Abstract
Importance
Duration of untreated psychosis (DUP) has been associated with poor outcomes in schizophrenia, but the mechanism responsible for this association is not known.
Objectives
To determine whether hippocampal volume loss occurs during the initial 8 weeks of antipsychotic treatment and whether it is associated with DUP, and to examine molecular biomarkers in association with hippocampal volume loss and DUP.
Design, Setting, and Participants
A naturalistic longitudinal study with matched healthy controls was conducted at Shanghai Mental Health Center. Between March 5, 2013, and October 8, 2014, 71 medication-naive individuals with nonaffective first-episode psychosis (FEP) and 73 age- and sex-matched healthy controls were recruited. After approximately 8 weeks, 31 participants with FEP and 32 controls were reassessed.
Exposures
The participants with FEP were treated according to standard clinical practice with second-generation antipsychotics.
Main Outcomes and Measures
Hippocampal volumetric integrity (HVI) (an automated estimate of the parenchymal fraction in a standardized hippocampal volume of interest), DUP, 13 peripheral molecular biomarkers, and 14 single-nucleotide polymorphisms from 12 candidate genes were determined.
Results
The full sample consisted of 71 individuals with FEP (39 women and 32 men; mean [SD] age, 25.2 [7.7] years) and 73 healthy controls (40 women and 33 men; mean [SD] age, 23.9 [6.4] years). Baseline median left HVI was lower in the FEP group (n = 57) compared with the controls (n = 54) (0.9275 vs 0.9512; difference in point estimate, −0.020 [95% CI, −0.029 to −0.010]; P = .001). During approximately 8 weeks of antipsychotic treatment, left HVI decreased in 24 participants with FEP at a median annualized rate of −.03791 (–4.1% annualized change from baseline) compared with an increase of 0.00115 (0.13% annualized change from baseline) in 31 controls (difference in point estimate, −0.0424 [95% CI, −0.0707 to −0.0164]; P = .001). The change in left HVI was inversely associated with DUP (r = −0.61; P = .002). Similar results were found for right HVI, although the association between change in right HVI and DUP did not achieve statistical significance (r = −0.35; P = .10). Exploratory analyses restricted to the left HVI revealed an association between left HVI and markers of inflammation, oxidative stress, brain-derived neurotrophic factor, glial injury, and markers reflecting dopaminergic and glutamatergic transmission.
Conclusions and Relevance
An association between longer DUP and accelerated hippocampal atrophy during initial treatment suggests that psychosis may have persistent, possibly deleterious, effects on brain structure. Additional studies are needed to replicate these exploratory findings of molecular mechanisms by which untreated psychosis may affect hippocampal volume and to determine whether these effects account for the known association between longer DUP and poor outcome.
This longitudinal case-control study examines whether hippocampal volume loss occurs during the initial 8 weeks of antipsychotic treatment and whether it is associated with duration of untreated psychosis, and examines molecular biomarkers in association with hippocampal volume loss and duration of untreated psychosis.
Key Points
Question
Does hippocampal volume loss occur during initial antipsychotic treatment and is it associated with duration of untreated psychosis?
Findings
In this longitudinal case-control study of individuals with first-episode nonaffective psychosis before and after initiation of antipsychotic medication, patients had significantly greater hippocampal atrophy compared with healthy controls at baseline. Moreover, at 8-week follow-up, hippocampal atrophy increased to a greater extent in patients compared with healthy controls, and the rate of progression of left hippocampal atrophy was significantly correlated with the duration of untreated psychosis.
Meaning
Early hippocampal volume loss may play a role in mediating the association between duration of untreated psychosis and poor outcomes in schizophrenia.
Introduction
Hippocampal volume reduction is prominent in schizophrenia, correlates with poor outcome, and has been linked to early progression of illness. A long duration of untreated psychosis (DUP) is also associated with poor outcome, but a biological basis for this association has not been identified, to our knowledge. Several factors, including elevated cortisol levels, inflammation, oxidative stress, decreased neurotrophic factors, and glutamatergic excitotoxic effects, have been linked to early psychosis and could mediate an association between DUP and hippocampal volume loss, but evidence from longitudinal studies is lacking. Whereas the negative association of DUP with clinical course is attenuated by the initiation of antipsychotic treatment, the evidence is mixed as to whether antipsychotics contribute to loss of brain volume or protect against it. Hence, the degree to which loss of brain volume early in treatment reflects an illness effect, a drug effect, or both is not known.
To address the question of whether hippocampal volume reduction occurs early in treatment and is associated with DUP, we measured hippocampal volumetric integrity (HVI) (inversely associated with hippocampal atrophy) before and after initial antipsychotic treatment in medication-naive patients with first-episode psychosis (FEP) and in matched healthy controls. We hypothesized that HVI would be reduced at baseline in participants with FEP relative to controls, would decrease at a faster rate, and would be correlated with DUP. As an exploratory approach, we also measured peripheral biomarkers that reflect factors hypothesized to affect hippocampal volume in schizophrenia and examined whether these biomarkers interact with DUP to predict HVI.
Methods
Study Design and Setting
Between March 5, 2013, and October 8, 2014, individuals with nonaffective FEP were recruited from the Shanghai Mental Health Center early psychosis program. Healthy controls group-matched by sex, age, and educational level were recruited by advertisement. All participants were Mandarin-speaking Han Chinese individuals living in the Shanghai metropolitan area. Eligible participants with FEP met the criteria for schizophrenia or schizophreniform disorder based on the Structured Clinical Interview for DSM-IV-TR, were medication naive, were experiencing a first episode of psychosis, and did not meet the criteria for any other Axis I disorder. Healthy controls were assessed using the Structured Clinical Interview for DSM-IV-TR (nonpatient version) to exclude any Axis I disorder and were also psychotropic medication naive. All participants were between the ages of 16 and 40 years; were right-handed; completed at least 9 years of school; were free of substance abuse, suicidal ideation, and unstable medical illness; and had no contraindications to magnetic resonance imaging. After baseline assessments, participants with FEP were treated by their clinicians with second-generation antipsychotics according to standard clinical practice (eAppendix 1 in the Supplement) and were invited to return for clinical assessment and magnetic resonance imaging after 8 weeks. Matched healthy controls were also invited to return at 8 weeks for a follow-up evaluation. Participants with FEP who were outpatients were contacted weekly by telephone to reinforce medication adherence; baseline and follow-up visits were scheduled as closely as possible to 8 weeks apart. The study protocol was approved by the Shanghai Mental Health Center institutional review board and the New York University Medical Center institutional review board, and all participants provided written informed consent.
Study Measures
The primary outcome was annualized change in HVI. Because the left and right hippocampi are asymmetrical in volume and may differ in vulnerability to disease, we examined both left HVI (LHVI) and right HVI (RHVI). Participants with FEP and controls underwent imaging at Shanghai Mental Health Center at baseline and after approximately 8 weeks on a 3.0-T Siemens Verio magnetic resonance imaging scanner (Siemens) with a 32-channel head coil (eAppendix 2 in the Supplement). Hippocampal volumetric integrity, defined as the parenchymal fraction of a standardized volume of interest that is expected to encompass the hippocampus in a healthy brain, was calculated using a fully automated procedure in which the volume of interest is estimated for each hemisphere using automatically detected landmarks and the tissue fraction is estimated by histogram analysis (eAppendix 3 in the Supplement). This procedure has demonstrated excellent test-retest reliability (intraclass correlation coefficient, 0.998) and superior performance compared with FreeSurfer, version 5.3.0, in differentiating individuals with Alzheimer disease from healthy age- and sex-matched controls on the basis of hippocampal volume change. To compare approaches, measurement of hippocampal volume was also performed using the longitudinal stream of FreeSurfer, version 6. All participants received a full psychiatric and medical screening by a research psychiatrist (B.Z.) (eAppendix 4 in the Supplement), including calculation of DUP based on history provided by the participant and family members. Duration of untreated psychosis was defined as the number of weeks elapsed since the onset of at least 1 persistent psychotic symptom of moderate or greater severity. Participants with FEP also completed the Brief Psychiatric Rating Scale (BPRS) and Scale for Assessment of Negative Symptoms administered by a single postdoctoral rater at baseline and 8 weeks. All participants completed the MATRICS Consensus Cognitive Battery at baseline and 8 weeks.
Molecular Biomarkers
Fasting blood samples and 3 saliva samples (collected in the morning, at noon, and in the evening) were obtained at baseline from the participants with FEP and controls and at week 8 from the participants with FEP (eAppendices 5-8 in the Supplement). Plasma biomarkers were selected to measure inflammation (C-reactive protein, interleukin 1B, interleukin 8 [IL-8], tumor necrosis factor, and interferon γ [IFN-γ] values), oxidative stress (thioredoxin and S100 calcium binding protein B [S100B] values), excitotoxicity (glutamate, aspartate, and homocysteine concentrations), brain-derived neurotrophic factor (BDNF) levels, stress (salivary cortisol levels), and mitochondrial injury (lactate values). Genotyping included 14 single-nucleotide polymorphisms associated with these pathways (eAppendix 9 in the Supplement).
Statistical Analysis
Statistical analyses were performed with SPSS software, version 23.0 (IBM Corp) and JMP Pro, version 12.2.0 (SAS Institute). All data were tested for normality of distribution using the Kolmogorov-Smirnov test. Duration of untreated psychosis, HVI, and serum biomarkers were not normally distributed. To examine between-group median differences in HVI, we used a Mann-Whitney test. To explore potential associations between DUP and biomarkers, we used Spearman rank order correlations. Normally distributed data were evaluated using the Pearson correlation coefficient, independent samples t tests, and χ2 tests for independence. Because 4 participants received medication briefly before biomarker collection, a sensitivity analysis was performed by excluding participants who were not medication free at the time of assessment. Because the interval between imaging differed between individuals, the change from baseline in HVI was converted to an annualized rate of change.
Two primary analyses were performed: comparison of HVI between groups at baseline and comparison of the annualized rate of change in HVI from baseline to follow-up between groups. Secondary analyses examined correlations between DUP and baseline HVI and between DUP and change in HVI. Confirmatory analyses were performed to examine the association of laterality with baseline HVI and change in HVI (eAppendix 10 in the Supplement). Additional exploratory analyses to assess associations between clinical measures, biomarkers, and HVI were performed without correction for multiple comparisons. These analyses were restricted to the LHVI to minimize type I error. Given the large number of predictors, and to avoid model overfitting, we applied least absolute shrinkage and selection operator (LASSO) regression (eAppendix 11 in the Supplement) and limited biomarkers for multivariate modeling to those that were associated with baseline LHVI or with change in LHVI at P < .10. Least absolute shrinkage and selection operator regression is a modified form of least-squares regression that shrinks noninfluential coefficients to zero, excluding them from the final model. This approach was used owing to its robustness under conditions of nonnormality and large variable to sample size ratios, which allow for the examination of multiple interactions while minimizing the risk of type I error. For the 2 primary analyses, P < .03 (2-tailed) was considered significant.
Results
Enrollment and Retention of Participants
A total of 71 patients with FEP (19 inpatients and 52 outpatients) and 73 matched healthy controls met the inclusion criteria and were enrolled in the study. Participants with FEP and their parents reported fewer years of education, and participants with FEP had lower rates of employment and marriage and lower MATRICS Consensus Cognitive Battery composite scores compared with controls (Table 1). A total of 31 participants with FEP and 32 controls completed week 8 assessments. Dropout rates were 32% for inpatients with FEP (6 of 19) and 65% for outpatients with FEP (34 of 52); 18 participants with FEP refused the offer for a second assessment, 15 outpatients were unable to travel back to Shanghai Mental Health Center for follow-up assessment, and 7 were unavailable for follow-up. Individuals with FEP who completed the study had higher BPRS total scores and BPRS agitation subscale scores at baseline compared with individuals with FEP who did not complete the study (eTable 1 in the Supplement). At baseline, 57 participants with FEP and 54 controls had imaging results that met quality standards (eTable 2 in the Supplement); 24 participants with FEP and 32 controls had imaging results at both baseline and follow-up that met quality standards (eTables 2 and 3 in the Supplement). An image analysis expert (B.A.A.) blinded to group status performed quality control for HVI analysis by visual inspection for motion and other artifacts (eAppendix 12 in the Supplement).
Table 1. Baseline Characteristics of Study Participants.
| Characteristic | Total No. | Participants With FEPa | Total No. | Healthy Controlsa | Test Statistic | P Value |
|---|---|---|---|---|---|---|
| Age, mean (SD), y | 71 | 25.2 (7.7) | 73 | 23.9 (6.4) | t = 1.13 | .26 |
| Women, No. (%) | 71 | 39 (55) | 73 | 40 (55) | χ21 = .012 | .52 |
| Educational level, mean (SD), y | 71 | 12.0 (3.1) | 73 | 12.9 (2.3) | t = −1.95 | .05 |
| Parental educational level, mean (SD), y | 71 | 8.0 (5.8) | 73 | 10.0 (4.1) | t = −2.36 | .02 |
| Married, No. (%) | 71 | 17 (24) | 73 | 19 (26) | χ21 = .059 | .48 |
| Employed, No. (%) | 71 | 15 (21) | 73 | 34 (47) | χ22 = 11.61 | .003 |
| Tobacco use, No. (%) | 71 | 2 (3) | 73 | 0 | χ22 = 3.30 | .19 |
| DUP, mean (SD), wk | 61 | 25.4 (21.6) | NA | NA | NA | NA |
| BPRS total score, mean (SD) | 70 | 48.01 (10.97) | NA | NA | NA | NA |
| BPRS agitation subscale score, mean (SD) | 70 | 9.54 (4.24) | NA | NA | NA | NA |
| BPRS positive subscale score, mean (SD) | 70 | 19.11 (4.80) | NA | NA | NA | NA |
| BPRS negative subscale score, mean (SD) | 70 | 5.81 (3.27) | NA | NA | NA | NA |
| SANS composite score, mean (SD) | 70 | 20.74 (17.95) | NA | NA | NA | NA |
| MCCB composite score, mean (SD) | 69 | 35.43 (13.69) | 66 | 45.09 (9.43) | t = 4.79 | .001 |
| CRP, median (IQR), FI | 69 | 7631.41 (6778.62-9456.46) | 61 | 7610.07 (6937.90-9025.92) | U = 2065 | .85 |
| IL-1B, median (IQR), FI | 69 | 68.82 (35.35-166.58) | 61 | 76.77 (44.41-225.11) | U = 1902 | .34 |
| IL-8, median (IQR), FI | 69 | 71.30 (49.79-83.26) | 61 | 63.41 (50.77-82.46) | U = 2034 | .74 |
| IFN-γ, median (IQR), FI | 69 | 89.42 (70.87-113.86) | 61 | 88.96 (64.69-131.47) | U = 2054 | .81 |
| TNF, median (IQR), FI | 69 | 93.60 (76.02-105.44) | 61 | 92.03 (74.28-103.94) | U = 1984 | .57 |
| Salivary cortisol, median (IQR), μg/dL | 46 | 0.24 (0.15-0.33) | 54 | 0.18 (0.12-0.27) | U = 910 | .02 |
| Aspartate, median (IQR), nmol/mL | 69 | 134.32 (86.67-198.31) | 61 | 102.32 (79.33-141.65) | U = 1666 | .04 |
| Glutamate, median (IQR), nmol/mL | 69 | 111.00 (98.20-128.00) | 61 | 105.00 (92.00-121.60) | U = 1845 | .23 |
| Lactate, median (IQR), mg/dL | 69 | 15.08 (11.27-20.34) | 60 | 13.28 (9.83-17.75) | U = 1770 | .16 |
| HCY, median (IQR), mg/L | 69 | 0.65 (0.61-0.71) | 61 | 0.65 (0.60-0.73) | U = 2091 | .95 |
| BDNF, median (IQR), FI | 69 | 340.83 (142.52-340.83) | 62 | 287.37 (145.99-722.44) | U = 2009 | .55 |
| Thioredoxin, median (IQR), pg/mL | 69 | 420.17 (323.58-609.50) | 61 | 492.33 (324.17-637.67) | U = 1956 | .49 |
| S100B, median (IQR), FI | 69 | 125.13 (69.76-211.93) | 61 | 130.44 (61.14-265.52) | U = 2044 | .79 |
Abbreviations: BDNF, brain-derived neurotrophic factor; BPRS, Brief Psychiatric Rating Scale; CRP, C-reactive protein; DUP, duration of untreated psychosis; FEP, first episode of psychosis; FI, fluorescence intensity; HCY, homocysteine; IL, interleukin; IFN-γ, interferon γ; IQR, interquartile range; MCCB, MATRICS Consensus Cognitive Battery; NA, not applicable; SANS, Scale for Assessment of Negative Symptoms; S100B, S100 calcium binding protein B; TNF, tumor necrosis factor.
SI conversion factor: To convert lactate to millimoles per liter, multiply by 0.111.
Comparisons between participants with FEP and healthy controls were adjusted for parental educational level.
HVI and DUP
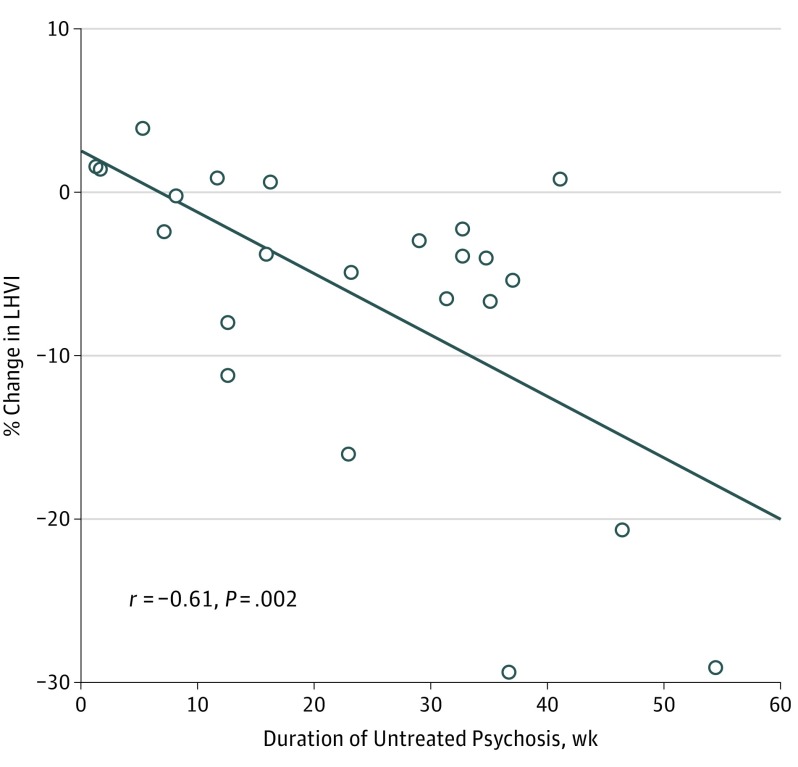
At baseline, 57 participants with FEP had lower median LHVI (0.9275 [interquartile range (IQR), 0.8256-0.9689] vs 0.9512 [IQR, 0.8501-0.9825]; P = .001) and lower RHVI (0.9237 [IQR, 0.8525-0.9661] vs 0.9412 [IQR, 0.8681-0.9690]; P = .001) compared with 54 controls (Table 2; eFigures 1 and 2 in the Supplement). The change in LHVI and RHVI from baseline to follow-up in the controls did not differ significantly from zero. In the participants with FEP, LHVI decreased at a median annualized rate of −4.1%, and RHVI decreased at a median annualized rate of −3.3%, which differed significantly from the control group (P = .001; eFigures 3-6 in the Supplement). At baseline, neither LHVI (r = 0.04; P = .80) nor RHVI (r = 0.07; P = .64) was significantly correlated with DUP; however, DUP was significantly correlated with the reduction from baseline to follow-up in LHVI (r = −0.61; P = .002) (Figure 1), but not with the change in RHVI (r = −0.35; P = .10). The effect of laterality was not significant for any HVI finding in participants with FEP, but baseline RHVI was larger than LHVI in controls (eAppendix 10 in the Supplement). Left and right hippocampal volume as measured by the longitudinal stream of FreeSurfer, version 6, did not differ between groups at baseline, nor did change in hippocampal volume at follow-up (eTable 4 in the Supplement).
Table 2. Comparisons Between Groups: Baseline, Week 8, and Change in Hippocampal Volumetric Integrity.
| Characteristic | Total No. | Participants With FEP, Median (IQR) | Total No. | Healthy Controls, Median (IQR) | Test Statistic | P Value | Effect Size (r)a |
|---|---|---|---|---|---|---|---|
| Baseline LHVI | 57 | 0.9275 (0.8256 to 0.9689) | 54 | 0.9512 (0.8501 to 0.9825) | 841.00 | .001 | .391 |
| Baseline RHVI | 57 | 0.9237 (0.8525 to 0.9661) | 54 | 0.9412 (0.8681 to 0.9690) | 967.00 | .001 | .320 |
| Baseline LHVI (completers) | 24 | 0.9323 (0.8343 to 0.9789) | 31 | 0.9633 (0.9138 to 0.9876) | 157.00 | .001 | .481 |
| Baseline RHVI (completers) | 24 | 0.9277 (0.8623 to 0.9678) | 31 | 0.9537 (0.8795 to 0.9722) | 445.00 | .001 | .476 |
| Week 8 LHVI | 24 | 0.9297 (0.8083 to 0.9763) | 32 | 0.9636 (0.9149 to 0.9908) | 111.00 | .001 | .654 |
| Week 8 RHVI | 24 | 0.9239 (0.8575 to 0.9597) | 32 | 0.9548 (0.8795 to 0.9722) | 88.50 | .001 | .604 |
| Median annualized change in LHVI | 24 | −0.03791 (−0.0758 to 0.0049) | 31 | 0.001151 (−0.0165 to 0.0207) | 179.00 | .001 | .441 |
| Median annualized change in RHVI | 24 | −0.03027 (−0.0817 to 0.00004) | 31 | 0.004535 (−0.0151 to 0.0350) | 184.00 | .001 | .430 |
Abbreviations: FEP, first episode of psychosis; LHVI, left hippocampal volumetric integrity; RHVI, right hippocampal volumetric integrity.
Effect sizes were calculated using the z score statistic from each Mann-Whitney test comparison of median values, divided by the square root of the sample size.
Figure 1. Duration of Untreated Psychosis (DUP) and Annualized Percentage Change in Left Hippocampal Volumetric Integrity (LHVI) From Baseline to Follow-up in Participants With First-Episode Psychosis.
The solid line represents the association between ranked values for LHVI and DUP. Spearman rank correlation coefficient is shown.
Peripheral Biomarkers
At baseline, median plasma aspartate and salivary cortisol concentrations were elevated in participants with FEP compared with controls; no other biomarker differed significantly between groups (Table 1). Results did not differ when analysis was restricted to participants with FEP who were medication free at the time that blood samples were obtained (eTable 5 in the Supplement). Plasma aspartate and homocysteine concentrations increased at follow-up in participants with FEP, although the elevation in aspartate concentrations was not significant when the analysis was restricted to participants with FEP who were medication free at baseline (eTable 6 in the Supplement) and no difference between groups in biomarkers at baseline or after treatment would survive correction for multiple comparisons. Change in homocysteine concentrations was associated with change in S100B (r = 0.52; P = .004), IFN-γ (r = 0.54; P = .002), IL-8 (r = 0.47; P = .009), and tumor necrosis factor (r = 0.41; P = .03) concentrations but was not significantly associated with change in LHVI.
Antipsychotic Effects
After a mean duration of 62 days of antipsychotic treatment, individuals with FEP who completed the study showed significant improvement on the BPRS total score, the BPRS positive subscale score, the BPRS agitation subscale score, and the MATRICS Consensus Cognitive Battery composite score (eTable 7 in the Supplement). Mean daily antipsychotic dose was not significantly associated with DUP or with baseline HVI or change in HVI (eTable 8 in the Supplement).
Associations Between LHVI, Biomarkers, and Clinical Variables
In participants with FEP, baseline LHVI was significantly correlated with RHVI (r = 0.72; P < .001), with the BPRS total score (r = −0.33; P = .014), and with the BPRS agitation subscale score (r = −0.31; P = .021) (eTable 9 in the Supplement). The change in LHVI was inversely associated with the BPRS agitation subscale score (r = −0.45; P = .03) and with change in the BPRS negative symptoms subscale score (r = −0.41; P = .05), such that greater baseline agitation and less response of negative symptoms were associated with greater reduction in LHVI (eTable 10 in the Supplement). However, correlations between clinical variables and LHVI would not survive correction for multiple comparisons.
Significant results of the LASSO regression models are summarized in Table 3; complete results can be found in eTables 11 and 12 in the Supplement, and correlational results from which factors for the LASSO regression were selected can be found in eTables 13 and 14 in the Supplement. In the first model, significant main effects associated with baseline LHVI included INF-γ, IL-8, BDNF GG genotype, and nitric oxide synthase 1 (NOS1) CC genotype. Significant interactions with DUP associated with baseline LHVI were found for INF-γ, IL-8, NOS1 CC genotype, BDNF AA genotype, zinc finger protein 804A (ZNF804A) GG genotype, and catechol-O-methyltransferase (COMT) GA genotype. In the second model, a significant main effect associated with the change in LHVI was found for S100B; interactions between DUP and S100B, thioredoxin, and NOS1 CC genotype were also associated with change in LHVI.
Table 3. Significant Results From LASSO Multivariate Association Model of Baseline and Change in LHVI.
| Baseline LHVI Factorsa | Parameter Estimate | SE | 95% CI | P Value |
|---|---|---|---|---|
| IL-8 | −0.000273 | 0.0001146 | −0.00049 to 4.816E−5 | .02 |
| IFN-γ | 0.0003771 | 0.0001557 | 7.1923E−5 to 0.0006822 | .02 |
| NOS1 (CC) | 0.0295961 | 0.0093529 | 0.0112649 to 0.0479274 | .002 |
| BDNF (GG) | 0.0235826 | 0.0091729 | 0.005604 to 0.0415612 | .01 |
| IFN-γ × DUP | 1.3282E-5 | 6.3559E-6 | 8.2421E−7 to 2.574E−5 | .04 |
| IL-8 × DUP | 7.2689E-5 | 0.0000127 | 4.7779E−5 to 0.0000976 | .001 |
| NOS1 (CC) × DUP | 0.0021139 | 0.0005374 | 0.0010606 to 0.0031673 | .001 |
| BDNF (AA) × DUP | 0.002997 | 0.0009478 | −0.004855 to 0.001139 | .002 |
| ZNF804A (GG) × DUP | 0.0012497 | 0.0002708 | 0.000719 to 0.0017803 | .001 |
| COMT (GA) × DUP | −0.00142 | 0.000502 | −0.002404 to 0.000437 | .005 |
| Change in LHVI factors | ||||
| S100B | −0.016025 | 0.0016714 | −0.019301 to −0.012749 | .001 |
| S100B × DUP | −0.000801 | 0.000159 | −0.001112 to −0.000489 | .001 |
| Thioredoxin × DUP | −0.000159 | 2.7019E-5 | −0.000212 to −0.000106 | .001 |
| NOS1 (CC) × DUP | −0.241284 | 0.0811758 | −0.400386 to −0.082182 | .003 |
Abbreviations: BDNF, brain-derived neurotrophic factor; COMT, catechol-O-methyltransferase; DUP, duration of untreated psychosis; IFN-γ, interferon γ; IL-8, interleukin 8; LASSO, least absolute shrinkage and selection operator; LHVI, left hippocampal volumetric integrity; NOS1, nitric oxide synthase 1; S100B, S100 calcium binding protein B; ZNF804A, zinc finger protein 804A.
Parenthetic capital letters CC, GG, AA, and GA refer to genotypes.
Discussion
Left hippocampal volumetric integrity and RHVI were reduced at baseline in participants with FEP compared with controls and decreased further during 8 weeks of treatment; this reduction in LHVI during treatment was correlated with DUP. Our exploratory analysis identified several biomarkers and genetic markers that interacted with DUP and were associated with baseline LVHI and with the change in LHVI during treatment. However, none of the plasma biomarkers that were associated with LHVI differed between patients with FEP and controls at baseline or significantly changed with treatment, so the factors that we identified as possible mediators of the effect of untreated psychosis on hippocampal volume are not clearly associated with illness or treatment.
Hippocampal Volumetric Integrity
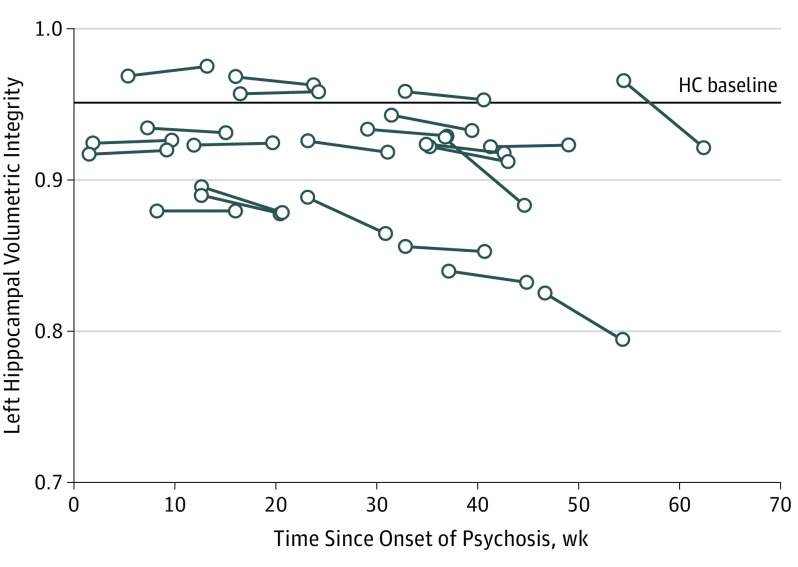
Previous studies have not found consistent evidence of progressive hippocampal volume loss after onset of illness. We found a median annualized rate of LHVI reduction of 4.1%, whereas the mean annualized reduction was 6.2%, reflecting the effect of 2 outliers with annualized LHVI reductions of 20.6% and 29.1% (Figure 2; eFigure 3 in the Supplement). Although the annualized rate of change in LHVI was used as a standardized unit of measure, we do not know if this rate of change would remain constant beyond the 8-week interval measured in this study. Our detection of significant change with our automated measure of HVI and not with hippocampal volume measured by the longitudinal stream of FreeSurfer, version 6, reflects the high reliability and sensitivity of the HVI method for detecting pathologic change, as previously demonstrated in Alzheimer disease. We confirmed the superior test-retest reliability of HVI in a publicly available set of repeated scans of healthy individuals; the total coefficient of variation for HVI was 0.43% vs 1.03% using the longitudinal stream of FreeSurfer, version 6 (eAppendix 13 in the Supplement). In addition, HVI is an estimate of relative (percentage) hippocampal atrophy rather than of absolute hippocampal volume and, hence, is less influenced by interindividual variability in brain volume. For example, in the current data set, HVI was markedly less correlated with intracranial volume (r = −0.19; P = .05) than was hippocampal volume measured by FreeSurfer, version 6 (r = 0.69; P = 1.0 × 10−16) (eAppendix 14 in the Supplement).
Figure 2. Change From Baseline to Follow-up in Left Hippocampal Volumetric Integrity (LHVI) by Time Since Onset of Psychosis in Participants With First-Episode Psychosis (FEP).
The solid lines indicate individual participants with FEP; HC indicates the median LHVI value for healthy controls. Hippocampal volumetric integrity, defined as the parenchymal fraction of a standardized volume of interest that is expected to encompass the hippocampus in a healthy brain, was calculated using a fully automated procedure in which the volume of interest is estimated for each hemisphere using automatically detected landmarks and the tissue fraction is estimated by histogram analysis.
DUP and HVI
We found that the annualized rate of LHVI reduction increased by 0.4% per week of DUP across the full range of DUP values (Figure 1). Previous studies have largely failed to identify morphologic correlates of DUP, although in a cross-sectional study, Guo and colleagues found an inverse correlation between DUP and hippocampal volume. Duration of untreated psychosis was not independently associated with baseline HVI in our sample, but DUP interacted with BDNF, NOS1, COMT, and ZNF804A genotypes and with 2 inflammatory serum biomarkers in association with baseline LHVI.
HVI and Clinical Measures
At baseline, higher scores on the BPRS agitation subscale were associated with greater hippocampal atrophy (eTable 9 in the Supplement) and with greater reduction of LHVI during treatment (eTable 10 in the Supplement). Although reduced hippocampal volume may have contributed to agitation, it is also possible that stress, expressed behaviorally as agitation, contributed to hippocampal volume loss. A rapid decrease in LHVI was also associated with less response of negative symptoms, suggesting a potential clinical consequence of hippocampal volume change early in treatment. However, these findings must be considered exploratory because they would not survive correction for multiple comparisons.
Peripheral Biomarkers
At baseline, we did not find differences in measures of inflammation and oxidative stress between patients and healthy controls, contrary to some other studies. We did find elevation of salivary cortisol concentrations in individuals with FEP, but unlike the finding by Mondelli and colleagues, salivary cortisol concentration was not associated with hippocampal volume, nor were genes involved in cortisol regulation (FKBP5 and NR3C2; eTables 15 and 16 in the Supplement). In addition, aspartate concentrations were elevated at baseline among individuals with FEP. Aspartate is an excitatory neurotransmitter that acts primarily at extrasynaptic N-methyl-d-aspartate receptors that are associated with neurotoxicity; however, neither baseline aspartate concentrations nor the increase in aspartate concentrations with treatment were correlated with LHVI.
Homocysteine
Antipsychotic treatment was associated with an elevation of plasma homocysteine concentrations. Homocysteine is a neurotoxic amino acid that has been associated with hippocampal volume loss in healthy elderly individuals and with hippocampal injury and sensitization to oxidative stress and excitotoxic effects in animal models. Homocysteine is a by-product of dopamine metabolism by COMT, which is localized primarily in astrocytes. Although the elevation of homocysteine during treatment was not correlated with LHVI change, homocysteine concentrations were correlated with a marker for astrocytic injury (S100B) and with inflammatory factors (IFN-γ and IL-8) that, in turn, were correlated with LHVI.
Biomarkers Associated With Baseline LHVI
The interaction of DUP with several biomarkers was significantly associated with baseline LHVI, suggesting that they may play a role in the negative association of psychosis with hippocampal volume. These biomarkers included COMT genotype and ZNF804A genotype; ZNF804A regulates expression of COMT and of dopamine D2 receptors in the hippocampus. The interaction of DUP with other biomarkers, including the inflammatory biomarkers IL-8 and IFN-γ, the growth factor BDNF genotype, and the NOS1 genotype, was associated with baseline LHVI. Neuronal nitric oxide synthase is coupled to the N-methyl-d-aspartate receptor and regulates the balance between neuroplasticity, neurotoxicity, and pruning by regulating inhibitory and excitatory transmitter release as well as the generation of nitrosyl free radicals. Brain-derived neurotrophic factor directly influences hippocampal neurogenesis and supports dendritic arborization and plasticity. Thus, the association between cumulative exposure to psychosis (DUP) and hippocampal volume at baseline was mediated by biomarkers associated with dopamine and glutamate transmission, inflammation, oxidative stress, and BDNF genotype, consistent with current models of schizophrenia.
Factors Associated With Change in LHVI
The reduction in LHVI during treatment was associated with baseline concentrations of S100B and thioredoxin. S100 calcium binding protein B is a small calcium-binding molecule released by glia in response to inflammation or oxidative stress. Thioredoxin is an antioxidant molecule released by astrocytes and neurons in response to oxidative stress. One potential source of oxidative stress in psychosis is excessive release of dopamine, which produces free radicals when metabolized by COMT. Antipsychotics may initially increase dopamine levels in the hippocampus by antagonism of presynaptic autoreceptors in the CA1 subfield. The increased hippocampal dopamine release associated with antipsychotic treatment may be time limited, however, since increased antipsychotic-induced midbrain dopamine neuron firing is attenuated after several weeks of sustained exposure.
Limitations
Our sample of participants with FEP was homogenous with respect to race/ethnicity and the absence of substance abuse, so these results may not generalize to more heterogeneous populations. Furthermore, 56% of participants with FEP did not complete follow-up at week 8 —this may have introduced a bias toward hospitalized patients, as indicated by the higher ratings of symptom severity in those who completed the study. It is unclear to what degree peripheral biomarkers inform us about the biochemical environment of the hippocampus because the origin of these biomarkers is not limited to the central nervous system and access to the peripheral circulation is limited by the blood-brain barrier. Finally, in the absence of a placebo control, we are unable to determine from these data whether the change in HVI was the result of illness progression or a medication effect, or both. Although our results from peripheral biomarkers provide promising directions for further research on mechanisms contributing to hippocampal volume loss, these results must be considered exploratory, and the considerable heterogeneity among the participants with FEP suggests that much larger samples are needed.
Conclusions
We found significantly lower HVI at baseline in participants with FEP compared with healthy controls and additional HVI reduction during antipsychotic treatment that correlated with DUP, consistent with a persistent, possibly deleterious, effect of untreated psychosis on brain structure. Our exploratory analysis identified molecular mechanisms by which DUP may affect hippocampal volume. Larger longitudinal studies of longer duration are needed to examine the association between DUP, hippocampal volume, and clinical outcomes.
eAppendix 1. Antipsychotic medication
eAppendix 2. Imaging
eAppendix 3. Computation of hippocampal volumetric integrity
eAppendix 4. Medical and psychiatric assessments
eAppendix 5. Sample collection and preparation
eAppendix 6. Blood biomarker assays
eAppendix 7. KASP (Kompetitive Allele Specific PCR) genotyping assays
eAppendix 8. Salivary cortisol
eAppendix 9. SNPs and associated pathways
eAppendix 10. Tests of effects of hemispheric laterality on HVI findings
eAppendix 11. Lasso regression
eAppendix 12. Excluded subjects
eAppendix 13. Test-retest reliability comparison between HVI and FreeSurfer v.6
eAppendix 14. Correlations between intra-cranial volume and FreeSurfer v.6 hippocampal volumes and HVI
eTable 1. Comparison of baseline measures between FEP completers and non-completers
eTable 2. Baseline characteristics of study participants with baseline imaging that met quality standards
eTable 3. Comparison of FEP and HC participants who completed follow up imaging
eTable 4. Comparison between FEP participants and HC in baseline hippocampal volume and change from baseline of hippocampal volume calculated by the longitudinal stream of FreeSurfer v. 6
eTable 5. Comparison of peripheral biomarkers at baseline between healthy controls and FEP patients who were medication free
eTable 6. Change in peripheral biomarkers with analysis restricted to FEP participants who were medication free at baseline
eTable 7. Change from baseline to week 8 in FEP participants
eTable 8. Spearman correlations between antipsychotic dose, DUP and HVI in FEP participants
eTable 9. Spearman correlations between LHVI and clinical variables at baseline
eTable 10. Spearman correlations between change in LHVI and clinical assessments
eTable 11. Lasso regression – baseline LHVI
eTable 12. Lasso regression – change in LHVI
eTable 13. Spearman correlations between change in LHVI and baseline biomarkers
eTable 14. Spearman correlations between change in LHVI and change in biomarkers
eTable 15. Comparison of LHVI between FEP participants grouped by genotype
eTable 16. Comparison of percent change in LHVI between FEP participants grouped by genotype
eFigure 1. Histogram of LHVI baseline for FEP participants and HC
eFigure 2. Histogram of RHVI baseline for FEP participants and HC
eFigure 3. Histogram of LHVI annualized change rate for FEP participants and HC
eFigure 4. Histogram of RHVI annualized change rate for FEP participants and HC
eFigure 5. Line plots of LHVI Change from Baseline to Follow Up in FEP Participants and HC
eFigure 6. Line plots of RHVI Change from Baseline to Follow Up in FEP Participants and HC
References
- 1.Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:529-553. [DOI] [PubMed] [Google Scholar]
- 2.van Erp TG, Hibar DP, Rasmussen JM, et al. . Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium [published correction appears in Mol Psychiatry. 2016;21(4):585]. Mol Psychiatry. 2016;21(4):547-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho NF, Iglesias JE, Sum MY, et al. . Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol Psychiatry. 2017;22(1):142-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anvari AA, Friedman LA, Greenstein D, Gochman P, Gogtay N, Rapoport JL. Hippocampal volume change relates to clinical outcome in childhood-onset schizophrenia. Psychol Med. 2015;45(12):2667-2674. [DOI] [PubMed] [Google Scholar]
- 5.Lappin JM, Morgan C, Chalavi S, et al. . Bilateral hippocampal increase following first-episode psychosis is associated with good clinical, functional and cognitive outcomes. Psychol Med. 2014;44(6):1279-1291. [DOI] [PubMed] [Google Scholar]
- 6.Schobel SA, Chaudhury NH, Khan UA, et al. . Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70(12):1294-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penttilä M, Jääskeläinen E, Hirvonen N, Isohanni M, Miettunen J. Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2014;205(2):88-94. [DOI] [PubMed] [Google Scholar]
- 9.Rund BR. Does active psychosis cause neurobiological pathology? a critical review of the neurotoxicity hypothesis. Psychol Med. 2014;44(8):1577-1590. [DOI] [PubMed] [Google Scholar]
- 10.Goff DC, Romero K, Paul J, Mercedes Perez-Rodriguez M, Crandall D, Potkin SG. Biomarkers for drug development in early psychosis: current issues and promising directions. Eur Neuropsychopharmacol. 2016;26(6):923-937. [DOI] [PubMed] [Google Scholar]
- 11.Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goff DC, Falkai P, Fleischhacker WW, et al. . The long-term effects of antipsychotic medication on clinical course in schizophrenia. Am J Psychiatry. 2017;174(9):840-849. [DOI] [PubMed] [Google Scholar]
- 13.Ardekani BA, Bermudez E, Mubeen AM, Bachman AH; Alzheimer’s Disease Neuroimaging Initiative . Prediction of incipient Alzheimer’s disease dementia in patients with mild cognitive impairment. J Alzheimers Dis. 2017;55(1):269-281. [DOI] [PubMed] [Google Scholar]
- 14.Ardekani BA, Convit A, Bachman AH. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. J Alzheimers Dis. 2016;50(3):847-857. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 16.Woolard AA, Heckers S. Anatomical and functional correlates of human hippocampal volume asymmetry. Psychiatry Res. 2012;201(1):48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada N, Fukunaga M, Yamashita F, et al. . Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry. 2016;21(10):1460-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Gaby M, Shipton OA, Paulsen O. Synaptic plasticity and memory: new insights from hippocampal left-right asymmetries. Neuroscientist. 2015;21(5):490-502. [DOI] [PubMed] [Google Scholar]
- 19.Ho BC, Alicata D, Mola C, Andreasen NC. Hippocampus volume and treatment delays in first-episode schizophrenia. Am J Psychiatry. 2005;162(8):1527-1529. [DOI] [PubMed] [Google Scholar]
- 20.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS). Psychol Rep. 1962;10(3):799-812. [Google Scholar]
- 21.Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1981. [Google Scholar]
- 22.Nuechterlein KH, Green MF, Kern RS, et al. . The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203-213. [DOI] [PubMed] [Google Scholar]
- 23.Mondelli V, Cattaneo A, Murri MB, et al. . Stress and inflammation reduce brain-derived neurotrophic factor expression in first-episode psychosis: a pathway to smaller hippocampal volume. J Clin Psychiatry. 2011;72(12):1677-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Bueno B, Bioque M, Mac-Dowell KS, et al. . Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull. 2014;40(2):376-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang XY, Chen DC, Xiu MH, et al. . The novel oxidative stress marker thioredoxin is increased in first-episode schizophrenic patients. Schizophr Res. 2009;113(2-3):151-157. [DOI] [PubMed] [Google Scholar]
- 26.Aleksovska K, Leoncini E, Bonassi S, Cesario A, Boccia S, Frustaci A. Systematic review and meta-analysis of circulating S100B blood levels in schizophrenia. PLoS One. 2014;9(9):e106342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruman II, Mouton PR, Emokpae R Jr, Cutler RG, Mattson MP. Folate deficiency inhibits proliferation of adult hippocampal progenitors. Neuroreport. 2005;16(10):1055-1059. [DOI] [PubMed] [Google Scholar]
- 28.Grima G, Benz B, Parpura V, Cuénod M, Do KQ. Dopamine-induced oxidative stress in neurons with glutathione deficit: implication for schizophrenia. Schizophr Res. 2003;62(3):213-224. [DOI] [PubMed] [Google Scholar]
- 29.Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59(9):812-815. [DOI] [PubMed] [Google Scholar]
- 30.Rizos E, Papathanasiou MA, Michalopoulou PG, et al. . A longitudinal study of alterations of hippocampal volumes and serum BDNF levels in association to atypical antipsychotics in a sample of first-episode patients with schizophrenia. PLoS One. 2014;9(2):e87997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regenold WT, Phatak P, Marano CM, Sassan A, Conley RR, Kling MA. Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: implications for the mitochondrial dysfunction hypothesis. Biol Psychiatry. 2009;65(6):489-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol. 1996;58(1):267-288. [Google Scholar]
- 33.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning. 2nd ed New York, NY: Springer-Verlag; 2009. [Google Scholar]
- 34.Kubota M, van Haren NE, Haijma SV, et al. . Association of IQ changes and progressive brain changes in patients with schizophrenia. JAMA Psychiatry. 2015;72(8):803-812. [DOI] [PubMed] [Google Scholar]
- 35.Ho BC, Alicata D, Ward J, et al. . Untreated initial psychosis: relation to cognitive deficits and brain morphology in first-episode schizophrenia. Am J Psychiatry. 2003;160(1):142-148. [DOI] [PubMed] [Google Scholar]
- 36.Guo X, Li J, Wei Q, et al. . Duration of untreated psychosis is associated with temporal and occipitotemporal gray matter volume decrease in treatment naïve schizophrenia. PLoS One. 2013;8(12):e83679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(suppl):E38-E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradford SE, Nadler JV. Aspartate release from rat hippocampal synaptosomes. Neuroscience. 2004;128(4):751-765. [DOI] [PubMed] [Google Scholar]
- 40.Hooshmand B, Mangialasche F, Kalpouzos G, et al. . Association of vitamin B12, folate, and sulfur amino acids with brain magnetic resonance imaging measures in older adults: a longitudinal population-based study. JAMA Psychiatry. 2016;73(6):606-613. [DOI] [PubMed] [Google Scholar]
- 41.den Heijer T, Vermeer SE, Clarke R, et al. . Homocysteine and brain atrophy on MRI of non-demented elderly. Brain. 2003;126(pt 1):170-175. [DOI] [PubMed] [Google Scholar]
- 42.Kruman II, Culmsee C, Chan SL, et al. . Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20(18):6920-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang G, Dragan M, Freeman D, Wilson JX. Activation of catechol-O-methyltransferase in astrocytes stimulates homocysteine synthesis and export to neurons. Glia. 2005;51(1):47-55. [DOI] [PubMed] [Google Scholar]
- 44.Zesiewicz TA, Wecker L, Sullivan KL, Merlin LR, Hauser RA. The controversy concerning plasma homocysteine in Parkinson disease patients treated with levodopa alone or with entacapone: effects of vitamin status. Clin Neuropharmacol. 2006;29(3):106-111. [DOI] [PubMed] [Google Scholar]
- 45.Hess JL, Quinn TP, Akbarian S, Glatt SJ. Bioinformatic analyses and conceptual synthesis of evidence linking ZNF804A to risk for schizophrenia and bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2015;168B(1):14-35. [DOI] [PubMed] [Google Scholar]
- 46.Hardingham N, Dachtler J, Fox K. The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front Cell Neurosci. 2013;7:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cocchi E, Drago A, Serretti A. Hippocampal pruning as a new theory of schizophrenia etiopathogenesis. Mol Neurobiol. 2016;53(3):2065-2081. [DOI] [PubMed] [Google Scholar]
- 48.Chen HJ, Spiers JG, Sernia C, Lavidis NA. Acute restraint stress induces specific changes in nitric oxide production and inflammatory markers in the rat hippocampus and striatum. Free Radic Biol Med. 2016;90:219-229. [DOI] [PubMed] [Google Scholar]
- 49.Magariños AM, Li CJ, Gal Toth J, et al. . Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21(3):253-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steiner J, Bielau H, Bernstein HG, Bogerts B, Wunderlich MT. Increased cerebrospinal fluid and serum levels of S100B in first-onset schizophrenia are not related to a degenerative release of glial fibrillar acidic protein, myelin basic protein and neurone-specific enolase from glia or neurones. J Neurol Neurosurg Psychiatry. 2006;77(11):1284-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rocchetti J, Isingrini E, Dal Bo G, et al. . Presynaptic D2 dopamine receptors control long-term depression expression and memory processes in the temporal hippocampus. Biol Psychiatry. 2015;77(6):513-525. [DOI] [PubMed] [Google Scholar]
- 52.Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983;3(8):1607-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Antipsychotic medication
eAppendix 2. Imaging
eAppendix 3. Computation of hippocampal volumetric integrity
eAppendix 4. Medical and psychiatric assessments
eAppendix 5. Sample collection and preparation
eAppendix 6. Blood biomarker assays
eAppendix 7. KASP (Kompetitive Allele Specific PCR) genotyping assays
eAppendix 8. Salivary cortisol
eAppendix 9. SNPs and associated pathways
eAppendix 10. Tests of effects of hemispheric laterality on HVI findings
eAppendix 11. Lasso regression
eAppendix 12. Excluded subjects
eAppendix 13. Test-retest reliability comparison between HVI and FreeSurfer v.6
eAppendix 14. Correlations between intra-cranial volume and FreeSurfer v.6 hippocampal volumes and HVI
eTable 1. Comparison of baseline measures between FEP completers and non-completers
eTable 2. Baseline characteristics of study participants with baseline imaging that met quality standards
eTable 3. Comparison of FEP and HC participants who completed follow up imaging
eTable 4. Comparison between FEP participants and HC in baseline hippocampal volume and change from baseline of hippocampal volume calculated by the longitudinal stream of FreeSurfer v. 6
eTable 5. Comparison of peripheral biomarkers at baseline between healthy controls and FEP patients who were medication free
eTable 6. Change in peripheral biomarkers with analysis restricted to FEP participants who were medication free at baseline
eTable 7. Change from baseline to week 8 in FEP participants
eTable 8. Spearman correlations between antipsychotic dose, DUP and HVI in FEP participants
eTable 9. Spearman correlations between LHVI and clinical variables at baseline
eTable 10. Spearman correlations between change in LHVI and clinical assessments
eTable 11. Lasso regression – baseline LHVI
eTable 12. Lasso regression – change in LHVI
eTable 13. Spearman correlations between change in LHVI and baseline biomarkers
eTable 14. Spearman correlations between change in LHVI and change in biomarkers
eTable 15. Comparison of LHVI between FEP participants grouped by genotype
eTable 16. Comparison of percent change in LHVI between FEP participants grouped by genotype
eFigure 1. Histogram of LHVI baseline for FEP participants and HC
eFigure 2. Histogram of RHVI baseline for FEP participants and HC
eFigure 3. Histogram of LHVI annualized change rate for FEP participants and HC
eFigure 4. Histogram of RHVI annualized change rate for FEP participants and HC
eFigure 5. Line plots of LHVI Change from Baseline to Follow Up in FEP Participants and HC
eFigure 6. Line plots of RHVI Change from Baseline to Follow Up in FEP Participants and HC