressive disorder in youth. JAMA Psychiatry. 2015;72(10):1045-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ. Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clin. 2013;4:209-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychological Association. The road to resilience. http://www.apa.org/helpcenter/road-resilience.aspx. Accessed January 15, 2017.
- 7.Southwick SM, Bonanno GA, Masten AS, Panter-Brick C, Yehuda R. Resilience definitions, theory, and challenges: interdisciplinary perspectives. [published online October 1, 2014]. Eur J Psychotraumatol. 2014;5. doi: 10.3402/ejpt.v5.25338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202-214. [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861-863. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116-130. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS. The brain on stress: toward an integrative approach to brain, body, and behavior. Perspect Psychol Sci. 2013;8(6):673-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlman G, Simmons AN, Wu J, et al. Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. J Affect Disord. 2012;139(1):75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly CG, Ho TC, Blom EH, et al. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J Affect Disord. 2017;207:86-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cullen KR, Westlund MK, Klimes-Dougan B, et al. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 2014;71(10):1138-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ordaz SJ, LeMoult J, Colich NL, et al. Ruminative brooding is associated with salience network coherence in early pubertal youth. Soc Cogn Affect Neurosci. 2017;12(2):298-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchheim A, Viviani R, Kessler H, et al. Changes in prefrontal-limbic function in major depression after 15 months of long-term psychotherapy. PLoS One. 2012;7(3):e33745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Straub J, Plener PL, Sproeber N, et al. Neural correlates of successful psychotherapy of depression in adolescents. J Affect Disord. 2015;183:239-246. [DOI] [PubMed] [Google Scholar]
- 20.Tao R, Calley CS, Hart J, et al. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. Am J Psychiatry. 2012;169(4):381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erk S, Mikschl A, Stier S, et al. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30(47):15726-15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bermpohl F, Walter M, Sajonz B, et al. Attentional modulation of emotional stimulus processing in patients with major depression–alterations in prefrontal cortical regions. Neurosci Lett. 2009;463(2):108-113. [DOI] [PubMed] [Google Scholar]
- 23.Crowther A, Smoski MJ, Minkel J, et al. Resting-state connectivity predictors of response to psychotherapy in major depressive disorder. Neuropsychopharmacology. 2015;40(7):1659-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clasen PC, Beevers CG, Mumford JA, Schnyer DM. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Dev Cogn Neurosci. 2014;7:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai XJ, Hirshfeld-Becker D, Biederman J, et al. Altered intrinsic functional brain architecture in children at familial risk of major depression. Biol Psychiatry. 2016;80(11):849-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blakemore SJ. Development of the social brain in adolescence. J R Soc Med. 2012;105(3):111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988. [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM–IV–TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN). New York, NY: New York State Psychiatric Institute; 2002. [Google Scholar]
- 29.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 30.Kovacs M. Children’s Depression Inventory: Manual. New York: Multi-Health Systems; 1992. [Google Scholar]
- 31.Swearingen EM, Cohen LH. Measurement of adolescents’ life events: the junior high life experiences survey. Am J Community Psychol. 1985;13(1):69-85. [DOI] [PubMed] [Google Scholar]
- 32.Whitfield-Gabrieli S, Nieto-Castanon A. CONN: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125-141. [DOI] [PubMed] [Google Scholar]
- 33.Ho TC, Connolly CG, Henje Blom E, et al. Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biol Psychiatry. 2015;78(9):635-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hulvershorn LA, Cullen KR, Francis M, Westlund M. Developmental Resting State Functional Connectivity for Clinicians. Curr Behav Neurosci Rep. 2014;1(3):161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55(1):8-23. [DOI] [PubMed] [Google Scholar]
- 36.Fair DA, Cohen AL, Power JD, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37(2):579-588. [DOI] [PubMed] [Google Scholar]
- 39.Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1(3):210-220. [DOI] [PubMed] [Google Scholar]
- 40.Siegel JS, Mitra A, Laumann TO, et al. Data quality influences observed links between functional connectivity and behavior. Cereb Cortex. 2017;27(9):4492-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mears D, Pollard HB. Network science and the human brain: Using graph theory to understand the brain and one of its hubs, the amygdala, in health and disease. J Neurosci Res. 2016;94(6):590-605. [DOI] [PubMed] [Google Scholar]
- 42.Vijayakumar N, Allen NB, Youssef G, et al. Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 2016;37(6):2027-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51(4):356-367. [DOI] [PubMed] [Google Scholar]
- 44.Vijayakumar N, Allen NB, Dennison M, Byrne ML, Simmons JG, Whittle S. Cortico-amygdalar maturational coupling is associated with depressive symptom trajectories during adolescence. Neuroimage. 2017;156:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheuer H, Alarcón G, Demeter DV, Earl E, Fair DA, Nagel BJ. Reduced fronto-amygdalar connectivity in adolescence is associated with increased depression symptoms over time. Psychiatry Res. 2017;266:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1(1):59-65. [DOI] [PubMed] [Google Scholar]
- 47.Craig AD. How do you feel—now? the anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59-70. [DOI] [PubMed] [Google Scholar]
- 48.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51(9):693-707. [DOI] [PubMed] [Google Scholar]
- 49.Sliz D, Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front Hum Neurosci. 2012;6:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henje Blom E, Connolly CG, Ho TC, et al. Altered insular activation and increased insular functional connectivity during sad and happy face processing in adolescent major depressive disorder. J Affect Disord. 2015;178:215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gotlib IH, Ordaz SJ. The importance of assessing neural trajectories in pediatric depression. JAMA Psychiatry. 2016;73(1):9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix
eAppendix 1. Subjects
eAppendix 2. fMRI and Resting-State fMRI Data Preprocessing
eAppendix 3. Artifact and Motion Correction
eAppendix 4. No Main Effects of CDI or WAIS on Group Differences in Functional Connectivity
eAppendix 5. Additional Study Limitations, Alternative Explanations, and Future Directions