Abstract
Objectives
A previous randomized placebo-controlled trial in military veterans posttraumatic stress disorder (PTSD) found that quetiapine improved global PTSD symptoms severity, depression and anxiety as well as the re-experiencing and hypearousal clusters. However, it is not known if individual symptoms had a preferential response to this medication. The goal of this study was to analyze the individual symptom response in this group of patients.
Methods
Data from a previous trial was re-analyzed. Each of the of the scale items was analyzed individually using Repeated Measures Analysis of Variance.
Results
Compared to placebo, there was a significant decline in the Clinician-Administered PTSD Scale intrusive memories and insomnia questions. In the Davidson Trauma Scale, greater improvements were observed on irritability, difficulty concentrating, hyperstartle and a trend was observed on avoiding thoughts or feelings about the event. Greater improvements compared with placebo were noted on the Hamilton Depression (HAM-D) middle and late insomnia items. On the Hamilton Anxiety scale (HAM-A), the insomnia item was significantly improved.
Conclusions
Quetiapine demonstrated greater effect than placebo on several symptoms. The strongest response was seen on insomnia, which the highest significance level on the CAPS. The insomnia items of both the HAM-D and HAM-A also demonstrated improvement with quetiapine. These finding indicate quetiapine improved sleep measure. Insomnia can be a difficult problem to treat in PTSD patients, therefore quetiapine should be considered in difficult cases.
Keywords: stress disorders, post-traumatic, antipsychotic agents, combat disorders, veterans, psychopharmacology
Introduction
Posttraumatic stress disorder (PTSD) develops in a significant proportion of people exposed to traumatic events.1 PTSD rates are particularly high in military veterans.2 DSM 5 criteria distinguishes 5 symptom clusters in PTSD: Re-experiencing the event, avoidance of reminders of the trauma, feelings of numbness or detachment; changes in cognition and symptoms of hyperarousal.1 To meet diagnostic criteria symptoms need to be present for at least one month and cause impairment in functioning.1 PTSD tends to be a chronic condition.3 The Selective serotonin reuptake inhibitors (SSRI’s) are considered first line treatment for PTSD4,5 although venlafaxine is also effective.6 However, only sertraline and paroxetine have FDA approval for treatment of PTSD. Antidepressants have been shown to improve most PTSD symptoms.7–9 Post hoc analyses of sertraline and venlafaxine trials have shown differential symptom response with the strongest effect on “psychologically mediated symptoms” (anger, emotional distress with exposure to reminders, anhedonia, detachment and numbing) and less effect on “somatic symptoms” (nightmares, insomnia, physiological distress at exposure to trauma and hyperstartle).8,9 Of interest was the early and strong response seen in anger,8,9 that partly predicted improvement in other symptoms.8,9 However, these medications had little effect on insomnia.8,9 In general, military veterans tend to have little or no response to antidepressant medication.10–13
Atypical antipsychotics are often the used in the treatment of military veterans with PTSD, particularly for sleep.14 Olanzapine has been reported to be beneficial for PTSD both as adjunctive15 or single agent.16,17 Risperidone was found effective in PTSD mostly as adjunctive treatment in several randomized placebo controlled trials in both civilians and veterans.18–21 However, a large Veterans Administration (VA) funded trial of risperidone as an adjunct in military veterans with no previous response to SSRI’s found it was no better than placebo for global PTSD severity.22 A post-hoc analysis did show improvement in re-experiencing and hyperarousal symptoms, although this was not considering clinical significant.22
In a previous randomized, placebo-controlled trial of quetiapine as single agent in PTSD, significant improvement was seen on the clinician administered PTSD scale (CAPS) total score, as well as re-experiencing and hyperarousal clusters.23 In addition, significant improvement was seen on the following clinical scales: Davidson Trauma Scale, Hamilton Depression (HAM D), Hamilton Anxiety (HAM A), Positive and Negative Syndrome Scale (PANSS) global psychopathology and positive symptom subscales.23 A surprising finding was the lack of effect on sleep measures as rated with the Pittsburgh Sleep Quality Index (PSQI). The purpose of the current study was to conduct a post hoc analysis of data from that previous quetiapine PTSD trial23 to examine the time course of individual item response of the scales utilized. The analysis of the instrument’s individual item response is important to determine the effects of quetiapine on specific symptoms. This information may inform how quetiapine could supplement the symptom response observed with antidepressants.8,9
Materials and Methods
Full details of the study are provided elsewhere;23 Briefly, participants were outpatient military veterans ages 18 years or older recruited from 2 VA medical centers (Albuquerque NM and Charleston SC). Participants met DSM IV PTSD diagnostic criteria as determined by the Clinician’s Administered PTSD scale (CAPS24) and were off psychiatric medications for at least 1 week. Participants had a CAPS total score of at least 50 after a 1-week placebo run-in. Both sites were approved by their local IRB’s and subjects signed informed consent forms before starting the study. For further demographic and clinical characteristics please see.23 The main outcome measure was change on CAPS total score. Additional evaluations included the Davidson Trauma Scale (DTS), the Positive and Negative Syndrome Scale (PANSS), HAM-D, HAM-A and the Pittsburgh Sleep Quality Index (PSQI). Eighty patients were randomized to quetiapine (N = 42) or placebo (N = 38). Patients were evaluated at weeks 0 (baseline), 2, 4, 8, and 12. The study medication was started at of 25 mg at bedtime and increased as tolerated to a 400 mg/day by the end of week 2. The allowed dose range was 50–800 mg/day with an average of 258 mg for quetiapine and 463 mg for placebo.23
Since baseline CAPS cluster B scores (re-experiencing) were higher in the quetiapine group compared to placebo (21 ± 7 vs 17 ± 15, t = 2.4, p = 0.018), we conducted Repeated Measures Analysis of Variance (RM ANOVA) adjusting for baseline CAPS score, using drug as a fix factor and visit as a repeated factor. The analyses were done separately by item in the individual CAPS clusters: B (re-experiencing) and D (hyperarousal). In the previous study the avoidance/numbing symptom cluster (C) was not found to improve with quetiapine, therefore this was not included in this analysis. All analyses of individual items for the different scales were also adjusted for baseline values.
Individual items of the following scales were analyzed: Davidson Trauma Scale, HAM-D, HAM-A, PANSS positive symptom cluster and PSQI. All statistical tests are two-sided with a significance level of 0.05 using SAS version: 9.2.
Results
For cluster B (re-experiencing symptoms), we found a significant difference between treatment groups (visit by treatment interaction) on item B1, intrusive memories, [F = 2.54 (4,240), p = 0.0403] (see Figure 1). This difference was evident by week 2 and sustained by week 12. We observed a trend toward visit by treatment interaction on item B4, distress by reminders [F = 2.34 (4,240), p = 0.055]. This was only evident by week 12.
Figure 1.
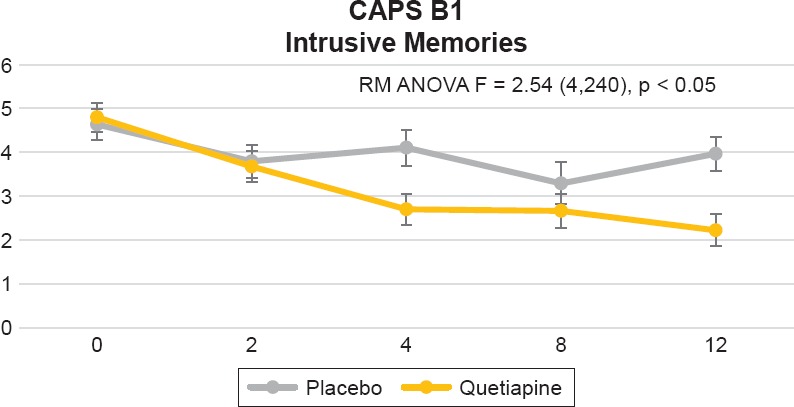
Clinician-Administered PTSD Scale (CAPS) for DSM-IV B1 Item, “Intrusive Memories.” Repeated Measures Analysis of Variance (RM ANOVA) Adjusting for Baseline CAPS Score, Using Drug as a Fix Factor and Visit as a Repeated Factor [F = 2.54 (4,240), p = 0.0403]. Figure Displays Least Square Means With Error Bars
There were no significant differences between the quetiapine and placebo groups on the following items: B2, dreams [F = 1.52 (4,240), p = 0.1965], B3, flashbacks [F = 0.41 (4,240), p = 0.7995] or B5, physiological reactivity to reminders [F = 1.57 (4,240), p = 0.1835]. For cluster D (hyperarousal symptoms), we found a significant difference between treatment groups (visit by treatment interaction) for item D1, problems falling or staying asleep [F = 5.11 (4,240), p = 0.0006] (see Figure 2). This difference was evident by week 2 and sustained by week 12. We did not find a difference between treatment groups for item D2 (irritability) [F = 0.6064 (4,240), p = 0.6064], D3, difficulty concentrating [F = 1.68 (4,240), p = 0.1554], D4 (hypervigilance) [F = 0.69 (4,240), p = 0.6028] or D5, exaggerated startle response [F = 1.10 (4,240), p = 0.3572].
Figure 2.
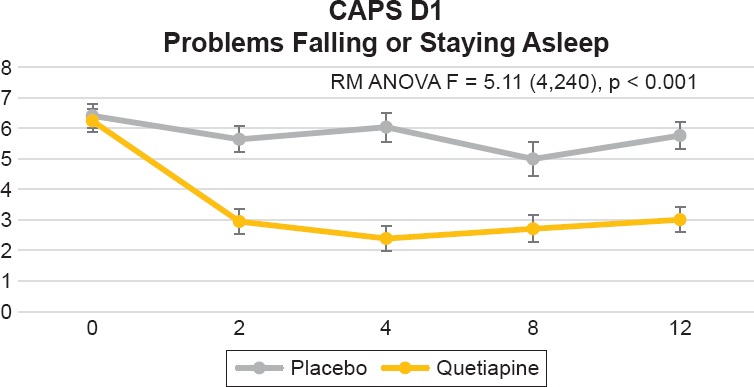
Clinician-Administered PTSD Scale (CAPS) for DSM-IV Item D1, “Problems Falling or Staying Asleep.” Repeated Measures Analysis of Variance (RM ANOVA) Adjusting for Baseline CAPS Score, Using Drug as a Fix Factor and Visit as a Repeated Factor [F = 5.11 (4,240), p = 0.0006]. Figure Displays Least Square Means
In the Davidson Trauma Scale, we found significant differences between treatment groups (visit by treatment interaction) on the following items: 1) Item 2, distressing dreams of the event [F = 5.18 (4,240), p = 0.0005], this was evident by week 2 and sustained by week 12 (see Figure 3); 2) Item 3, been irritable or had outbursts of anger [F = 4.20 (4,240), p = 0.0027], which was evident by week 2 and sustained by week 12 (see Figure 4); 3) Item 14, difficulty concentrating [F = 2.63 (4,240), p = 0.0350], this was only evident by week 12 (see Figure 5); and item 16, been jumpy or easily startled [F = 3.27 (4,240), p = 0.0123] that was only evident by week 12 (Figure 6). Additionally, we found a trend toward significance on item 5, avoiding any thoughts or feelings about the event [F = 2.30 (4,240) 0.0597].
Figure 3.
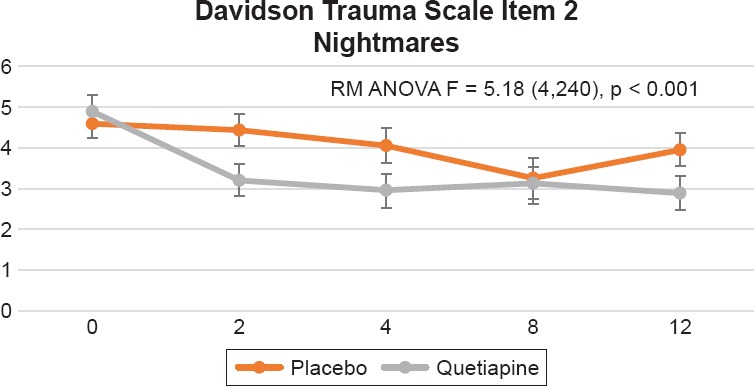
Davidson Trauma Scale, Item 2, “Distressing Dreams of The Event.” Repeated Measures Analysis of Variance (RM ANOVA) Adjusting for Baseline CAPS Score, Using Drug as a Fix Factor and Visit as a Repeated Factor [F = 5.18 ( 4,240), p = 0.0005]. Figure Displays Least Square Means with Error Bars
Figure 4.
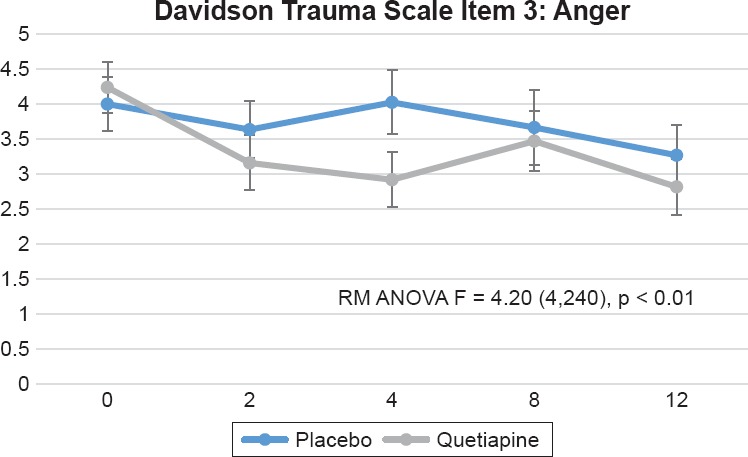
Davidson Trauma Scale item 3, “Been Irritable or Had Outbursts of Anger.” Repeated Measures Analysis of Variance (RM ANOVA) Adjusting for Baseline CAPS Score [F = 4.20 (4,240), p = 0.0027]. Figure Displays Least Square Means with Error Bars
Figure 5.
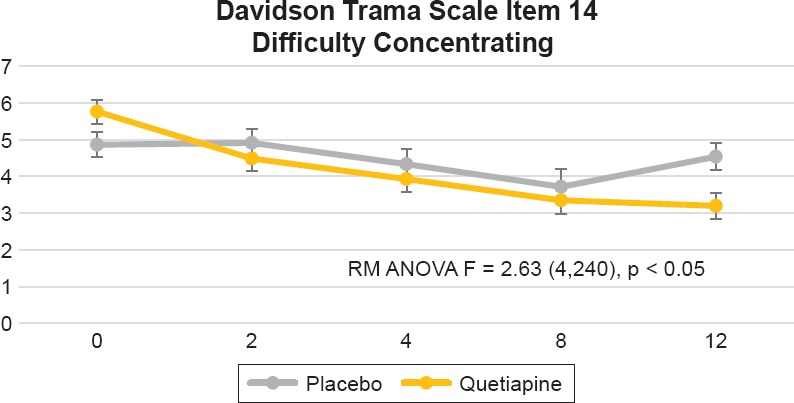
Davidson Trauma Scale item 14, “Had Difficulty Concentrating.” Repeated Measures Analysis of Variance (RM ANOVA) Adjusting for Baseline CAPS Score [F = 2.63 (4,240), p = 0.0350]. Figure Displays Least Square Means with Error Bars
Figure 6.
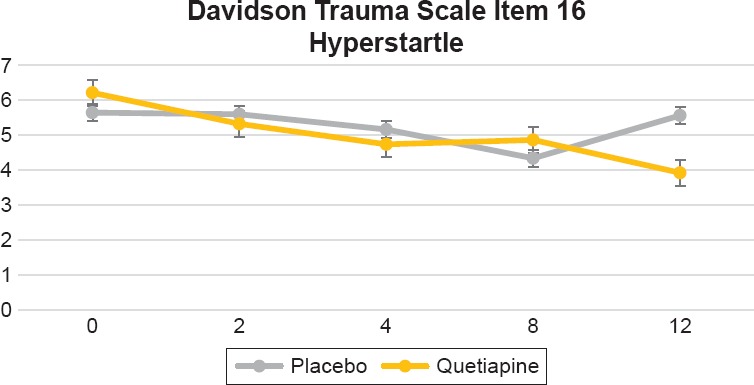
Davidson Trauma Scale Item 16, “Been Jumpy or Easily Startled.” Repeated Measures Analysis of Variance (RM ANOVA) Adjusting for Baseline CAPS Score [F = 3.27 (4,240), p = 0.0123]. Figure Displays Least Square Means with Error Bars
The HAM-D was administered at baseline and week 12. We found significant differences between treatment groups (visit by treatment interaction) on the following items: Item 5 insomnia, middle [F = 5.58 (4,240), p = 0.0003] and item 6, insomnia late [F = 3.62 (4,240), p = 0.0069]. However, there were no significant differences between groups on item 4 insomnia, early [F = 0.56 (4,240), p = 0.6914].
The HAM-A was administered at baseline and week 12. We found a significant differences between treatment groups (visit by treatment interaction) in item, 4 insomnia [F = 10.18 (1, 63), p = 0.0022] and a trend toward significance on item 19, cardiovascular symptoms (tachycardia, pain in chest, throbbing of vessels, fainting feelings, missing beat), [F = 2.87 (1, 63), p = 0.0951].
The PANSS Positive symptoms was administered at baseline and week 12. We found trend differences between treatment groups (visit by treatment interaction) on the following items: Item 2, conceptual disorganization [F = 2.79 (1, 63), p = 0.0998] and item 6 suspiciousness/persecution [F = 3.59 (1, 63), p = 0.0626].
The Pittsburgh Sleep Quality Index (PSQI) was administered at baseline, weeks 4, 8 and 12. We analyzed each of the 7 components and only found trend significance on sleep latency [F = 2.45 (3, 174), p = 0.0653]. None of the other components were significantly different between the quetiapine and placebo groups: Subjective sleep quality [F = 0.81 (3, 175), p = 0.4875]; sleep duration [F = 1.06 (3, 175), p = 0.3675]; habitual sleep efficiency [F = 1.89 (3, 174), p = 0.1339]; sleep disturbances [F = 1.06 (3, 174), p = 0.5530]; use of sleep medication [F = 1.17 (3, 175), p = 0.9169]; daytime dysfunction [F = 0.69 (3, 175), p = 0.5566].
Discussion
In a previous report, we found that quetiapine monotherapy improved PTSD severity measured by the total CAPS and Davidson Trauma Scale scores.23 Additionally we also observed greater improvement on the CAPS re-experiencing and hyperarousal clusters but not on avoidance/numbing.23 However, in that trial if was not clear if different symptoms had a preferential response to this medication. Our current findings show that compared to placebo, quetiapine demonstrated greater improvement on the following CAP items: Intrusive memories (B2) and insomnia (D1). This difference was evident by week 2 and maintained over the 12 week trial. Improved insomnia had the highest significance level. There was a trend toward more improvement on distress by reminders (B4) that was evident by week 12. On the Davidson Trauma Scale the fastest response was seen on distressing dreams of the event (item 2) and irritability (item 3), which were evident by week 2. Improvements in difficulty concentrating and hyperstartle were seen by week 12. These findings confirm our previous result that quetiapine is most effective in re-experiencing and hyperarousal symptoms.
In our prior report, both the total HAM-A and HAM-B scores had greater improvement on quetiapine. In the present analysis, the quetiapine group had more improvement only on the insomnia items of both scales. We previously found no differences on the total PSQI scores. In this subsequent analysis, there were no group differences on any of the 7 PSQI sleep components, we only found a trend improvement on sleep latency. Lastly, a trend improvement was also seen on conceptual disorganization and suspiciousness as measured by the PANSS.
Quetiapine is a second generation antipsychotic FDA-approved for the treatment of schizophrenia and bipolar disorder. Quetiapine has antagonistic properties of the following receptors: Dopamine D2, serotonin 5-HT2, serotonin 5HT2-A, histamine 1 (H1), noradrenergic alpha 1/alpha 2 receptors and partial agonist of the 5-HT2A receptor.25 In addition, norquetiapine, the main quetiapine metabolite, is a norepinephrine reuptake inhibitor.26 There is also evidence that quetiapine increases levels of neuropeptide Y, a resiliency peptide and lowers corticotropine releasing hormone (CRH) a stress peptide, in cerebrospinal fluid (CSF).27 This pharmacological profile may explain the observed benefits of quetiapine on PTSD symptom severity, anxiety, depression and sleep.
The lack of improvement observed on the PSQI is puzzling, we only found a trend improvement on sleep latency. However, the results from individual CAPS, HAM-A and HAM-D sleep items suggest that quetiapine did have indeed an effect on sleep. In fact, the CAPS sleep item had the highest significance level. This is consistent with anecdotal evidence. For example, a survey of 2613 Veterans Administration providers found that quetiapine was the most frequently prescribed atypical antipsychotic to veterans with PTSD (47%), and that the reasons for prescribing it was perceived efficacy, particularly for sleep and sedation.14
These findings suggest quetiapine is a good option to supplement the effect if antidepressants in PTSD, which tend to have a more global effect.7,8 Sertraline and venlafaxine demonstrated early improvement in anger but no effect on sleep.8,9 On their pooled analysis of individual symptom response they also found improvement on avoidance and numbing symptoms.8,9 Our findings suggest quetiapine may complement the antidepressant effects on symptoms of irritability and avoidance/numbing with its effects on insomnia. Due to the risk of metabolic side-effects, quetiapine should be reserved for treatment-resistance and severe PTSD cases and metabolic parameters should be monitored closely. Future studies should investigate quetiapine augmentation of antidepressants.
Acknowledgments
This study was funded by an investigator-initiated grant from AstraZeneca to Dr Hamner.
Footnotes
Disclosure
Dr. Villarreal reports no competing interests.
Dr. Hamner has been recipient of research grant support, honoraria and/or served as a consultant for the following pharmaceutical companies: Abbott, Alkermes, AstraZeneca, Bristol-Myers Squib, Eli Lilly, Forest Laboratories, Janssen, Organon, Pfizer, Otsuka, and Sanofi-Synthlabo.
Dr. Qualls reports no competing interests.
Dr. Canive has been the recipient of research grant support, honoraria, and/or served as a consultant for the following pharmaceutical companies Abbott, AstraZeneca, Bristol-Myers Squib, Eli Lilly, Organon, Otsuka, and Sanofi-Synthlabo.
References
- 1.American, Psychiatric and Association, Diagnostic and Statistic Manual for Mental Disorders. Fifth Edition. A.P. Association; Washington D.C.: 2013. [Google Scholar]
- 2.Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(Suppl 5):4–12. discussion 13-14. [PubMed] [Google Scholar]
- 4.Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. Jama. 2000;283(14):1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 5.Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry. 2001;158(12):1982–1988. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- 6.Davidson J, Baldwin D, Stein DJ, Kuper E, Benattia I, Ahmed S, Pedersen R, Musgnung J. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry. 2006;63(10):1158–1165. doi: 10.1001/archpsyc.63.10.1158. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer-Brody S, Connor KM, Churchill E, Davidson JR. Symptom-specific effects of fluoxetine in post-traumatic stress disorder. Int Clin Psychopharmacol. 2000;15(4):227–231. doi: 10.1097/00004850-200015040-00006. [DOI] [PubMed] [Google Scholar]
- 8.Davidson JR, Landerman LR, Farfel GM, Clary CM. Characterizing the effects of sertraline in post-traumatic stress disorder. Psychol Med. 2002;32(4):661–670. doi: 10.1017/s0033291702005469. [DOI] [PubMed] [Google Scholar]
- 9.Stein DJ, Pedersen R, Rothbaum BO, Baldwin DS, Ahmed S, Musgnung J, Davidson J. Onset of activity and time to response on individual CAPS-SX17 items in patients treated for post-traumatic stress disorder with venlafaxine ER: a pooled analysis. Int J Neuropsychopharmacol. 2009;12(1):23–31. doi: 10.1017/S1461145708008961. [DOI] [PubMed] [Google Scholar]
- 10.van der Kolk BA, Dreyfuss D, Michaels M, Shera D, Berkowitz R, Fisler R, Saxe G. Fluoxetine in posttraumatic stress disorder. J Clin Psychiatry. 1994;55(12):517–522. [PubMed] [Google Scholar]
- 11.Hertzberg MA, Feldman ME, Beckham JC, Kudler HS, Davidson JR. Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Ann Clin Psychiatry. 2000;12(2):101–105. doi: 10.1023/a:1009076231175. [DOI] [PubMed] [Google Scholar]
- 12.Zohar J, Amital D, Miodownik C, Kotler M, Bleich A, Lane RM, Austin C. Double-blind placebo-controlled pilot study of sertraline in military veterans with posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22(2):190–195. doi: 10.1097/00004714-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Friedman MJ, Marmar CR, Baker DG, Sikes CR, Farfel GM. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68(5):711–720. doi: 10.4088/jcp.v68n0508. [DOI] [PubMed] [Google Scholar]
- 14.Hermes E, Sernyak M, Rosenheck R. The use of second generation antipsychotics for post-traumatic stress disorder in a US Veterans Health Administration Medical Center. Epidemiol Psychiatr Sci. 2014;23(3):281–288. doi: 10.1017/S2045796013000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry. 2002;159(10):1777–1779. doi: 10.1176/appi.ajp.159.10.1777. [DOI] [PubMed] [Google Scholar]
- 16.Butterfield MI, Becker ME, Connor KM, Sutherland S, Churchill LE, Davidson JR. Olanzapine in the treatment of post-traumatic stress disorder: a pilot study. Int Clin Psychopharmacol. 2001;16(4):197–203. doi: 10.1097/00004850-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Carey P, Suliman S, Ganesan K, Seedat S, Stein DJ. Olanzapine monotherapy in posttraumatic stress disorder: efficacy in a randomized, double-blind, placebo-controlled study. Hum Psychopharmacol. 2012;27(4):386–391. doi: 10.1002/hup.2238. [DOI] [PubMed] [Google Scholar]
- 18.Reich DB, Winternitz S, Hennen J, Watts T, Stanculescu C. A preliminary study of risperidone in the treatment of posttraumatic stress disorder related to childhood abuse in women. J Clin Psychiatry. 2004;65(12):1601–1606. doi: 10.4088/jcp.v65n1204. [DOI] [PubMed] [Google Scholar]
- 19.Bartzokis G, Lu PH, Turner J, Mintz J, Saunders CS. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry. 2005;57(5):474–479. doi: 10.1016/j.biopsych.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Hamner MB, Faldowski RA, Ulmer HG, Frueh BC, Huber MG, Arana GW. Adjunctive risperidone treatment in post-traumatic stress disorder: a preliminary controlled trial of effects on comorbid psychotic symptoms. Int Clin Psychopharmacol. 2003;18(1):1–8. doi: 10.1097/00004850-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Padala PR, Madison J, Monnahan M, Marcil W, Price P, Ramaswamy S, Din AU, Wilson DR, Petty F. Risperidone monotherapy for post-traumatic stress disorder related to sexual assault and domestic abuse in women. Int Clin Psychopharmacol. 2006;21(5):275–280. doi: 10.1097/00004850-200609000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Krystal JH, Rosenheck RA, Cramer JA, Vessicchio JC, Jones KM, Vertrees JE, Horney RA, Huang GD, Stock C. Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: a randomized trial. JAMA. 2011;306(5):493–502. doi: 10.1001/jama.2011.1080. [DOI] [PubMed] [Google Scholar]
- 23.Villarreal G, Hamner MB, Canive JM, Robert S, Calais LA, Durklaski V, Zhai Y, Qualls C. Efficacy of Quetiapine Monotherapy in Posttraumatic Stress Disorder: A Randomized, Placebo-Controlled Trial. Am J Psychiatry. 2016;173(12):1205–1212. doi: 10.1176/appi.ajp.2016.15070967. [DOI] [PubMed] [Google Scholar]
- 24.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 25.Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68(1):29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Munoz F, Alamo C. Active metabolites as antidepressant drugs: the role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders. Front Psychiatry. 2013;4:102. doi: 10.3389/fpsyt.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikisch G, Baumann P, Liu T, Mathe AA. Quetiapine affects neuropeptide Y and corticotropin-releasing hormone in cerebrospinal fluid from schizophrenia patients: relationship to depression and anxiety symptoms and to treatment response. Int J Neuropsychopharmacol. 2012;15(8):1051–1061. doi: 10.1017/S1461145711001556. [DOI] [PubMed] [Google Scholar]


