Key Points
Question
What is the contribution of nonimaging clinical, behavioral, lifestyle, and cardiometabolic factors to multimodal neuroimaging phenotypes in psychosis?
Findings
In this imaging study of 92 patients with schizophrenia, 37 patients with bipolar disorder, and 48 healthy volunteers, nonimaging and imaging data have substantial covariation. General intelligence, body mass index, positive psychotic symptoms, substance use, and antipsychotic medication were major contributors to variation in brain structure and function.
Meaning
The results of this study underscore the importance of including these key factors in future research to derive a more accurate characterization of brain alterations associated with psychosis.
Abstract
Importance
Alterations in multiple neuroimaging phenotypes have been reported in psychotic disorders. However, neuroimaging measures can be influenced by factors that are not directly related to psychosis and may confound the interpretation of case-control differences. Therefore, a detailed characterization of the contribution of these factors to neuroimaging phenotypes in psychosis is warranted.
Objective
To quantify the association between neuroimaging measures and behavioral, health, and demographic variables in psychosis using an integrated multivariate approach.
Design, Setting, and Participants
This imaging study was conducted at a university research hospital from June 26, 2014, to March 9, 2017. High-resolution multimodal magnetic resonance imaging data were obtained from 100 patients with schizophrenia, 40 patients with bipolar disorder, and 50 healthy volunteers; computed were cortical thickness, subcortical volumes, white matter fractional anisotropy, task-related brain activation (during working memory and emotional recognition), and resting-state functional connectivity. Ascertained in all participants were nonimaging measures pertaining to clinical features, cognition, substance use, psychological trauma, physical activity, and body mass index. The association between imaging and nonimaging measures was modeled using sparse canonical correlation analysis with robust reliability testing.
Main Outcomes and Measures
Multivariate patterns of the association between nonimaging and neuroimaging measures in patients with psychosis and healthy volunteers.
Results
The analyses were performed in 92 patients with schizophrenia (23 female [25.0%]; mean [SD] age, 27.0 [7.6] years), 37 patients with bipolar disorder (12 female [32.4%]; mean [SD] age, 27.5 [8.1] years), and 48 healthy volunteers (20 female [41.7%]; mean [SD] age, 29.8 [8.5] years). The imaging and nonimaging data sets showed significant covariation (r = 0.63, P < .001), which was independent of diagnosis. Among the nonimaging variables examined, age (r = −0.53), IQ (r = 0.36), and body mass index (r = −0.25) were associated with multiple imaging phenotypes; cannabis use (r = 0.23) and other substance use (r = 0.33) were associated with subcortical volumes, and alcohol use was associated with white matter integrity (r = −0.15). Within the multivariate models, positive symptoms retained associations with the global neuroimaging (r = −0.13), the cortical thickness (r = −0.22), and the task-related activation variates (r = −0.18); negative symptoms were mostly associated with measures of subcortical volume (r = 0.23), and depression/anxiety was associated with measures of white matter integrity (r = 0.12).
Conclusions and Relevance
Multivariate analyses provide a more accurate characterization of the association between brain alterations and psychosis because they enable the modeling of other key factors that influence neuroimaging phenotypes.
This imaging study quantifies the association between neuroimaging measures and behavioral, health, and demographic variables in psychosis using an integrated multivariate approach.
Introduction
The main psychotic disorders, schizophrenia and bipolar disorder (BD), are genetically related, have overlapping clinical phenomenology, and rank among the leading causes of disability worldwide. Current models of psychotic disorders are heavily influenced by brain magnetic resonance imaging (MRI) findings that collectively show that patients differ from healthy volunteers on multiple structural and functional measures. The results of large-scale studies and meta-analyses suggest that schizophrenia and BD are associated with partially overlapping and distributed abnormalities in cortical thickness, subcortical volumes, and white matter integrity. Patients also show altered patterns of brain activation, commonly involving inefficient engagement of regulatory cortical regions, across a wide range of experimental conditions. Similarly, the intrinsic functional architecture of the brain is also altered in psychotic disorders, with hypoconnectivity within and between resting-state networks being the most prevalent observation.
These neuroimaging findings have been associated with clinical variables of schizophrenia and BD, including illness severity and symptomatic burden, indicating a link between the anatomical and functional brain changes and disease expression. However, the MRI signal can be influenced by lifestyle choices (eg, smoking, substance use, and physical activity), general health (eg, body mass index [BMI, calculated as weight in kilograms divided by height in meters squared]), and antipsychotic exposure, which are known to differ systematically between patients and healthy volunteers. These findings have led to calls for caution in ascribing case-control differences in neuroimaging measures to psychosis-related mechanisms and for integrated analyses of multimodal imaging as well as clinical and behavioral variables. There is a notable paucity of multivariate approaches that address this issue despite increased interest in diagnostic classification and patient stratification based on neuroimaging data.
The aim of the present study was to discover patterns of multivariate covariation between nonimaging and imaging variables and identify the most salient features that drive these associations. To achieve this aim, we obtained multimodal MRI data from patients with psychosis (n = 140) and healthy volunteers (n = 50) to derive measures of cortical thickness, subcortical volume, task-related brain activation, resting-state functional connectivity, and white matter fractional anisotropy (FA). In the same sample, we ascertained nonimaging factors known to be associated with the MRI signal that relate to psychopathology, cognition, lifestyle factors, and physical health. To go beyond simple correlations, we used sparse canonical correlation analyses (sCCAs), a powerful multivariate method. Sparse canonical correlation analysis is tailored to the analyses of high-dimensional data sets in which variables are expected to be correlated and does not require data reduction.
Methods
Participants
This imaging study was conducted at a university research hospital (Mount Sinai Health System, New York, New York) from June 26, 2014, to March 9, 2017. One hundred ninety participants were recruited at the Icahn School of Medicine at Mount Sinai (details of recruitment and assessment are provided in eMethods in the Supplement). The sample comprised 100 patients with schizophrenia, 40 patients with psychotic BD type 1, and 50 healthy volunteers (Table). The diagnostic status of all participants was ascertained via personal interview using the Structured Clinical Interview for DSM-5, Research Version, supplemented by information from medical records in the case of patients. All participants provided written informed consent, and the study was approved by the institutional review board of the Icahn School of Medicine at Mount Sinai.
Table. Study Sample Characteristics.
| Variable | HV (n = 48) |
BD (n = 37) |
SCZ (n = 92) |
|---|---|---|---|
| Age, mean (SD), y | 29.8 (8.5) | 27.5 (8.1) | 27.0 (7.6) |
| Female sex, No. (%) | 20 (41.7) | 12 (32.4) | 23 (25.0) |
| IQ, mean (SD)a | 115.2 (16.6) | 105.2 (18.1) | 93.5 (15.2) |
| BMI, mean (SD)b | 24.7 (4.4) | 26.1 (6.7) | 27.2 (6.4) |
| Overweight (BMI>25), No. (%) | 18 (37.5) | 20 (54.1) | 51 (55.4) |
| Physical activity per week, mean (SD), d | 1.7 (2.2) | 2.8 (2.5) | 2.3 (2.7) |
| Sedentary time, mean (SD)c | 2.8 (0.5) | 2.9 (0.2) | 2.6 (0.7) |
| Lifetime cannabis use, No. (%)d | 10 (20.8) | 22 (59.5) | 40 (43.5) |
| Lifetime substance use, No. (%)d | 10 (20.8) | 24 (64.9) | 46 (50.0) |
| Current alcohol use, mean (SD)e | 5.3 (2.2) | 6.0 (2.9) | 7.4 (2.2) |
| Currently smoking, meand | 1.3 | 1.6 | 1.6 |
| Family history of depression, No. (%)d | 10 (20.8) | 24 (64.9) | 46 (50.0) |
| Family history of psychosis, No. (%)e | 0 | 5 (13.5) | 53 (57.6) |
| Experience of trauma, No. (%)e | 6 (12.5) | 18 (48.6) | 36 (39.1) |
| Age at onset of BD or SCZ, mean (SD), y | NA | 20.9 (5.0) | 21.5 (5.3) |
| BPRS score, mean (SD)f | |||
| Positive symptomsg | 4.2 (1.4) | 9.6 (5.5) | 12.4 (6.2) |
| Negative symptomsg | 3.4 (2.3) | 4.0 (1.8) | 6.8 (3.8) |
| Depression/anxietyd | 4.1 (0.6) | 8.4 (4.3) | 9.0 (5.3) |
| Mania/disorganizationd | 6.1 (0.3) | 12.3 (8.5) | 9.5 (5.2) |
| Antipsychotic dosage, mean (SD), chlorpromazine equivalentsh | NA | 267.5 (326.3) | 264.5 (251.1) |
| Any antipsychotic, No. (%) | NA | 30 (81.1) | 86 (93.5) |
| First-generation antipsychotic, No. (%)i | NA | 13 (35.1) | 31 (33.7) |
| Second-generation antipsychotic, No. (%)i | NA | 26 (70.3) | 77 (83.7) |
| Antidepressant, No. (%)h | NA | 7 (18.9) | 12 (13.0) |
| Lithium, No. (%)c | NA | 15 (40.5) | 9 (9.8) |
| Mood stabilizers (various), No. (%)h | NA | 5 (13.5) | 15 (16.3) |
| >2 Medication classes, No. (%)h | NA | 28 (75.7) | 51 (55.4) |
Abbreviations: BD, patients with bipolar disorder; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BPRS, Brief Psychiatric Rating Scale; HV, healthy volunteers; NA, not applicable; SCZ, patients with schizophrenia.
HV>BD>SCZ based on appropriate tests at P < .05, false discovery rate corrected.
SCZ>HV based on appropriate tests at P < .05, false discovery rate corrected.
BD>SCZ based on appropriate tests at P < .05, false discovery rate corrected.
SCZ = BD>HV based on appropriate tests at P < .05, false discovery rate corrected.
SCZ>BD = HV based on appropriate tests at P < .05, false discovery rate corrected.
In the BPRS, symptoms are coded as 1 (absent) to 7 (extremely severe). The BPRS Positive symptoms are the sum of scores for hallucination, unusual thought content, and bizarre behavior subscales. The BPRS Negative symptoms are the sum of scores for blunted affect, emotional withdrawal, and motor retardation subscales. The BPRS Depression/anxiety scores are the sum of scores for anxiety, depression, suicidality, and guilt subscales. The BPRS Mania/disorganization scores are the sum of scores for motor hyperactivity, elevated mood, excitement, distractibility, and grandiosity subscales. For detailed definitions of other measures, see eTable 1 in the Supplement.
SCZ>BD>HV based on appropriate tests at P < .05, false discovery rate corrected.
SCZ = BD based on appropriate tests at P < .05, false discovery rate corrected.
Some patients were taking both first-generation and second-generation antipsychotics.
Nonimaging Data Set
The nonimaging data set comprised 18 variables and included demographic information (age and sex), personal and family psychopathology, IQ, substance use, psychological trauma, physical activity, BMI, and medication (detailed definitions are listed in eTable 1 in the Supplement). Duration of illness was not considered separately because it correlated highly with age (r = 0.74). In all participants, psychopathology was assessed using the 24-item Brief Psychiatric Rating Scale. This scale was chosen because it is suitable for the assessment of nonclinical populations; provides reliable severity estimates for negative and positive symptoms, mania/disorganization, and depression/anxiety; and is relevant to clinical practice because of its high interrater reliability even when administered by less experienced professionals. Medication type and dosage were recorded in patients, and the daily antipsychotic dose was converted to chlorpromazine equivalents. The Wechsler Abbreviated Scale of Intelligence II was used to obtain an estimate of current IQ. We focused on IQ because it indexes the covariance between cognitive tests while accounting for most of the variance in cognitive ability. We chose BMI as an index of cardiometabolic health because it is simple to measure in clinical practice and is widely used in determining public health policies. BMI is highly correlated with metabolic syndrome and insulin resistance and is a reliable predictor of all-cause morbidity and mortality.
MRI Modalities and Neuroimaging Data Set
We acquired structural, diffusion-weighted imaging (DWI) as well as task-related and resting-state functional MRI (fMRI) data on a 3-T scanner (Skyra; Siemens) in all participants (eMethods in the Supplement). After the analyses described below in the “Structural and DWI Analyses” and “Functional Imaging Analyses” subsections, we defined 167 variables that comprised regional brain measures from each modality, including measures of head motion (defined in eTable 2 in the Supplement). Univariate case-control differences in these neuroimaging phenotypes are reported in the eResults in the Supplement (subsidiary analyses, including eTable 3, eTable 4, and eFigures 1, 2, 3, and 4 in the Supplement).
Structural and DWI Analyses
Structural and DWI data analyses, including quality assessment, are fully described in the eMethods in the Supplement. Detailed definitions of all the variables derived from these analyses are listed in eTable 2 in the Supplement. Briefly, 64 cortical thickness measures and 18 subcortical volumetric measures were derived from each data set using standard procedures and tools from an image analysis suite (FreeSurfer; http://surfer.nmr.mgh.harvard.edu/). The DWI data were preprocessed using standard tools from FSL. We used tract-based spatial statistics to compute 38 measures of regional FA based on The John Hopkins University white matter atlas.
Functional Imaging Analyses
Task and resting-state fMRI data were processed using the Statistical Parametric Mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and the Data Processing & Analysis for (Resting-State) Brain Imaging toolbox to extract measures of task-related activation and resting-state connectivity as detailed in eMethods in the Supplement. For the task fMRI, we focused on working memory (WM) and emotion recognition (ER) based on prior evidence that dysfunction in these processes is a core dimension of psychosis. We used the 2-back WM task and the emotional face-matching ER task utilized by the Human Connectome Project (HCP) (humanconnectomeproject.org) to facilitate reproducibility and because the HCP tasks are similar to versions widely used in neuroimaging studies in psychosis. We identified 12 WM task–related and 14 ER task–related activation peaks (eTable 5, eTable 6, and eFigure 5 in the Supplement). Separately for each task, we defined spherical, 5-mm-radius volumes of interest centered on the coordinates of each activation peak from which we extracted average β values from each participant (see eMethods and eTable 2 in the Supplement for alternative analyses). For the resting-state fMRI, we identified 6 major networks in each data set. Specifically, we defined the default mode, central executive, salience, sensorimotor, visual, and auditory networks using validated masks available through the Functional Imaging in Neuropsychiatric Disorders Laboratory at Stanford University (http://findlab.stanford.edu/functional_ROIs.html) (eFigure 6 in the Supplement) and calculated within-network and between-network functional connectivity using Fisher z-transformed Pearson correlation (eTable 2 in the Supplement).
Sparse Canonical Correlation Analyses
We used an sCCA with an L1 penalty function that does not assume that the variables in the 2 data sets are uncorrelated (more details are provided in eMethods in the Supplement). Before data entry, we explored the univariate correlations between imaging and nonimaging variables (eFigure 7 in the Supplement) and tested for linearity (details are provided in eMethods in the Supplement). Nonimaging and imaging variables were standardized to a mean (SD) of 0 (1) and entered into sCCAs implemented in a computer program (MATLAB, version R2015b; MathWorks) using an in-house script. We performed a global sCCA that included all nonimaging variables (n = 18) (eTable 1 in the Supplement) and all imaging variables (n = 167) (eTable 2 in the Supplement). To increase granularity, we also conducted modular sCCAs to ascertain whether the nonimaging data set showed distinct patterns of covariation with each imaging subset (cortical thickness, subcortical volumes, FA, task-related activation, and resting-state connectivity). For each sCCA, (1) we computed the sparse parameters for a range of candidate values from 0.1x√p (high sparsity) to 1x√p (low sparsity at increments of 0.1, where p is the number of features in each data set) and fitted the resulting models; (2) we selected the optimal sparse criteria combination based on the parameters that corresponded to the values of the model that maximized the sCCA correlation value; and (3) we determined the optimal sCCA model and established its significance using permutations (n = 5000). The P value was defined as the number of permutations that resulted in a higher correlation than the original data divided by the total number of permutations. Therefore, the P value is explicitly corrected for multiple testing because it is compared against the null distribution of maximal correlation values across all estimated sCCAs. Only significant and reliable sCCA modes were reported based on the reliability analyses as detailed in the eMethods in the Supplement. In statistically significant modes, we calculated correlations between the individual variables and the opposite variate (ie, variable-to-variate correlations). We then ranked the variable-to-variate correlations based on their strength and reliability.
Reliability Analyses
The results reported herein were shown to be reliable after comprehensive analyses detailed in the eMethods and the eResults in the Supplement. Briefly, we tested the stability of the overall sCCA correlations, the sCCA modes, and the variable-to-variate correlations by repeating the analyses after randomly splitting the sample into equal training and test sets 5000 times (eTables 7, 8, 9, and 10 and eFigures 8, 9, 10, 11, and 12 in the Supplement). We also tested the stability of the overall sCCA using the leave-one-out method (eResults in the Supplement). Furthermore, we examined the reliability of the overall sCCA correlation as a function of sample size and composition and showed that outcomes of the sCCA were stable for any sample larger than approximately 70% of the original (ie, from n >124) (eFigure 9 in the Supplement). Finally, we confirmed that the overall sCCA correlations (eFigure 10 in the Supplement) and the variable-to-variate correlations (eFigure 11 in the Supplement) were similar when each diagnostic group was considered separately. We used Fisher r-to-z transformation to assess the significance of the difference between pairs of correlation coefficients.
Results
Global sCCA
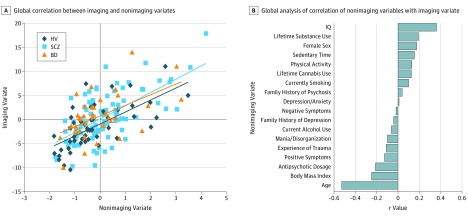
Only the first mode was both significant and reliable (eFigure 8 in the Supplement). The nonimaging (18 variables) and imaging (167 variables) data sets were significantly correlated (r = 0.63, P < .001) (Figure 1A). All groups contributed equally to the overall correlation (r = 0.66 for healthy volunteers, r = 0.64 for patients with schizophrenia, and r = 0.54 for patients with BD; P > .40 for all pairwise comparisons, with z < 1). Figure 1B shows the variable-to-variate correlations of the nonimaging measures; those with the highest correlations with the imaging variate were IQ (r = 0.36), age (r = −0.53), BMI (r = −0.25), antipsychotic dosage (r = −0.21), female sex (r = 0.18), and positive symptoms (r = −0.13) (eTable 7 and eFigure 12 in the Supplement). The imaging measures that were highly associated with the nonimaging variate mainly involved frontal, superior and inferior temporal, parietal (including the posterior cingulate cortex and precuneus), and visual regional measures of cortical thickness (eTable 8 in the Supplement). Among the other imaging measures, notable correlations with the nonimaging variate were seen for the FA of the posterior thalamic radiation bilaterally and for the dorsolateral prefrontal and parietal activation during the WM task.
Figure 1. Global Analysis of the Imaging and Nonimaging Data Sets.
A, Scatterplot of the imaging and nonimaging variates. The overall correlation was r = 0.63 (P < .001). Correlations were similar across groups (r = 0.54 for BD, r = 0.64 for SCZ, and r = 0.66 for HV). B, Correlation of nonimaging variables with the imaging variate. The y-axis shows the r value of each imaging variable with the global imaging variate. BD indicates patients with bipolar disorder; HV, healthy volunteers; and SCZ, patients with schizophrenia.
Modular sCCAs
The modular sCCAs were significant for cortical thickness (r = 0.64, P < .001), subcortical volumes (r = 0.50, P < .001), white matter FA (r = 0.44, P = .009), and task activation (r = 0.44, P = .009) but not for resting-state connectivity (r = 0.39, P = .91). Based on the reliability analyses, the sCCA for the resting-state connectivity module was not stable and appeared to be dependent on the size and composition of the sample (eFigure 5 and eDiscussion in the Supplement).
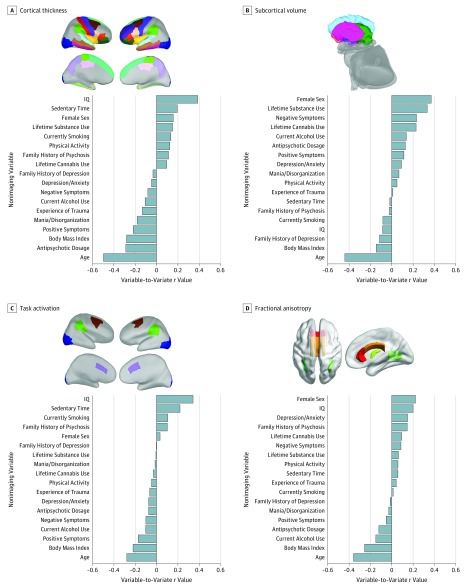
In each significant modular sCCA, only the first mode was both statistically significant and reliable (eFigure 8 in the Supplement). All groups contributed equally to each modular correlation (eFigure 10.1, eFigure 10.2, and eDiscussion in the Supplement). Figure 2 shows the variable-to-variate correlations for each significant modular sCCA.
Figure 2. Results of the Modular Sparse Canonical Correlation Analyses.
A, Top: Regional cortical thickness measures correlated most highly with nonimaging variate. Bottom: Correlations between nonimaging variables and cortical thickness variate. B, Top: Subcortical volumetric measures correlated most highly with nonimaging variate. Bottom: Correlations between nonimaging variables and subcortical volumes variate. C, Top: Regional task-related brain activation correlated most highly with nonimaging variate. Bottom: Correlations between nonimaging variables and task-related brain activation variate. D, Top: Regional fractional anisotropy measures correlated most highly with nonimaging variate. Bottom: Correlations between nonimaging variables and fractional anisotropy variate.
Cortical Thickness Module
Figure 2A shows the variable-to-variate correlations of the nonimaging measures; those with the highest correlations with the cortical thickness variate were age (r = −0.50), IQ (r = 0.39), BMI (r = −0.28), antipsychotic dosage (r = −0.29), and positive symptoms (r = −0.22) (Figure 2A and eTable 7 and eFigure 12 in the Supplement). Cortical thickness measures most highly correlated with the nonimaging variate were widely distributed in the frontal, insular, temporal, parietal, and visual cortex (Figure 2A and eTable 9 in the Supplement).
Subcortical Volume Module
Figure 2B shows the variable-to-variate correlations of the nonimaging measures; those with the highest correlations with the subcortical volume variate were age (r = −0.44), female sex (r = 0.37), negative symptoms (r = 0.23), other substance use (r = 0.33), and cannabis use (r = 0.23) (Figure 2B and eTable 7 in the Supplement). The volume of the caudate, putamen, and pallidum was most highly correlated with the nonimaging variate (Figure 2B and eTable 10 in the Supplement).
Task Activation Module
Figure 2C shows the variable-to-variate correlations of the nonimaging measures; those with the highest correlations with the task activation variate were age (r = −0.28), IQ (r = 0.35), BMI (r = −0.22), positive symptoms (r = −0.18), and sedentary time (r = 0.23) (Figure 2C and eTable 7 in the Supplement). The WM task activation in the dorsolateral prefrontal cortex, inferior parietal lobule, and dorsal anterior cingulate cortex were most highly correlated with the nonimaging variate (Figure 2C and eTable 11 in the Supplement).
White Matter FA Module
Figure 2D shows the variable-to-variate correlations of the nonimaging measures; those with the highest correlations with the white matter FA variate were age (r = −0.36), BMI (r = −0.26), female sex (r = 0.22), IQ (r = 0.20), and alcohol use (r = −0.15) (Figure 2D and eTable 7 in the Supplement). Depression/anxiety symptoms had the only psychopathology score that showed a correlation with the FA variate (r = 0.12). The regional FA of the posterior thalamic radiations, the fornix, and the body and genu of the corpus callosum were the most highly correlated with the nonimaging variate (Figure 2D and eTable 12 in the Supplement).
Discussion
In this study, we demonstrate a substantial and significant covariation between multimodal imaging phenotypes relating to brain structure, white matter integrity, task-related activation, and intrinsic functional connectivity and nonimaging variables of psychopathology, cognition, BMI, substance use, psychological trauma, and medication. Diagnostic status did not appear to alter the association between imaging phenotypes and nonimaging factors known to influence MRI signals (eDiscussion in the Supplement). Among the clinical dimensions of psychosis, positive symptoms were retained in the multivariate global, cortical thickness, and brain activation models while negative symptoms were mostly associated with measures of subcortical volume.
The present results reinforce the importance of age and IQ for multiple neuroimaging measures and highlight the association between BMI and neuroimaging phenotypes relating to cortical thickness, task-related brain activation, and white matter integrity. Higher BMI has been previously associated with lower cortical thickness and cortical gray matter volume in healthy volunteers. Investigations into the mechanisms underlying the association between the brain, weight regulation, and cardiometabolic health should be considered a priority, particularly because they may lead to interventions that can mitigate potential risk.
Recreational cannabis and substance use showed a positive covariation with the subcortical volume variate. The available information about the pattern of the association between brain metrics and substance use is inconsistent, especially in the case of recreational and occasional users. The present results align with reports of a modest increase in subcortical volumes in users, particularly in the basal ganglia. Alcohol use was negatively correlated with the FA variate, adding to previous observations that even light and recreational use of alcohol may affect white matter microstructure.
Positive and negative symptoms maintained substantial correlations with neuroimaging variates even when considered in the context of multiple other variables that influence the MRI signal. Specifically, we found negative correlations between positive symptoms and the multimodal, cortical thickness, and task-related brain activation variates. The neuroimaging literature to date has mainly supported an association between positive symptoms and cortical thinning. The present study extends these findings to suggest that positive symptoms have multimodal associations with neuroimaging phenotypes that relate both to structure and function. The positive association between negative symptoms and the subcortical volume variate was unexpected because even large studies on psychosis have failed to identify symptomatic correlates of subcortical volumetric measures. Conversely, the brain correlates of negative symptoms have not been reliably identified either; some studies have found that patients with chronic schizophrenia and persistent negative symptoms have reduced caudate volume, although other reports have found otherwise. However, the results of 2 previous studies have suggested subtle but detectable increases in caudate volume in the early stages of psychosis in patients with persistent negative symptoms or apathy. This observation could also apply to the present study sample, which comprised mostly young patients with an approximate mean age of 27 years.
Depression/anxiety was correlated with the white matter FA variate. This finding conforms with the results of a large DWI study on psychosis that reported a positive association between depressive symptoms and FA of the thalamic radiation and of the corpus callosum. Of note, FA measures in these regions had the highest correlation with the nonimaging variate in the present study.
Finally, antipsychotic dosage was negatively associated with the global and cortical thickness variates. Antipsychotic medication has been repeatedly linked to cortical thinning. The functional consequences of this association are unclear; however, the observational nature of most of the evidence precludes causal attributions.
Limitations
Our study has several limitations. Neuroimaging techniques include other modalities (eg, magnetic resonance spectroscopy), other analytic methods (eg, graph theory), and a multitude of other fMRI tasks that were not considered herein. Nevertheless, our study examined those modalities, analytic methods, and paradigms that are most commonly used in neuroimaging studies of psychosis. In future studies, the laboratory tests or other measures (eg, adiposity) of cardiometabolic health that were not included in the present study may provide more mechanistic insights about the association between BMI and imaging phenotypes. We did not use an extensive cognitive battery, which may have identified more detailed associations between cognition and imaging phenotypes. However, measures of general intelligence, such as IQ used herein, not only are correlated with individual task performance but also have been shown to have the most robust association with imaging phenotypes even when other cognitive measures are simultaneously considered. Future work should replicate and extend these results by examining the association with nonimaging and imaging phenotypes in larger samples.
Conclusions
We found significant covariation between multimodal imaging phenotypes and behavioral, clinical, lifestyle, and general health-related measures that was comparable in healthy volunteers and patients with psychosis. The results suggest that the severity of positive symptoms, arguably the hallmark of psychosis, is likely to be meaningfully related to reductions in cortical thickness and brain activation even when other nonimaging factors are also modeled. In addition, IQ and BMI had significant contributions to neuroimaging findings; because both measures are easy to obtain, their routine inclusion in neuroimaging studies is both feasible and advisable.
eMethods. Supplemental Methods
eTable 1. Definitions of the Nonimaging Variables
eTable 2. Definitions of the Nonimaging Variables
eTable 3. Suprathreshold Clusters of Task-Related Activation in the Working Memory Task
eTable 4. Suprathreshold Clusters of Task-Related Activation in the Emotion Recognition Task
eTable 5. Correlations Between the Individual Nonimaging Variables and the Imaging Variates for the Global and Modular Analyses in the Original Data Set (r) and Resampled Test-Sets (rTS)
eTable 6. Global Analysis: Correlations Between the Individual Imaging Variables and the Nonimaging Variate in the Original Data Set (r) and the Resampled Test-Sets (rTS)
eTable 7. Cortical Thickness Module: Correlations Between the Individual Cortical Thickness Variables and the Nonimaging Variate in the Original Data Set (r) and Resampled Test-Sets (rTS)
eTable 8. Subcortical Volume Module: Correlations Between the Individual Subcortical Volume Variables and the Nonimaging Variate in the Original Data Set (r) and Resampled Test-Sets (rTS)
eTable 9. Task Activation Module: Correlations Between the Individual Task-Related Brain Activation Variables and the Nonimaging Variate in the Original Data Set (r) and Resampled Test-Sets (rTS)
eTable 10. White Matter Fractional Anisotropy Module: Correlations Between the Individual Fractional Anisotropy Variables With the Nonimaging Variate in the Original Data Set (r) and Resampled Test-Sets (rTS)
eTable 11. Case-Control Differences in Brain Activation in the Working Memory Task (2-back >0-back)
eTable 12. Case-Control Differences in Brain Activation in the Emotional Recognition Task (Emotional Face Matching>Shape Matching)
eResults. Supplemental Results
eFigure 1. Activation Patterns Associated With the fMRI Tasks in the Study and Human Connectome Project (HCP) Samples
eFigure 2. Resting-State Networks
eFigure 3. Manhattan Plots of Univariate Correlations Between Imaging and Nonimaging Variables
eFigure 4. Histogram of the Redundancy-Reliability Score (RR-Scores) of the Global and Modular sCCAs
eFigure 5. Effect of Sample Size on sCCA Correlations
eFigure 6.1. Scatterplots for Each Modular sCCA, by Diagnostic Group
eFigure 6.2. Scatterplots for Each sCCA, for Healthy Volunteers vs Patients
eFigure 7. Results of the sCCAs Top Nonimaging Variables, Computed for Each Diagnostic Group
eFigure 8. Reliability of the Top Nonimaging Variables
eFigure 9. Case-Control Differences in Cortical Thickness
eFigure 10. Case-Control Differences in Subcortical Volumes
eFigure 11. Case-Control Differences in Fractional Anisotropy Measures
eFigure 12. Case-Control Differences in Resting-State Functional Connectivity
eDiscussion. Supplemental Discussion
References
- 1.Cross-Disorder Group of the Psychiatric Genomics Consortium Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purcell SM, Wray NR, Stone JL, et al. ; International Schizophrenia Consortium . Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtenstein P, Yip BH, Björk C, et al. . Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peralta V, Moreno-Izco L, Calvo-Barrena L, Cuesta MJ. The low- and higher-order factor structure of symptoms in patients with a first episode of psychosis. Schizophr Res. 2013;147(1):116-124. [DOI] [PubMed] [Google Scholar]
- 5.Russo M, Levine SZ, Demjaha A, et al. . Association between symptom dimensions and categorical diagnoses of psychosis: a cross-sectional and longitudinal investigation. Schizophr Bull. 2014;40(1):111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamminga CA, Ivleva EI, Keshavan MS, et al. . Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170(11):1263-1274. [DOI] [PubMed] [Google Scholar]
- 7.Whiteford HA, Degenhardt L, Rehm J, et al. . Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. [DOI] [PubMed] [Google Scholar]
- 8.Frangou S. A systems neuroscience perspective of schizophrenia and bipolar disorder. Schizophr Bull. 2014;40(3):523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bora E, Fornito A, Radua J, et al. . Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127(1-3):46-57. [DOI] [PubMed] [Google Scholar]
- 10.Gupta CN, Calhoun VD, Rachakonda S, et al. . Patterns of gray matter abnormalities in schizophrenia based on an international mega-analysis. Schizophr Bull. 2015;41(5):1133-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hibar DP, Westlye LT, Doan NT, et al. . Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group [published online May 2, 2017]. Mol Psychiatry. doi: 10.1038/mp.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hibar DP, Westlye LT, van Erp TG, et al. ; Costa Rica/Colombia Consortium for Genetic Investigation of Bipolar Endophenotypes . Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry. 2016;21(12):1710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Erp TG, Hibar DP, Rasmussen JM, et al. . Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly S, Jahanshad N, Zalesky A, et al. . Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group [published online October 17, 2017]. Mol Psychiatry. doi: 10.1038/mp.2017.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donoghue S, Holleran L, Cannon DM, McDonald C. Anatomical dysconnectivity in bipolar disorder compared with schizophrenia: a selective review of structural network analyses using diffusion MRI. J Affect Disord. 2017;209:217-228. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13(1):1-15. [DOI] [PubMed] [Google Scholar]
- 17.Delvecchio G, Sugranyes G, Frangou S. Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychol Med. 2013;43(3):553-569. [DOI] [PubMed] [Google Scholar]
- 18.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sprooten E, Rasgon A, Goodman M, et al. . Addressing reverse inference in psychiatric neuroimaging: meta-analyses of task-related brain activation in common mental disorders. Hum Brain Mapp. 2017;38(4):1846-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birur B, Kraguljac NV, Shelton RC, Lahti AC. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder: a systematic review of the magnetic resonance neuroimaging literature. NPJ Schizophr. 2017;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr Bull. 2012;38(3):608-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karbasforoushan H, Woodward ND. Resting-state networks in schizophrenia. Curr Top Med Chem. 2012;12(21):2404-2414. [DOI] [PubMed] [Google Scholar]
- 23.Meda SA, Ruaño G, Windemuth A, et al. . Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci U S A. 2014;111(19):E2066-E2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vargas C, López-Jaramillo C, Vieta E. A systematic literature review of resting state network–functional MRI in bipolar disorder. J Affect Disord. 2013;150(3):727-735. [DOI] [PubMed] [Google Scholar]
- 25.Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44(1):168-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martino M, Magioncalda P, Huang Z, et al. . Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc Natl Acad Sci U S A. 2016;113(17):4824-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nestor PG, Kubicki M, Nakamura M, et al. . Neuropsychological variability, symptoms, and brain imaging in chronic schizophrenia. Brain Imaging Behav. 2013;7(1):68-76. [DOI] [PubMed] [Google Scholar]
- 28.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walton E, Hibar DP, van Erp TGM, et al. ; Karolinska Schizophrenia Project Consortium (KaSP) . Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychol Med. 2018;48(1):82-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walton E, Hibar DP, van Erp TG, et al. ; Karolinska Schizophrenia Project Consortium (KaSP) . Positive symptoms associate with cortical thinning in the superior temporal gyrus via the ENIGMA Schizophrenia consortium. Acta Psychiatr Scand. 2017;135(5):439-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller KL, Alfaro-Almagro F, Bangerter NK, et al. . Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19(11):1523-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moser DA, Doucet GE, Ing A, et al. . An integrated brain-behavior model for working memory [published online December 5, 2017]. Mol Psychiatry. doi 10.1038/mp.2017.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith SM, Nichols TE, Vidaurre D, et al. . A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18(11):1565-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansell BR, Dwyer DB, Wood SJ, et al. . Divergent effects of first-generation and second-generation antipsychotics on cortical thickness in first-episode psychosis. Psychol Med. 2015;45(3):515-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lesh TA, Tanase C, Geib BR, et al. . A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry. 2015;72(3):226-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vita A, De Peri L, Deste G, Barlati S, Sacchetti E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? a meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry. 2015;78(6):403-412. [DOI] [PubMed] [Google Scholar]
- 38.Abramovic L, Boks MP, Vreeker A, et al. . The association of antipsychotic medication and lithium with brain measures in patients with bipolar disorder. Eur Neuropsychopharmacol. 2016;26(11):1741-1751. [DOI] [PubMed] [Google Scholar]
- 39.Kani AS, Shinn AK, Lewandowski KE, Öngür D. Converging effects of diverse treatment modalities on frontal cortex in schizophrenia: a review of longitudinal functional magnetic resonance imaging studies. J Psychiatr Res. 2017;84:256-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper J, Mancuso SG, Borland R, Slade T, Galletly C, Castle D. Tobacco smoking among people living with a psychotic illness: the second Australian Survey of Psychosis. Aust N Z J Psychiatry. 2012;46(9):851-863. [DOI] [PubMed] [Google Scholar]
- 41.Gurillo P, Jauhar S, Murray RM, MacCabe JH. Does tobacco use cause psychosis? systematic review and meta-analysis. Lancet Psychiatry. 2015;2(8):718-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore E, Mancuso SG, Slade T, Galletly C, Castle DJ. The impact of alcohol and illicit drugs on people with psychosis: the second Australian National Survey of Psychosis. Aust N Z J Psychiatry. 2012;46(9):864-878. [DOI] [PubMed] [Google Scholar]
- 43.Brunette MF, Mueser KT, Babbin S, et al. . Demographic and clinical correlates of substance use disorders in first episode psychosis [published online July 8, 2017]. Schizophr Res. doi: 10.1016/j.schres.2017.06.039 [DOI] [PubMed] [Google Scholar]
- 44.Strassnig M, Kotov R, Cornaccio D, Fochtmann L, Harvey PD, Bromet EJ. Twenty-year progression of body mass index in a county-wide cohort of people with schizophrenia and bipolar disorder identified at their first episode of psychosis. Bipolar Disord. 2017;19(5):336-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberger DR, Radulescu E. Finding the elusive psychiatric “lesion” with 21st-century neuroanatomy: a note of caution. Am J Psychiatry. 2016;173(1):27-33. [DOI] [PubMed] [Google Scholar]
- 46.Eickhoff SB, Etkin A. Going beyond finding the “lesion”: a path for maturation of neuroimaging. Am J Psychiatry. 2016;173(3):302-303. [DOI] [PubMed] [Google Scholar]
- 47.Cabral C, Kambeitz-Ilankovic L, Kambeitz J, et al. . Classifying schizophrenia using multimodal multivariate pattern recognition analysis: evaluating the impact of individual clinical profiles on the neurodiagnostic performance. Schizophr Bull. 2016;42(suppl 1):S110-S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocha-Rego V, Jogia J, Marquand AF, Mourao-Miranda J, Simmons A, Frangou S. Examination of the predictive value of structural magnetic resonance scans in bipolar disorder: a pattern classification approach. Psychol Med. 2014;44(3):519-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.First MB, Williams JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5, Research Version. Arlington, VA: American Psychiatric Association; 2015. [Google Scholar]
- 50.Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green M, Shaner A. Appendix 1: Brief Psychiatric Rating Scale (BPRS) expanded version (4.0) scales, anchor points and administration manual. Int J Methods Psychiatr Res. 1993;3:227-244. [Google Scholar]
- 51.Kopelowicz A, Ventura J, Liberman RP, Mintz J. Consistency of Brief Psychiatric Rating Scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41(2):77-84. [DOI] [PubMed] [Google Scholar]
- 52.Ruggeri M, Koeter M, Schene A, et al. ; EPSILON Study Group . Factor solution of the BPRS–expanded version in schizophrenic outpatients living in five European countries. Schizophr Res. 2005;75(1):107-117. [DOI] [PubMed] [Google Scholar]
- 53.Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res. 2000;97(2-3):129-135. [DOI] [PubMed] [Google Scholar]
- 54.Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale. Int J Methods Psychiatr Res. 1993;3:221-226. [Google Scholar]
- 55.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686-693. [DOI] [PubMed] [Google Scholar]
- 56.Wechsler D. Wechsler Abbreviated Scale of Intelligence. 2nd ed San Antonio, TX: NCS Pearson; 2011. [Google Scholar]
- 57.Carroll JB. Human Cognitive Abilities. New York, NY: Cambridge University Press; 1993. [Google Scholar]
- 58.Johnson W, Bouchard TJ Jr, Krueger RF, McGue M, Gottesman II. Just one g: consistent results from three test batteries. Intelligence. 2004;32:95-107. [Google Scholar]
- 59.Plomin R, Deary IJ. Genetics and intelligence differences: five special findings. Mol Psychiatry. 2015;20(1):98-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esteghamati A, Khalilzadeh O, Anvari M, Ahadi MS, Abbasi M, Rashidi A. Metabolic syndrome and insulin resistance significantly correlate with body mass index. Arch Med Res. 2008;39(8):803-808. [DOI] [PubMed] [Google Scholar]
- 61.Ryan MC, Fenster Farin HM, Abbasi F, Reaven GM. Comparison of waist circumference versus body mass index in diagnosing metabolic syndrome and identifying apparently healthy subjects at increased risk of cardiovascular disease. Am J Cardiol. 2008;102(1):40-46. [DOI] [PubMed] [Google Scholar]
- 62.Aune D, Sen A, Prasad M, et al. . BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Angelantonio E, Bhupathiraju SN, Wormser D, et al. ; Global BMI Mortality Collaboration . Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glasser MF, Sotiropoulos SN, Wilson JA, et al. ; WU-Minn HCP Consortium . The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith SM, Jenkinson M, Johansen-Berg H, et al. . Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487-1505. [DOI] [PubMed] [Google Scholar]
- 67.Mori S, Oishi K, Jiang H, et al. . Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14(3):339-351. [DOI] [PubMed] [Google Scholar]
- 69.Hill SK, Reilly JL, Keefe RS, et al. . Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170(11):1275-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee RS, Hermens DF, Scott J, et al. . A meta-analysis of neuropsychological functioning in first-episode bipolar disorders. J Psychiatr Res. 2014;57:1-11. [DOI] [PubMed] [Google Scholar]
- 71.Ruocco AC, Reilly JL, Rubin LH, et al. . Emotion recognition deficits in schizophrenia-spectrum disorders and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Schizophr Res. 2014;158(1-3):105-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bora E, Pantelis C. Meta-analysis of cognitive impairment in first-episode bipolar disorder: comparison with first-episode schizophrenia and healthy controls. Schizophr Bull. 2015;41(5):1095-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Damoiseaux JS, Rombouts SA, Barkhof F, et al. . Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848-13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith SM, Fox PT, Miller KL, et al. . Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040-13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Witten DM, Tibshirani R, Hastie T. A penalized matrix decomposition, with applications to sparse principal components and canonical correlation analysis. Biostatistics. 2009;10(3):515-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Franke K, Ziegler G, Klöppel S, Gaser C; Alzheimer’s Disease Neuroimaging Initiative . Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50(3):883-892. [DOI] [PubMed] [Google Scholar]
- 77.Taki Y, Kinomura S, Sato K, et al. . Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring). 2008;16(1):119-124. [DOI] [PubMed] [Google Scholar]
- 78.Kurth F, Levitt JG, Phillips OR, et al. . Relationships between gray matter, body mass index, and waist circumference in healthy adults. Hum Brain Mapp. 2013;34(7):1737-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Medic N, Ziauddeen H, Ersche KD, et al. . Increased body mass index is associated with specific regional alterations in brain structure. Int J Obes (Lond). 2016;40(7):1177-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doucet GE, Rasgon N, McEwen BS, Micali N, Frangou S. Elevated body mass index is associated with increased integration and reduced cohesion of sensory-driven and internally guided resting-state functional brain networks [published online January 23, 2017]. Cereb Cortex. doi: 10.1093/cercor/bhx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martín-Santos R, Fagundo AB, Crippa JA, et al. . Neuroimaging in cannabis use: a systematic review of the literature. Psychol Med. 2010;40(3):383-398. doi: 10.1093/cercor/bhx008 [DOI] [PubMed] [Google Scholar]
- 82.Batalla A, Bhattacharyya S, Yücel M, et al. . Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8(2):e55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lorenzetti V, Solowij N, Fornito A, Lubman DI, Yucel M. The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Curr Pharm Des. 2014;20(13):2138-2167. [DOI] [PubMed] [Google Scholar]
- 84.Koenders L, Machielsen MW, van der Meer FJ, et al. . Brain volume in male patients with recent onset schizophrenia with and without cannabis use disorders. J Psychiatry Neurosci. 2015;40(3):197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilman JM, Kuster JK, Lee S, et al. . Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 2014;34(16):5529-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chye Y, Solowij N, Suo C, et al. . Orbitofrontal and caudate volumes in cannabis users: a multi-site mega-analysis comparing dependent versus non-dependent users. Psychopharmacology (Berl). 2017;234(13):1985-1995. [DOI] [PubMed] [Google Scholar]
- 87.Topiwala A, Allan CL, Valkanova V, et al. . Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357:j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sprooten E, Papmeyer M, Smyth AM, et al. . Cortical thickness in first-episode schizophrenia patients and individuals at high familial risk: a cross-sectional comparison. Schizophr Res. 2013;151(1-3):259-264. [DOI] [PubMed] [Google Scholar]
- 89.Li Y, Li WX, Xie DJ, Wang Y, Cheung EF, Chan RC. Grey matter reduction in the caudate nucleus in patients with persistent negative symptoms: an ALE meta-analysis [published online April 5, 2017]. Schizophr Res. doi: 10.1016/j.schres.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 90.Galderisi S, Quarantelli M, Volpe U, et al. . Patterns of structural MRI abnormalities in deficit and nondeficit schizophrenia. Schizophr Bull. 2008;34(2):393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fischer BA, Keller WR, Arango C, et al. . Cortical structural abnormalities in deficit versus nondeficit schizophrenia. Schizophr Res. 2012;136(1-3):51-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Voineskos AN, Foussias G, Lerch J, et al. . Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry. 2013;70(5):472-480. [DOI] [PubMed] [Google Scholar]
- 93.Buchanan RW, Breier A, Kirkpatrick B, et al. . Structural abnormalities in deficit and nondeficit schizophrenia. Am J Psychiatry. 1993;150(1):59-65. [DOI] [PubMed] [Google Scholar]
- 94.Mørch-Johnsen L, Nesvåg R, Faerden A, et al. . Brain structure abnormalities in first-episode psychosis patients with persistent apathy. Schizophr Res. 2015;164(1-3):59-64. [DOI] [PubMed] [Google Scholar]
- 95.Sarrazin S, Poupon C, Linke J, et al. . A multicenter tractography study of deep white matter tracts in bipolar I disorder: psychotic features and interhemispheric disconnectivity. JAMA Psychiatry. 2014;71(4):388-396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eTable 1. Definitions of the Nonimaging Variables
eTable 2. Definitions of the Nonimaging Variables
eTable 3. Suprathreshold Clusters of Task-Related Activation in the Working Memory Task
eTable 4. Suprathreshold Clusters of Task-Related Activation in the Emotion Recognition Task
eTable 5. Correlations Between the Individual Nonimaging Variables and the Imaging Variates for the Global and Modular Analyses in the Original Data Set (r) and Resampled Test-Sets (rTS)
eTable 6. Global Analysis: Correlations Between the Individual Imaging Variables and the Nonimaging Variate in the Original Data Set (r) and the Resampled Test-Sets (rTS)
eTable 7. Cortical Thickness Module: Correlations Between the Individual Cortical Thickness Variables and the Nonimaging Variate in the Original Data Set (r) and Resampled Test-Sets (rTS)
eTable 8. Subcortical Volume Module: Correlations Between the Individual Subcortical Volume Variables and the Nonimaging Variate in the Original Data Set (r) and Resampled Test-Sets (rTS)
eTable 9. Task Activation Module: Correlations Between the Individual Task-Related Brain Activation Variables and the Nonimaging Variate in the Original Data Set (r) and Resampled Test-Sets (rTS)
eTable 10. White Matter Fractional Anisotropy Module: Correlations Between the Individual Fractional Anisotropy Variables With the Nonimaging Variate in the Original Data Set (r) and Resampled Test-Sets (rTS)
eTable 11. Case-Control Differences in Brain Activation in the Working Memory Task (2-back >0-back)
eTable 12. Case-Control Differences in Brain Activation in the Emotional Recognition Task (Emotional Face Matching>Shape Matching)
eResults. Supplemental Results
eFigure 1. Activation Patterns Associated With the fMRI Tasks in the Study and Human Connectome Project (HCP) Samples
eFigure 2. Resting-State Networks
eFigure 3. Manhattan Plots of Univariate Correlations Between Imaging and Nonimaging Variables
eFigure 4. Histogram of the Redundancy-Reliability Score (RR-Scores) of the Global and Modular sCCAs
eFigure 5. Effect of Sample Size on sCCA Correlations
eFigure 6.1. Scatterplots for Each Modular sCCA, by Diagnostic Group
eFigure 6.2. Scatterplots for Each sCCA, for Healthy Volunteers vs Patients
eFigure 7. Results of the sCCAs Top Nonimaging Variables, Computed for Each Diagnostic Group
eFigure 8. Reliability of the Top Nonimaging Variables
eFigure 9. Case-Control Differences in Cortical Thickness
eFigure 10. Case-Control Differences in Subcortical Volumes
eFigure 11. Case-Control Differences in Fractional Anisotropy Measures
eFigure 12. Case-Control Differences in Resting-State Functional Connectivity
eDiscussion. Supplemental Discussion