Abstract
Progressive non-familial adult onset cerebellar degeneration has been rarely associated with hypothyroidism and is known to be reversible after therapy. We report a case of cerebellar atrophy in a 31 year old female whose detailed evaluation had revealed sub-clinical hypothyroidism secondary to autoimmune thyroiditis with a very high anti-TPO (anti-thyroid peroxidase) antibody levels. MRI (Magnetic Resonanace Imaging) of brain showed diffuse bilateral cerebellar atrophy. She was treated with thyroid hormone supplementation and after one year of follow up, cerebellar signs had disappeared completely with significant reduction in anti-TPO antibody levels. Imaging of the brain post one year of follow-up revealed normal cerebellum. Hence, we opine that thyroid dysfunction should always be kept in mind while evaluating patients presenting with acute onset cerebellar ataxia as it can be easily reversed with thyroid hormone replacement therapy.
Keywords: Cerebellar atrophy, sub-clinical hypothyroidism, reversible, anti-TPO antibodies, autoimmune thyroiditis
To The Editor,
An extensive scrutiny of the medical literature revealed that the development of cerebellar degeneration secondary to Hashimoto’s thyroiditis is a rarity.1,2 It was interesting to note from these reports that an elementary therapeutic measure in the form of thyroxine supplementation can go a long way in ensuring partial or in some instances a complete resolution of symptoms. Till date, the paucity of literature in this domain has only further compounded the ignorance with respect to the scientific community’s understanding of the implicated underlying mechanisms here. Hence, it is our endeavour here to bring to notice one such case where following an intelligent evaluation, the patient underwent a complete recovery using thyroxine. We have further attempted to understand the disease pathogenesis in our patient in light of the various existent theories.
A 31 year old lady presented to the internal medicine outpatient department of our hospital with an acute onset, progressive dysarthria, gait disturbance, vertigo and postural tremors, which can be dated back to three weeks. There were no complaints of associated sensory, cognitive or additional motor deficits. The patient did not suffer from any other co-morbidities. There were no instances of similar complaints among her family members.
A detailed systemic evaluation did not reveal any other anomalies barring positive cerebellar signs of dysarthria, dysdiadochokinesia, dysmetria, ataxia and intentional tremors. Her cognitive and sensory evaluation was observed to be normal. Ophthalmological evaluation did not throw up any anomalies as well. Finally, at the conclusion of our history taking and systemic evaluation, our patient appeared to suffer from only cerebellar symptoms without any other anomalies. Neither the patient’s personal history nor the familial history threw up any hints as to the cause for this constellation of symptoms.
In view of the myriad possibilities for the underlying cause, a battery of laboratory investigations were carried out so as to pinpoint the causative element for the patient’s symptoms. Normal blood cell counts ruled out any infections. Liver and renal functions were observed to be within normal limits, hence precluding out any toxicological reasons for the cerebellar manifestations. Serum vitamin B12 and ceruloplasmin evaluation was carried out to rule out nutritional causes. Anti-nuclear antibody was found to be negative. However, it was the thyroid function test that helped us finally crack the diagnosis. With serum T3 at 1.12 ng/mL (normal range – 0.6–1.8 ng/mL), T4 at 8.4 mcg/mL (normal range – 4.0–11.0 mcg/mL) and thyroid stimulating hormone (TSH) levels at 8.35 microunits/mL (0.4–4.0 microunits/mL), it was suspected that thyroid dysfunction manifesting as sub-clinical hypothyroidism could be a possible reason for cerebellar signs and symptoms.
Further investigations revealed remarkably high anti-thyroid peroxidase (anti-TPO) antibodies – 3092 (<35 IU/mL), indicating an active autoimmune thyroid disorder. Fine needle aspiration cytology (FNAC) of thyroid showed evidences of lymphocytic thyroiditis. Magnetic resonance imaging (MRI) of brain revealed features of diffuse bilateral cerebellar atrophy (Figure 1). No evidence of cerebellar haemorrhage and ischemia was noted.
Figure 1.
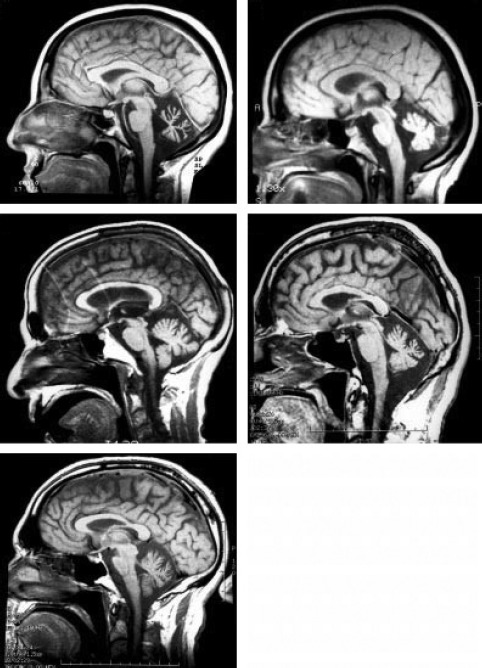
Images of MRI of Brain Indicating Diffuse Cerebellar Atrophy Obtained During the Initial Admission
Additionally, the possibility of spino-cerebellar ataxia was considered. However, in view of financial constraints, genetic evaluation for SCA was not performed. Moreover, the course of disease in our patient did not concur with the commonly observed slowly progressing, prolonged course of disease seen with SCA. Thus, keeping in mind the laboratory results, imaging findings and patient’s clinical presentation, a diagnosis of Hashimoto’s thyroiditis induced sub-clinical hypothyroidism leading to cerebellar degeneration was arrived upon.
In the backdrop of the diagnosis and the sub-clinical hypothyroid state of the patient, thyroxine replacement therapy at a dose of 25 mcg was initiated. The patient was regularly followed up for a period of one year, during which her symptoms resolved completely and the anti-TPO antibody titres declined to 80.12 IU/mL. The latest MRI scan revealed a normal picture of the brain.
Hashimoto’ thyroiditis, an auto-immune disorder of thyroid gland, featuring the presence of auto-antibodies against thyroid peroxidase, TSH receptors and thyroglobulin, has been implicated as the causative element for cerebellar degeneration in a few instances.1,2 However, the underlying mechanism is still shrouded under a lot of controversy. One line of thought suggests that the resulting hypothyroidism due to Hashimoto’s thyroiditis may reduce the cardiac output, producing decreased cerebral blood flow and decline in oxygen and glucose utilization by the cerebellar neurons.3 If one abides by this theory, a mere restoration of the euthyroid status by hormone supplementation could help reverse cerebellar signs and symptoms, which again seems to be the case in our patient.
However, in a second class of patients with similar symptomatology, who have not responded to thyroid hormone supplementation, it has been hypothesized that Hashimoto’s thyroiditis may just be a ‘marker’ for the underlying generalized autoimmune disease in the patient and that cerebellar manifestations may be produced either due to a separate auto-antibody, i.e. anti-Purkinje cell antibody, glutamate acid decarboxylase (GAD) or due to cross reactivity between thyroid and cerebellar antigens.4
Additionally, another theory to come to light is the autoimmune mediated calcium channelopathy that might result in cerebellar damage besides Hashimoto’s thyroiditis.5
Hence, in conclusion, we believe that though cerebellar manifestations are a rarity with Hashimoto’s thyroiditis, the possibility should always be kept in mind while treating patients with similar symptoms as a simple therapeutic measure of thyroxine supplementation could help mitigate the further progress of the disease, if initiated in time.
Further, sub-clinical hypothyroidism in patients with similar symptomatology should not be neglected and treated at the earliest. Besides thyroid hormone supplementation, a trial of other treatment modalities like plasmapheresis, intravenous immunoglobulin (IVIg) can be attempted in patients with a high suspicion index of autoimmune disorders.
Acknowledgments
Nil.
Footnotes
Source of Funding
This report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
None declared.
References
- 1.Pavan MR, Deepak M, Basavaprabhu A, Gupta A. Doctor I Am Swaying: An Interesting Case of Ataxia. J Clin Diagn Res. 2012;6(4):702–703. [Google Scholar]
- 2.Shneyder NV, Lyons MK, Driver-Dunckley E, Evidente VGH. Cerebellar ataxia from multiple potential causes: hypothyroidism, Hashimoto’s thyroiditis, thalamic stimulation, and essential tremor, Tremor Other Hyperkinet Mov. 2012;2 doi: 10.7916/D8BP01H5. tre-02-44-309-2 http://dx.doi.org/10.7916/D8BP01H5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka M, Kawarabayashi T, Okamoto K, Morimatsu M, Hirai S. Reduction of cerebral blood flow and metabolic rate of oxygen in a case of hypothyroidism presenting with cerebellar ataxia. Rinsho Shinkeigaku. 1987;27:1262–1265. [PubMed] [Google Scholar]
- 4.Chiu A, Sherman S. Clinical manifestations and differential diagnosis of hypothyroidism. In: Falk SA, editor. Thyroid disease: endocrinology, surgery, nuclear medicine, and Radiotherapy. Philadelphia: Lippincott-Raven; 1997. pp. 379–391. [Google Scholar]
- 5.Zuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the á 1A-voltage-dependent calcium channel. Nature Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]


