Abstract
Background
Depressive disorders are expected to be the second highest cause of morbidity in the world until few years. Moreover, patients with depression frequently show many side effects and low compliance to therapy. To find a more tolerated and more efficacy therapy is a growing need.
Objective
This observational study investigates the efficacy, safety and tolerability of paroxetine hydrochloride comparing slow versus standard titration in a population affected by Depressive Disoders (according to DSM 5).
Methods
186 outpatients were assessed throught the following scales: Hamilton Depression Rating Scale (HDRS) for depression and World Health Organization Quality of Life Scale Bref for the perceived quality of life (WHOQOL BREF). Treatment-emerged Adverse Events (TEAEs) were recorded throught self-reports. Statystical analysys was performed by GraphPad Prism Version 5.1.
Results
The efficacy of paroxetine was confirmed in both titrations by the number of clinical remitters (HDRS ≤ 7 at 12 weeks for 53% of the standard titration group and 58% of the slow titration group), without differences. About safety and tolerability there were more frequent TEAEs among the standard titration group (p < 0.01). Comparing WHOQOL BREF between the two groups at the recruitment and at the twelth week emerged a statistically significant difference (p = 0.003), with highest scores reached in slow titration group.
Conclusions
Although the short observation period is an evident limit, this study is consistent to the literature about the efficacy of both titrations of paroxetine to improve depression and shows promising results about the increased tolerability of paroxetine slow titration.
Keywords: antidepressants, serotonin reuptake inhibitors, drug tolerance, adverse effects, paroxetine hydrochloride
Introduction
By 2020, depressive disorders are expected to be the second highest cause of morbidity in the world.1 Considering the impact of depression on public health, it is estimated that the treatment of mood disorders in Europe costs 113.4 billion euro per year;2 effective treatments of this disorder are an important objective to reach.
In the last years, in keeping to literature, epidemiological data have shown an increase of patients with diagnosis of depressive disorders, reactive adaptation disorders with mixed anxiety and depressed mood.3 According to the guidelines for the treatment of depressive disorders associated or not with anxiety, antidepressants are the first choice,4 including selective serotonin reuptake inhibitors (SSRIs). Sometimes these therapies are associated to the onset of adverse events that used to disappear after the first 2 weeks of treatment.5 These side effects are caused by the neurotransmission deficiency due to the pharmacological action that produces an up-regulation in the post-synaptic receptor: when SSRIs cause the rapid increase of 5HT, 5HT systemic response is expanded, with the appearance of adverse events up to when the serotoninergic system is not down-regulated.6 The medication-related side effects may be associated with antidepressant discontinuation before reaching a period of exposure believed to result in effectiveness.7 It also affects the confidence in the efficacy of psychopharmacological treatment and lack of perception of the benefit before the improvement of mood.8 The main cause of interruption in the first stage of treatment, although of proven efficacy, appears to be the presence of side effects, which influence the quality of life of the patient and consequently induce him/her to interrupt the therapy.9 The formulation in drops of SSRIs is equally effective to tablets, but it may allow patients to have a higher cognition, consciousness and control on drug assumption; patients who accepted to change tablets with drops sustain that the latter formulation is simpler to administer than the former, making them sure to assume the appropriate dose.9 An advantage of the liquid formulation is that it allows adopting slow titration, reaching the effective plasmatic concentration of SSRIs in a very gradual way. This formulation will allow the clinicians to design individualized tapering off regimens to avoid withdrawal symptoms.10
In line with these considerations our study aims to observe the efficacy, safety and tolerability of paroxetine hydrochloride, comparing slower versus standard up titration in an outpatient population with a diagnosis of depressive disorders (according to DSM 5), in the routine clinical practice.
Primary efficacy assessment was performed by comparing the clinical remission, defined by a Hamilton Depression Rating Scale (HDRS) total score ≤ 7 at 12 weeks between the two titration groups. Secondary, safety and tolerability were evaluated monitoring and recording the incidence of any treatment-emergent adverse event (TEAE)11 during the study period through an unstructured global approach based on self-reports. Secondary efficacy assessment was evaluated by comparing the benefits of therapies through the rating scale World Health Organization Quality of Life Scale Bref (WHOQOL BREF) between the two groups.
Methods
Sample
This retrospective and observational study evaluated 186 patients. Data of patients corresponding to the following criteria were included: age ≥ 18 years old; have a diagnosis of depressive disorder (according to DSM 5 criteria for Depressive Disorders); be an outpatient of the “Anxiety and Depression Clinic” of the “ASST Settelaghi of Varese”, Italy; be in monotherapy with paroxetine hydrochloride slow or standard titration (drug naive or switch from another treatment); to sign a written informed consent for the use of data in an anonymous form. Data from patients presenting the following exclusion criteria were excluded: have a diagnosis of substance-related and alcohol addictive disorders.
The period of observation went from December 2014 to October 2016. In order to compare the 2 types of titration we have evaluated data dividing patients into 2 groups: the “slow titration group” (95 patients) and the “standard titration group” (91 patients), basing on the kind of titration given by clinicians.
In the “standard titration group” were included data of patients receiving a therapy from a starting dose of 10 mg/die; instead the “slow titration group” included data about patients receiving a therapy starting from a lower dose of 5 mg, increased by 5 mg on up to 10 mg on the 7th day. The maximum dose reached was 40 mg/die.
Data about the clinical of patients and about the theraphy were taken from the control done by the clinicians at the start of the therapy, after 4 weeks and after 12 weeks, as the clinicians tend to visit patients suffering of depression at the outpatient service in their routine clinical practice.
We have defined these appointments respectively as T0, T1 and T2 in order to make the interpretation of data clearer.
Scales
During each evaluation clinicians tested patients through different assessment tools. We collected data from the following scales, administered by clinicians at the starting of the therapy, 4 weeks later and 12 weeks later:
–Hamilton Depression Rating Scale (HDRS): it is a rating scale developed to quantify depression. It consists of items representing different levels of gravity: no depressive symptoms (0–7), mild symptoms (8–17), moderate symptoms (17–24), severe symptoms (> 25).12
–World Health Organization Quality of Life Scale Bref, (WHOQOL-BREF) assesses the individual’s perceptions in the context of their culture and value systems, and their personal goals, standards and concerns. The WHOQOL-BREF instrument comprises 26 items, which measure the following 4 broad domains: physical health (items 3, 4, 10, 15, 16, 17, 18), psychological health (items 5, 6, 7, 19, 26), social relationships (items 20, 21 and 22), and environment (items 8, 9, 12, 13, 14, 23, 24, 25); items 1 and 2 do not fall under any domain but are included in the calculation of the total score. Each item is given a score on a Likert scale from 1 to 5 expressing increasingly satisfaction of the subject. Exceptions are items 3, 4 and 26, where the scoring is decreasing.13
The total scores of the scales were counted as the sum of the items’ scores.
Statistical Analysis
As the number of patients amounted to 186, normal trend could be assumed. Approximating the normal distribution of the data we have seen fit to use parametric t-tests.
Fisher’S exact test was used for proportions between the two groups, including the evaluation of the primary endpoint (the number of patients with HDRS’ score ≤ 7).
All statistical tests were two-tailed, with p < 0.05 considered statistically significant. Statistical analysis was performed by GraphPad Prism Version 5.1 for Windows (GraphPad software, San Diego, CA).
Results
Sample
Data from 186 patients meeting the inclusion criteria were enrolled consecutively. Basing on the type of titration, they were assorted into 2 groups: 95 into the “slow titration group” and 91 into the “standard titration group”. During the observation period 10 patients dropped out (5.6% of all patients): 3 in the “slow titration group” and 7 in the “rapid titration group”. Among these 10 patients, 5 patients dropped out for reported edverse events (as explained in secondary tolerability results), while 5 patients did not tend all the appointments (3 of them answered to phone-call referring that they were fine; 2 did not answer to the phone call). Demographic and clinical characteristics of the sample are shown in Table 1. No significant differences in age, sex distribution or severity of depression between the two groups at T0 emerged.
Table 1. Baseline Demographics and Clinical Characteristics of the Two Titration Groups of Patients.
| SAMPLE CHARACTERISTICS | SLOW TITR. GROUP | STANDARD TITR. GROUP | P VALUE |
|---|---|---|---|
| Age, Mean (SD) | 50.23 (11.61) | 48.26 (11.20) | 0.26 |
| Sex | |||
| Females N, (%) | 56 (61%) | 31 (37%) | 1 |
| Males N, (%) | 36 (39%) | 53 (43%) | |
| HDRS total score, mean (SD) | 26.63 (6.84) | 28.54 (10.63) | 0.1 |
Abbreviations: Titr., Titration; SD, standard deviation.
Efficacy Assessment
At the 12th week patients achieving a score ≤ 7 at the HDRS, index of clinical remission, were 53% of the “standard titration group” and 58% of the “slow titration group”, without differences between the two populations (p = 0.54).
Tolerability Assessment
TEAEs emerged in both groups, more frequently in the “standard titration” one (35.7% vs 9.7%, p < 0.001). Gastrointestinal disorders and sexual disorders were the most commonly TEAEs, with a statistically significant difference between the two groups (15% vs 2%, respectively, p < 0.005 for sexual dysfunction and 15% vs 4%, p < 0.03 for nausea). Particularly, sexual dysfunction was reported as responsible of 4 dropouts while headache of 1. For the other TEAEs reported there were no statistically significant differences between the two groups of patients, as shown in Table 2.
Table 2. Treatment Emergent Adverse Events.
| TEAE | STANDARD TITRATION N (%) | SLOW TITRATION N (%) | P VALUE |
|---|---|---|---|
| Diarrhea | 5 (6%) | 1 (1%) | 0.11 |
| Nausea | 13 (15%) | 4 (4%) | 0.03 |
| Sexual disfunction | 13 (15%) | 2 (2%) | 0.005 |
| Sedation | 3 (3.5%) | 1 (1%) | 0.35 |
| Restlessness | 1 (1%) | 0 | 0.48 |
| Headache | 10 (12%) | 5 (5.4%) | 0.1 |
Secondary Efficacy Assessment
Despite the short-term treatment period evaluated “slow titration group” achieved greater scores in WHOQOL BREF at 4 and 12 weeks (p < 0.00 and p < 0.00), as shown in Table 3. These results, based on broad measurements of patient well-being, indicate a greater quality of life perceived.
Table 3. Comparison between the Scales’ Scores in the Two Groups at T1 and at T2.
| WHOQOL | SLOW TITRATION MEAN, (SD) | STANDARD TITRATION MEAN, (SD) | P VALUE |
|---|---|---|---|
| T1 | 49.12 (9.1) | 43.58 (8.8) | 0.0001 |
| T2 | 65.42 (10.42) | 57.64 (11.04) | 0.0001 |
Abbreviation: SD, standard deviation.
Discussion
The measurements performed by the HDRS scores throughout the observation period (Figures 1 and 2) and the number of remitters in the two groups confirmed the documented efficacy of paroxetine, without differences in the two types of titration, as already demostrated by Amodeo et al8 in a cancer population. Differently, in an elderly population study, the slow titration gave a larger number of remitters (84% vs 54.5%, p = 0.02) and, similarly to our study, fewer dropouts (20% vs 73”, p < 0.00).14 Also in this paper, a preponderance of dropouts among the “standard titration group” emerged, probably due to the higher incidence of treatment-emerged adverse events in this kind of titration. It can be estimated that 35.7% of the population with rapid titling presented TEAEs versus 9.7% of the “slow titration group” (p < 0.001); this data is confirmed also in the professional literature.
Figure 1.
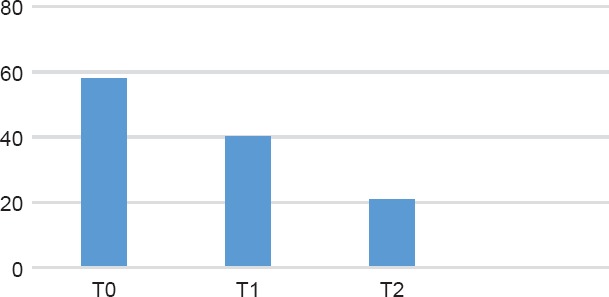
HDRS Trand Among the Standard Titration Group
Figure 2.
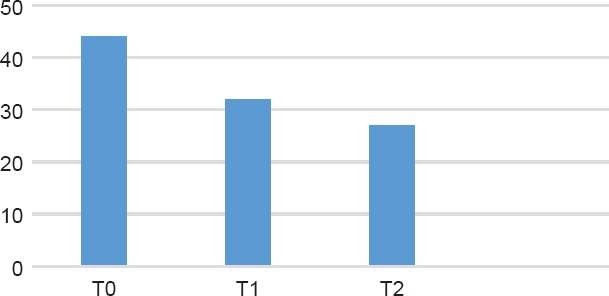
HDRS Trand Among the Slow Titration Group
Despite the extreme individuality of the drug response in terms of efficacy and tolerability,15–17 it is known that antidepressants are generally associated with a significant burden of side effects affecting treatment adherence and quality of life. Slow titration may be needed to reduce the impact of side effects and to improve effectiveness, resulting from the balance between efficacy and safety.18,19 In our population the most commonly affected system organ classes were gastrointestinal disorders and sexual disorders, with a statistically significant difference between the two groups (respectively p < 0.005 for sexual dysfunction and p < 0.03 for nausea). Particularly sexual dysfunction was reported as responsible of 4 drop out while 1 was due to headache. It is known and confirmed by the literature that many psychiatric medications, including SSRI and SNRI, can adversely affect normal sexual response,20 and that this adverse effect rapresent a problem particularly among male patients.
Depression has a negative impact on quality of life and a negative impact on social functioning.21 Despite the short-term treatment period observed “slow titration group” achieved greater scores in WHOQOL BREF at 4 and 12 weeks (respectively p < 0.00 and p < 0.00). These results, based on broad measurements of patient well-being, could be due to the fewer side effects experienced by these patients, but obviously this last consideration need a larger population and other trials to be confirmed. As for other hot topics in clinical psychopharmacology, such as the comparison of first-generation and second-generation antipsychotics and the use of antidepressants depressive disturbs, the ability to integrate more recent evidence with previous knowledge should improve the clinician’s therapeutic abilities and guide the treatment choice towards a more individualized approach.22
Conclusions
This study aimed to observe and compare two different paroxetine titrations in the treatment of depression in a “real world” outpatient population. Our results suggested that slow titration can reduce the number and severity of side effects, thus reducing the drug-related dropouts compared to standard titration (p < 0.001) in a routine clinical setting. In addition, slow titration was found to be as effective as standard titration: both titrations groups highlighted a significant improvement of depression.
In conclusion, the results of this study confirmed previous evidence on the efficacy and safety of paroxetine. Going further than previous studies, our results suggest that slow titration is better tolerated than standard paroxetine titration for the treatment of depression.
The limits of this study are the short duration of the observation and the lack of a scale about the development of adverse events. Further controlled trials are needed to confirm our evidences.
References
- 1.Stahl SM, Fava M, Trivedi MH et al. Agomelatine in the tratment of Majore Depressive Disorder: an 8 week, multicenter, randomized, Placebo-Controlled Trial. J Clin Psichiatry. 2010;71:5. doi: 10.4088/JCP.09m05471blu. [DOI] [PubMed] [Google Scholar]
- 2.Olesen J, Gustavsson A, Svensson M et al. The economic cost of brain disorders in Europe. European Journal of Neurology. Eur J Neurol. 2012;19:155–162. doi: 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- 3.Poloni N, Callegari C, Buzzi A et al. The Italian version of ISOS and RSQ, two suitable scales for investigating recovery style from psychosis. 2010;19:352–359. [PubMed] [Google Scholar]
- 4.APA. The American Journal of Psychiatry. Third. American Psychiatric Publishing, Inc; 2010. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. [Google Scholar]
- 5.James M, Ferguson M.D. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim Care Companion. J Clin Psychiatry. 2001;3:22–27. doi: 10.4088/pcc.v03n0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson HD, Pace WD, Libby AM et al. Rates of 5 common antidepressant side effects among new adult and adolescent cases of depression: a retrospective US claims study. Clin Ther. 2012;34:113–123. doi: 10.1016/j.clinthera.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Rosholm J, Andersen M, Gram L. Are there differences in the use of selective serotonin reuptake inhibitors and tricyclic antidepressants? A prescription database study. Eur J Clin Pharmacol. 2001;56:923–929. doi: 10.1007/s002280000234. [DOI] [PubMed] [Google Scholar]
- 8.Amodeo L, Castelli L, Leombruni P et al. Slow versus standard up-titration of paroxetine for the treatment of depression in cancer patients: a pilot study. International Review of Psychiatry. 2012;20:375–384. doi: 10.1007/s00520-011-1118-8. [DOI] [PubMed] [Google Scholar]
- 9.Zanardi R, Colombo L, Marcheggiani E et al. Paroxetine drops versus paroxetine tablets: evaluation of compliance in a six-month study. Riv Psichiatr. 2013;48:261–267. doi: 10.1708/1292.14294. [DOI] [PubMed] [Google Scholar]
- 10.Van den Tweel ERW, Relleke M, Muniz Piniella P. Paroxetine oral solution is bioequivalent to paroxetine tablets: advantages of the solution. Int J Clin Pharmacol Ther. 2007;45:611–6. doi: 10.5414/cpp45611. [DOI] [PubMed] [Google Scholar]
- 11.Callegari C, Ielmini M, Bianchi L et al. Antiepileptic drug use in a nursing home setting: a retrospective study in older adults. Funct Neurol. 2016;31:87–93. doi: 10.11138/FNeur/2016.31.2.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller I, Bishop S, Norman W et al. The modified Hamilton rating scale for depression: Reliability and validity. Psychiatry Research. 1985;4:23–28. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 13.De Girolamo, Becchi - Coppa - De Leo - Neri Whoqol e salute e qualita’della vita. AAVV; Centro Scientifico Editore. 2001 [Google Scholar]
- 14.Olgiati P, Bajo E, Serretti A et al. C.E.A.P Cost-Effectiveness Analysis on Psychiatric Disorders Group. Benefit of slow titration of paroxetine to treat depression in the elderly. Hum Psychopharmacol. 2014;29:544–51. doi: 10.1002/hup.2433. [DOI] [PubMed] [Google Scholar]
- 15.Bolla E, Bortolaso P, Ferrari M et al. Are CYP1A2*1F and *1C associated with clozapine tolerability? a preliminary investigation. Psychiatry Res. 2011;189(3):483. doi: 10.1016/j.psychres.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Ielmini M, Poloni N, Caselli I et al. The utility of pharmacogenetic testing to support the treatment of bipolar disorder. Pharmacogenomics and Personalized Medicine 2018. doi: 10.2147/PGPM.S160967. Article in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ielmini M, Poloni N, Caselli I, Diurni M, Grecchi A, Callegari C. The role of pharmacogenetic testing in the treatment of bipolar disorder: preliminary results. Minerva Psichiatr. 2018;59(1):10–5. [Google Scholar]
- 18.Zanardi R, Colombo L, Marcheggiani E et al. Paroxetine drops versus paroxetine tablets: evaluation of compliance in a six-month study. Riv Psichiatr. 2013;48:261–7. doi: 10.1708/1292.14294. [DOI] [PubMed] [Google Scholar]
- 19.Olgiati P, Serretti A et al. Persistent benefits of slow titration of paroxetine in a six-monyh follow-up. Human psychopharmacology. 2015;10:2472–78. doi: 10.1002/hup.2478. [DOI] [PubMed] [Google Scholar]
- 20.Portman DJ, Kaunitz AM, Kazempour K et al. Effects of low dose paroxetine 7.5 mg on weight and sexual function during treatment of vasomotor symptoms associated with menopause. Menopause. 2014;21:1082–90. doi: 10.1097/GME.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iren Akbyyik D, Berkusun OE, Sumbuloglu V et al. Quality of life of Turkish patients with depression in Ankara and in Berlin. EurPsychiatry. 2008;23:41–49. doi: 10.1016/S0924-9338(08)70055-5. [DOI] [PubMed] [Google Scholar]
- 22.Dell’Osso B, Albert U, Atti AR et al. Bridging the gap between education and appropriate use of benzodiazepines in psychiatric clinical practice. Neuropsychiatr Dis Treat. 2015;30(11):1885–909. doi: 10.2147/NDT.S83130. [DOI] [PMC free article] [PubMed] [Google Scholar]


